DEPP Deficiency Contributes to Browning of White Adipose Tissue
Abstract
:1. Introduction
2. Results
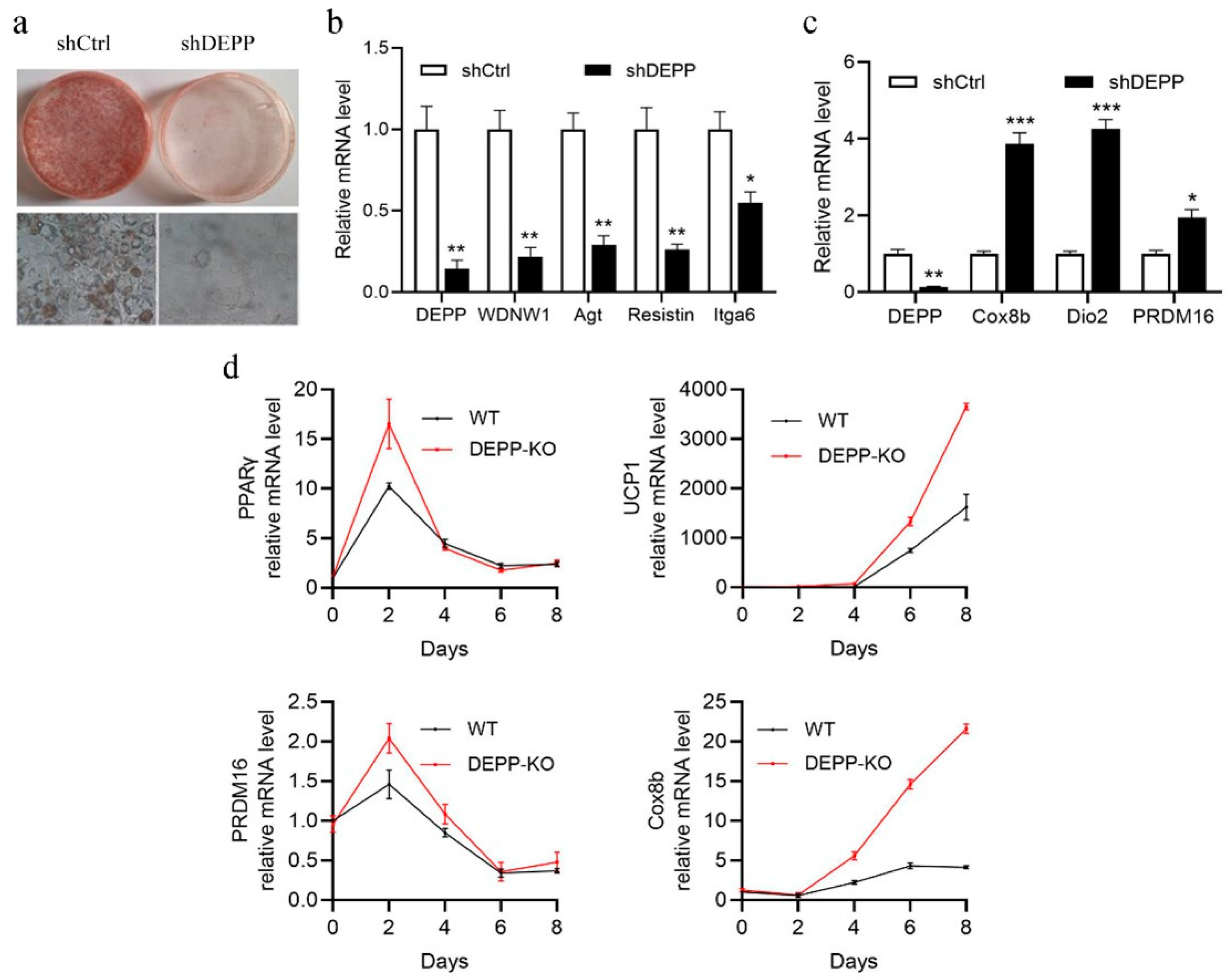
2.1. DEPP Deficiency Reduces Lipid Storage in White Adipocytes and Induces Browning of Adipocytes In Vitro
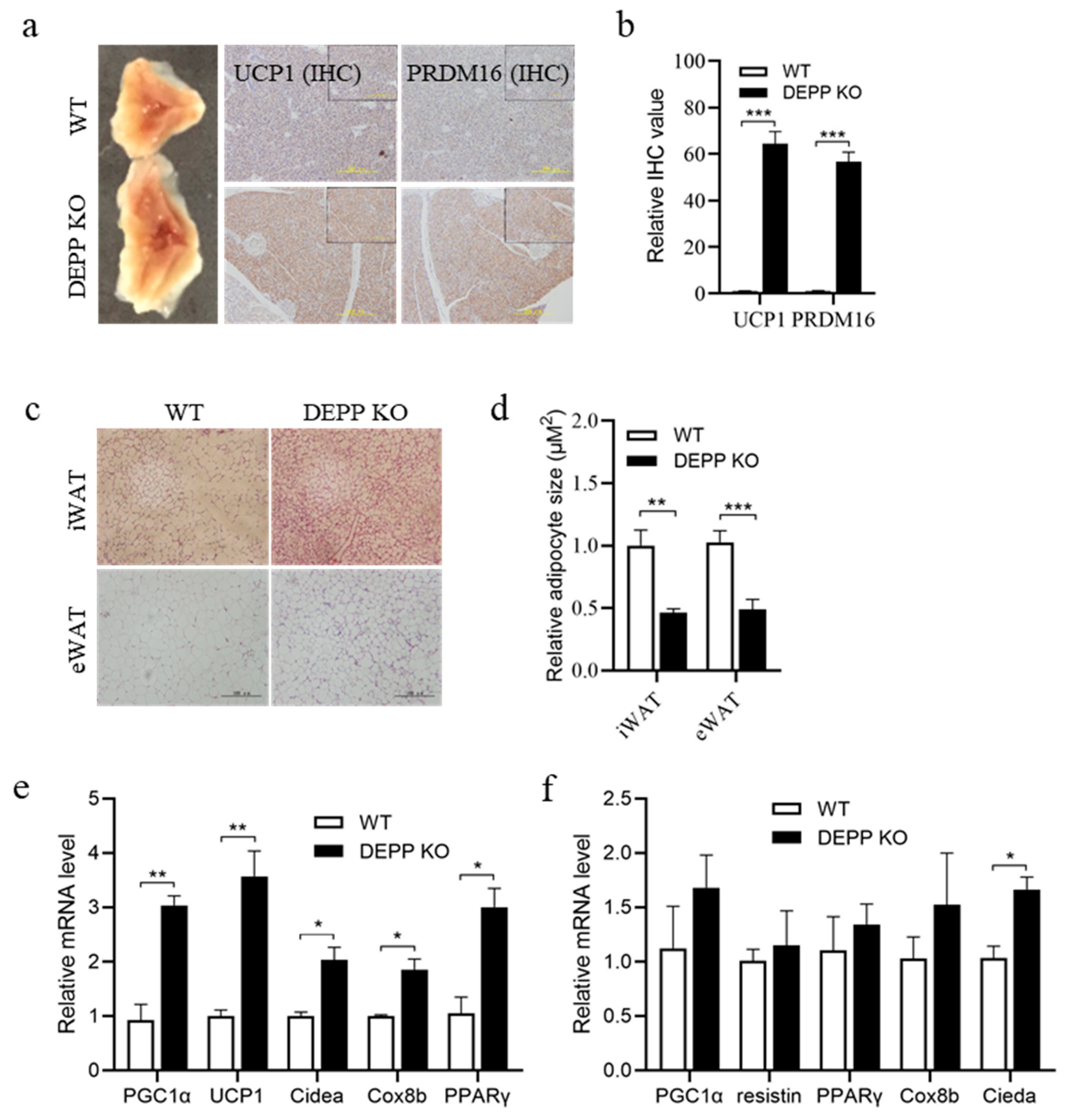
2.2. DEPP Deficiency Enhances BAT Activity and Induces White Adipocyte Browning in Mice
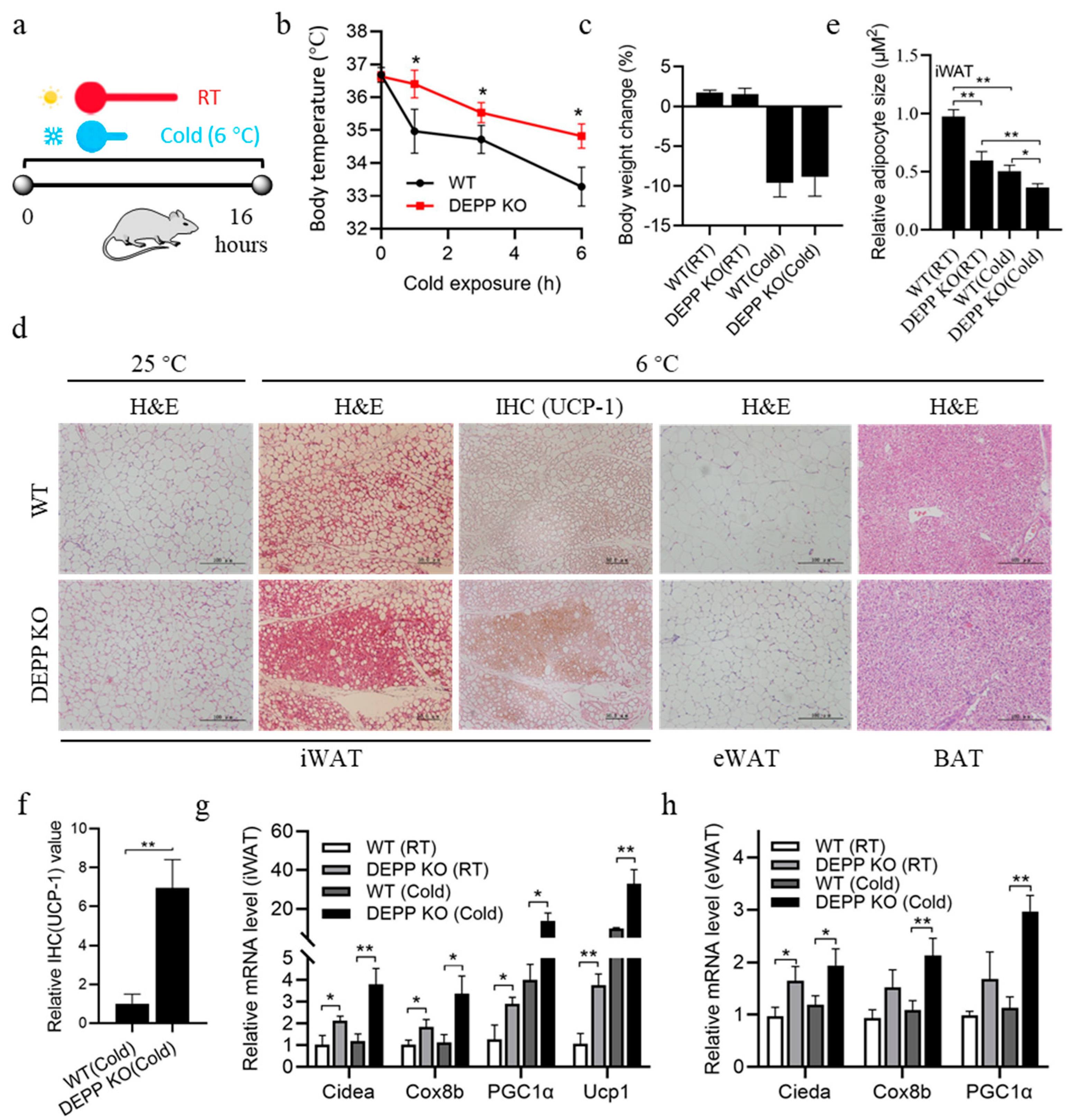
2.3. Cold Exposure Stimulates More Browning of WAT in DEPP KO Mice
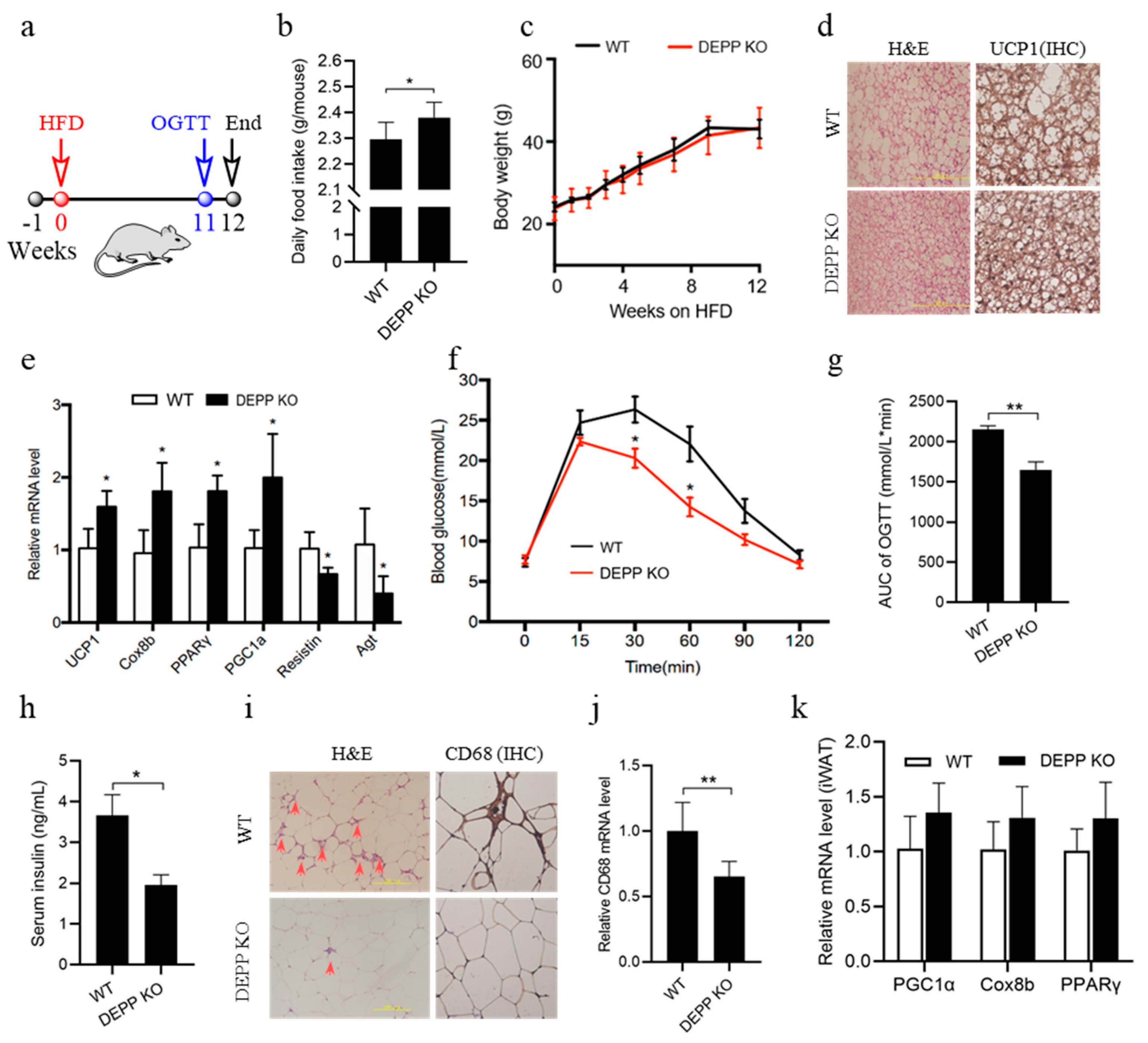
2.4. DEPP Deficiency Improves Insulin Sensitivity under HFD Feeding in Mice
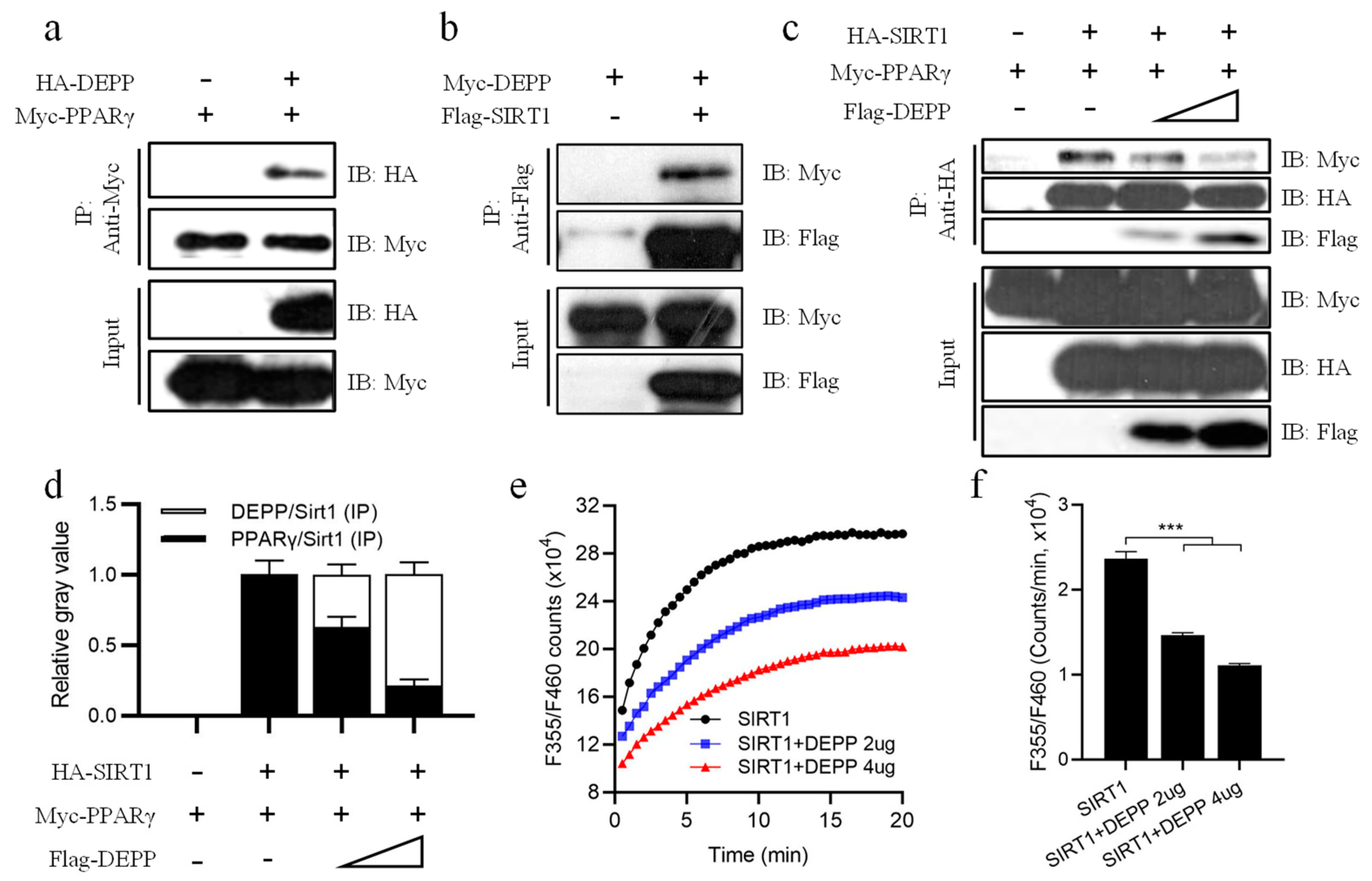
2.5. DEPP Competitively Binds SIRT1 with PPARγ
3. Discussion
4. Materials and Methods
4.1. mDEPP Protein Purification and Primary Antibody Production
4.2. SIRT1 Deacetylase Assay
4.3. T3-L1 Culture and Differentiation Induction
4.4. Mice Embryonic Fibroblasts’ (MEFs) Isolation and Differentiation
4.5. Oil Red O Staining
4.6. Lentivirus-Mediated DEPP Knockdown
4.7. CO-IP and Western Blot
4.8. DEPP Knockout Mice Production
4.9. Animal Treatment
4.10. H&E Staining and IHC Staining
4.11. OGTT and Insulin Test
4.12. Total RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravussin, E.; Galgani, J.E. The implication of brown adipose tissue for humans. Annu. Rev. Nutr. 2011, 31, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurylowicz, A.; Puzianowska-Kuznicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Picard, F.; Gehin, M.; Annicotte, J.; Rocchi, S.; Champy, M.F.; O’Malley, B.W.; Chambon, P.; Auwerx, J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 2002, 111, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Cederberg, A.; Gronning, L.M.; Ahren, B.; Tasken, K.; Carlsson, P.; Enerback, S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001, 106, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Powelka, A.M.; Seth, A.; Virbasius, J.V.; Kiskinis, E.; Nicoloro, S.M.; Guilherme, A.; Tang, X.; Straubhaar, J.; Cherniack, A.D.; Parker, M.G.; et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J. Clin. Investig. 2006, 116, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Tsukiyama-Kohara, K.; Poulin, F.; Kohara, M.; DeMaria, C.T.; Cheng, A.; Wu, Z.; Gingras, A.C.; Katsume, A.; Elchebly, M.; Spiegelman, B.M.; et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat. Med. 2001, 7, 1128–1132. [Google Scholar] [CrossRef]
- Scime, A.; Grenier, G.; Huh, M.S.; Gillespie, M.A.; Bevilacqua, L.; Harper, M.E.; Rudnicki, M.A. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005, 2, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Vernochet, C.; Peres, S.B.; Davis, K.E.; McDonald, M.E.; Qiang, L.; Wang, H.; Scherer, P.E.; Farmer, S.R. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol. Cell. Biol. 2009, 29, 4714–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Feng, X.; Qiu, L.; Pan, Z.; Wang, R.; Lin, S.; Hou, D.; Jin, L.; Li, Y. Identification of the antibiotic ionomycin as an unexpected peroxisome proliferator-activated receptor gamma (PPARgamma) ligand with a unique binding mode and effective glucose-lowering activity in a mouse model of diabetes. Diabetologia 2013, 56, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Banks, A.S.; Kon, N.; Knight, C.; Matsumoto, M.; Gutierrez-Juarez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Nonoguchi, K.; Sakurai, T.; Masuda, T.; Itoh, K.; Fujita, J. A novel protein Depp, which is induced by progesterone in human endometrial stromal cells activates Elk-1 transcription factor. Mol. Hum. Reprod. 2005, 11, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Anderson, D.J. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1589–1604. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kuriyama, H.; Kihara, S.; Kishida, K.; Maeda, N.; Hibuse, T.; Nishizawa, H.; Matsuda, M.; Funahashi, T.; Shimomura, I. Insulin-mediated regulation of decidual protein induced by progesterone (DEPP) in adipose tissue and liver. Horm. Metab. Res. Horm.-Und Stoffwechs. Horm. Metab. 2010, 42, 173–177. [Google Scholar] [CrossRef]
- Omran, F.; Christian, M. Inflammatory Signaling and Brown Fat Activity. Front. Endocrinol. 2020, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Koza, R.A.; Nikonova, L.; Hogan, J.; Rim, J.S.; Mendoza, T.; Faulk, C.; Skaf, J.; Kozak, L.P. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006, 2, e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, T.P.; Kim, H.Y.; Saxton, A.M.; Kim, J.H. Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J x TALLYHO/JngJ) F2 mice. BMC Genom. 2010, 11, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, C.M.; Gomez-Llorente, C.; Tofe, I.; Gil-Campos, M.; Canete, R.; Gil, A. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int. J. Mol. Sci. 2015, 16, 7723–7737. [Google Scholar] [CrossRef]
- Li, W.; Ji, M.; Lin, Y.; Miao, Y.; Chen, S.; Li, H. DEPP/DEPP1/C10ORF10 regulates hepatic glucose and fat metabolism partly via ROS-induced FGF21. FASEB J. 2018, 32, 5459–5469. [Google Scholar] [CrossRef] [Green Version]
- Schlogl, M.; Piaggi, P.; Pannacciuli, N.; Bonfiglio, S.M.; Krakoff, J.; Thearle, M.S. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated With Weight Change. Diabetes 2015, 64, 3680–3689. [Google Scholar] [CrossRef] [Green Version]
- Chatamra, K.; Daniel, P.M.; Lam, D.K. The effects of fasting on core temperature, blood glucose and body and organ weights in rats. Q. J. Exp. Physiol. 1984, 69, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, C.M.; Hojlund, K.; Hansen, L.; Oakeley, E.J.; Hemmings, B.; Abdallah, B.M.; Brusgaard, K.; Beck-Nielsen, H.; Gaster, M. Transcriptional profiling of myotubes from patients with type 2 diabetes: No evidence for a primary defect in oxidative phosphorylation genes. Diabetologia 2008, 51, 2068–2077. [Google Scholar] [CrossRef] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, M.; Ammerpohl, O.; von Schonfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, H.; et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Mercader, J.; Palou, A.; Bonet, M.L. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity 2010, 18, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Abella, A.; Dubus, P.; Malumbres, M.; Rane, S.G.; Kiyokawa, H.; Sicard, A.; Vignon, F.; Langin, D.; Barbacid, M.; Fajas, L. Cdk4 promotes adipogenesis through PPAR gamma activation. Cell Metab. 2005, 2, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Ng, W.W.; Jin, L.H.; Ye, Z.; Han, J.; Lin, S.C. Axin utilizes distinct regions for competitive MEKK1 and MEKK4 binding and JNK activation. J. Biol. Chem. 2003, 278, 37451–37458. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Zhu, Y.; Han, Y.; Feng, X.; Pan, Z.; He, Y.; Li, Y.; Jin, L. DEPP Deficiency Contributes to Browning of White Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 6563. https://doi.org/10.3390/ijms23126563
Guo F, Zhu Y, Han Y, Feng X, Pan Z, He Y, Li Y, Jin L. DEPP Deficiency Contributes to Browning of White Adipose Tissue. International Journal of Molecular Sciences. 2022; 23(12):6563. https://doi.org/10.3390/ijms23126563
Chicago/Turabian StyleGuo, Fusheng, Yanlin Zhu, Yaping Han, Xuhui Feng, Zhifu Pan, Ying He, Yong Li, and Lihua Jin. 2022. "DEPP Deficiency Contributes to Browning of White Adipose Tissue" International Journal of Molecular Sciences 23, no. 12: 6563. https://doi.org/10.3390/ijms23126563
APA StyleGuo, F., Zhu, Y., Han, Y., Feng, X., Pan, Z., He, Y., Li, Y., & Jin, L. (2022). DEPP Deficiency Contributes to Browning of White Adipose Tissue. International Journal of Molecular Sciences, 23(12), 6563. https://doi.org/10.3390/ijms23126563





