Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins
Abstract
:1. Introduction
2. Results
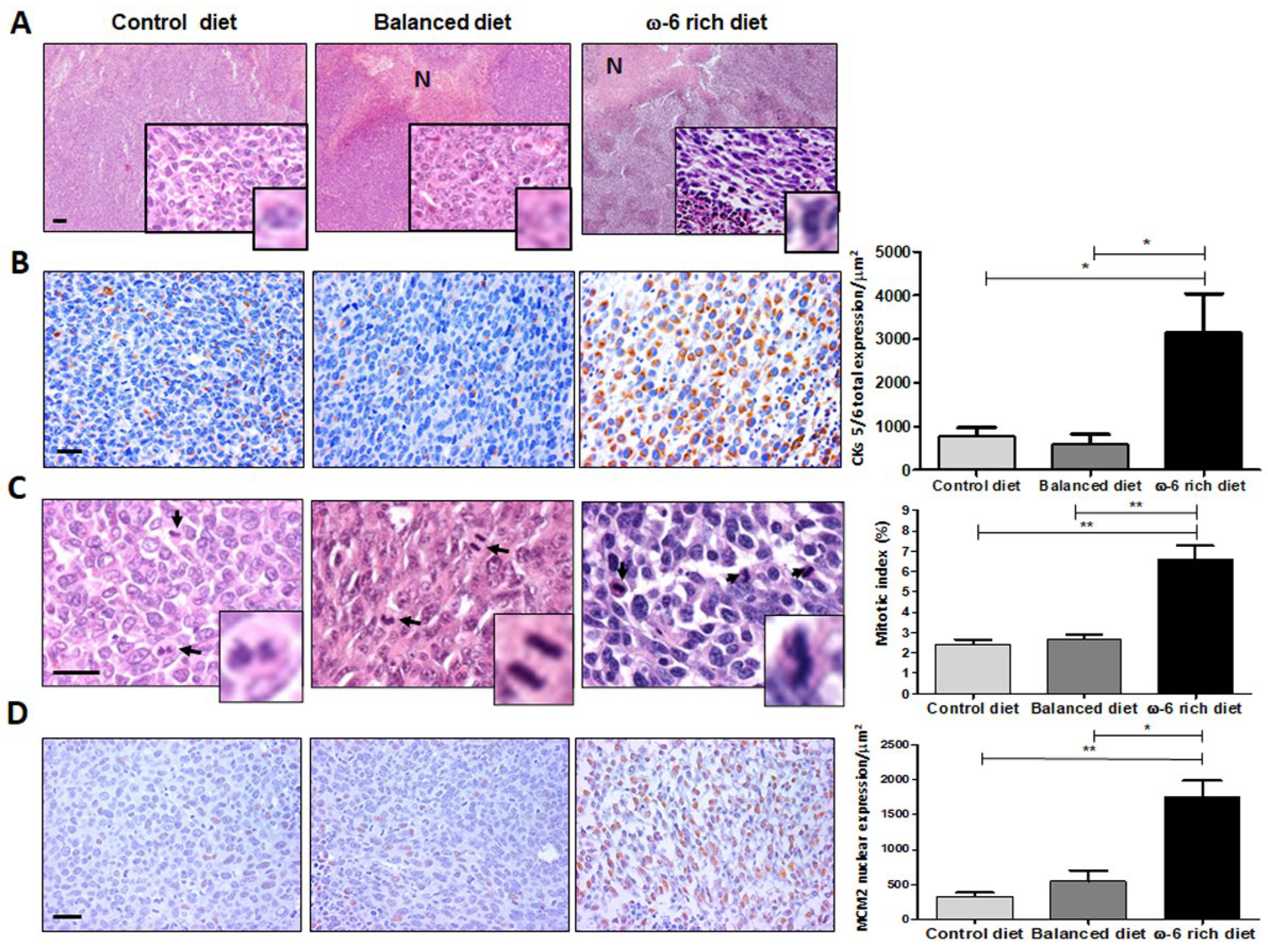
2.1. ω-6 Feeding Induces Higher Lung Cancer Aggressiveness, De-Differentiation and Increase in Proliferation
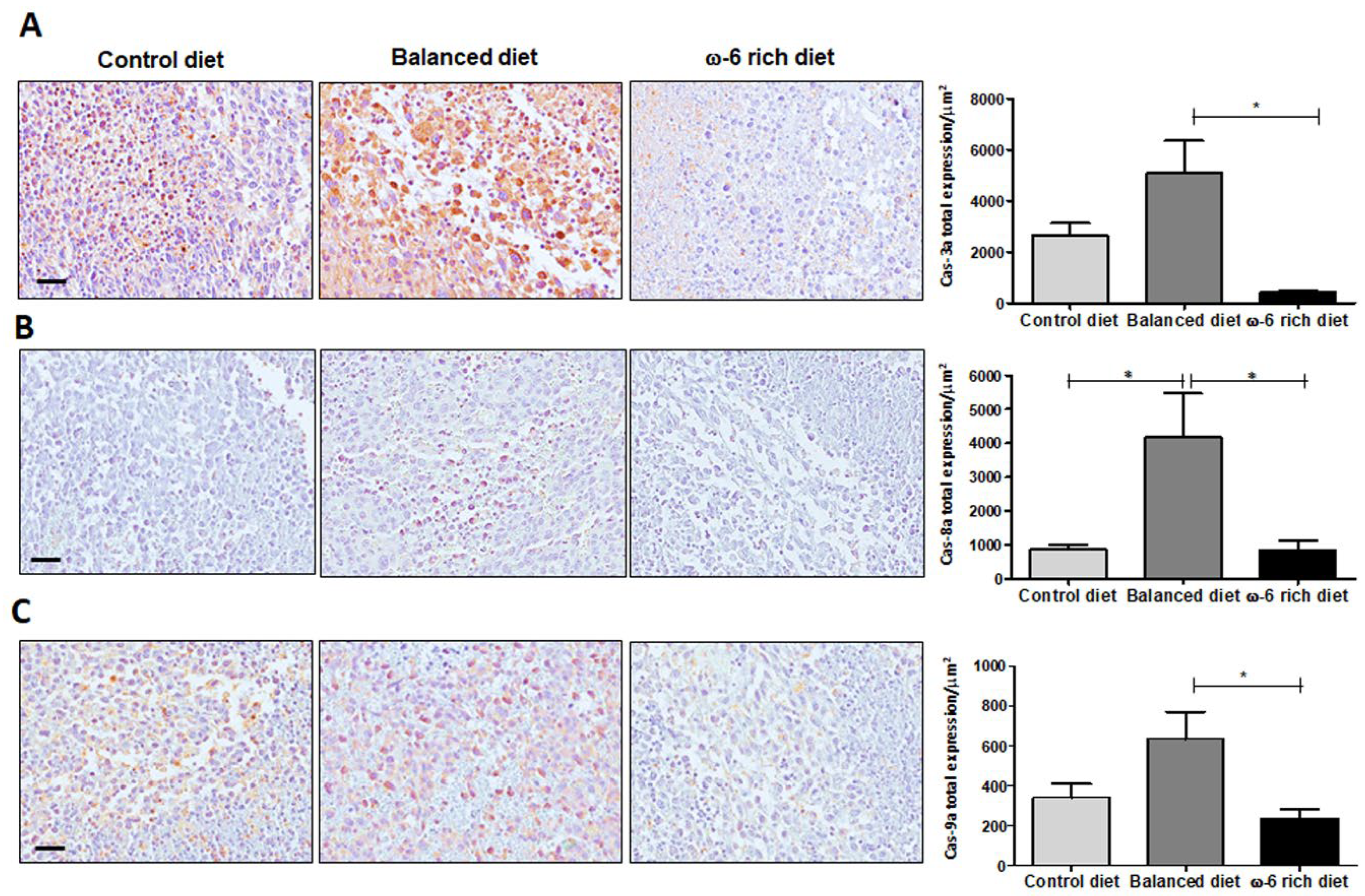
2.2. High Intake of ω-6 PUFA Inhibits Caspase-Dependent Apoptosis
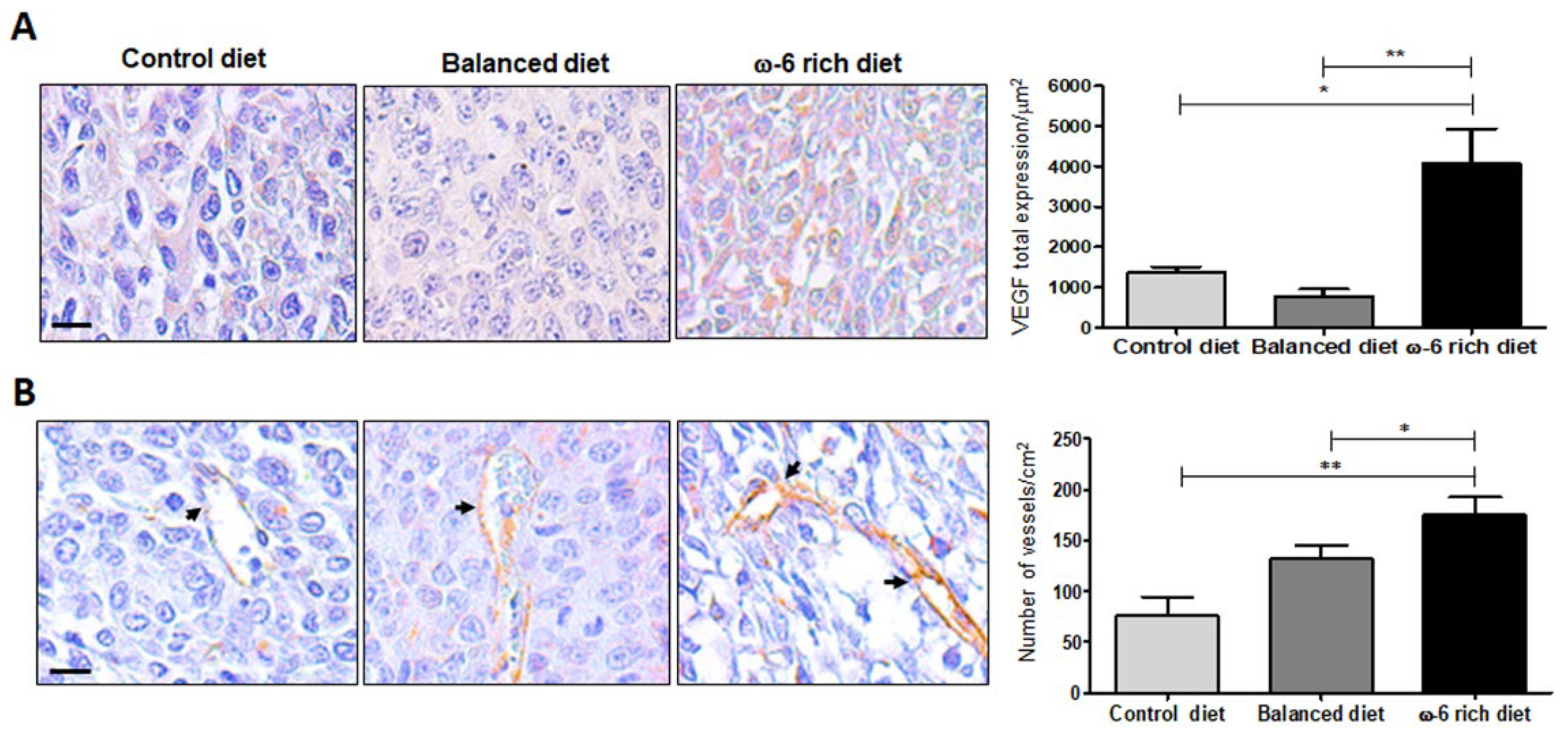
2.3. ω-6-Rich Diet Increases Angiogenesis of Tumor Cells
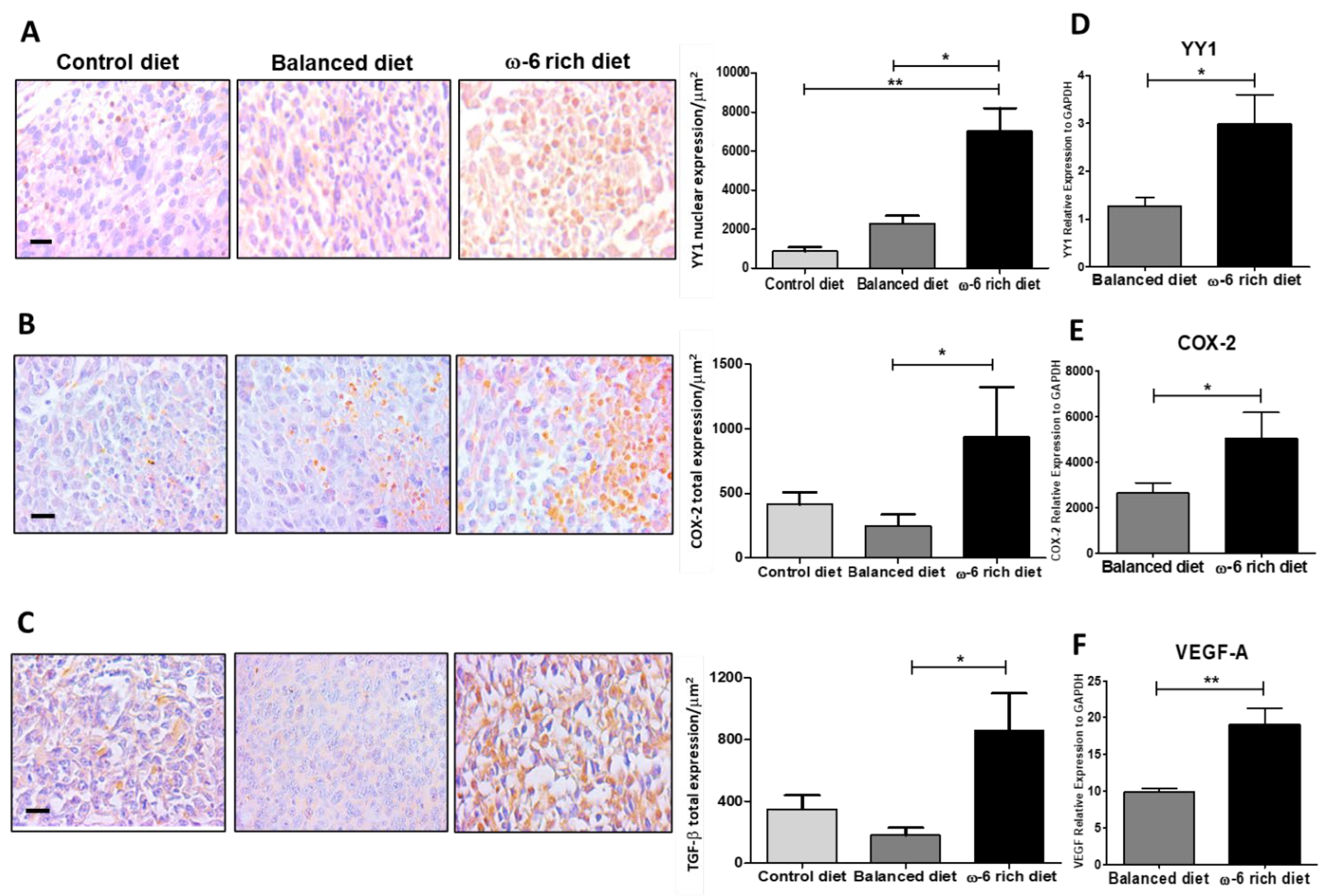
2.4. ω-6 Rich Diet Induces Oxylipin Generation Associated with Tumor Aggressiveness
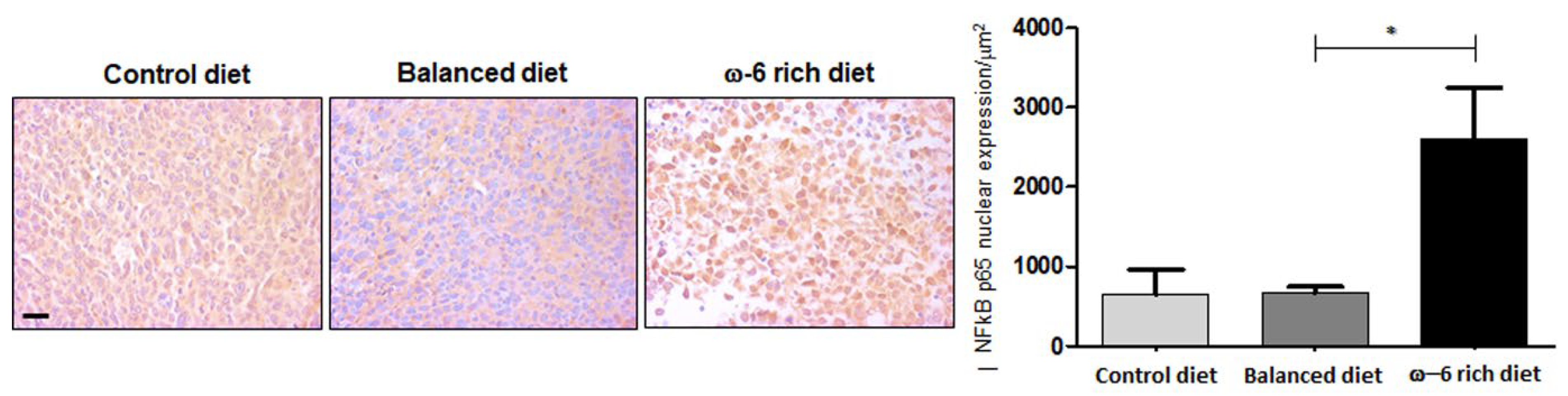
2.5. ω-6-Rich Diet Increases the Aggressiveness of Tumor Cells
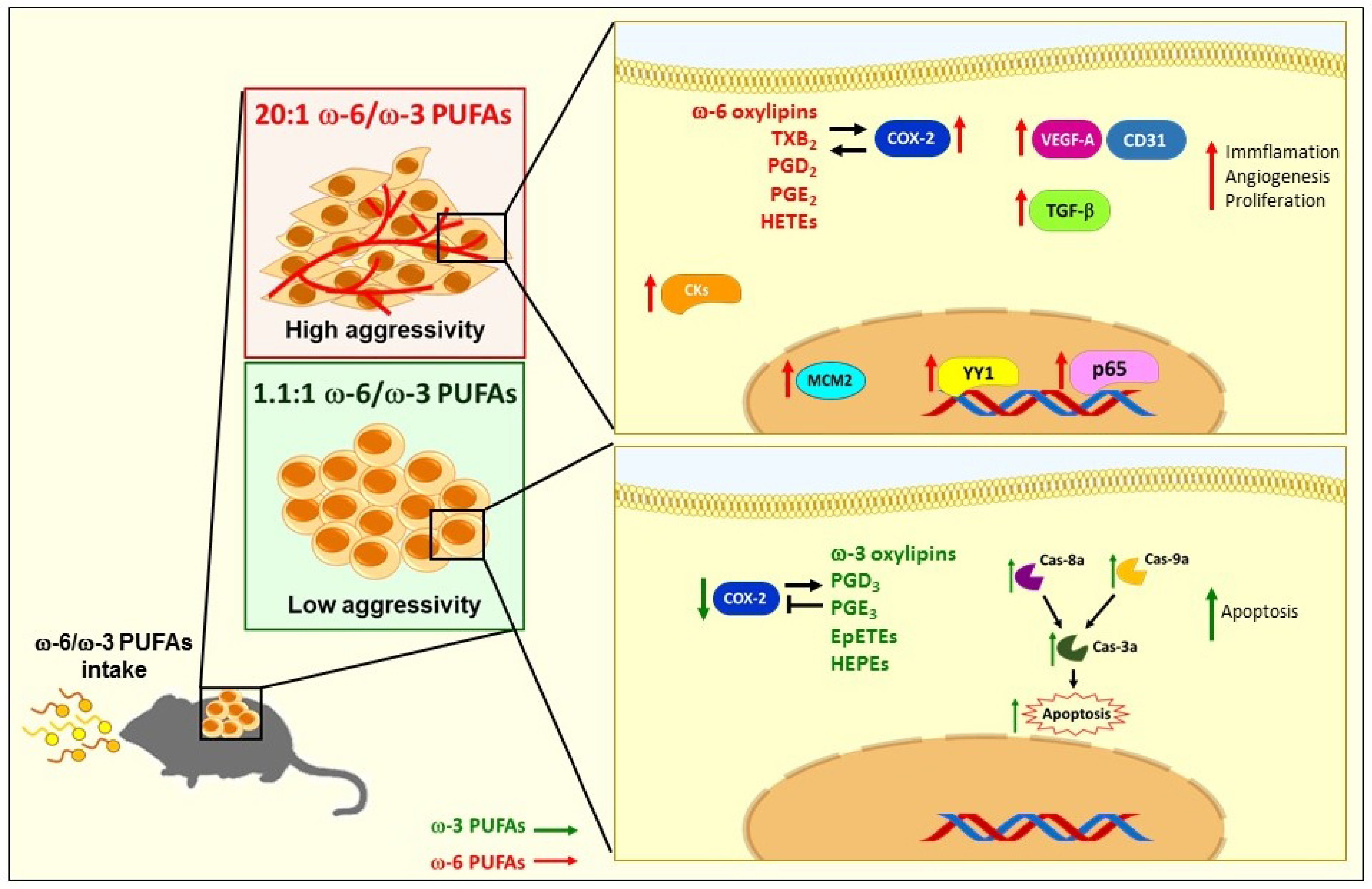
3. Discussion
4. Materials and Methods
4.1. Cell Line
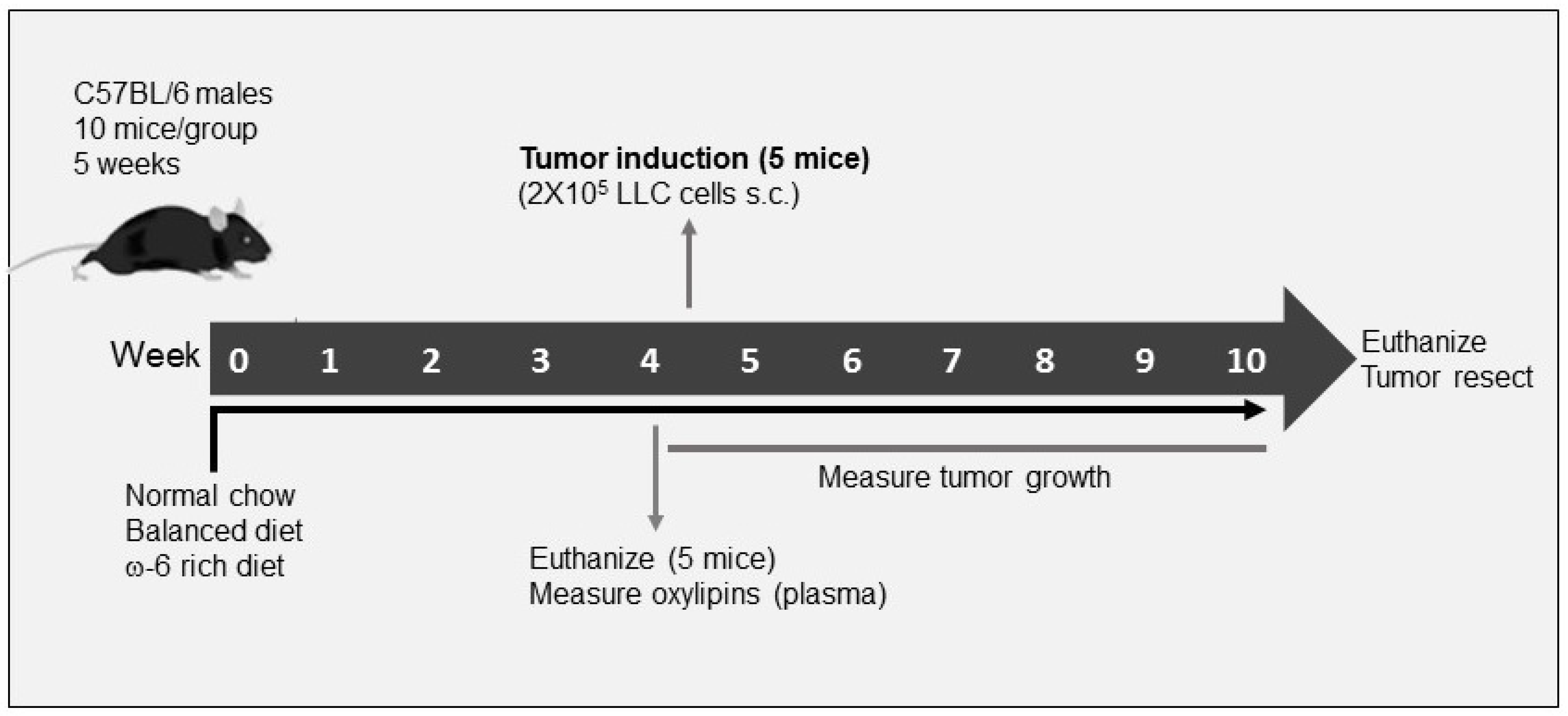
4.2. Experimental Model
4.3. Hematoxylin and Eosin Staining
4.4. Mitotic Index
4.5. Immunohistochemical Analysis and Digital Pathology
4.6. Oxylipin Profiling
4.7. RT-PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- International Agency for Research on Cancer. Globocan. All cancers incidence worldwide. Int. Agency Res. Cancer 2019, 876, 2018–2019. [Google Scholar]
- Fujimoto, J.; Wistuba, I.I. Current concepts on the molecular pathology of non-small cell lung carcinoma. Semin. Diagn. Pathol. 2014, 31, 306–313. [Google Scholar]
- Ko, E.C.; Raben, D.; Formenti, S.C. The integration of radiotherapy with immunotherapy for the treatment of non–small cell lung cancer. Clin. Cancer Res. 2018, 24, 5792–5806. [Google Scholar]
- Lemjabbar-Alaoui, H.; Hassan, O.U.I.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta-Rev. Cancer 2015, 1856, 189–210. [Google Scholar]
- Muñiz-Hernández, S.; Huerta-Yepez, S.; Hernández-Pedro, N.; Ramírez-Tirado, L.A.; Aviles-Salas, A.; Maldonado, A.; Hernández-Cueto, D.; Baay-Guzmán, G.; Arrieta, O. Association between nuclear expression of retinoic acid receptor alpha and beta and clinicopathological features and prognosis of advanced non-small cell lung cancer. Int. J. Clin. Oncol. 2016, 21, 1051–1061. [Google Scholar]
- Willett, W.C. Diet and cancer. Oncologist 2000, 5, 393–404. [Google Scholar]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. An Int. Rev. J. 2015, 6, 513–540. [Google Scholar]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front. Oncol. 2013, 3, 224. [Google Scholar]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Papel de las dietas ricas en omega-3 y omega-6 en el desarrollo del cáncer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 446–456. [Google Scholar]
- Liu, J.; Mazzone, P.J.; Cata, J.P.; Kurz, A.; Bauer, M.; Mascha, E.J.; Sessler, D.I. Serum free fatty acid biomarkers of lung cancer. Chest 2014, 146, 670–679. [Google Scholar]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Lee, K.S.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar]
- Ling, H.; Jia, X.; Zhang, Y.; Gapter, L.A.; Lim, Y.S.; Agarwal, R.; Ng, K.Y. Pachymic acid inhibits cell growth and modulates arachidonic acid metabolism in nonsmall cell lung cancer A549 cells. Mol. Carcinog 2010, 49, 271–282. [Google Scholar]
- Xia, S.H.; Wang, J.; Kang, J.X. Decreased n-6/n-3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes. Carcinogenesis 2005, 26, 779–784. [Google Scholar]
- Yu, W.; Chen, L.; Yang, Y.Q.; Falck, J.R.; Guo, A.M.; Li, Y.; Yang, J. Cytochrome P450 ω-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother. Pharmacol. 2010, 68, 619–629. [Google Scholar]
- Huerta-Yepez, S.; Tirado-Rodriguez, A.; Montecillo-Aguado, M.R.; Yang, J.; Hammock, B.D.; Hankinson, O. Aryl Hydrocarbon Receptor-Dependent inductions of omega-3 and omega-6 polyunsaturated fatty acid metabolism act inversely on tumor progression. Sci. Rep. 2020, 10, 7843. [Google Scholar]
- Lewis, J.S.; Ritter, J.H.; El-Mofty, S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod. Pathol. 2005, 18, 1471–1481. [Google Scholar]
- Antoine, M.; Vieira, T.; Fallet, V.; Hamard, C.; Duruisseaux, M.; Cadranel, J.; Wislez, M. Carcinomes sarcomatoïdes pulmonaires. Ann. Pathol. 2016, 36, 44–54. [Google Scholar]
- Lucas, D.R.; Pass, H.I.; Madan, S.K.; Adsay, N.V.; Wali, A.; Tabaczka, P.; Lonardo, F. Sarcomatoid mesothelioma and its histological mimics: A comparative immunohistochemical study. Histopathology 2003, 42, 270–279. [Google Scholar]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar]
- Huang, T.; Wang, G.; Yang, L.; Peng, B.; Wen, Y.; Ding, G.; Wang, Z. Transcription Factor YY1 Modulates Lung Cancer Progression by Activating lncRNA-PVT1. DNA Cell Biol. 2017, 36, 947–958. [Google Scholar]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar]
- Aoki, T.; Frȍsen, J.; Fukuda, M.; Bando, K.; Shioi, G.; Tsuji, K.; Ollikainen, E.; Nozaki, K.; Laakkonen, J.; Narumiya, S. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci. Signal. 2017, 10, eaah6037. [Google Scholar]
- Huerta-Yepez, S.; Vega, M.; Garban, H.; Bonavida, B. Involvement of the TNF-α autocrine-paracrine loop, via NF-κB and YY1, in the regulation of tumor cell resistance to Fas-induced apoptosis. Clin. Immunol. 2006, 120, 297–309. [Google Scholar]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar]
- Wick, M.R.; Swanson, P.E. Carcinosarcomas: Current perspectives and an historical review of nosological concepts. Semin. Diagn. Pathol. 1993, 10, 118–127. [Google Scholar]
- Weissferdt, A. Pulmonary sarcomatoid carcinomas: A review. Adv. Anat. Pathol. 2018, 25, 304–313. [Google Scholar]
- Martin, L.W.; Correa, A.M.; Ordonez, N.G.; Roth, J.A.; Swisher, S.G.; Vaporciyan, A.A.; Walsh, G.L.; Rice, D.C. Sarcomatoid carcinoma of the lung: A predictor of poor prognosis. Ann. Thorac. Surg. 2007, 84, 973–980. [Google Scholar]
- Bae, H.M.; Min, H.S.; Lee, S.H.; Kim, D.W.; Chung, D.H.; Lee, J.S.; Kim, Y.W.; Heo, D.S. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007, 58, 112–115. [Google Scholar]
- Chaft, J.E.; Sima, C.S.; Ginsberg, M.S.; Huang, J.; Kris, M.G.; Travis, W.D.; Azzoli, C.G. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J. Thorac. Oncol. 2012, 7, 1400–1405. [Google Scholar]
- Raveglia, F.; Mezzetti, M.; Panigalli, T.; Furia, S.; Giuliani, L.; Conforti, S.; Meda, S. Personal experience in surgical management of pulmonary pleomorphic carcinoma. Ann. Thorac. Surg. 2004, 78, 1742–1747. [Google Scholar]
- Dudderidge, T.J.; Stoeber, K.; Loddo, M.; Atkinson, G.; Fanshawe, T.; Griffiths, D.F.; Williams, G.H. Mcm2, geminin, and KI67 define proliferative state and are prognostic markers in renal cell carcinoma. Clin. Cancer Res. 2005, 11, 2510–2517. [Google Scholar]
- Going, J.J.; Keith, W.N.; Neilson, L.; Stoeber, K.; Stuart, R.C.; Williams, G.H. Aberrant expression of minichromosome maintenance proteins 2 and 5, and ki-67 in dysplastic squamous oesophageal epithelium and barrett’s mucosa. Gut 2002, 50, 373–377. [Google Scholar]
- Abe, S.; Yamamoto, K.; Kurata, M.; Abe-Suzuki, S.; Horii, R.; Akiyama, F.; Kitagawa, M. Targeting MCM2 function as a novel strategy for the treatment of highly malignant breast tumors. Oncotarget 2015, 6, 34892–34909. [Google Scholar]
- Aihemaiti, G.; Kurata, M.; Nogawa, D.; Yamamoto, A.; Mineo, T.; Onishi, I.; Kinowaki, Y.; Jin, X.-H.; Tatsuzawa, A.; Miyasaka, N.; et al. Subcellular localization of MCM2 correlates with the prognosis of ovarian clear cell carcinoma. Oncotarget 2018, 9, 28213–28225. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Serini, S.; Piccioni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar]
- Skendera, B.; Vaculova, A.H.; Hofmanova, J. Docosahexaenoic fatty acid (DHA) in the regulation of colon cell growth and cell death: A review. Biomed. Pap. 2012, 156, 186–199. [Google Scholar]
- Song, E.A.; Kim, H. Docosahexaenoic acid induces oxidative DNA damage and apoptosis, and enhances the chemosensitivity of cancer cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar]
- Arita, K.; Kobuchi, H.; Utsumi, T.; Takehara, Y.; Akiyama, J.; Horton, A.A.; Utsumi, K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 2001, 62, 821–828. [Google Scholar]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N.; et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 8, 70049–70071. [Google Scholar]
- Dyari, H.R.E.; Rawling, T.; Bourget, K.; Murray, M. Synthetic ω-3 epoxyfatty acids as antiproliferative and pro-apoptotic agents in human breast cancer cells. J. Med. Chem. 2014, 57, 7459–7464. [Google Scholar]
- Dyari, H.R.E.; Rawling, T.; Chen, Y.; Sudarmana, W.; Bourget, K.; Dwyer, J.M.; Allison, S.E.; Murray, M. A novel synthetic analogue of v-3 17,18-epoxyeicosatetraenoic acid activates TNF receptor-1/ASK1/JNK signaling to promote apoptosis in human breast cancer cells. FASEB J. 2017, 31, 5246–5257. [Google Scholar]
- Dymkowska, D.; Szczepanowska, J.; Wiȩckowski, M.R.; Wojtczak, L. Short-term and long-term effects of fatty acids in rat hepatoma AS-30D cells: The way to apoptosis. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2006, 1763, 152–163. [Google Scholar]
- Dymkowska, D.; Wojtczak, L. Arachidonic acid-induced apoptosis in rat hepatoma AS-30D cells is mediated by reactive oxygen species. Acta Biochim. Pol. 2009, 56, 711–715. [Google Scholar]
- Vento, R.R.; D’alessandro, N.N.; Giuliano, M.M.; Lauricella, M.M.; Carabillò, M.M.; Tesoriere, G.G. Induction of apoptosis by arachidonic acid in human retinoblastoma Y79 cells: Involvement of oxidative stress. Exp. Eye Res. 2000, 70, 503–517. [Google Scholar]
- Polavarapu, S.; Dwarakanath, B.S.; Das, U.N. Arachidonic acid activates extrinsic apoptotic pathway to enhance tumoricidal action of bleomycin against IMR-32 cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 16–22. [Google Scholar]
- Cui, L.; Zhao, Y.; Pan, Y.; Zheng, X.; Shao, D.; Jia, Y.; He, K.; Li, K.; Chen, L. Chemotherapy induces ovarian cancer cell repopulation through the caspase 3-mediated arachidonic acid metabolic pathway. Onco. Targets. Ther. 2017, 10, 5817–5826. [Google Scholar]
- Yang, J.; Zaman, M.M.; Vlasakov, I.; Roy, R.; Huang, L.; Martin, C.R.; Freedman, S.D.; Serhan, C.N.; Moses, M.A. Adipocytes promote ovarian cancer chemoresistance. Sci. Rep. 2019, 9, 13316. [Google Scholar]
- Monjazeb, A.M.; High, K.P.; Koumenis, C.; Chilton, F.H. Inhibitors of arachidonic acid metabolism act synergistically to signal apoptosis in neoplastic cells. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 463–474. [Google Scholar]
- Panigrahy, D.; Kaipainen, A.; Greene, E.R.; Huang, S. Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010, 29, 723–735. [Google Scholar]
- Avila-Román, J.; Talero, E.; Alcaide, A.; de Los Reyes, C.; Zubía, E.; García-Mauriño, S.; Motilva, V. Preventive effect of the microalga Chlamydomonas debaryana on the acute phase of experimental colitis in rats. Br. J. Nutr. 2014, 112, 1055–1064. [Google Scholar]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U. Effects of dietary n–3 and n–6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar]
- Calder, P.C. N-3 Fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar]
- Krysan, K.; Reckamp, K.; Sharma, S.; Dubinett, S. The potential and rationale for COX-2 inhibitors in lung cancer. Anti-Cancer Agents Med. Chem. 2008, 6, 209–220. [Google Scholar]
- Zhong, X.; Fan, Y.; Ritzenthaler, J.D.; Zhang, W.; Wang, K.; Zhou, Q.; Roman, J. Novel link between prostaglandin E2 (PGE2) and cholinergic signaling in lung cancer: The role of c-Jun in PGE2-induced α7 nicotinic acetylcholine receptor expression and tumor cell proliferation. Thorac. Cancer 2015, 6, 488–500. [Google Scholar]
- Yang, P.; Chan, D.; Felix, E.; Cartwright, C.; Menter, D.G.; Madden, T.; Klein, R.D.; Fischer, S.M.; Newman, R.A. Formation and antiproliferative effect of prostaglandin E(3) from eicosapentaenoic acid in human lung cancer cells. J. Lipid Res. 2004, 45, 1030–1039. [Google Scholar]
- Moreno, J.J. New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem. Pharmacol. 2009, 77, 1–10. [Google Scholar]
- Vang, K.; Ziboh, V.A. 15-Lipoxygenase metabolites of γ-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: Possible implications of dietary fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 363–372. [Google Scholar]
- Bergmann, C.B.; Mcreynolds, C.B. sEH-derived metabolites of linoleic acid drive pathologic in fl ammation while impairing key innate immune cell function in burn injury. Proc. Natl. Acad. Sci. USA 2022, 119, e2120691119. [Google Scholar]
- McReynolds, C.B.; Cortes-Puch, I.; Ravindran, R.; Khan, I.H.; Hammock, B.G.; Shih, P.-A.B.; Yang, J. Plasma linoleate diols are potential biomarkers for severe COVID-19 infections. Front. Physiol. 2021, 12, 663869. [Google Scholar]
- Cui, P.H.; Petrovic, N.; Murray, M. The ω-3 epoxide of eicosapentaenoic acid inhibits endothelial cell proliferation by p38 MAP kinase activation and cyclin D1/CDK4 down-regulation. Br. J. Pharmacol. 2011, 162, 1143–1155. [Google Scholar]
- Wagner, K.; Inceoglu, B.; Hammock, B.D. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat. 2011, 96, 76–83. [Google Scholar]
- Wang, W.; Zhu, J.; Lyu, F.; Panigrahy, D.; Ferrara, K.W.; Hammock, B.; Zhang, G. ω-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014, 113–115, 13–20. [Google Scholar]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar]
- Xia, R.; Sun, L.; Liao, J.; Li, H.; You, X.; Xu, D.; Yang, J.; Hwang, S.H.; Jones, R.D.; Hammock, B.; et al. Inhibition of pancreatic carcinoma growth through enhancing ω-3 epoxy polyunsaturated fatty acid profile by inhibition of soluble epoxide hydrolase. Anticancer Res. 2019, 39, 3651–3660. [Google Scholar]
- Zhang, Q.; Stovall, D.B.; Inoue, K.; Sui, G. The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 2011, 16, 163–197. [Google Scholar]
- Joo, M.; Wright, J.G.; Hu, N.N.; Sadikot, R.T.; Park, G.Y.; Blackwell, T.S.; Christman, J.W. Yin Yang 1 enhances cyclooxygenase-2 gene expression in macrophages. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L1219–L1226. [Google Scholar]
- Gye, Y.P.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L797–L805. [Google Scholar]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Sawaoka, H.; Hori, M.; DuBois, R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93, 705–716. [Google Scholar]
- Marrogi, A.J.; Travis, W.D.; Welsh, J.A.; Khan, M.A.; Rahim, H.; Tazelaar, H.; Pairolero, P.; Trastek, V.; Jett, J.; Caporaso, N.E.; et al. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin. Cancer Res. 2000, 6, 4739–4744. [Google Scholar]
- Wu, Y.-C.; Su, L.-J.; Wang, H.-W.; Lin, C.-F.J.; Hsu, W.-H.; Chou, T.-Y.; Huang, C.-Y.F.; Lu, C.-L.; Hsueh, C.-T. Co-overexpression of cyclooxygenase-2 and microsomal prostaglandin e Synthase-1 adversely affects the postoperative survival in non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1167–1174. [Google Scholar]
- Giaginis, C.; Alexandrou, P.; Tsoukalas, N.; Sfiniadakis, I.; Kavantzas, N.; Agapitos, E.; Patsouris, E.; Theocharis, S. Hu-antigen receptor (HuR) and cyclooxygenase-2 (COX-2) expression in human non-small-cell lung carcinoma: Associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. Tumor Biol. 2014, 36, 315–327. [Google Scholar]
- Zhang, C.; Zhu, X.; Hua, Y.; Zhao, Q.; Wang, K.; Zhen, L.; Wang, G.; Lü, J.; Luo, A.; Cho, W.C.; et al. YY1 mediates TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial cells. Respir. Res. 2019, 20, 249. [Google Scholar]
- Rodríguez-Barbero, A.; Dorado, F.; Velasco, S.; Pandiella, A.; Banas, B.; López-Novoa, J.M. TGF-β1 induces COX-2 expression and PGE2 synthesis through MAPK and PI3K pathways in human mesangial cells. Kidney Int. 2006, 70, 901–909. [Google Scholar]
- Colak, S.; ten Dijke, P. Targeting TGF-β signaling in cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar]
- Zhang, X.; Yan, K.; Deng, L.; Liang, J.; Liang, H.; Feng, D.; Ling, B. Cyclooxygenase 2 promotes proliferation and invasion in ovarian cancer cells via the PGE2/NF-κB pathway. Cell Transplant. 2019, 28, 1S–13S. [Google Scholar]
- Sugiura, K.; Stock, C.C.; Sugiura, M.M. Studies in a tumor spectrum: III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res. 1955, 15, 38–51. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar]
- Yang, J.; Schmelzer, K.; Georgi, K.; Hammock, B.D. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Chem. 2009, 81, 8085–8093. [Google Scholar]
- Zivkovic, A.M.; Yang, J.; Georgi, K.; Hegedus, C.; Nording, M.L.; O’Sullivan, A.; German, J.B.; Hogg, R.J.; Weiss, R.H.; Bay, C.; et al. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics 2012, 8, 1102–1113. [Google Scholar]
- Yang, J.; Solaimani, P.; Dong, H.; Hammock, B.; Hankinson, O. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin markedly increases the levels of a number of cytochrome P450 metabolites of omega-3 polyunsaturated fatty acids in the liver and lung. J. Toxicol. Sci. 2013, 38, 833–836. [Google Scholar]
| ω-6 PUFAs | Enzyme | Oxilypins | Balanced Diet (μmol/L) | ω-6 Rich Diet (μmol/L) | p Value |
|---|---|---|---|---|---|
| LA | COX | --- | --- | --- | --- |
| LOX | 9-HODE | 2654.3 ± 174.1 | 10,285 ± 612.4 | *** | |
| 13-HODE | 2097.4 ± 112.5 | 8985.5 ± 473.2 | *** | ||
| CYP450 | --- | --- | --- | --- | |
| ARA | COX | TXB2 | 11.0 ± 3.5 | 28.2 ± 9.4 | * |
| PGD2 | 0.2 ± 0.06 | 0.5 ± 0.1 | * | ||
| LOX | LXA4 | 3.0 ± 1.2 | 7.9 ± 2.5 | * | |
| 12-oxo-ETE | 142.5 ± 60.4 | 534.6 ± 177.1 | * | ||
| 9-HETE | 0.01 ± 0.001 | 1.2 ± 0.4 | *** | ||
| 12-HETE | 325.1 ± 201.1 | 1751.8 ± 400.1 | ** | ||
| CYP450 | 5,6-DiHETrE | 0.6 ± 0.1 | 1.2 ± 0.1 | ** | |
| 8,9-DiHETrE | 0.7 ± 0.2 | 1.6 ± 0.1 | * | ||
| 14,15-DiHETrE | 0.8 ± 0.2 | 1.5 ± 0.2 | * |
| ω-3 PUFAs | Enzyme | Oxilypins | Balanced Diet (μmol/L) | ω-6 Rich Diet (μmol/L) | p Value |
|---|---|---|---|---|---|
| ALA | COX | --- | --- | --- | --- |
| LOX | 13-HOTrE | 7.6 ± 1.6 | 0.2 ± 0.03 | ** | |
| CYP450 | --- | --- | --- | --- | |
| EPA | COX | PGD3 | 0.3 ± 0.1 | 0.08 ± 0.02 | * |
| LOX | 5-HEPE | 42.3 ± 19.5 | 4.1 ± 1.4 | * | |
| 8-HEPE | 33.4 ± 12.4 | 1.5 ± 0.7 | * | ||
| 15-HEPE | 73.4 ± 33.2 | 10.0 ± 2.7 | * | ||
| CYP450 | 8,9-EpETE | 131.7 ± 34.4 | 5.2 ± 1.7 | ** | |
| 11,12-EpETE | 106.7 ± 27.1 | 6.0 ± 1.2 | ** | ||
| 14,15-EpETE | 184.4 ± 44.0 | 10.2 ± 1.7 | ** | ||
| 17,18-EpETE | 226.2 ± 55.8 | 14.4 ± 3.0 | *** | ||
| 11,12-DiHETE | 0.6 ± 0.05 | 0.09 ± 0.02 | *** | ||
| 14,15-DiHETE | 0.8 ± 0.1 | 0.1 ± 0.02 | ** | ||
| 17,18-DiHETE | 3.9 ± 0.7 | 1.4 ± 0.2 | ** | ||
| DHA | COX | --- | --- | --- | --- |
| LOX | --- | --- | ---- | --- | |
| CYP450 | 7,8-EpDPE | 5353.7 ± 1520.7 | 1207.2 ± 319.2 | * | |
| 10,11-EpDPE | 398.5 ± 117.1 | 91.0 ± 22.6 | * | ||
| 13,14-EpDPE | 256.5 ± 74.3 | 58.2 ± 14.5 | * | ||
| 16,17-EpDPE | 253.3 ± 79.4 | 54.4 ± 13.7 | * | ||
| 19,20-EpDPE | 325.1 ± 102.2 | 61.0 ± 17.2 | * | ||
| 7,8-DiHDPE | 4550.1 ± 771.2 | 463.9 ± 165.8 | ** | ||
| 10,11-DiHDPE | 1.2 ± 0.2 | 0.4 ± 0.1 | * | ||
| 13,14-DiHDPE | 1.0 ± 0.2 | 0.4 ± 0.02 | * | ||
| 16,17-DiHDPE | 2.0 ± 0.4 | 0.7 ± 0.09 | * | ||
| 19,20-DiHDPE | 17.7 ± 7.1 | 4.9 ± 0.4 | * |
| Control Diet | Balanced Diet | ω-6 Rich Diet | |
|---|---|---|---|
| Saturated Fatty Acids | 15.6 | 50.2 | 48.2 |
| Monounsaturated Fatty Acids | 16.0 | 8.2 | 8.9 |
| Polyunsaturated Fatty Acids | 15.2 | 10.5 | 12.8 |
| Total of ω-3 PUFAs | 1.9 * | 4.1 | 0.5 |
| Alpha-Linolenic acid (ALA) | --- | 0.3 | 0.2 |
| Eicosapentaenoic acid (EPA) | --- | 2.3 | 0.2 |
| Docosahexaenoic acid (DHA) | --- | 1.5 | 0.1 |
| Total of ω-6 PUFAs | 12.3 | 5 | 12.2 |
| Linoleic acid (LA) | 12.2 | 4.7 | 11.9 |
| Arachidonic acid (ARA) | 0.1 | 0.3 | 0.3 |
| ω-6/ω-3 ratio | 6.4:1 | 1.1:1 | 20:1 |
| Antigen | Dilution | Company | Catalog Number |
|---|---|---|---|
| Active Caspase-3 | 1:125 | Abcam (Cambridge, UK) | ab32042 |
| Active Caspase-8 | 1:125 | Novus (Saint Charles, MO, USA) | NB100-56116 |
| Active Caspase-9 | 1:125 | Abgent (San Diego, CA, USA) | AP7974a |
| CD31 | 1:1000 | Abcam | ab28364 |
| Cytokeratin 5/6 | 1:800 | Diagnostic Biosystems (Pleasanton, CA, USA) | PDM123 |
| COX-2 | 1:500 | Novus | NB100-689 |
| MCM2 | 1:500 | Abcam | ab4461 |
| NFkB p65 | 1:100 | Abcam | ab7970 |
| TGF-β | 1:500 | Abcam | ab92486 |
| VEGF-A | 1:500 | Abcam | ab46154 |
| YY1 | 1:500 | Novus | NBP2-20932 |
| Probe | ID | Company |
|---|---|---|
| COX-2 | Mm03294838_g1 | Applied Biosystems (Waltham, MA, USA) |
| VEGF-A | Mm00437306_m1 | Applied Biosystems |
| YY1 | Mm00456392_m1 | Applied Biosystems |
| GAPDH | Mm05724508_g1 | Applied Biosystems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. https://doi.org/10.3390/ijms23116179
Montecillo-Aguado M, Tirado-Rodriguez B, Antonio-Andres G, Morales-Martinez M, Tong Z, Yang J, Hammock BD, Hernandez-Pando R, Huerta-Yepez S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. International Journal of Molecular Sciences. 2022; 23(11):6179. https://doi.org/10.3390/ijms23116179
Chicago/Turabian StyleMontecillo-Aguado, Mayra, Belen Tirado-Rodriguez, Gabriela Antonio-Andres, Mario Morales-Martinez, Zhen Tong, Jun Yang, Bruce D. Hammock, Rogelio Hernandez-Pando, and Sara Huerta-Yepez. 2022. "Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins" International Journal of Molecular Sciences 23, no. 11: 6179. https://doi.org/10.3390/ijms23116179
APA StyleMontecillo-Aguado, M., Tirado-Rodriguez, B., Antonio-Andres, G., Morales-Martinez, M., Tong, Z., Yang, J., Hammock, B. D., Hernandez-Pando, R., & Huerta-Yepez, S. (2022). Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. International Journal of Molecular Sciences, 23(11), 6179. https://doi.org/10.3390/ijms23116179







