Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways
Abstract
:1. Introduction
2. Results
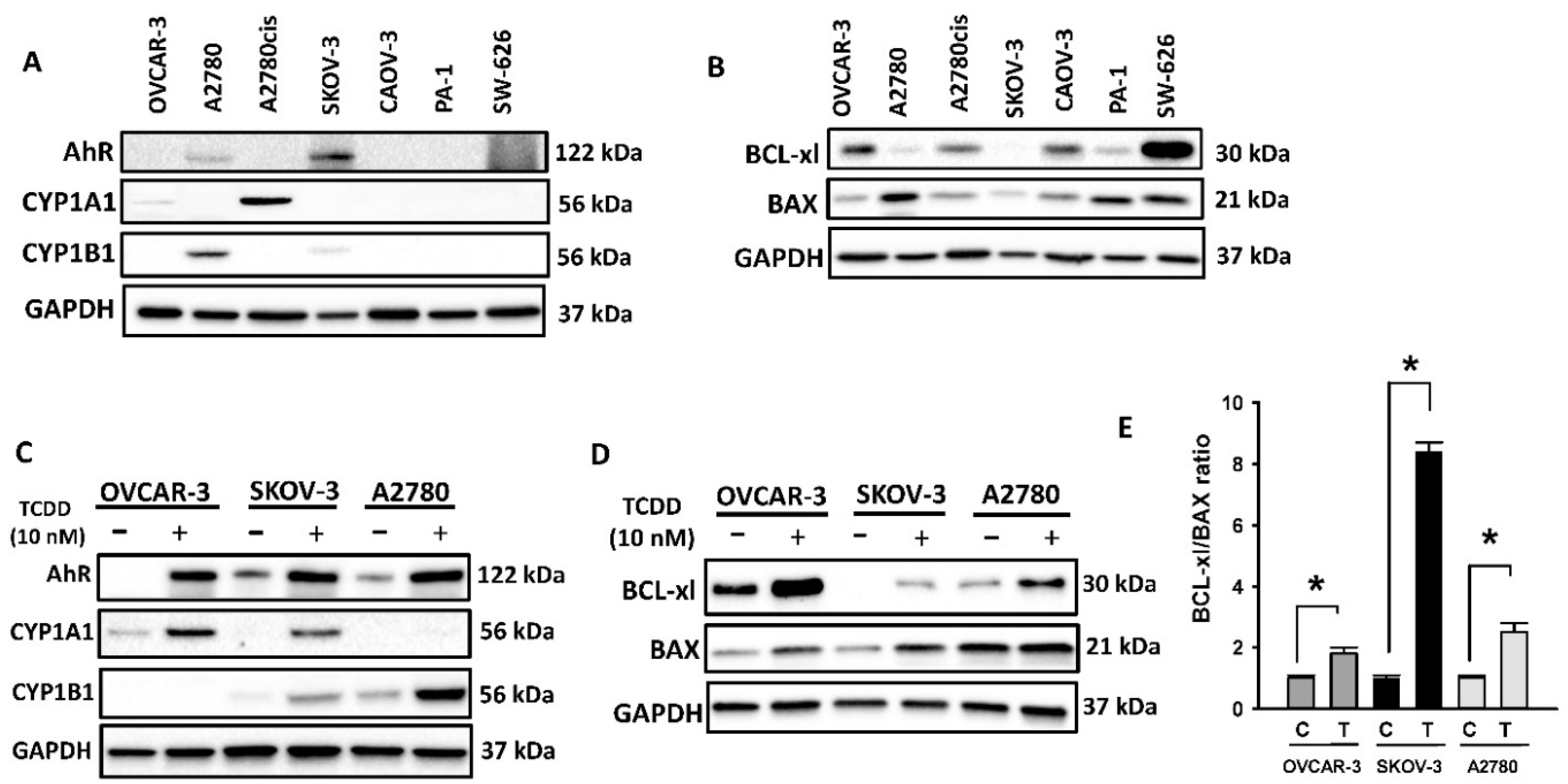
2.1. Constitutive and Inducible Expression of AhR and BCL Family Proteins in OC Cells
2.1.1. Basal Expression of AhR and BCL-2 Proteins in OC Cell Lines
2.1.2. TCDD-Inducible Expression of AhR and BCL-2 Proteins in OC Cells
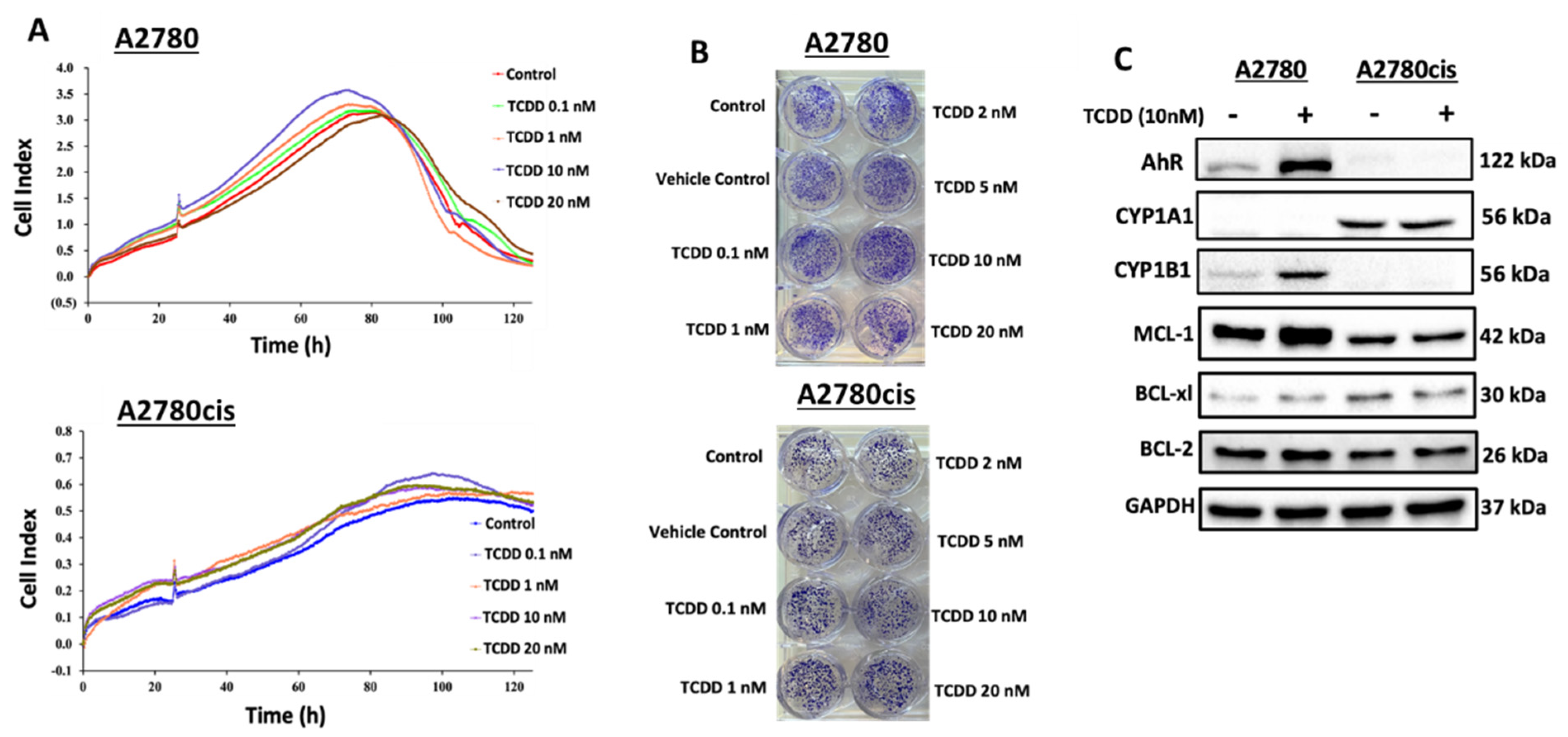
2.2. Effect of AhR Activation on the Chemoresistance of OC
2.2.1. Effect of AhR Activation on Cell Viability and Clonogenicity of Wild-Type and Resistant A2780 Cells
2.2.2. Constitutive and TCDD-Inducible Expression of AhR and BCL-2 Family Proteins in Wild Type and Resistant A2780 Cells
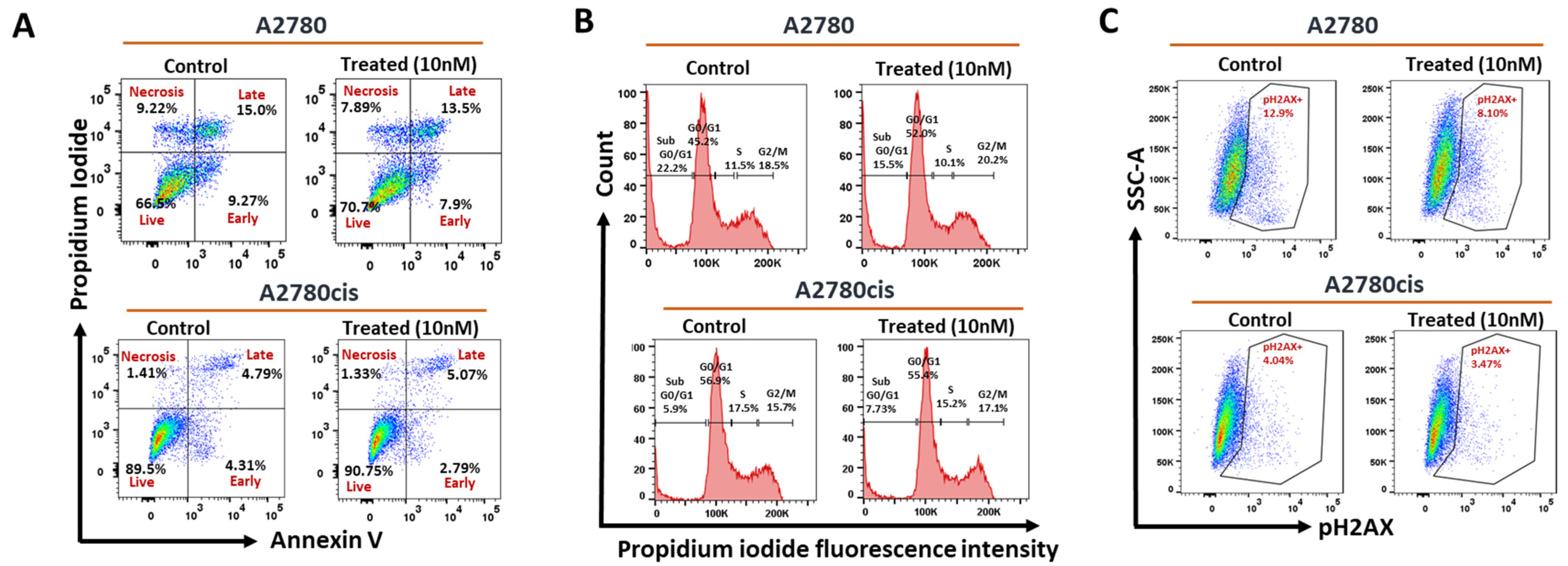
2.2.3. Effect of AhR Induction on Apoptosis, Cell Cycle Phases, and DNA Breaks in Wild-Type and Resistant A2780 Cells
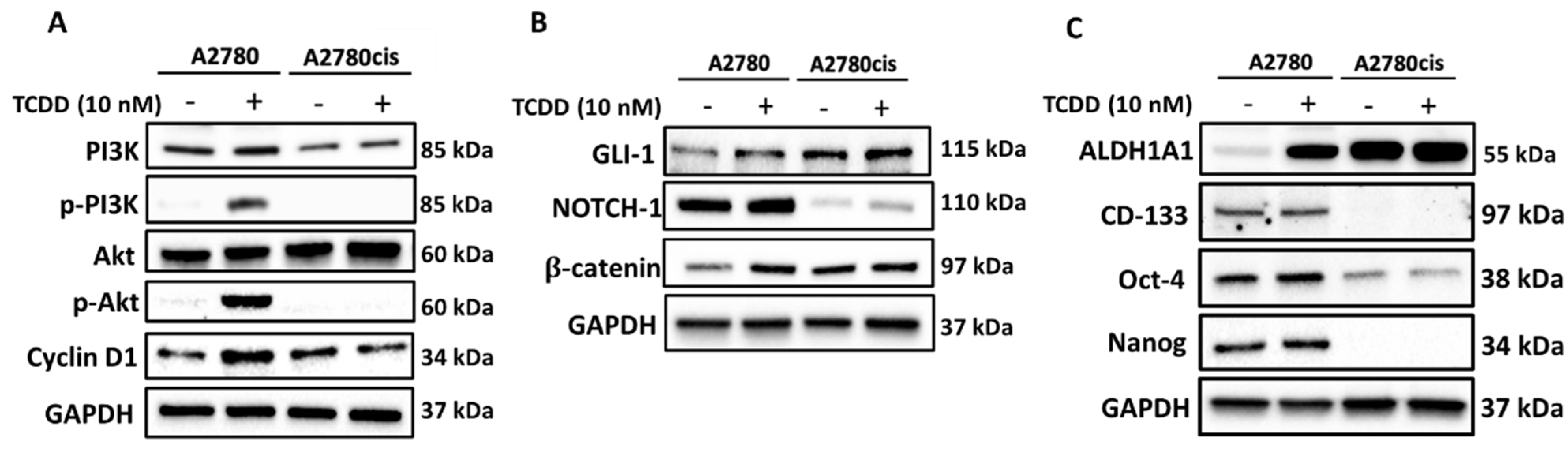
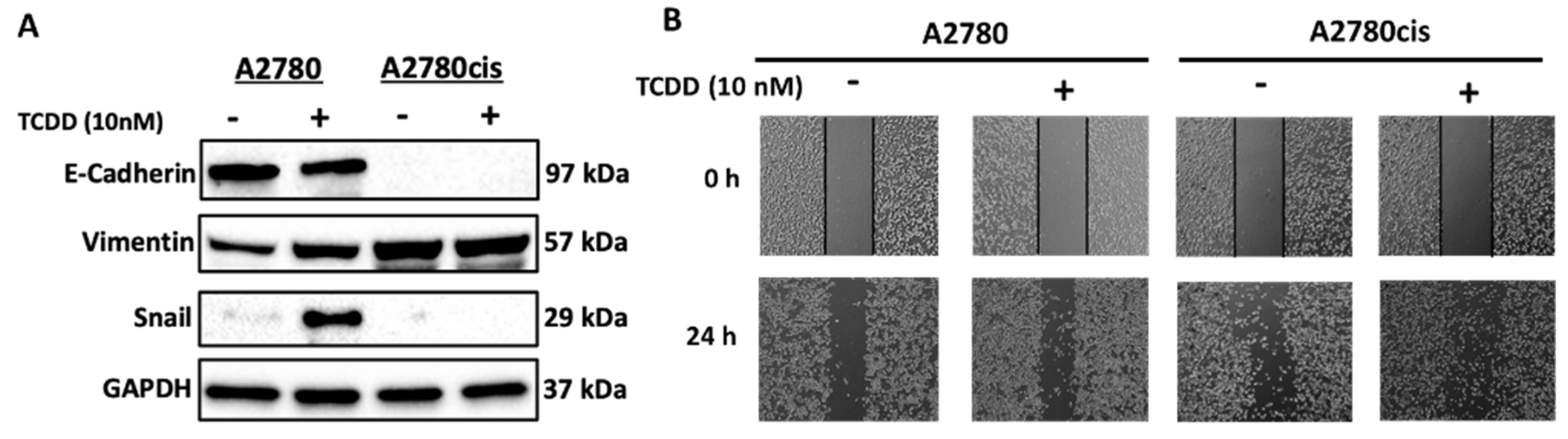
2.3. Molecular Mechanisms Regulating the Effect and Involvement of AhR in OC Chemoresistance
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. OC Cell Lines and Cell Culture
4.3. Protein Extraction and Western Blot Analysis
4.4. Real-Time Cell Analyzer (RTCA) Analysis
4.5. Clonogenic Assay
4.6. Apoptosis Assay
4.7. Cell Cycle Analysis
4.8. H2AX Phosphorylation Assay
4.9. Wound Healing/Scratch Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Society, A.C. Key Statistics for Ovarian Cancer. Am. Cancer Soc. 2021. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 1 May 2022).
- Guo, C.; Song, C.; Zhang, J.; Gao, Y.; Qi, Y.; Zhao, Z.; Yuan, C. Revisiting chemoresistance in ovarian cancer: Mechanism, biomarkers, and precision medicine. Genes Dis. 2020, 3, 668–681. [Google Scholar] [CrossRef]
- Ahmed, N.; Abubaker, K.; Findlay, J.K. Ovarian cancer stem cells: Molecular concepts and relevance as therapeutic targets. Mol. Asp. Med. 2014, 39, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-De-Mendoza, A.; Graves, K.D.; Gómez-Trillos, S.; Song, M.; Anderson, L.; Campos, C.; Carrera, P.; Ostrove, N.; Peshkin, B.N.; Schwartz, M.D.; et al. Developing a culturally targeted video to enhance the use of genetic counseling in Latina women at increased risk for hereditary breast and ovarian cancer. J. Community Genet. 2019, 11, 85–99. [Google Scholar] [CrossRef]
- Mosgaard, B.J.; Meaidi, A.; Høgdall, C.; Noer, M.C. Risk factors for early death among ovarian cancer patients: A nationwide cohort study. J. Gynecol. Oncol. 2020, 31, e30. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Yu, H.; Kanerva, A.; Försti, A.; Sundquist, K.; Hemminki, K. Familial Ovarian Cancer Clusters with Other Cancers. Sci. Rep. 2018, 8, 11561. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Yu, S.; Tan, Q.; Guo, P.; Liu, H. Role of AhR in regulating cancer stem cell–like characteristics in choriocarcinoma. Cell Cycle 2018, 17, 2309–2320. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhfyan, A.; Alhoshani, A.; Korashy, H.M. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation. Mol. Cancer 2017, 16, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Stanford, E.A.; Wang, Z.; Novikov, O.; Mulas, F.; Landesman-Bollag, E.; Monti, S.; Smith, B.W.; Seldin, D.C.; Murphy, G.J.; Sherr, D.H. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef] [Green Version]
- Vacher, S.; Castagnet, P.; Chemlali, W.; Lallemand, F.; Meseure, D.; Pocard, M.; Bieche, I.; Perrot-Applanat, M. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS ONE 2018, 13, e0190619. [Google Scholar] [CrossRef] [Green Version]
- Silginer, M.; Burghardt, I.; Gramatzki, D.; Bunse, L.; Leske, H.; Rushing, E.J.; Hao, N.; Platten, M.; Weller, M.; Roth, P. The aryl hydrocarbon receptor links integrin signaling to the TGF-β pathway. Oncogene 2016, 35, 3260–3271. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.; Carney, P.; Bradfield, C.A.; Kennedy, G.D. The Aryl Hydrocarbon Receptor is a Repressor of Inflammation-associated Colorectal Tumorigenesis in Mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Bunaciu, R.P.; Yen, A. Activation of the aryl hydrocarbon receptor ahr promotes retinoic acid-induced differentiation of myelo-blastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011, 71, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Ronnekleiv-Kelly, S.M.; Nukaya, M.; Díaz-Díaz, C.J.; Megna, B.W.; Carney, P.R.; Geiger, P.G.; Kennedy, G.D. Aryl hydrocarbon receptor-dependent apoptotic cell death induced by the flavonoid chrysin in human colorectal cancer cells. Cancer Lett. 2016, 370, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Contador-Troca, M.; Alvarez-Barrientos, A.; Barrasa, E.; Rico-Leo, E.M.; Catalina-Fernández, I.; Menacho-Márquez, M.; Bustelo, X.R.; Garcia-Borron, J.C.; Gómez-Durán, A.; Sáenz-Santamaría, J.; et al. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis 2013, 34, 2683–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuster, E.; Mayr, D.; Hester, A.; Kolben, T.; Zeder-Göß, C.; Burges, A.; Mahner, S.; Jeschke, U.; Trillsch, F.; Czogalla, B. Correlation of the Aryl Hydrocarbon Receptor with FSHR in Ovarian Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2862. [Google Scholar] [CrossRef] [Green Version]
- Saygin, C.; Samour, M.; Chumakova, A.; Jarrar, A.; Lathia, J.D.; Reizes, O. Reporter Systems to Study Cancer Stem Cells. Methods Mol. Biol. 2016, 1516, 319–333. [Google Scholar] [CrossRef]
- Sari, I.N.; Phi, L.T.H.; Jun, N.; Wijaya, Y.T.; Lee, S.; Kwon, H.Y. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells 2018, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-S.; Ma, J.; Wong, A.S.T. Chemoresistance in ovarian cancer: Exploiting cancer stem cell metabolism. J. Gynecol. Oncol. 2018, 29, e32. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F.; Dunfield, L.; Phillips, K.P.; Krewski, D.; Vanderhyden, B.C. Risk Factors for Ovarian Cancer: An Overview with Emphasis on Hormonal Factors. J. Toxicol. Environ. Health B 2008, 11, 301–321. [Google Scholar] [CrossRef]
- Bekki, K.; Vogel, H.; Li, W.; Ito, T.; Sweeney, C.; Haarmann-Stemmann, T.; Matsumura, F.; Vogel, C.F.A. The aryl hydrocarbon receptor (AhR) mediates re-sistance to apoptosis induced in breast cancer cells. Pestic. Biochem. Physiol. 2015, 120, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Frauenstein, K.; Sydlik, U.; Tigges, J.; Majora, M.; Wiek, C.; Hanenberg, H.; Abel, J.; Esser, C.; Fritsche, E.; Krutmann, J.; et al. Evidence for a novel anti-apoptotic pathway in human keratinocytes involving the aryl hydrocarbon receptor, E2F1, and checkpoint kinase 1. Cell Death Differ. 2013, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, M.; Gährs, M.; Haben, M.; Michels, C.; Schrenk, D. Inhibition of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin depends on protein biosynthesis. Cell Biol. Toxicol. 2010, 26, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.; De Oliveira, M.B.; Peng, C.; Ailles, L.E.; Liu, G.; Covens, A.; Krylov, S.N. Multi-drug-resistance efflux in cisplatin-naive and cisplatin-exposed A2780 ovarian cancer cells responds differently to cell culture dimensionality. Mol. Clin. Oncol. 2021, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.; Castellano, G.; Stallone, G.; Netti, G.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Min, Y.H.; Eom, J.; Cheong, J.W.; Maeng, H.; Kim, J.Y.; Jeung, H.K.; Lee, S.T.; Lee, M.H.; Hahn, J.S.; Ko, Y.W. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: Its significance as a prognostic variable. Leukemia 2003, 17, 995–997. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Vashishta, M.; Kong, L.; Wu, X.; Lu, J.J.; Guha, C.; Dwarakanath, B.S. The Role of Notch, Hedgehog, and Wnt Signaling Pathways in the Resistance of Tumors to Anticancer Therapies. Front. Cell Dev. Biol. 2021, 9, 650772. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.P. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer 2011, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Li, Q.; Wu, F.; Lin, J.; Chen, J.; Zheng, H.; Guo, L. Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons from Somatic Cell Reprogramming. Front. Cell Dev. Biol. 2020, 8, 760. [Google Scholar] [CrossRef]
- Therachiyil, L.; Haroon, J.; Sahir, F.; Siveen, K.S.; Uddin, S.; Kulinski, M.; Buddenkotte, J.; Steinhoff, M.; Krishnankutty, R. Dysregulated Phosphorylation of p53, Autophagy and Stemness Attributes the Mutant p53 Harboring Colon Cancer Cells Impaired Sensitivity to Oxaliplatin. Front. Oncol. 2020, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Leo, R.; Therachiyil, L.; Siveen, S.K.; Uddin, S.; Kulinski, M.; Buddenkotte, J.; Steinhoff, M.; Krishnankutty, R. Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells. Cancers 2019, 11, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberg, H.-H.; Peters, C.; Kabelitz, D.; Wesch, D. Real-time cell analysis (RTCA) to measure killer cell activity against adherent tumor cells in vitro. Methods Enzym. 2019, 631, 429–441. [Google Scholar] [CrossRef]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chgn, W.J. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634. [Google Scholar] [CrossRef] [Green Version]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Akhtar, S.; Mateo, J.M.; Merhi, M.; Taha, R.; El Omri, H.; Mraiche, F.; et al. Sanguinarine suppresses growth and induces apoptosis in childhood acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 782–794. [Google Scholar] [CrossRef]
- Krishnankutty, R.; Iskandarani, A.; Therachiyil, L.; Uddin, S.; Azizi, F.; Kulinski, M.; Bhat, A.A.; Mohammad, R.M. Anticancer Activity of Camel Milk via Induction of Autophagic Death in Human Colorectal and Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2018, 19, 3501–3509. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Therachiyil, L.; Krishnankutty, R.; Ahmad, F.; Mateo, J.M.; Uddin, S.; Korashy, H.M. Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways. Int. J. Mol. Sci. 2022, 23, 6395. https://doi.org/10.3390/ijms23126395
Therachiyil L, Krishnankutty R, Ahmad F, Mateo JM, Uddin S, Korashy HM. Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways. International Journal of Molecular Sciences. 2022; 23(12):6395. https://doi.org/10.3390/ijms23126395
Chicago/Turabian StyleTherachiyil, Lubna, Roopesh Krishnankutty, Fareed Ahmad, Jericha M. Mateo, Shahab Uddin, and Hesham M. Korashy. 2022. "Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways" International Journal of Molecular Sciences 23, no. 12: 6395. https://doi.org/10.3390/ijms23126395
APA StyleTherachiyil, L., Krishnankutty, R., Ahmad, F., Mateo, J. M., Uddin, S., & Korashy, H. M. (2022). Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways. International Journal of Molecular Sciences, 23(12), 6395. https://doi.org/10.3390/ijms23126395








