Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview
Abstract
1. Introduction
| Genetic Factors | Environmental Factors | Existential Factors |
|---|---|---|
| Histocompatibility genes (HLA class I and II) | Iodine | Sex |
| Immunoregulatory genes (SNPs in HLA, CTLA-4, PTPN22, CD40 genes) | Medications (e.g., interferon-α, lithium, amiodarone) | Associated diseases (e.g., type 1 diabetes mellitus, pernicious anaemia, coeliac disease, myasthenia gravis) |
| Thyroid-specific genes | Infections (e.g., hepatitis C virus) | Age |
| Genes associated with thyroid peroxidase antibody synthesis | Smoking | Pregnancy |
| Selenium | Down’s syndrome | |
| Vitamin D | Microbiome composition | |
| Alcohol | Familial aggregation | |
| Radiation Exposure |
2. Methods
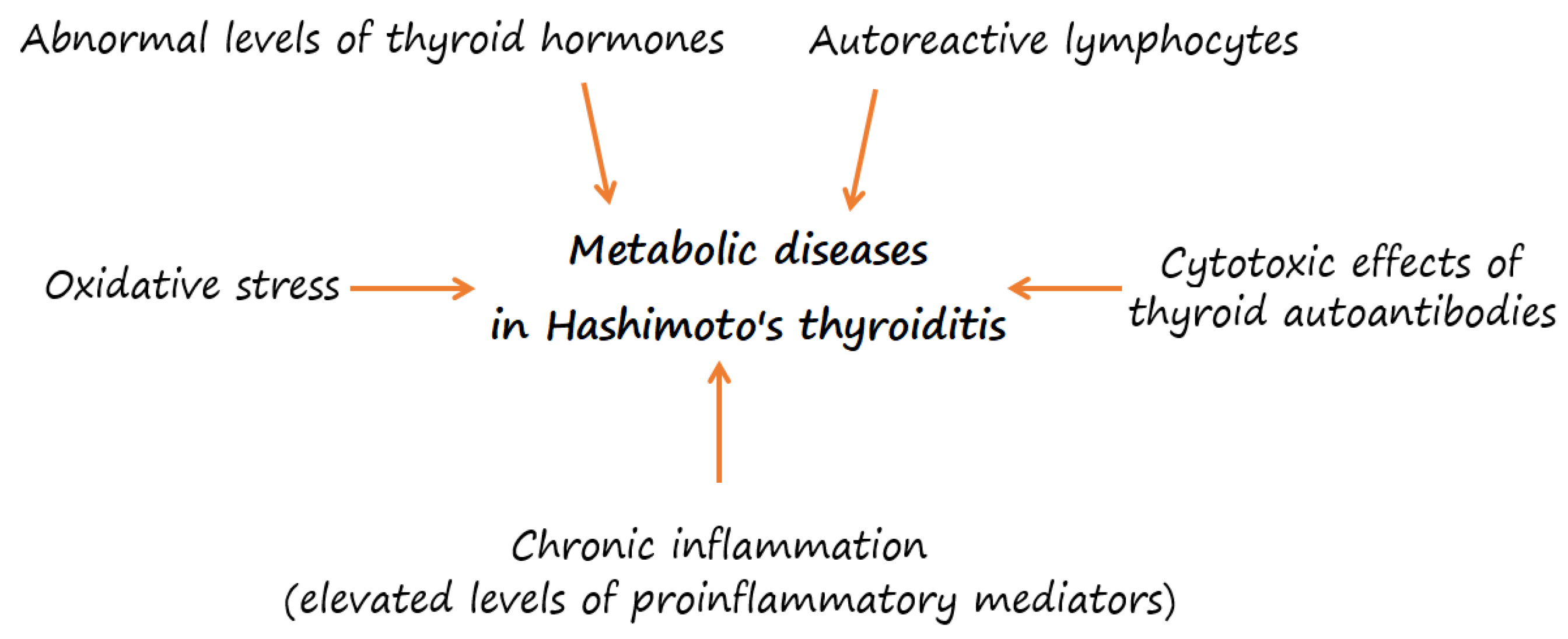
3. Overweight or Obesity and Their Association with Metabolic Risk in HT
4. Oxidative Stress in HT
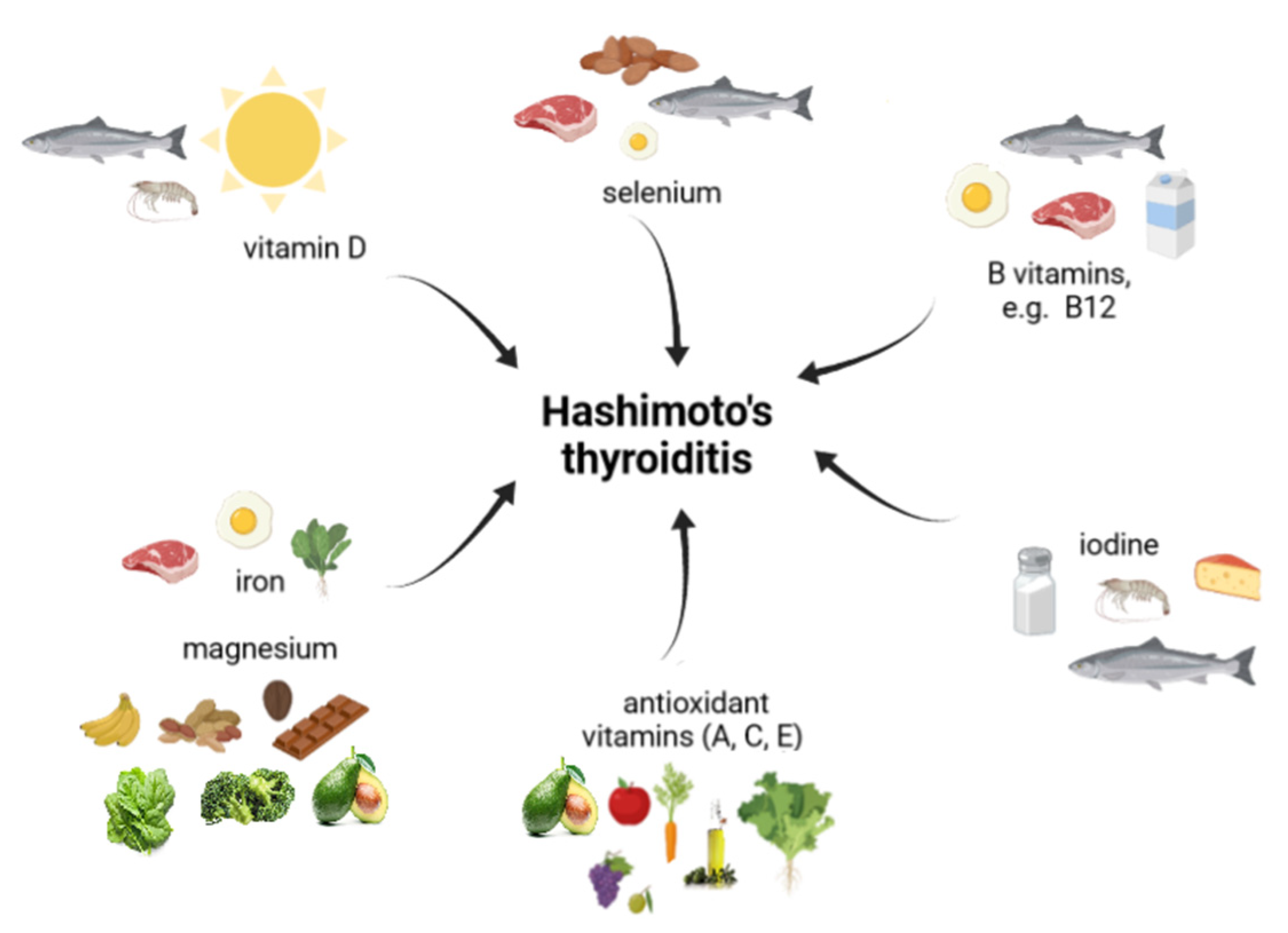
5. Micronutrients in HT
5.1. Iodine
5.2. Selenium
5.3. Iron and Magnesium
5.4. Vitamin D
| Study | Study Population | Dose/Form/Supplementation Time | Evaluated Diagnostic Parameters | Key Results | Ref. |
|---|---|---|---|---|---|
| Clinical trial | 218 euthyroid HT patients | 186 HT patients with 25(OH)D < 30 ng/mL received 1200–4000 IU vitamin D daily for four months | Anthropometric characteristics, SBP, DBP, serum concentrations of vitamin D, TSH, fT4, calcium, phosphorus, titers of TPOAb and TgAb | - significant negative correlation between 25(OH)D and TPOAb levels among all HT patients; - TPOAb levels were significantly higher in vitamin D deficient HT patients compared to no vitamin D deficiency; - supplementation of vitamin D in vitamin D deficient HT patients caused a significant decrease (20.3%) in serum TPOAb levels; - after supplementation BMI, serum TgAb and TSH levels decreased not significant. | [27] |
| 75 patients with HT and 43 healthy individuals | Vitamin D deficient patients (<20 ng/mL): 50,000 IU of 25(OH)D3 weekly for eight weeks | Serum levels of vitamin D, TSH, HDL cholesterol and thyroid autoantibodies titers | - patients with HT had significantly lower vitamin D level compared to controls; - titers of thyroid autoantibodies significantly decreased after vitamin D supplementation in euthyroid HT patients; - after supplementation HDL cholesterol level improved in the euthyroid HT group. | [119] | |
| Prospective study | 82 patients with AIT and vitamin D deficiency (<20 ng/mL) | Study group (n = 46): patients treated with 1000 IU/day vitamin D for one month; Control group (n = 36): patients without vitamin D therapy | Serum levels of vitamin D, TSH, fT4, titers of TPOAb and TgAb | - TPOAb and TgAb levels were significantly decreased in the study group, this evaluated parameters did not significantly change in the control group; - thyroid function tests did not significantly change in two groups. | [120] |
| Randomized controlled trial | 100 newly diagnosed AIT patients | Study group (n = 50): 60,000 IU 25(OH)D weekly and 500 mg/day calcium for eight weeks; Control group (n = 50): 500 mg/day calcium for eight weeks | Serum levels of vitamin D and titers of TPOAb | - 74% of HT patients were vitamin D deficient; - significant decrease of TPOAb level in the study group compared to the control group. | [121] |
| Retrospective study | 198 euthyroid subjects with vitamin D deficiency (<30 ng/mL) | Study group (n = 95): patients with AIT; Control group (n = 103): subjects without AIT. The groups were also divided into subgroups depending on the dose and period of supplementation | Serum levels of vitamin D and TSH | - in the study group TSH level significantly decreased after 100,000 IU vitamin D monthly; - no significant changes in TSH level in the control group, regardless of treatment dose and duration; - 25(OH)D level significantly improved with all monthly doses and especially in the group receiving 100,000 IU/month. | [122] |
| Clinical Trial | 34 euthyroid or mild subclinical hypothyroid HT women with 25(OH)D levels >30 ng/mL, treated ≥6 months with LT4 | Study group (n = 18): women with LT4 and 2000 IU vitamin D daily for six months; Control group (n = 16): women with LT4 treatment | Serum levels of vitamin D, TSH, fT4, fT3, titers of TPOAb and TgAb | - in the study group 25(OH)D levels increased, TPOAb titers reduced and tended to reduce TgAb; - vitamin D supplementation did not affect serum levels of TSH, fT3 and fT4; - 25(OH)D level inversely correlated with titers of thyroid antibodies. | [123] |
| Randomized clinical trial | 42 women with HT | Study group: 50,000 IU vitamin D weekly for three months; Control group: placebo for three months | Serum levels of vitamin D, Ca2+ ion, T4, T3, TSH titers of TPOAb and TgAb | - significant decrease of TgAb and TSH levels in the study group; - no significant reduction of TPOAb level in the study group compared to controls; - no significant changes in the serum levels of T3 and T4 levels in both groups. | [125] |
| Double-blind, randomized, placebo-controlled clinical trial | 56 patients with HT and vitamin D deficiency (≤20 ng/mL) | Study group (n = 30): 50,000 IU vitamin D weekly for 12 weeks; Control group (n = 26): placebo for 12 weeks | Serum levels of vitamin D, TSH, calcium, parathormone, creatinine and TPOAb titers | - vitamin D level increased in the study group; - TPOAb and TSH levels did not significantly change in both groups; - significant decrease for PTH level in study group. | [126] |
| Double-blind, randomized, placebo-controlled clinical trial | 65 vitamin D deficient euthyroid or hypothyroid patients with positive TPOAb | Study group (n = 33): 50,000 IU vitamin D3 weekly for 12 weeks; Control group (n = 32): placebo for 12 weeks | Serum levels of calcium, hsCRP, insulin, albumin, phosphorus, TG, TC and HDL cholesterol, IFG, glycated hemoglobin (HbA1c), blood urea nitrogen, creatinine | - levels of vitamin D increased significantly in study group; - HbA1c and insulin levels was increased significantly in both groups; - other variables did not change a significantly after trial. | [127] |
| A Randomized Open-label Trial | 23 patients with HT | Weekly supplementation of 60,000 IU vitamin D for eight weeks followed by once a month for four months | Serum levels of vitamin D, TSH, fT4, and TPOAb titers | - serum vitamin D level was increased significantly after trial (87% patients had normal levels); - significant increase in the TPOAb and fT4 levels and significant reduction of TSH level after six months of therapy. | [128] |
| Comparative Study | 47 euthyroid women with HT and low vitamin D status | Study group (n = 23): 200 μg selenomethionine daily for at least 12 months before the study and 4000 IU vitamin D daily for six months Control group (n = 24): 4000 IU vitamin D daily for six months | Serum levels of TSH, fT4, fT3, vitamin D, titers of TPOAb and TgAb | - in both groups, 25(OH)D levels were increased, TPOAb and TgAb titers were reduced; - the effects on antibody titers were more pronounced in women receiving vitamin D and selenomethionine; | [129] |
5.5. Vitamin B12
6. Diet in HT
6.1. Different Eating Patterns in HT
| Characteristics of the Diet | Duration of the Study | Cohort Studied | Examined Parameters | Results | Ref. |
|---|---|---|---|---|---|
| Lactose-restricted diet | eight weeks | 83 HT patients taking LT4: euthyroid (n = 53), subclinical (n = 19), overt hypothyroidism (n = 3), subclinical hyperthyroidism (n = 8) | Serum levels of TSH, fT4, calcium and parathormone, titers of TPOAb | - level of TSH significantly decreased in the euthyroid and subclinical hypothyroid patients with lactose intolerance following lactose restriction; - the level of TSH in the euthyroid patients without lactose intolerance remained unchanged; - the levels of PTH, fT4 and calcium did not significantly change after lactose restriction. | [148] |
| Gluten-free diet | six months | 34 women with HT: Study group (n = 16): gluten-free diet; Control group (n = 18): without any dietary treatment | Serum titers of TPOAb and TgAb, levels of TSH, fT3, fT4 and 25(OH)D | - in the control group serum TSH, fT3, fT4 and 25(OH)D levels remained at the similar levels; - in the study group the gluten-free diet reduced serum titers of TPOAb and TgAb and slightly increased 25(OH)D levels; - no differences in TSH, fT3 and fT4 levels in the study group after gluten-free diet; - in the study group TPOAb titers correlated with 25(OH)D levels. | [149] |
| Gluten-free diet with selenium supplementation | six months | 98 drug-naive HT women with subclinical hypothyroidism Study group (n = 50): 200 µg selenium and gluten-free diet Control group (n = 48): selenium supplementation without any dietary treatment. | Serum titers of TPOAb and TgAb, levels of TSH, fT4 and fT3 | - euthyroidism was restored in 74% of the study group and in 58.3% of the control group; - TSH, TPOAb and TgAb levels were significantly reduced in both groups; - serum TPOAb titer in the study group had a more significant decrease (by 49%) than those in the control group (by 34%). | [150] |
| Gluten-free diet connected with a healthy lifestyle promotion and education by our expert dietitian | 12 months | 62 euthyroid HT women with LT4 treatment: Study group (n = 31): gluten-free diet, healthy lifestyle education; Control group (n = 31): no changes in the diet, diet containing gluten | Serum levels of TSH, fT3, fT4, titers of TPOAb and TgAb, body weight and BMI | - a reduction in TSH levels after three, six and 12 months in the study group; - an increase in fT4 concentrations after six and 12 months; - no differences in TPOAb and TgAb, fT3 or fT4 levels in both groups after 12 months. | [151] |
| Elimination/reducing diets with selenium and zinc supplementation 1400–1600 kcal/day (with a deficit of about 1000 kcal/day) Macronutrient content: 25% protein, 30% fat, and 45% carbohydrate | six months | 100 women previously diagnosed with HT, obesity and receiving L-thyroxine, 200 mcg of 1selenomethionine/day and 30 mg of zinc gluconate/day: Study group: 50 women following individually adjusted elimination/reducing diets, Control group: 50 women following reducing diets with the same caloric content (without ingredient elimination) | Serum levels of TSH, fT3, fT4, titers of TPOAb and TgAb, body weight and BMI | - the decrease in BMI, body fat percentage, TSH concentration, TPOAb and TgAb levels in the study group were significantly greater compared to the control group; - the study group showed significantly greater increases in fT4 and fT3 levels than the control group; - after six months there was a positive correlation between the difference in body fat content and TSH levels and between TPOAb levels and BMI and a negative correlation between serum fT4 or fT3 and BMI. | [153] |
| Paleo-style diet without grains and dairy products with micronutrients supplementation | 15 months | A case study of 23-year-old euthyroid woman diagnosed eight months prior with HT | Serum levels of TSH, fT4, zinc, ferritin, vitamin D and B12, titers of TgAb and TPOAb | - a significant reduction in TPOAb and TgAb; - improvement in symptoms. | [154] |
| Autoimmune Protocol Diet, Supported Lifestyle Intervention | 10 weeks | 17 normal or overweight female subjects with a prior diagnosis of HT | Blood cell count, metabolic profile, levels of TSH, fT4, fT3, hsCRP, titers of TPOAb and TgAb, Short Form Health Survey, Medical Symptoms Questionnaire | - no statistically significant changes in TSH, fT4, fT3 and thyroid antibodies; - a statistically significant improvement in health-related quality of life; - the clinical symptom burden decreased significantly; - hsCRP significantly decrease by 29%. | [155] |
| Modified auto- immune Paleo low-calorie diet (1200 kcal) | six months | A case study of newly diagnosed 49-year-old obese woman with HT, medically free from any chronic diseases | levels of fT4, fT3, TSH, IFG, insulin, TG, non-HDL and HDL cholesterol titers of TPOAb, body composition, anthropometric measurements | - a significant reduction in body weight, BMI, waist and hip circumference, WHR, fat mass, levels of TC, TG, LDL and non-HDL cholesterol, insulin, IFG, TSH, and TPOAb; - fT3 and fT4 remained within normal reference range; - significant increase in HDL cholesterol level. | [156] |
6.2. Gut Microbiota in HT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Radetti, G. Clinical Aspects of Hashimoto’s Thyroiditis. Endocr. Dev. 2014, 26, 158–170. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in Our Understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef]
- Dong, Y.H.; Fu, D.G. Autoimmune Thyroid Disease: Mechanism, Genetics and Current Knowledge. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3611–3618. [Google Scholar]
- Iddah, M.A.; Macharia, B.N. Autoimmune Thyroid Disorders. ISRN Endocrinol. 2013, 2013, e509764. [Google Scholar] [CrossRef]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- McLeod, D.S.A.; Cooper, D.S. The Incidence and Prevalence of Thyroid Autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef]
- Sur, M.L.; Gaga, R.; Lazăr, C.; Lazea, C. Genetic and Environmental Factors in the Pathophysiology of Hashimoto’s Thyroiditis. Pediatr. Endocrinol. Rev. PER 2020, 17, 343–348. [Google Scholar] [CrossRef]
- Rydzewska, M.; Jaromin, M.; Pasierowska, I.E.; Stożek, K.; Bossowski, A. Role of the T and B Lymphocytes in Pathogenesis of Autoimmune Thyroid Diseases. Thyroid Res. 2018, 11, 2. [Google Scholar] [CrossRef]
- Kawicka, A.; Regulska-Ilow, B.; Regulska-Ilow, B. Metabolic Disorders and Nutritional Status in Autoimmune Thyroid Diseases. Postepy Hig. Med. Doswiadczalnej Online 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Yalcin, M.M.; Altinova, A.E.; Cavnar, B.; Bolayir, B.; Akturk, M.; Arslan, E.; Ozkan, C.; Cakir, N.; Balos Toruner, F. Is Thyroid Autoimmunity Itself Associated with Psychological Well-Being in Euthyroid Hashimoto’s Thyroiditis? Endocr. J. 2017, 64, 425–429. [Google Scholar] [CrossRef]
- Giynas Ayhan, M.; Uguz, F.; Askin, R.; Gonen, M.S. The Prevalence of Depression and Anxiety Disorders in Patients with Euthyroid Hashimoto’s Thyroiditis: A Comparative Study. Gen. Hosp. Psychiatry 2014, 36, 95–98. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in Endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Weetman, A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Invest. 2021, 44, 883–890. [Google Scholar] [CrossRef]
- Shukla, S.K.; Singh, G.; Ahmad, S.; Pant, P. Infections, Genetic and Environmental Factors in Pathogenesis of Autoimmune Thyroid Diseases. Microb. Pathog. 2018, 116, 279–288. [Google Scholar] [CrossRef]
- Kust, D.; Matesa, N. The Impact of Familial Predisposition on the Development of Hashimoto’s Thyroiditis. Acta Clin. Belg. 2020, 75, 104–108. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef]
- Torino, F.; Barnabei, A.; Paragliola, R.; Baldelli, R.; Appetecchia, M.; Corsello, S.M. Thyroid Dysfunction as an Unintended Side Effect of Anticancer Drugs. Thyroid 2013, 23, 1345–1366. [Google Scholar] [CrossRef]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jørgensen, T.; Laurberg, P. Moderate Alcohol Consumption May Protect against Overt Autoimmune Hypothyroidism: A Population-Based Case-Control Study. Eur. J. Endocrinol. 2012, 167, 483–490. [Google Scholar] [CrossRef]
- Effraimidis, G.; Tijssen, J.G.P.; Wiersinga, W.M. Alcohol Consumption as a Risk Factor for Autoimmune Thyroid Disease: A Prospective Study. Eur. Thyroid J. 2012, 1, 99–104. [Google Scholar] [CrossRef]
- Effraimidis, G.; Tijssen, J.G.P.; Brosschot, J.F.; Wiersinga, W.M. Involvement of Stress in the Pathogenesis of Autoimmune Thyroid Disease: A Prospective Study. Psychoneuroendocrinology 2012, 37, 1191–1198. [Google Scholar] [CrossRef]
- Markomanolaki, Z.S.; Tigani, X.; Siamatras, T.; Bacopoulou, F.; Tsartsalis, A.; Artemiadis, A.; Megalooikonomou, V.; Vlachakis, D.; Chrousos, G.P.; Darviri, C. Stress Management in Women with Hashimoto’s Thyroiditis: A Randomized Controlled Trial. J. Mol. Biochem. 2019, 8, 3–12. [Google Scholar]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Tsekouras, K.C.; Evangelopoulos, A.D.; Kotsiris, D.A.; Tzortzinis, A.A. Is Vitamin D Related to Pathogenesis and Treatment of Hashimoto’s Thyroiditis? Hell. J. Nucl. Med. 2015, 18, 222–227. [Google Scholar]
- Rostami, R.; Nourooz-Zadeh, S.; Mohammadi, A.; Khalkhali, H.R.; Ferns, G.; Nourooz-Zadeh, J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants 2020, 9, 1070. [Google Scholar] [CrossRef]
- Wiebolt, J.; Achterbergh, R.; den Boer, A.; van der Leij, S.; Marsch, E.; Suelmann, B.; de Vries, R.; van Haeften, T.W. Clustering of Additional Autoimmunity Behaves Differently in Hashimoto’s Patients Compared with Graves’ Patients. Eur. J. Endocrinol. 2011, 164, 789–794. [Google Scholar] [CrossRef]
- Tian, X.; Li, N.; Su, R.; Dai, C.; Zhang, R. Selenium Supplementation May Decrease Thyroid Peroxidase Antibody Titer via Reducing Oxidative Stress in Euthyroid Patients with Autoimmune Thyroiditis. Int. J. Endocrinol. 2020, 2020, 9210572. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of Vitamin D on Thyroid Autoimmunity Markers in Hashimoto’s Thyroiditis: Systematic Review and Meta-Analysis. J. Int. Med. Res. 2021, 49, 03000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Zuo, Z.; Zhao, Y.; Wang, K. The Effect of Vitamin D Supplementation on Thyroid Autoantibody Levels in the Treatment of Autoimmune Thyroiditis: A Systematic Review and a Meta-Analysis. Endocrine 2018, 59, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation Obesity: A Chronic Relapsing Progressive Disease Process. A Position Statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 January 2022).
- Siemińska, L.; Wojciechowska, C.; Walczak, K.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Foltyn, W.; Kos-Kudła, B. Associations between Metabolic Syndrome, Serum Thyrotropin, and Thyroid Antibodies Status in Postmenopausal Women, and the Role of Interleukin-6. Endokrynol. Pol. 2015, 66, 394–403. [Google Scholar] [CrossRef][Green Version]
- McLeod, D.S.A. Autoimmune Thyroid Disease: A Novel Risk Factor for Atherosclerosis? Endocrine 2013, 44, 8–10. [Google Scholar] [CrossRef][Green Version]
- Ranganadane, R.; Sumathi, S.S.; Raghavan, S.A.; Shafiulla, A.; Subramanian, G.; Nachimuthu, M.K.S. Cardiovascular Risk in Hashimoto\’s Thyroiditis: Role of Thyroid Autoimmunity. SBV J. Basic Clin. Appl. Health Sci. 2021, 4, 20–22. [Google Scholar] [CrossRef]
- Tamer, G.; Mert, M.; Tamer, I.; Mesci, B.; Kilic, D.; Arik, S. Effects of Thyroid Autoimmunity on Abdominal Obesity and Hyperlipidaemia. Endokrynol. Pol. 2011, 62, 421–428. [Google Scholar]
- Tomczyńska, M.; Salata, I.; Saluk, J. Autoimmunizacyjne choroby tarczycy jako czynnik ryzyka chorób układu sercowo-naczyniowego. Chor. Serca Naczyń 2017, 14, 30–38. [Google Scholar]
- Zöller, B.; Li, X.; Sundquist, J.; Sundquist, K. Risk of Subsequent Coronary Heart Disease in Patients Hospitalized for Immune-Mediated Diseases: A Nationwide Follow-Up Study from Sweden. PLoS ONE 2012, 7, e33442. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, Y.-K.; Lin, C.-L.; Yeh, J.-H.; Kao, C.-H. Hashimoto’s Thyroiditis, Risk of Coronary Heart Disease, and L-Thyroxine Treatment: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2015, 100, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Kuh, D.; Pierce, M.; Franklyn, J.A. Childhood Weight Gain and Thyroid Autoimmunity at Age 60–64 Years: The 1946 British Birth Cohort Study. J. Clin. Endocrinol. Metab. 2013, 98, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Minocci, A.; Tagliaferri, M.A.; Guzzaloni, G.; Di Blasio, A.; De Medici, C.; Aimaretti, G.; Liuzzi, A. Investigations of Thyroid Hormones and Antibodies in Obesity: Leptin Levels Are Associated with Thyroid Autoimmunity Independent of Bioanthropometric, Hormonal, and Weight-Related Determinants. J. Clin. Endocrinol. Metab. 2010, 95, 3965–3972. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wang, B.; Yao, Q.; Li, Q.; Jia, X.; Zhang, J. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Fu, J.; Wang, G. Association between Thyroid Hormones, Thyroid Antibodies, and Cardiometabolic Factors in Non-Obese Individuals with Normal Thyroid Function. Front. Endocrinol. 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Bolal, M.; Ates, I.; Demir, B.F.; Altay, M.; Turhan, T.; Yılmaz, N. The Relationship between Homocysteine and Autoimmune Subclinical Hypothyroidism. Int. J. Med. Biochem. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Popławska-Kita, A.; Siewko, K.; Telejko, B.; Kościuszko-Zdrodowska, M.; Hryniewicka, J.; Szelachowska, M.; Milewski, R.; Górska, M. Body Mass Analysis in Patients with Hashimoto Thyroiditis. Prog. Health Sci. 2014, 4, 18–23. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, X.; Tang, X.; Li, Y.; Tong, N.; Wang, G.; Zhang, J.-A.; Wang, Y.; Ba, J.; Chen, B.; et al. The Correlation between Metabolic Disorders and Tpoab/Tgab: A Cross-Sectional Population-Based Study. Endocr. Pract. 2020, 26, 869–882. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, J.; Li, H.; Zhong, H.; Wan, Q. Changes in Glucose-lipid Metabolism, Insulin Resistance, and Inflammatory Factors in Patients with Autoimmune Thyroid Disease. J. Clin. Lab. Anal. 2019, 33, e22929. [Google Scholar] [CrossRef]
- Gierach, M.; Gierach, J.; Skowrońska, A.; Rutkowska, E.; Spychalska, M.; Pujanek, M.; Junik, R. Hashimoto’s Thyroiditis and Carbohydrate Metabolism Disorders in Patients Hospitalised in the Department of Endocrinology and Diabetology of Ludwik Rydygier Collegium Medicum in Bydgoszcz between 2001 and 2010. Endokrynol. Pol. 2012, 63, 14–17. [Google Scholar]
- Cerit, E.T.; Akturk, M.; Altinova, A.E.; Tavil, Y.; Ozkan, C.; Yayla, C.; Altay, M.; Demirtas, C.; Cakir, N. Evaluation of Body Composition Changes, Epicardial Adipose Tissue, and Serum Omentin-1 Levels in Overt Hypothyroidism. Endocrine 2015, 49, 196–203. [Google Scholar] [CrossRef] [PubMed]
- İşgüven, P.; Gündüz, Y.; Kılıç, M. Effects of Thyroid Autoimmunity on Early Atherosclerosis in Euthyroid Girls with Hashimoto’s Thyroiditis. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.N.; Elessawy, R.; Deghedy, A.; Hafez, A.; Elsherbiny, T.M. Hashimoto Thyroiditis Is an Independent Cardiovascular Risk Factor in Clinically Hypothyroid Patients. Alex. J. Med. 2011, 47, 267–276. [Google Scholar] [CrossRef][Green Version]
- Topaloglu, O.; Gokay, F.; Kucukler, K.; Burnik, F.S.; Mete, T.; Yavuz, H.C.; Berker, D.; Guler, S. Is Autoimmune Thyroiditis a Risk Factor for Early Atherosclerosis in Premenopausal Women Even If in Euthyroid Status? Endocrine 2013, 44, 145–151. [Google Scholar] [CrossRef]
- Collet, T.-H.; Bauer, D.C.; Cappola, A.R.; Asvold, B.O.; Weiler, S.; Vittinghoff, E.; Gussekloo, J.; Bremner, A.; den Elzen, W.P.J.; Maciel, R.M.B.; et al. Thyroid Antibody Status, Subclinical Hypothyroidism, and the Risk of Coronary Heart Disease: An Individual Participant Data Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3353–3362. [Google Scholar] [CrossRef]
- Mousa, U.; Bozkuş, Y.; Kut, A.; Demir, C.C.; Tutuncu, N.B. Fat distribution and metabolic profile in sujects with Hashimoto’s thyroiditis. Acta Endocrinol. Buchar. 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, Q.; Zhou, H.; Ma, Z.F. Prevalence of Components of Metabolic Syndrome among Adults with the Presence of Autoimmune Thyroid Condition in an Iodine-Sufficient Region. Biol. Trace Elem. Res. 2020, 199, 2837–2843. [Google Scholar] [CrossRef]
- Owecki, M.; Dorszewska, J.; Sawicka-Gutaj, N.; Oczkowska, A.; Owecki, M.K.; Michalak, M.; Fischbach, J.; Kozubski, W.; Ruchała, M. Serum Homocysteine Levels Are Decreased in Levothyroxine-Treated Women with Autoimmune Thyroiditis. BMC Endocr. Disord. 2014, 14, 18. [Google Scholar] [CrossRef]
- Solini, A.; Dardano, A.; Santini, E.; Polini, A.; Monzani, F. Adipocytokines Mark Insulin Sensitivity in Euthyroid Hashimoto’s Patients. Acta Diabetol. 2013, 50, 73–80. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, e6757154. [Google Scholar] [CrossRef]
- Giannakou, M.; Saltiki, K.; Mantzou, E.; Loukari, E.; Philippou, G.; Terzidis, K.; Stavrianos, C.; Kyprianou, M.; Psaltopoulou, T.; Karatzi, K.; et al. The Effect of Obesity and Dietary Habits on Oxidative Stress in Hashimoto’s Thyroiditis. Endocr. Connect. 2018, 7, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Aghasi, M.R.; Mohammadi, A.; Nourooz-Zadeh, J. Enhanced Oxidative Stress in Hashimoto’s Thyroiditis: Inter-Relationships to Biomarkers of Thyroid Function. Clin. Biochem. 2013, 46, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Baser, H.; Can, U.; Baser, S.; Yerlikaya, F.H.; Aslan, U.; Hidayetoglu, B.T. Assesment of Oxidative Status and Its Association with Thyroid Autoantibodies in Patients with Euthyroid Autoimmune Thyroiditis. Endocrine 2015, 48, 916–923. [Google Scholar] [CrossRef]
- Ates, I.; Yilmaz, F.M.; Altay, M.; Yilmaz, N.; Berker, D.; Güler, S. The Relationship between Oxidative Stress and Autoimmunity in Hashimoto’s Thyroiditis. Eur. J. Endocrinol. 2015, 173, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative Stress and Advanced Glycation End Products in Hashimoto’s Thyroiditis. Thyroid 2016, 26, 504–511. [Google Scholar] [CrossRef]
- Öztürk, Ü.; Vural, P.; Özderya, A.; Karadağ, B.; Doğru-Abbasoğlu, S.; Uysal, M. Oxidative Stress Parameters in Serum and Low Density Lipoproteins of Hashimoto’s Thyroiditis Patients with Subclinical and Overt Hypothyroidism. Int. Immunopharmacol. 2012, 14, 349–352. [Google Scholar] [CrossRef]
- Morawska, K.; Maciejczyk, M.; Popławski, Ł.; Popławska-Kita, A.; Krętowski, A.; Zalewska, A. Enhanced Salivary and General Oxidative Stress in Hashimoto’s Thyroiditis Women in Euthyreosis. J. Clin. Med. 2020, 9, 2102. [Google Scholar] [CrossRef]
- Korkmaz, H.; Tabur, S.; Ozkaya, M.; Oguz, E.; Elboga, U.; Aksoy, N.; Akarsu, E. Paraoxonase and Arylesterase Levels in Autoimmune Thyroid Diseases. Redox Rep. Commun. Free Radic. Res. 2016, 21, 227–231. [Google Scholar] [CrossRef][Green Version]
- Ates, I.; Arikan, M.F.; Altay, M.; Yilmaz, F.M.; Yilmaz, N.; Berker, D.; Guler, S. The Effect of Oxidative Stress on the Progression of Hashimoto’s Thyroiditis. Arch. Physiol. Biochem. 2018, 124, 351–356. [Google Scholar] [CrossRef]
- Ates, I.; Altay, M.; Yilmaz, F.M.; Topcuoglu, C.; Yilmaz, N.; Berker, D.; Guler, S. The Impact of Levothyroxine Sodium Treatment on Oxidative Stress in Hashimoto’s Thyroiditis. Eur. J. Endocrinol. 2016, 174, 727–734. [Google Scholar] [CrossRef][Green Version]
- Bjørklund, G.; Chirumbolo, S. Role of Oxidative Stress and Antioxidants in Daily Nutrition and Human Health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global Iodine Nutrition: Where Do We Stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Liontiris, M.I.; Mazokopakis, E.E. A Concise Review of Hashimoto Thyroiditis (HT) and the Importance of Iodine, Selenium, Vitamin D and Gluten on the Autoimmunity and Dietary Management of HT Patients. Points That Need More Investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; World Health Organization: Geneva, Switzerland, 2014; pp. 1–54. [Google Scholar]
- Krela-Kaźmierczak, I.; Czarnywojtek, A.; Skoracka, K.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Ruchała, M.; Dobrowolska, A. Is There an Ideal Diet to Protect against Iodine Deficiency? Nutrients 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Amino, N. Hashimoto’s Thyroiditis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., Eds.; MDTex: Dartmouth, MA, USA, 2017. [Google Scholar]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine Excess as an Environmental Risk Factor for Autoimmune Thyroid Disease. Int. J. Mol. Sci. 2014, 15, 12895–12912. [Google Scholar] [CrossRef]
- Teti, C.; Panciroli, M.; Nazzari, E.; Pesce, G.; Mariotti, S.; Olivieri, A.; Bagnasco, M. Iodoprophylaxis and Thyroid Autoimmunity: An Update. Immunol. Res. 2021, 69, 129–138. [Google Scholar] [CrossRef]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the Thyroid Gland: More Good News for Clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple Nutritional Factors and Thyroid Disease, with Particular Reference to Autoimmune Thyroid Disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Filipowicz, D.; Majewska, K.; Kalantarova, A.; Szczepanek-Parulska, E.; Ruchała, M. The Rationale for Selenium Supplementation in Patients with Autoimmune Thyroiditis, according to the Current State of Knowledge. Endokrynol. Pol. 2021, 72, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.H.; Papini, E.; Attanasio, R.; Negro, R.; Hegedüs, L. A 2018 European Thyroid Association Survey on the Use of Selenium Supplementation in Hashimoto’s Thyroiditis. Eur. Thyroid J. 2020, 9, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Prochownik, E.; Błażewska-Gruszczyk, A.; Słowiaczek, M.; Sun, Q.; Schomburg, L.; Ochab, E.; Bartyzel, M. Positive Effects of Selenium Supplementation in Women with Newly Diagnosed Hashimoto’s Thyroiditis in an Area with Low Selenium Status. Int. J. Clin. Pract. 2021, 75, e14484. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Sun, R.-X.; Li, C.-F.; Wang, X.-H. The Effects of Selenium Supplementation on Antibody Titres in Patients with Hashimoto’s Thyroiditis. Endokrynol. Pol. 2021, 72, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Pirola, I.; Rotondi, M.; Cristiano, A.; Maffezzoni, F.; Pasquali, D.; Marini, F.; Coperchini, F.; Paganelli, M.; Apostoli, P.; Chiovato, L.; et al. Selenium Supplementation in Patients with Subclinical Hypothyroidism Affected by Autoimmune Thyroiditis: Results of the SETI Study. Endocrinol. Diabetes Nutr. 2020, 67, 28–35. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of Selenium on Thyroid Autoimmunity and Regulatory T Cells in Patients with Hashimoto’s Thyroiditis: A Prospective Randomized-Controlled Trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef]
- Karimi, F.; Omrani, G.R. Effects of Selenium and Vitamin C on the Serum Level of Antithyroid Peroxidase Antibody in Patients with Autoimmune Thyroiditis. J. Endocrinol. Invest. 2019, 42, 481–487. [Google Scholar] [CrossRef]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of Short-Term Selenium Supplementation on the Natural Course of Hashimoto’s Thyroiditis: Clinical Results of a Blinded Placebo-Controlled Randomized Prospective Trial. J. Endocrinol. Invest. 2017, 40, 83–89. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Albusta, A.Y.; Fedorowicz, Z.; Carter, B.; Pijl, H. Selenium Supplementation for Hashimoto’s Thyroiditis: Summary of a Cochrane Systematic Review. Eur. Thyroid J. 2014, 3, 25–31. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Starchl, C.; Scherkl, M.; Amrein, K. Celiac Disease and the Thyroid: Highlighting the Roles of Vitamin D and Iron. Nutrients 2021, 13, 1755. [Google Scholar] [CrossRef] [PubMed]
- Koç, Ş.; Güngör, K.; Güngör, N.D.; Uzunlulu, M. Iron Deficiency in Women with Thyroid-Specific Autoantibodies: A Case Control Study. J. Exp. Clin. Med. 2022, 39, 194–198. [Google Scholar]
- Zhang, H.-Y.; Teng, X.-C.; Shan, Z.-Y.; Wang, Z.-J.; Li, C.-Y.; Yu, X.-H.; Mao, J.-Y.; Wang, W.-W.; Xie, X.-C.; Teng, W.-P. Association between Iron Deficiency and Prevalence of Thyroid Autoimmunity in Pregnant and Non-Pregnant Women of Childbearing Age: A Cross-Sectional Study. Chin. Med. J. 2019, 132, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Tan, L.; Liu, Y.; Liu, T.; Wang, H.; et al. Severely Low Serum Magnesium Is Associated with Increased Risks of Positive Anti-Thyroglobulin Antibody and Hypothyroidism: A Cross-Sectional Study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-Hydoxyvitamin D Concentrations in Relation to Hashimoto’s Thyroiditis: A Systematic Review, Meta-Analysis and Meta-Regression of Observational Studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Fang, F.; Chai, Y.; Wei, H.; Wang, K.; Tan, L.; Zhang, W.; Fan, Y.; Li, F.; Shan, Z.; Zhu, M. Vitamin D Deficiency Is Associated with Thyroid Autoimmunity: Results from an Epidemiological Survey in Tianjin, China. Endocrine 2021, 73, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and Thyroid Disorders: A Systematic Review and Meta-Analysis of Observational Studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Zhu, Y.; Fang, L. Correlation Between Hashimoto’s Thyroiditis–Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Mansournia, N.; Mansournia, M.A.; Saeedi, S.; Dehghan, J. The Association between Serum 25OHD Levels and Hypothyroid Hashimoto’s Thyroiditis. J. Endocrinol. Invest. 2014, 37, 473–476. [Google Scholar] [CrossRef]
- De Pergola, G.; Triggiani, V.; Bartolomeo, N.; Giagulli, V.A.; Anelli, M.; Masiello, M.; Candita, V.; De Bellis, D.; Silvestris, F. Low 25 Hydroxyvitamin D Levels Are Independently Associated with Autoimmune Thyroiditis in a Cohort of Apparently Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 646–652. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, K.J.; Kim, D.; Hwang, S.; Lee, E.J. Low Serum Vitamin D Is Associated with Anti-Thyroid Peroxidase Antibody in Autoimmune Thyroiditis. Yonsei Med. J. 2014, 55, 476–481. [Google Scholar] [CrossRef]
- Maciejewski, A.; Wójcicka, M.; Roszak, M.; Losy, J.; Łącka, K. Assessment of Vitamin D Level in Autoimmune Thyroiditis Patients and a Control Group in the Polish Population. Adv. Clin. Exp. Med. 2015, 24, 801–806. [Google Scholar] [CrossRef]
- Kim, D. Low Vitamin D Status Is Associated with Hypothyroid Hashimoto’s Thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Turashvili, N.; Javashvili, L.; Giorgadze, E. Vitamin D Deficiency Is More Common in Women with Autoimmune Thyroiditis: A Retrospective Study. Int. J. Endocrinol. 2021, 2021, 4465563. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.-Y.; Sun, H.; Xu, X.-Q.; Xu, S.-A.; Suo, Y.; Cao, L.-J.; Zhou, Q.; Yu, H.-J.; Cao, W.-Z. Low Vitamin D Levels Are Associated with Cognitive Impairment in Patients with Hashimoto Thyroiditis. BMC Endocr. Disord. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Badenhoop, K.; Tijssen, J.G.P.; Wiersinga, W.M. Vitamin D Deficiency Is Not Associated with Early Stages of Thyroid Autoimmunity. Eur. J. Endocrinol. 2012, 167, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Botelho, I.M.B.; Moura Neto, A.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s Thyroiditis and Its Relationship with Thyroid Function and Inflammatory Status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef]
- Nodehi, M.; Ajami, A.; Izad, M.; Asgarian Omran, H.; Chahardoli, R.; Amouzegar, A.; Yekaninejad, S.; Hemmatabadi, M.; Azizi, F.; Esfahanian, F.; et al. Effects of Vitamin D Supplements on Frequency of CD4+ T-Cell Subsets in Women with Hashimoto’s Thyroiditis: A Double-Blind Placebo-Controlled Study. Eur. J. Clin. Nutr. 2019, 73, 1236–1243. [Google Scholar] [CrossRef]
- Ucan, B.; Sahin, M.; Sayki Arslan, M.; Colak Bozkurt, N.; Kizilgul, M.; Güngünes, A.; Cakal, E.; Ozbek, M. Vitamin D Treatment in Patients with Hashimoto’s Thyroiditis May Decrease the Development of Hypothyroidism. Int. J. Vitam. Nutr. Res. 2016, 86, 9–17. [Google Scholar] [CrossRef]
- Simsek, Y.; Cakır, I.; Yetmis, M.; Dizdar, O.S.; Baspinar, O.; Gokay, F. Effects of Vitamin D Treatment on Thyroid Autoimmunity. J. Res. Med. Sci. 2016, 21, 85. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.A.; Kumar, A.; Mukhopadhyay, S. Vitamin D Supplementation Reduces Thyroid Peroxidase Antibody Levels in Patients with Autoimmune Thyroid Disease: An Open-Labeled Randomized Controlled Trial. Indian J. Endocrinol. Metab. 2016, 20, 391–398. [Google Scholar] [CrossRef]
- Villa, A.; Corsello, A.; Cintoni, M.; Papi, G.; Pontecorvi, A.; Corsello, S.M.; Paragliola, R.M. Effect of Vitamin D Supplementation on TSH Levels in Euthyroid Subjects with Autoimmune Thyroiditis. Endocrine 2020, 70, 85–91. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Selenomethionine Potentiates the Impact of Vitamin D on Thyroid Autoimmunity in Euthyroid Women with Hashimoto’s Thyroiditis and Low Vitamin D Status. Pharmacol. Rep. 2019, 71, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, R.; Saboor-Yaraghi, A.-A.; Amouzegar, A.; Khalili, D.; Vakili, A.Z.; Azizi, F. Can Supplementation with Vitamin D Modify Thyroid Autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, TSH) in Hashimoto’s Thyroiditis? A Double Blind, Randomized Clinical Trial. Horm. Metab. Res. Horm. 2019, 51, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Vahabi Anaraki, P.; Aminorroaya, A.; Amini, M.; Momeni, F.; Feizi, A.; Iraj, B.; Tabatabaei, A. Effect of Vitamin D Deficiency Treatment on Thyroid Function and Autoimmunity Markers in Hashimoto’s Thyroiditis: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Res. Med. Sci. 2017, 22, 103. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, P.V.; Aminorroaya, A.; Amini, M.; Feizi, A.; Iraj, B.; Tabatabaei, A. Effects of Vitamin D Deficiency Treatment on Metabolic Markers in Hashimoto Thyroiditis Patients. J. Res. Med. Sci. 2017, 22, 5. [Google Scholar] [CrossRef]
- Behera, K.K.; Saharia, G.K.; Hota, D.; Sahoo, D.P.; Sethy, M.; Srinivasan, A. Effect of Vitamin D Supplementation on Thyroid Autoimmunity among Subjects of Autoimmune Thyroid Disease in a Coastal Province of India: A Randomized Open-Label Trial. Niger. Med. J. 2020, 61, 237–240. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D and Selenomethionine on Thyroid Antibody Titers, Hypothalamic-Pituitary-Thyroid Axis Activity and Thyroid Function Tests in Men with Hashimoto’s Thyroiditis: A Pilot Study. Pharmacol. Rep. 2019, 71, 243–247. [Google Scholar] [CrossRef]
- Gao, X.-R.; Yu, Y.-G. Meta-Analysis of the Association between Vitamin D Receptor Polymorphisms and the Risk of Autoimmune Thyroid Disease. Int. J. Endocrinol. 2018, 2018, 2846943. [Google Scholar] [CrossRef]
- Maciejewski, A.; Kowalczyk, M.J.; Herman, W.; Czyżyk, A.; Kowalska, M.; Żaba, R.; Łącka, K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? BioMed Res. Int. 2019, 2019, e8197580. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Anemia in Thyroid Diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef]
- Ness-Abramof, R.; Nabriski, D.A.; Shapiro, M.S.; Shenkman, L.; Shilo, L.; Weiss, E.; Reshef, T.; Braverman, L.E. Prevalence and Evaluation of B12 Deficiency in Patients with Autoimmune Thyroid Disease. Am. J. Med. Sci. 2006, 332, 119–122. [Google Scholar] [CrossRef]
- Aktaş, H.Ş. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Med. Princ. Pract. 2020, 29, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake from Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Jaya Kumari, S.; Bantwal, G.; Devanath, A.; Aiyyar, V.; Patil, M. Evaluation of Serum Vitamin B12 Levels and Its Correlation with Anti-Thyroperoxidase Antibody in Patients with Autoimmune Thyroid Disorders. IJCB 2015, 30, 217–220. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.-P.; Lin, H.-P.; Chen, H.-M.; Kuo, Y.-S.; Lang, M.-J.; Sun, A. Hemoglobin, Iron, and Vitamin B12 Deficiencies and High Blood Homocysteine Levels in Patients with Anti-Thyroid Autoantibodies. J. Formos. Med. Assoc. 2014, 113, 155–160. [Google Scholar] [CrossRef]
- Wojtas, N.; Wadolowska, L.; Bandurska-Stankiewicz, E. Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 4841. [Google Scholar] [CrossRef] [PubMed]
- Matana, A.; Torlak, V.; Brdar, D.; Popović, M.; Lozić, B.; Barbalić, M.; Perica, V.B.; Punda, A.; Polašek, O.; Hayward, C.; et al. Dietary Factors Associated with Plasma Thyroid Peroxidase and Thyroglobulin Antibodies. Nutrients 2017, 9, 1186. [Google Scholar] [CrossRef]
- Kaličanin, D.; Brčić, L.; Ljubetić, K.; Barić, A.; Gračan, S.; Brekalo, M.; Torlak Lovrić, V.; Kolčić, I.; Polašek, O.; Zemunik, T.; et al. Differences in Food Consumption between Patients with Hashimoto’s Thyroiditis and Healthy Individuals. Sci. Rep. 2020, 10, 10670. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Wątor, P.; Gębski, J.; Frąckiewicz, J.; Drywień, M.E. Are Nutritional Patterns among Polish Hashimoto Thyroiditis Patients Differentiated Internally and Related to Ailments and Other Diseases? Nutrients 2021, 13, 3675. [Google Scholar] [CrossRef]
- Trofimiuk-Muldner, M.; Czubek, E.; Sztorc, J.; Skalniak, A.; Hubalewska-Dydejczyk, A. MON-013 Nutritional Approach to Autoimmune Thyroiditis (AIT)—The Patients’ and Medical Professionals’ View. J. Endocr. Soc. 2019, 3, MON-013. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated Fatty Acids Trigger TLR4-Mediated Inflammatory Response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhao, Y.; Song, Y.; Xu, C.; Yang, J.; Xuan, S.; Yan, H.; Yu, C.; Zhao, M.; Xu, J.; et al. Dietary High-Fat Lard Intake Induces Thyroid Dysfunction and Abnormal Morphology in Rats. Acta Pharmacol. Sin. 2014, 35, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Shao, S.; Xu, G.; Song, Y.; Xu, C.; Gao, L.; Hu, C.; Zhao, J. A High-Fat Diet Rich in Saturated and Mono-Unsaturated Fatty Acids Induces Disturbance of Thyroid Lipid Profile and Hypothyroxinemia in Male Rats. Mol. Nutr. Food Res. 2018, 62, 1700599. [Google Scholar] [CrossRef]
- Benvenga, S.; Vigo, M.T.; Metro, D.; Granese, R.; Vita, R.; Le Donne, M. Type of Fish Consumed and Thyroid Autoimmunity in Pregnancy and Postpartum. Endocrine 2016, 52, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Asik, M.; Gunes, F.; Binnetoglu, E.; Eroglu, M.; Bozkurt, N.; Sen, H.; Akbal, E.; Bakar, C.; Beyazit, Y.; Ukinc, K. Decrease in TSH Levels after Lactose Restriction in Hashimoto’s Thyroiditis Patients with Lactose Intolerance. Endocrine 2014, 46, 279–284. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Velija, A.Z.; Hadzovic-Dzuvo, A.; Al, T.D. The Effect of Selenium Supplementation and Gluten-Free Diet in Patients with Subclinical Hypothyroidism Affected by Autoimmune Thyroiditis. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2020; Volume 70. [Google Scholar]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef]
- Avard, N.; Grant, S. A Case Report of a Novel, Integrative Approach to Hashimoto’s Thyroiditis with Unexpected Results. Adv. Integr. Med. 2018, 5, 75–79. [Google Scholar] [CrossRef]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-Disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, e4556. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayyari, N.S. Successful Dietary Intervention Plan for Hashimoto’s Thyroiditis: A Case Study. Rom. J. Diabetes Nutr. Metab. Dis. 2020, 27, 381–385. [Google Scholar]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Mohammad, I.S.; Guo, H.; Shahzad, M.; Hou, Y.J.; Ma, C.; Naseem, Z.; Wu, X.; Shi, P.; Xu, J. Molecular Estimation of Alteration in Intestinal Microbial Composition in Hashimoto’s Thyroiditis Patients. Biomed. Pharmacother. 2017, 95, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Fallahi, P.; Antonelli, A.; Benvenga, S.; Centanni, M. Gut Microbiota and Hashimoto’s Thyroiditis. Rev. Endocr. Metab. Disord. 2018, 19, 293–300. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. TEM 2019, 30, 479–490. [Google Scholar] [CrossRef]
- Cayres, L.C.D.F.; de Salis, L.V.V.; Rodrigues, G.S.P.; Lengert, A.V.H.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients with Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef]
- Liu, S.; An, Y.; Cao, B.; Sun, R.; Ke, J.; Zhao, D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 5036959. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. https://doi.org/10.3390/ijms23126580
Mikulska AA, Karaźniewicz-Łada M, Filipowicz D, Ruchała M, Główka FK. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. International Journal of Molecular Sciences. 2022; 23(12):6580. https://doi.org/10.3390/ijms23126580
Chicago/Turabian StyleMikulska, Aniceta A., Marta Karaźniewicz-Łada, Dorota Filipowicz, Marek Ruchała, and Franciszek K. Główka. 2022. "Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview" International Journal of Molecular Sciences 23, no. 12: 6580. https://doi.org/10.3390/ijms23126580
APA StyleMikulska, A. A., Karaźniewicz-Łada, M., Filipowicz, D., Ruchała, M., & Główka, F. K. (2022). Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. International Journal of Molecular Sciences, 23(12), 6580. https://doi.org/10.3390/ijms23126580








