The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize
Abstract
:1. Introduction
2. Results
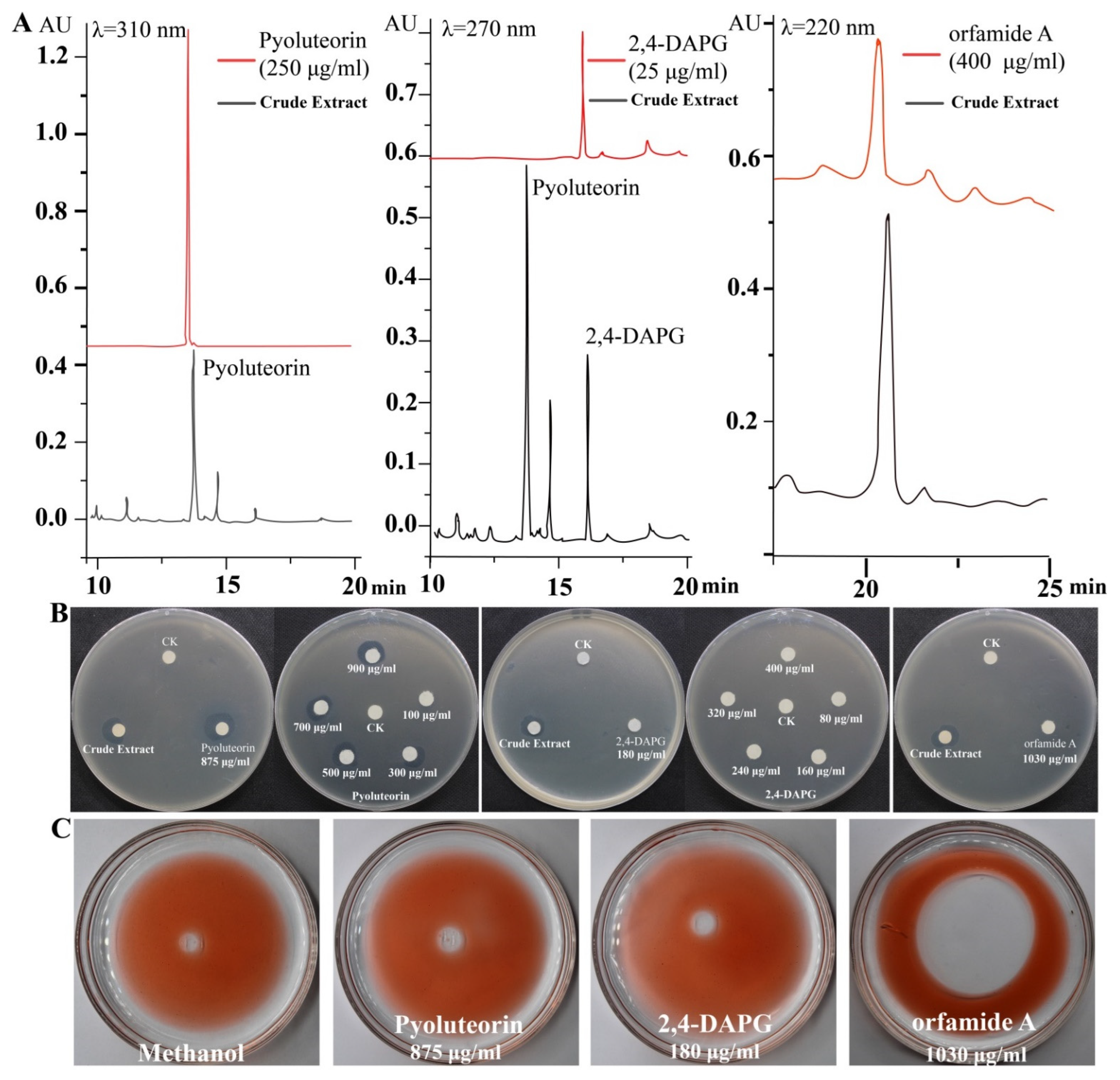
2.1. Antibacterial and Surface Activity of Crude Extract Prepared from P. protegens Pf-5
2.2. Pyoluteorin and Orfamide A produced by P. protegens Pf-5 Showed In Vitro Antibacterial and Surface Activity, Respectively
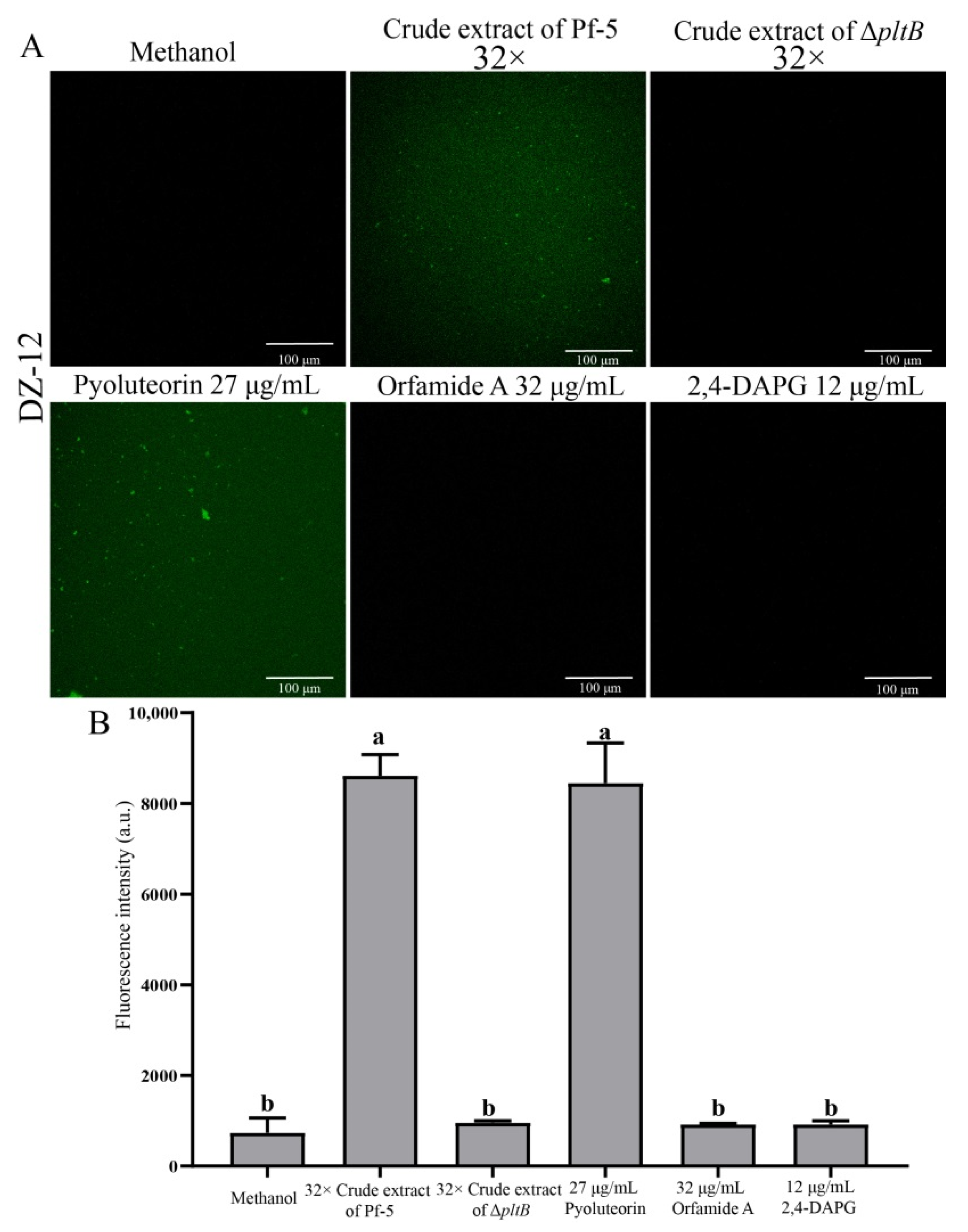
2.3. Pyoluteorin Produced by P. protegens Pf-5 Contributes to the Inhibitory Effect on Biofilm Formation of P. ananatis DZ-12
2.4. Induction of Reactive Oxygen Species in P. ananatis DZ-12 Exposed to Crude Extract Containing Pyoluteorin
2.5. Ultrastructural Changes in P. ananatis DZ-12 Caused by Crude Extract
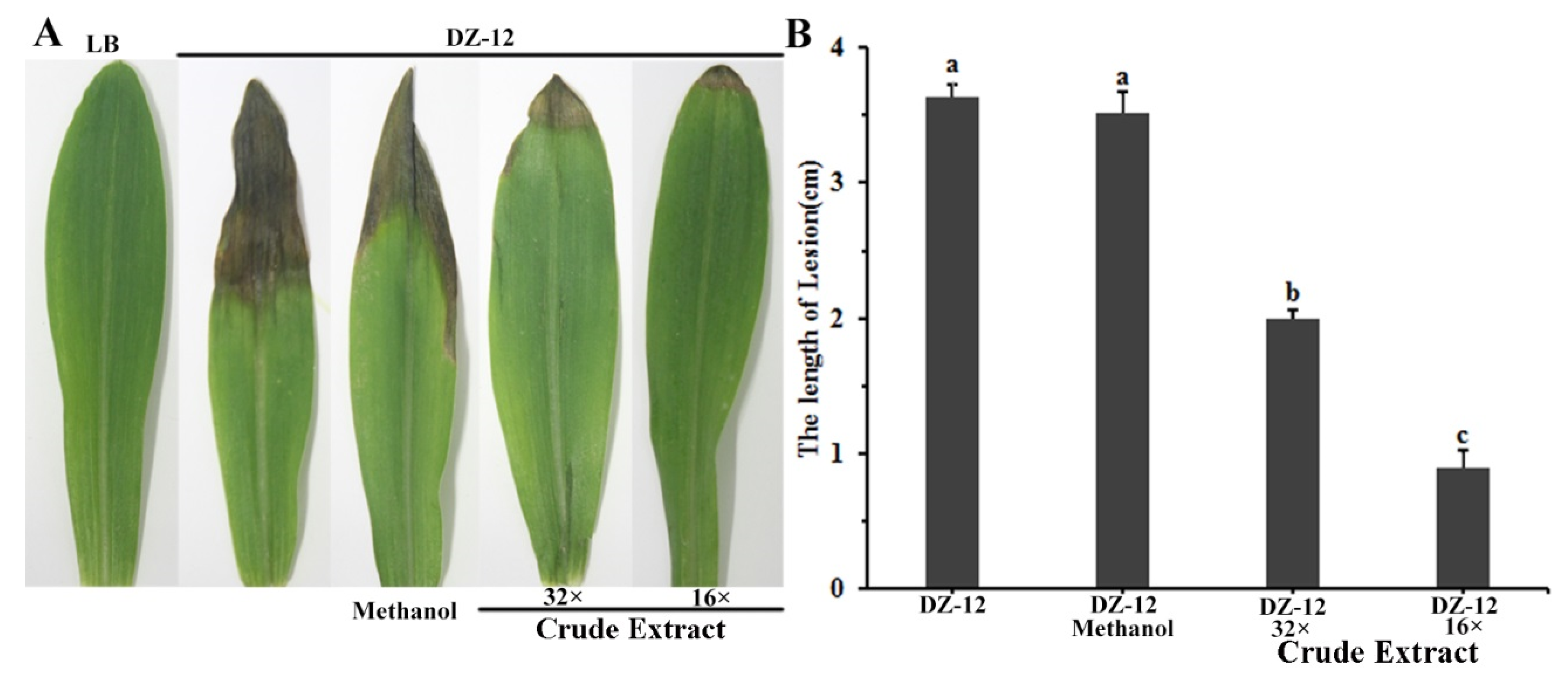
2.6. Crude Extract Suppresses the Virulence of Brown Rot of Maize Leaves Caused by P. ananatis DZ-12
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Preparation of Crude Extraction from P. protegens Pf-5
4.3. Construction of the Gene Knockout Mutants of P. protegens Pf-5
4.4. Antibacterial Activity Assay
4.5. Bio-Surfactant Activity Assay
4.6. Identification of Compounds Using High-Performance Liquid Chromatography Analysis
4.7. Observed Structural Changes in P. ananatis DZ-12 Caused by Crude Extract
4.8. Biofilm Formation
4.9. Reactive Oxygen Species Detection
4.10. Plant Infection Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Maayer, P.; Chan, W.Y.; Rubagotti, E.; Venter, S.N.; Toth, I.K.; Birch, P.R.J.; Coutinho, T.A. Analysis of the Pantoea ananatis pan-genome reveals factors underlying its ability to colonize and interact with plant, insect and vertebrate hosts. BMC Genom. 2014, 15, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Gao, L.; Huang, H.; Zhao, Y.; Hanif, A.; Wu, H.; Gu, Q.; Wu, L.; Gao, X. Exploring the pathogenic function of Pantoea ananatis endogenous plasmid by an efficient and simple plasmid elimination strategy. Microbiol. Res. 2021, 246, 126710. [Google Scholar] [CrossRef] [PubMed]
- Malagi, G.; Santos, I.D.; Camochena, R.C.; Moccellin, R. Diagrammatic scale for severity evaluation of maize white foliar spot. Rev. Cienc. Agron. 2011, 42, 797–804. [Google Scholar] [CrossRef]
- Mamede, M.C.; Tebaldi, N.D.; Mota, L.; Martins, O.M.; Coelho, L. Detection of Pantoea ananatis in corn seeds on semi-selective medium. Trop. Plant Pathol. 2018, 43, 254–256. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends. Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2019, 17, 119–128. [Google Scholar] [CrossRef]
- Walsh, U.F.; Morrissey, J.P.; O’Gara, F. Pseudomonas for biocontrol of phytopathogens: From functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 2001, 12, 289–295. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas Biocontrol Agents of Soilborne Pathogens: Looking Back Over 30 Years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.R.; Stipanovic, R.D. Control of Rhizoctonia solani on cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. Phytopathology 1979, 69, 480–482. [Google Scholar] [CrossRef] [Green Version]
- Shaukat, S.S.; Siddiqui, I.A. Impact of biocontrol agents Pseudomonas fluorescens CHA0 and its genetically modified derivatives on the diversity of culturable fungi in the rhizosphere of mungbean. J. Appl. Microbiol. 2003, 95, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gu, G.; Chen, W.; Gao, L.; Wu, X.; Zhang, L. The outer membrane protein OprF and the sigma factor SigX regulate antibiotic production in Pseudomonas fluorescens 2P24. Microbiol. Res. 2018, 206, 159–167. [Google Scholar] [CrossRef]
- Yue, S.; Huang, P.; Li, S.; Jan, M.; Hu, H.; Wang, W.; Zhang, X. Enhanced Production of 2-Hydroxyphenazine from Glycerol by a Two-Stage Fermentation Strategy in Pseudomonas chlororaphis GP72AN. J. Agric. Food Chem. 2020, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Slininger, P.J.; Burkhead, K.D.; Schisler, D.A.; Bothast, R.J. Isolation, identification, and accumulation of 2-acetamidophenol in liquid cultures of the wheat take-all biocontrol agent Pseudomonas fluorescens 2–79. Appl. Microbiol. Biotechnol. 2000, 54, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hu, H.; Wang, W.; Zhang, X. Genetic engineering of Pseudomonas chlororaphis GP72 for the enhanced production of 2-Hydroxyphenazine. Microb. Cell Fact. 2016, 15, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, S.L.F.; Everts, K.L.; Gardener, B.M.; Masler, E.P.; Abdelnabby, H.M.E.; Skantar, A.M. Assessment of DAPG-producing Pseudomonas fluorescens for Management of Meloidogyne incognita and Fusarium oxysporum on Watermelon. J. Nematol. 2016, 48, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Loper, J.E.; Henkels, M.D.; Shaffer, B.T.; Valeriote, F.A.; Gross, H. Isolation and identification of rhizoxin analogs from Pseudomonas fluorescens Pf-5 by using a genomic mining strategy. Appl. Environ. Microbiol. 2008, 74, 3085–3093. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, I.T.; Press, C.M.; Ravel, J.; Kobayashi, D.Y.; Myers, G.S.A.; Mavrodi, D.V.; DeBoy, R.T.; Seshadri, R.; Ren, Q.; Madupu, R.; et al. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 2005, 23, 873–878. [Google Scholar] [CrossRef]
- Gross, H.; Stockwell, V.O.; Henkels, M.D.; Nowak-Thompson, B.; Loper, J.E.; Gerwick, W.H. The Genomisotopic Approach: A Systematic Method to Isolate Products of Orphan Biosynthetic Gene Clusters. Chem. Biol. 2007, 14, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Qiao, J.; Li, P.; Zhang, L.; Qiao, Z.; Lin, L.; Yu, C.; Yang, Y.; Zubair, M.; Gu, Q.; et al. novel Rap-Phr system in Bacillus velezensis NAU-B3 regulates surfactin production and sporulation via interaction with ComA. Appl. Microbiol. Biotechnol. 2020, 104, 10059–10074. [Google Scholar] [CrossRef] [PubMed]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging11This article is dedicated to the memory of our dear friend, colleague, and mentor Lars Ernster (1920–1998), in gratitude for all he gave to us. Free Radical Bio. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Smith, D.D.N.; Kirzinger, M.W.B.; Stavrinides, J. Draft Genome Sequence of the Antibiotic-Producing Epiphytic Isolate Pantoea ananatis BRT175. Genome Announc. 2013, 1, e00902-13. [Google Scholar] [CrossRef] [Green Version]
- Kraus, J.; Loper, J.E. Characterization of a Genomic Region Required for Production of the Antibiotic Pyoluteorin by the Biological Control Agent Pseudomonas fluorescens Pf-5. Appl. Environ. Microb. 1995, 61, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Maurhofer, M.; Keel, C.; Haas, D.; Défago, G. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur. J. Plant Pathol. 1994, 100, 221–232. [Google Scholar] [CrossRef]
- Fan, D.; Yu, S.; Yang, Y.; Qu, S. Pyoluteorin Induces Apoptosis and Autophagy in NSCLC Cells. Biol. Pharm. Bull. 2021, 44, 976–983. [Google Scholar] [CrossRef]
- Ding, T.; Yang, L.; Zhang, W.; Shen, Y. Pyoluteorin induces cell cycle arrest and apoptosis in human triple-negative breast cancer cells MDA-MB-231. J. Pharm. Pharmacol. 2020, 72, 969–978. [Google Scholar] [CrossRef]
- Rose, M.M.; Scheer, D.; Hou, Y.; Hotter, V.S.; Komor, A.J.; Aiyar, P.; Scherlach, K.; Vergara, F.; Yan, Q.; Loper, J.E.; et al. The bacterium Pseudomonas protegens antagonizes the microalga Chlamydomonas reinhardtii using a blend of toxins. Environ. Microbiol. 2021, 23, 5525–5540. [Google Scholar] [CrossRef]
- Pellicciaro, M.; Padoan, E.; Lione, G.; Celi, L.; Gonthier, P. Pyoluteorin Produced by the Biocontrol Agent Pseudomonas protegens Is Involved in the Inhibition of Heterobasidion Species Present in Europe. Pathogens 2022, 11, 391. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G.; et al. 1,2,4-Oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem. 2021, 209, 112892. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.J.; Reed, C.S.; Melander, C. Effects of N-pyrrole substitution on the anti-biofilm activities of oroidin derivatives against Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2008, 18, 4325–4327. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Fang, F.; Li, Y. Synthesis and anti-biofilm activities of dihydro-pyrrol-2-one derivatives on Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2015, 25, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X.; et al. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microb. 2017, 83, e01075-17. [Google Scholar] [CrossRef] [Green Version]
- Meena, K.R.; Kanwar, S.S.; Freire, D. Lipopeptides as the Antifungal and Antibacterial Agents: Applications in Food Safety and Therapeutics. Biomed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.H.; Nybroe, O.; Koch, B.; Hansen, M.; Sørensen, J. Genes involved in cyclic lipopeptide production are important for seed and straw colonization by Pseudomonas sp. strain DSS73. Appl. Environ. Microb. 2005, 71, 4112–4116. [Google Scholar] [CrossRef] [Green Version]
- Aiyar, P.; Schaeme, D.; García-Altares, M.; Carrasco Flores, D.; Dathe, H.; Hertweck, C.; Sasso, S.; Mittag, M. Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Jang, J.Y.; Yang, S.Y.; Kim, Y.C.; Lee, C.W.; Park, M.S.; Kim, J.C.; Kim, I.S. Identification of Orfamide A as an Insecticidal Metabolite Produced by Pseudomonas protegens F6. J. Agric. Food Chem. 2013, 61, 6786–6791. [Google Scholar] [CrossRef]
- Hassan, K.A.; Johnson, A.; Shaffer, B.T.; Ren, Q.; Kidarsa, T.A.; Elbourne, L.D.; Hartney, S.; Duboy, R.; Goebel, N.C.; Zabriskie, T.M.; et al. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ. Microbiol. 2010, 12, 899–915. [Google Scholar] [CrossRef]
- Oni, F.E.; Geudens, N.; Omoboye, O.O.; Bertier, L.; Hua, H.; Adiobo, A.; Sinnaeve, D.; Martins, J.C.; Höfte, M. Fluorescent Pseudomonas and cyclic lipopeptide diversity in the rhizosphere of cocoyam (Xanthosoma sagittifolium). Environ. Microbiol. 2019, 21, 1019–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Gu, Q.; Xie, Y.; Lou, Z.; Xue, P.; Fang, L.; Yu, C.; Jia, D.; Huang, G.; Zhu, B.; et al. Cold-adapted Bacilli isolated from the Qinghai–Tibetan Plateau are able to promote plant growth in extreme environments. Environ. Microbiol. 2019, 21, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Vasil, M.L.; Greenberg, E.P. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 2005, 102, 11076. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X.; et al. The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. https://doi.org/10.3390/ijms23126431
Gu Q, Qiao J, Wang R, Lu J, Wang Z, Li P, Zhang L, Ali Q, Khan AR, Gao X, et al. The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. International Journal of Molecular Sciences. 2022; 23(12):6431. https://doi.org/10.3390/ijms23126431
Chicago/Turabian StyleGu, Qin, Junqing Qiao, Ruoyi Wang, Juan Lu, Zhengqi Wang, Pingping Li, Lulu Zhang, Qurban Ali, Abdur Rashid Khan, Xuewen Gao, and et al. 2022. "The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize" International Journal of Molecular Sciences 23, no. 12: 6431. https://doi.org/10.3390/ijms23126431
APA StyleGu, Q., Qiao, J., Wang, R., Lu, J., Wang, Z., Li, P., Zhang, L., Ali, Q., Khan, A. R., Gao, X., & Wu, H. (2022). The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. International Journal of Molecular Sciences, 23(12), 6431. https://doi.org/10.3390/ijms23126431




