Editorial to the Special Issue: “Dysregulation of Human Molecular and Metabolic Mechanisms Resulting in Oxidative Stress and Damage Generation in the Space Environment”
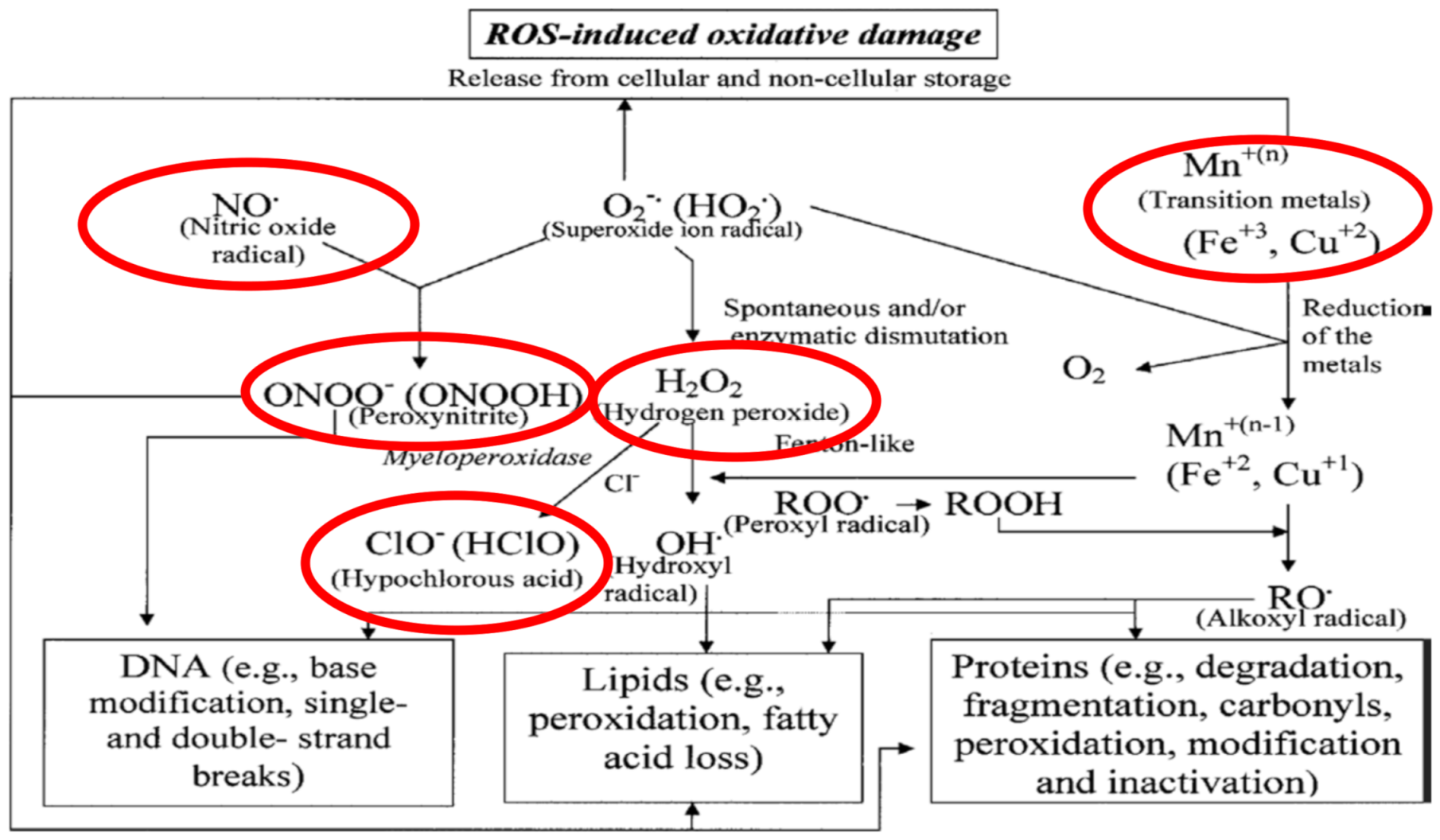
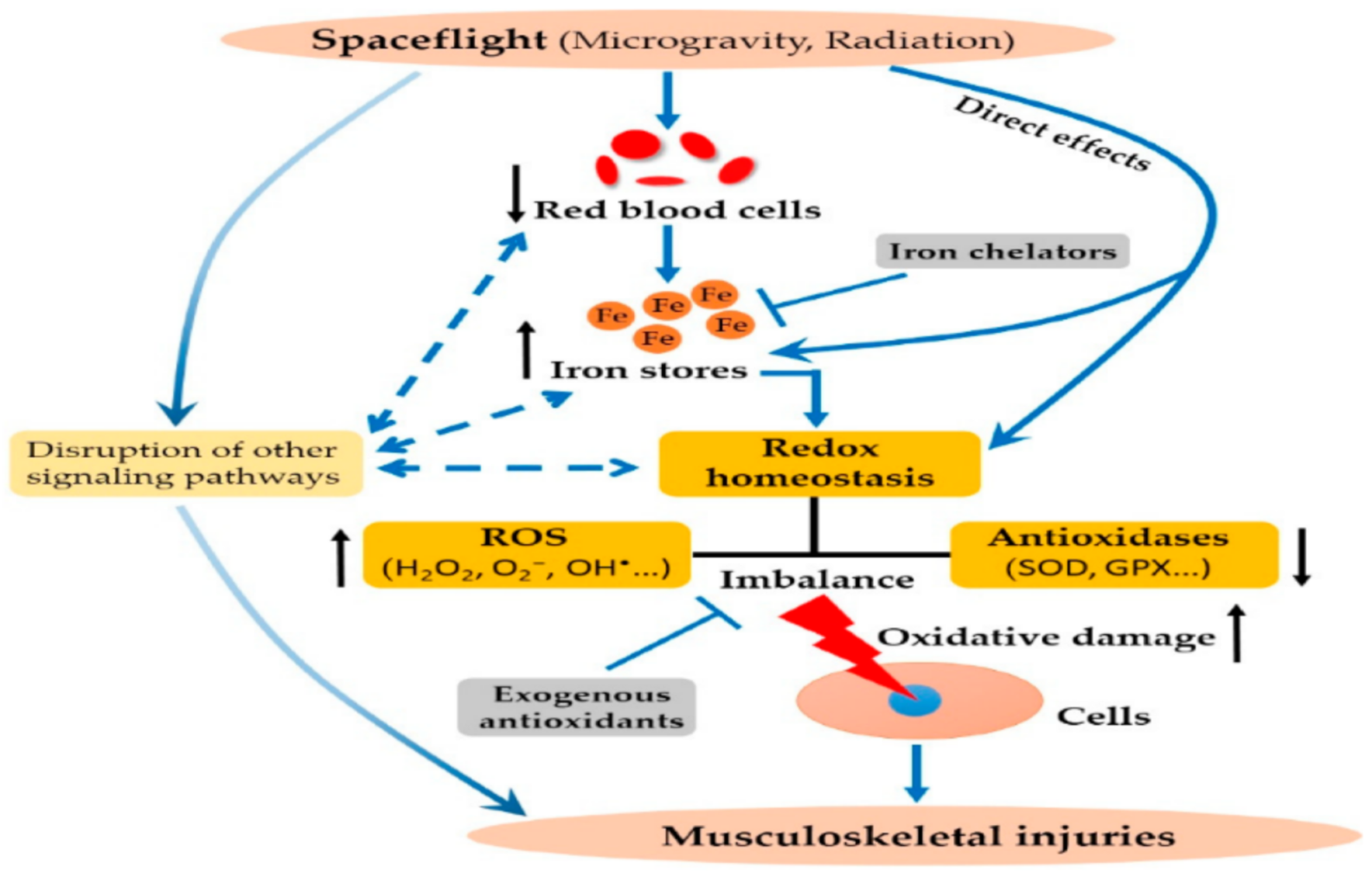
1. Introduction
Funding
Conflicts of Interest
References
- Weinzierl, M.; Sarang, M. The Commercial Space Age Is Here, Private Space Travel Is just the Beginning, Harvard Business Review. 12 February 2021. Available online: https://hbr.org/2021/02/the-commercial-space-age-is-here (accessed on 11 May 2022).
- Rayl, A.J.S. Wheels Down. In MARS: Secrets of the Planet Next Door; Popular Science Special Edition; Popular Science: New York, NY, USA, 2022. [Google Scholar]
- Rayl, A.J.S. The Real Challenges of Getting Humans to Mars. In MARS: Secrets of the Planet Next Door; Popular Science Special Edition; Popular Science: New York, NY, USA, 2022. [Google Scholar]
- Schmidt, M.A.; Goodwin, T.J. Personalized medicine in human space flight: Using Omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics 2013, 9, 1134–1156. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G.; Rodriguez, H. Critical Reviews in Oxidative Stress and Aging: Advances in Basic Science, Diagnostics and Intervention; World Scientific: Singapore, 2003; Volume 2, 1523p, ISBN 9789814490948. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Schmidt, C.M.; Goodwin, T.J. Pharmacogenomics in Spaceflight. In Handbook of Space Pharmaceuticals; Pathak, Y., Araújo dos Santos, M., Zea, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–39. [Google Scholar] [CrossRef]
- Goodwin, T.J.; Christofidou-Solomidou, M. Oxidative stress and space biology: An organ-based approach. Int. J. Mol. Sci. 2018, 19, 959. [Google Scholar] [CrossRef] [PubMed]
- Laiakis, E.C.; Shuryak, I.; Deziel, A.; Wang, Y.-W.; Barnette, B.L.; Yu, Y.; Ullrich, R.L.; Fornace, A.J., Jr.; Emmett, M.R. Effects of low dose space radiation exposures on the splenic metabolome. Int. J. Mol. Sci. 2021, 22, 3070. [Google Scholar] [CrossRef]
- Barnette, B.L.; Yu, Y.; Ullrich, R.L.; Emmett, M.R. Mitochondrial effects in the liver of C57BL/6 mice by low dose, high energy, high charge irradiation. Int. J. Mol. Sci. 2021, 22, 11806. [Google Scholar] [CrossRef]
- Kim, H.-N.; Richardson, K.K.; Krager, K.J.; Ling, W.; Simmons, P.; Allen, A.R.; Aykin-Burns, N. Simulated galactic cosmic rays modify mitochondrial metabolism in osteoclasts, increase osteoclastogenesis and cause trabecular bone loss in mice. Int. J. Mol. Sci. 2021, 22, 11711. [Google Scholar] [CrossRef]
- Cahill, T.; Cope, H.; Bass, J.J.; Overbey, E.G.; Gilbert, R.; da Silveira, W.A.; Paul, A.M.; Mishra, T.; Herranz, R.; Reinsch, S.S.; et al. Mammalian and invertebrate models as complementary tools for gaining mechanistic insight on muscle responses to spaceflight. Int. J. Mol. Sci. 2021, 22, 9470. [Google Scholar] [CrossRef]
- Kumar, A.; Tahimic, C.G.T.; Almeida, E.A.C.; Globus, R.K. Spaceflight modulates the expression of key oxidative stress and cell cycle related genes in heart. Int. J. Mol. Sci. 2021, 22, 9088. [Google Scholar] [CrossRef]
- Klein, P.M.; Alaghband, Y.; Doan, N.-L.; Ru, N.; Drayson, O.G.G.; Baulch, J.E.; Kramár, E.A.; Wood, M.A.; Soltesz, I.; Limoli, C.L. Acute, low-dose neutron exposures adversely impact central nervous system function. Int. J. Mol. Sci. 2021, 22, 9020. [Google Scholar] [CrossRef]
- Krishnan, B.; Natarajan, C.; Bourne, K.Z.; Alikhani, L.; Wang, J.; Sowa, A.; Groen, K.; Perry, B.; Dickstein, D.L.; Baulch, J.E.; et al. Chronic low dose neutron exposure results in altered neurotransmission properties of the hippocampus-prefrontal cortex axis in both mice and rats. Int. J. Mol. Sci. 2021, 22, 3668. [Google Scholar] [CrossRef]
- Beheshti, A.; McDonald, J.T.; Hada, M.; Takahashi, A.; Mason, C.E.; Mognato, M. Genomic changes driven by radiation-induced DNA damage and microgravity in human cells. Int. J. Mol. Sci. 2021, 22, 10507. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Goodwin, T.J.; Pelligra, R. Incorporation of omics analyses into artificial gravity research for space exploration countermeasure development. Metabolomics 2016, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Kurth, L.M.; McCawley, M.; Hendryx, M.; Lusk, S. Atmospheric particulate matter size distribution and concentration in West Virginia coal mining and non-mining areas. J. Expo Sci. Environ. Epidemiol. 2014, 24, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Salm, A.K.; Benson, M.J. Increased dementia mortality in west virginia counties with mountaintop removal mining? Int. J. Environ. Res. Public Health 2019, 16, 4278. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M. Personal and family health in rural areas of Kentucky with and without mountaintop coal mining. J. Rural Health 2013, 29 (Suppl. 1), s79–s88. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- McCawley, M.; Knuckles, T. Toxicology of nano- and ultrafine particles. In Toxicology Principles for the Industrial Hygienist, 2nd ed.; Lutrell, W.E., Jederberg, W.W., Still, K.R., Eds.; American Industrial Hygiene Association: Falls Church, VA, USA, 2019; Chapter 24. [Google Scholar]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM2.5 Exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspect 2016, 124, 23–29. Available online: https://ehp.niehs.nih.gov/doi/10.1289/ehp.1408973 (accessed on 11 May 2022). [CrossRef]
- Cauda, E.; Chubb, L.G.; Miller, A.L. Mining Publication: Silica Adds to Respirable Dust Concerns: What If You Could Know the Silica Dust Levels in a Coal Mine After Every Shift? The National Institute for Occupational Safety and Health (NIOSH). 2016. Available online: https://www.cdc.gov/niosh/mining/works/coversheet1967.html#:~:text=Exposure%20to%20silica%20in%20coal,in%20space%20and%20over%20time (accessed on 11 May 2022).
- Moreno, T.; Trechera, P.; Querol, X.; Lah, R.; Johnson, D.; Wrana, A.; Williamson, B. Trace element fractionation between PM10 and PM2.5 in coal mine dust: Implications for occupational respiratory health. Int. J. Coal Geol. 2019, 203, 52–59. [Google Scholar] [CrossRef]
- Lemmon, M.T.; Wolff, M.J.; Smith, M.D.; Clancy, R.T.; Banfield, D.; Landis, G.A.; Ghosh, A.; Smith, P.H.; Spanovich, N.; Whitney, B.; et al. Atmospheric imaging results from the Mars exploration rovers: Spirit and Opportunity. Science 2004, 306, 1753–1756. Available online: https://www.science.org/doi/10.1126/science.1104474 (accessed on 11 May 2022). [CrossRef]
- Krisanova, N.; Kasatkina, L.; Sivko, R.; Borysov, A.; Nazarova, A.; Slenzka, K.; Borisova, T. Neurotoxic potential of lunar and martian dust: Influence on em, proton gradient, active transport, and binding of glutamate in rat brain nerve terminals. Astrobiology 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Dong, D.; Shang, P. Effects of iron overload and oxidative damage on the musculoskeletal system in the space environment: Data from spaceflights and ground-based simulation models. Int. J. Mol. Sci. 2018, 19, 2608. [Google Scholar] [CrossRef] [PubMed]
- Eric, M. NASA Wants to Set a New Radiation Limit for Astronauts, Wired Science. Available online: https://www.wired.com/story/nasa-wants-to-set-a-new-radiation-limit-for-astronauts/ (accessed on 11 February 2021).
- NASA Technical Standard NASA-STD-3001; NASA Space Flight Human-System Standard Volume 1, Revision a: Crew Health. National Aeronautics and Space Administration: Washington, DC, USA, 2015; Volume 1. Available online: https://www.nasa.gov/sites/default/files/atoms/files/nasa-std-3001-vol-1a-chg1.pdf (accessed on 11 May 2022).
- Parihar, V.; Allen, B.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.; Chmielewski, N.; Giedzinski, E.; Acharya, M.; Britten, R.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Baulch, J.; Klein, P.; Baddour, A.; Apodaca, L.; Kramár, E.; Alikhani, E.; Garcia, C.; Angulo, M.; Batra, R.; et al. New concerns for neurocognitive function during deep space exposures to chronic, low dose-rate, neutron radiation. Eneuro 2019, 6, ENEURO.0094-19.2019. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodwin, T.J.; Christofidou-Solomidou, M. Editorial to the Special Issue: “Dysregulation of Human Molecular and Metabolic Mechanisms Resulting in Oxidative Stress and Damage Generation in the Space Environment”. Int. J. Mol. Sci. 2022, 23, 6466. https://doi.org/10.3390/ijms23126466
Goodwin TJ, Christofidou-Solomidou M. Editorial to the Special Issue: “Dysregulation of Human Molecular and Metabolic Mechanisms Resulting in Oxidative Stress and Damage Generation in the Space Environment”. International Journal of Molecular Sciences. 2022; 23(12):6466. https://doi.org/10.3390/ijms23126466
Chicago/Turabian StyleGoodwin, Thomas J., and Melpo Christofidou-Solomidou. 2022. "Editorial to the Special Issue: “Dysregulation of Human Molecular and Metabolic Mechanisms Resulting in Oxidative Stress and Damage Generation in the Space Environment”" International Journal of Molecular Sciences 23, no. 12: 6466. https://doi.org/10.3390/ijms23126466
APA StyleGoodwin, T. J., & Christofidou-Solomidou, M. (2022). Editorial to the Special Issue: “Dysregulation of Human Molecular and Metabolic Mechanisms Resulting in Oxidative Stress and Damage Generation in the Space Environment”. International Journal of Molecular Sciences, 23(12), 6466. https://doi.org/10.3390/ijms23126466





