Crosstalk between the Circadian Clock and Histone Methylation
Abstract
:1. Introduction
2. Results
2.1. SDG724 Has a Huge Influence on the Function of OsLHY
2.2. OsLHY Has a Huge Influence on the Function of SDG724
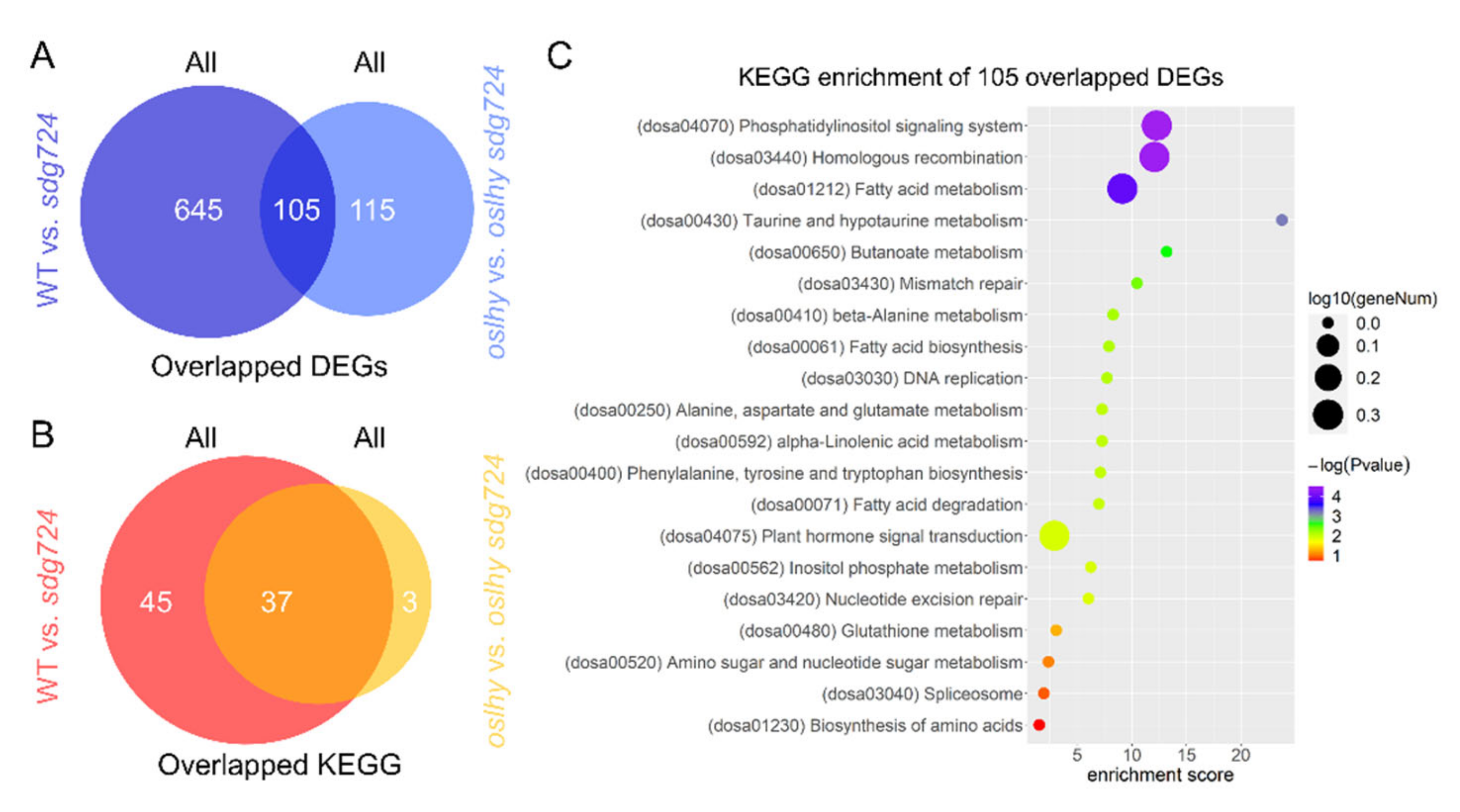
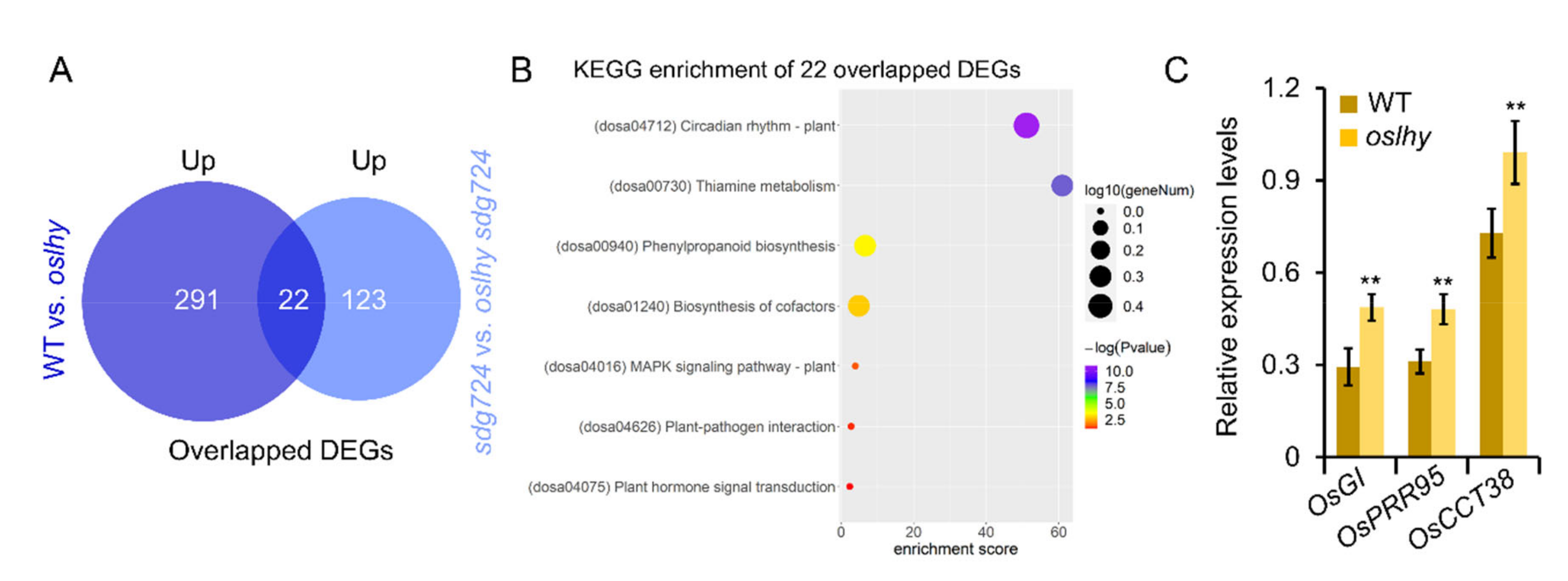
2.3. Exploring the Targets of OsLHY in the Clock Pathway
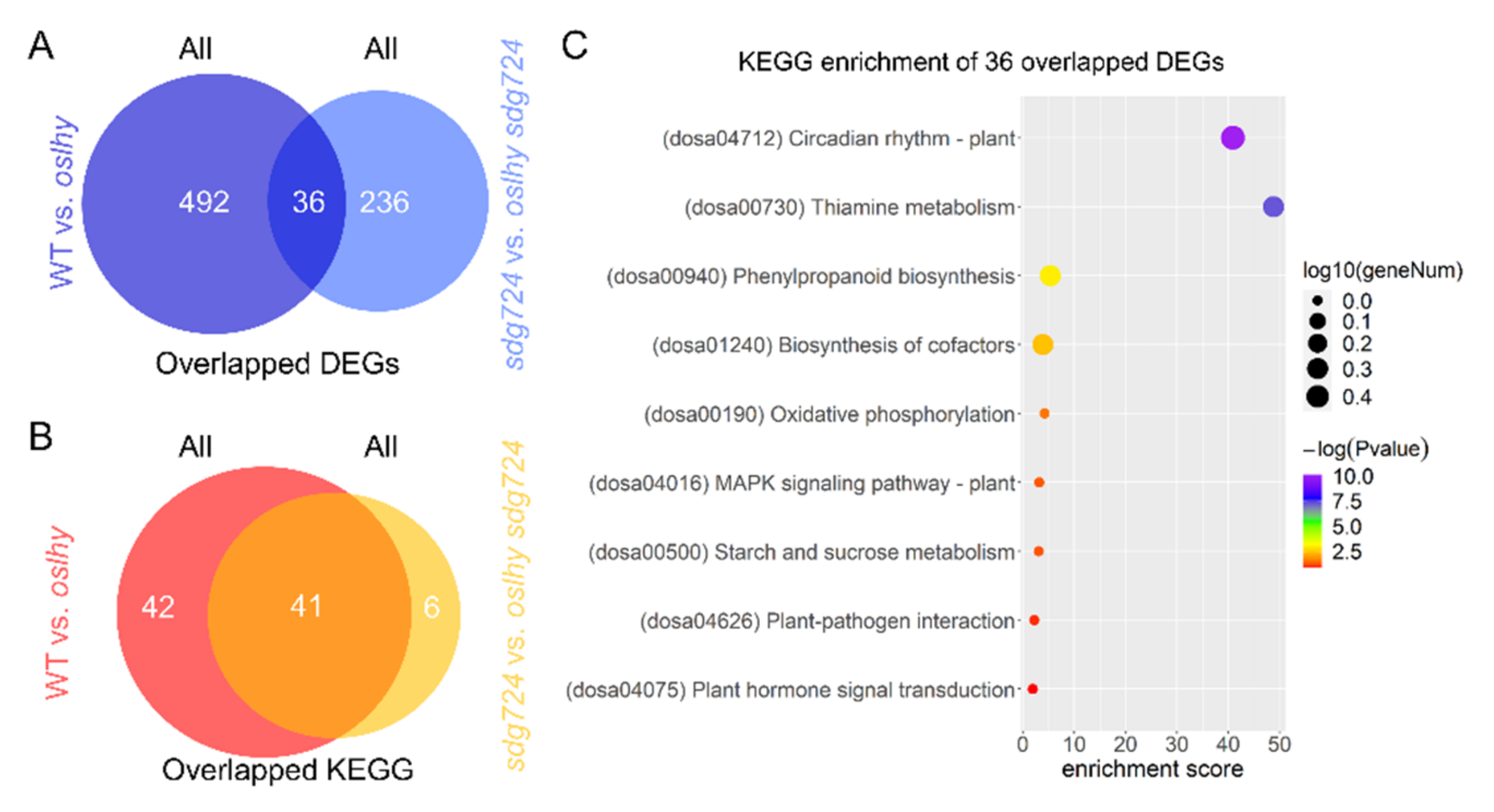
2.4. Exploring the Targets of SDG724 in the Clock Pathway
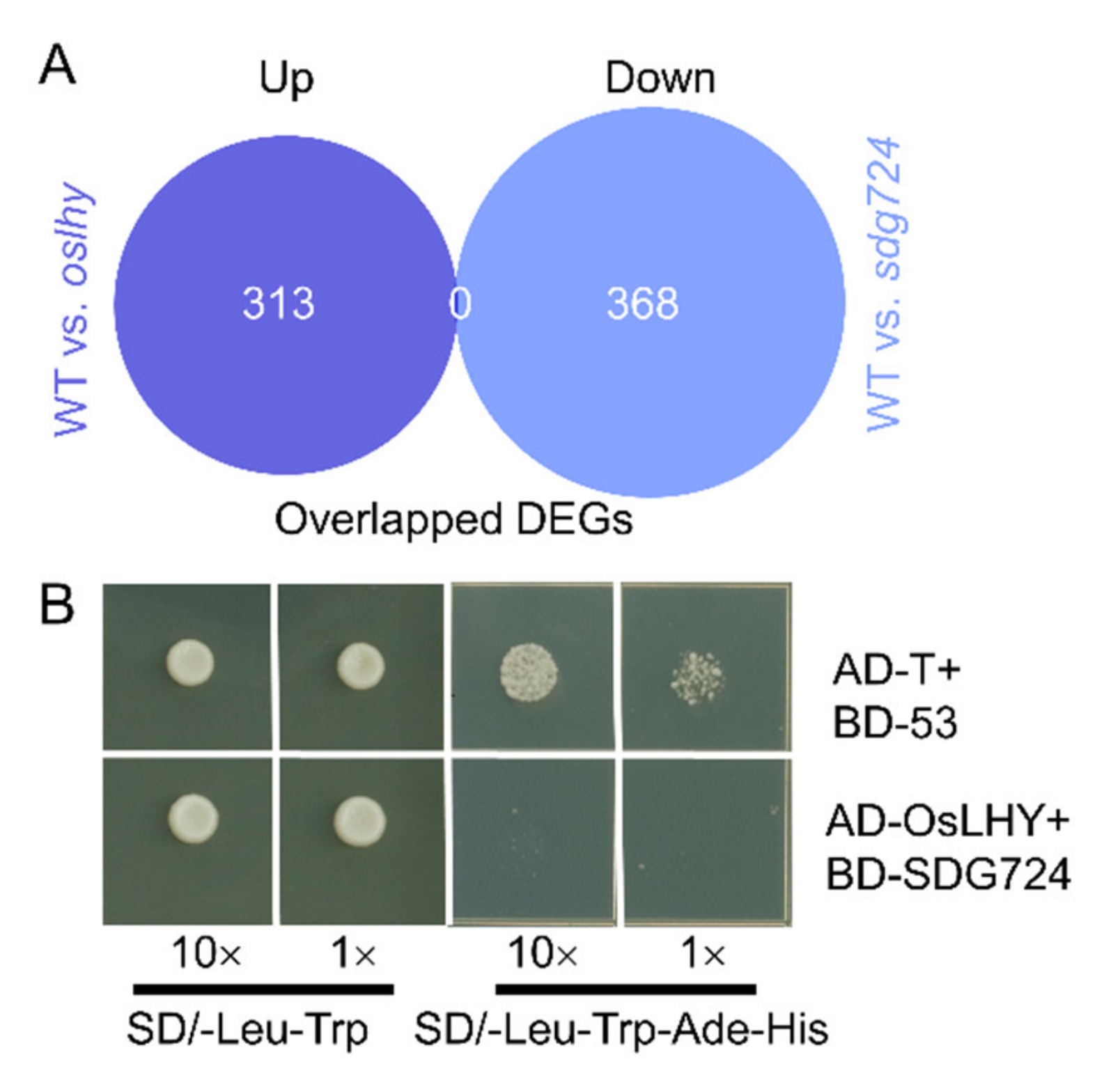
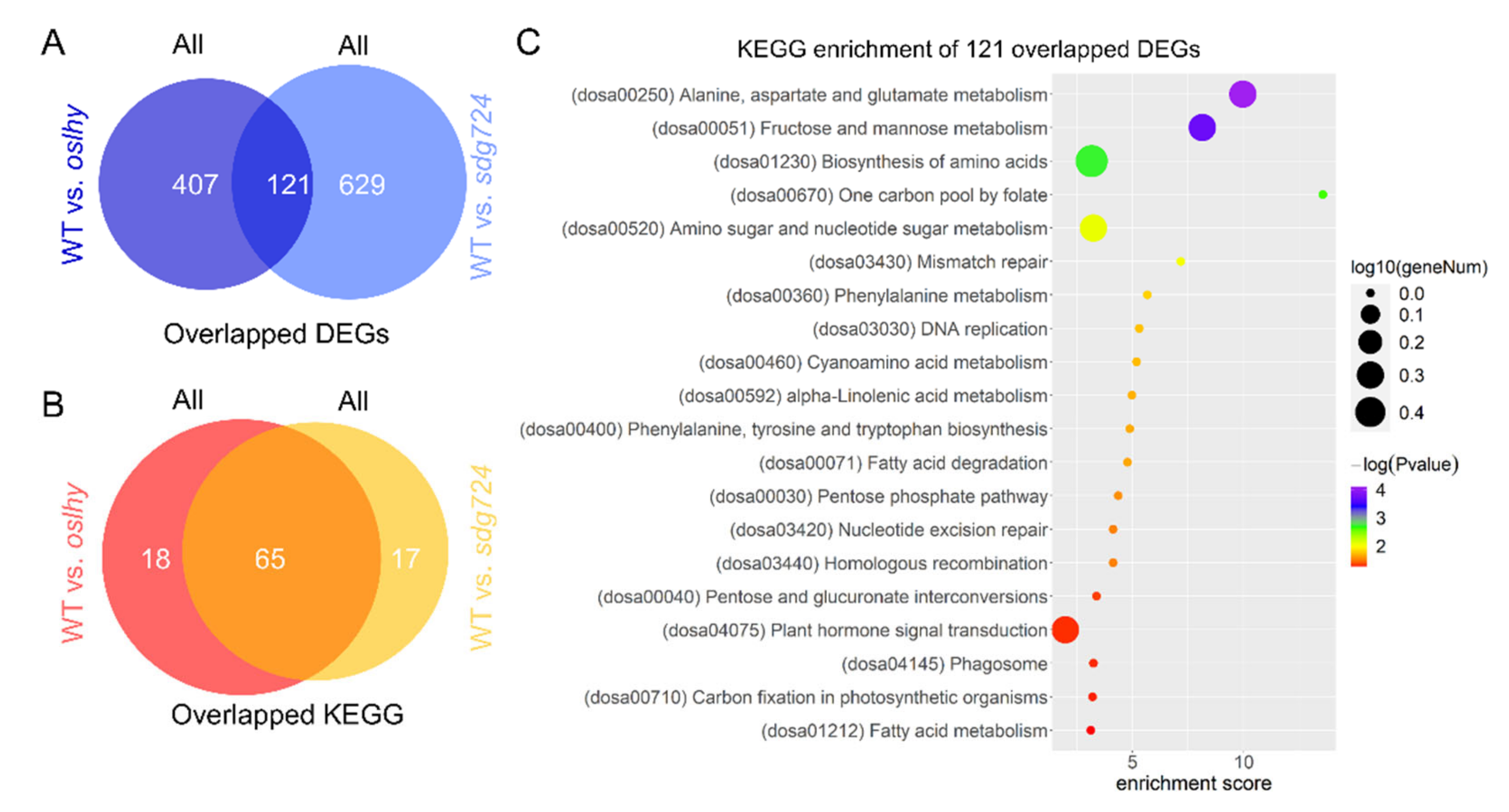
2.5. OsLHY and SDG724 Might Not Share the Same Target
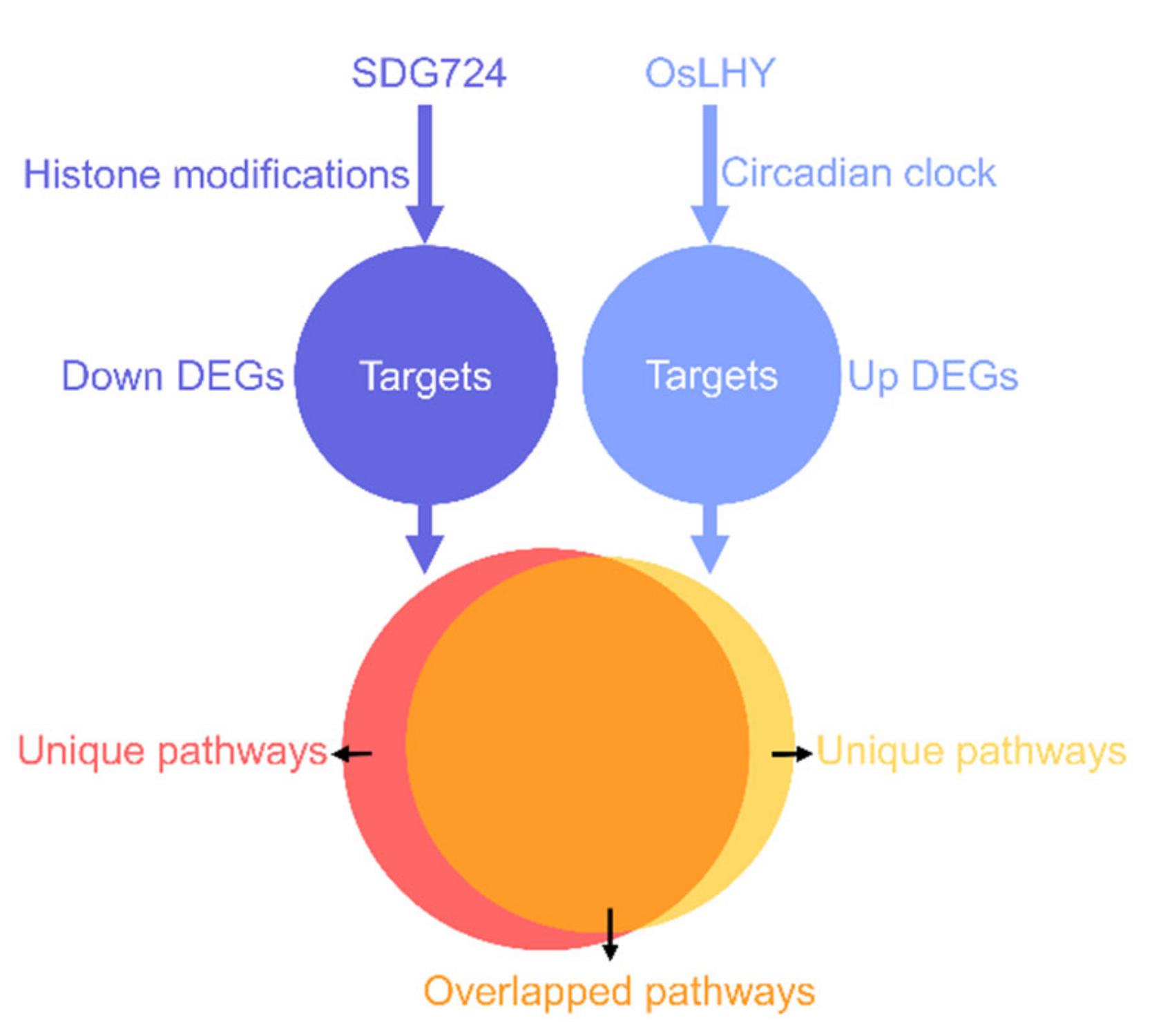
2.6. Most of the Pathways Controlled by OsLHY and SDG724 Merged
3. Discussion
4. Materials and Methods
4.1. RNA Sequencing and Analysis
4.2. DEGs and KEGG Pathway Investigation
4.3. qRT-PCR Analysis
4.4. Y2H Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClung, C.R. The Plant Circadian Oscillator. Biology 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.; Chen, L.; Ge, L.; Huang, W. A novel loop: Mutual regulation between epigenetic modification and the circadian clock. Front. Plant Sci. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Wang, J.; Huang, F.-Y.; Yang, S.; Wu, K. The plant circadian clock and chromatin modifications. Genes 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Zhang, K.; Zhou, Y.; Xiang, L.; He, C.; Zhong, C.; Li, K.; Wang, Q.; Yang, C.; Wang, Q.; et al. Dual function of clock component OsLHY sets critical day length for photoperiodic flowering in rice. Plant Biotechnol. J. 2021, 19, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, J.; Wagner, D. Histone modifications and dynamic regulation of genome accessibility in plants. Curr. Opin. Plant Biol. 2007, 10, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, F.-Y.; Chen, F.-F.; Li, C.; Chen, C.; Lai, Y.-C.; Chen, J.-H.; Cui, Y.; Wu, K. The Arabidopsis LDL1/2-HDA6 histone modification complex is functionally associated with CCA1/LHY in regulation of circadian clock genes. Nucleic Acids Res. 2018, 46, 10669–10681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Fang, J.; Zhao, T.; Xu, B.; Zhang, F.; Liu, L.; Tang, J.; Zhang, G.; Deng, X.; Chen, F.; et al. The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 2012, 24, 3235–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayama, R.; Yokoi, S.; Tamaki, S.; Yano, M.; Shimamoto, K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 2003, 422, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, X.; Hu, Y.; Zhou, X.; He, Q.; Liang, L.; Xing, Y. Global analysis of CCT family knockout mutants identifies four genes involved in regulating heading date in rice. J. Integr. Plant Biol. 2021, 63, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Sakakibara, H. Pseudo-response regulators 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 2010, 22, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, F.; Inagaki, N.; Hanada, A.; Yamaguchi, S.; Kamiya, Y.; Miyao, A.; Hirochika, H.; Takano, M. Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation. Plant Cell Physiol. 2012, 53, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Gong, S.F.; Li, Q.H.; Sang, Y.; Yang, H.Q. Functional and signaling mechanism analysis of rice Cryptochrome 1. Plant J. 2006, 46, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, T.; Song, Q.; Ye, W.; Song, X.; Chu, J.; Li, J.; Chen, Z.J. The rice circadian clock regulates tiller growth and panicle development through strigolactone signaling and sugar sensing. Plant Cell 2020, 32, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Li, K.; Yan, M.; Zhang, J.; Yu, M.; Tang, S.; Wang, L.; Qu, H.; Luo, L.; et al. Nitrogen Mediates Flowering Time and Nitrogen Use Efficiency via Floral Regulators in Rice. Curr. Biol. 2021, 31, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, H.; Su, C.; Wang, X.; Wang, L. Rice Circadian clock associated1 transcriptionally regulates ABA signaling to confer multiple abiotic stress tolerance. Plant Physiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.-J.; Yan, Y.; Alam, M.S.; Liu, Z.; Yang, Z.-K.; Tao, R.-F.; Yue, E.-K.; Duan, M.-H.; Xu, J.-H. OsLHY is involved in regulating flowering through the Hd1- and Ehd1-mediated pathways in rice (Oryza sativa L.). Plant Sci. 2022, 315, 111145. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Falcon, S.; Gentleman, R. Hypergeometric testing used for gene set enrichment analysis. In Bioconductor Case Studies; Springer: Berlin/Heidelberg, Germany, 2008; pp. 207–220. [Google Scholar]
- Chen, T.; Liu, Y.-X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Tian, X.; Li, X.; Zhou, W.; Ren, Y.; Wang, Z.; Liu, Z.; Tang, J.; Tong, H.; Fang, J.; Bu, Q. Transcription Factor OsWRKY53 Positively Regulates Brassinosteroid Signaling and Plant Architecture. Plant Physiol. 2017, 175, 1337–1349. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Liu, S.; He, C.; Zhong, C.; Liu, H.; Luo, X.; Li, K.; Zhang, K.; Wang, Q.; Chen, C.; et al. Crosstalk between the Circadian Clock and Histone Methylation. Int. J. Mol. Sci. 2022, 23, 6465. https://doi.org/10.3390/ijms23126465
Sun C, Liu S, He C, Zhong C, Liu H, Luo X, Li K, Zhang K, Wang Q, Chen C, et al. Crosstalk between the Circadian Clock and Histone Methylation. International Journal of Molecular Sciences. 2022; 23(12):6465. https://doi.org/10.3390/ijms23126465
Chicago/Turabian StyleSun, Changhui, Shihang Liu, Changcai He, Chao Zhong, Hongying Liu, Xu Luo, Ke Li, Kuan Zhang, Qian Wang, Congping Chen, and et al. 2022. "Crosstalk between the Circadian Clock and Histone Methylation" International Journal of Molecular Sciences 23, no. 12: 6465. https://doi.org/10.3390/ijms23126465
APA StyleSun, C., Liu, S., He, C., Zhong, C., Liu, H., Luo, X., Li, K., Zhang, K., Wang, Q., Chen, C., Tang, Y., Yang, B., Chen, X., Xu, P., Zou, T., Li, S., Qin, P., Wang, P., Chu, C., & Deng, X. (2022). Crosstalk between the Circadian Clock and Histone Methylation. International Journal of Molecular Sciences, 23(12), 6465. https://doi.org/10.3390/ijms23126465





