Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia
Abstract
1. Introduction
2. Results
2.1. Experimental Timeline
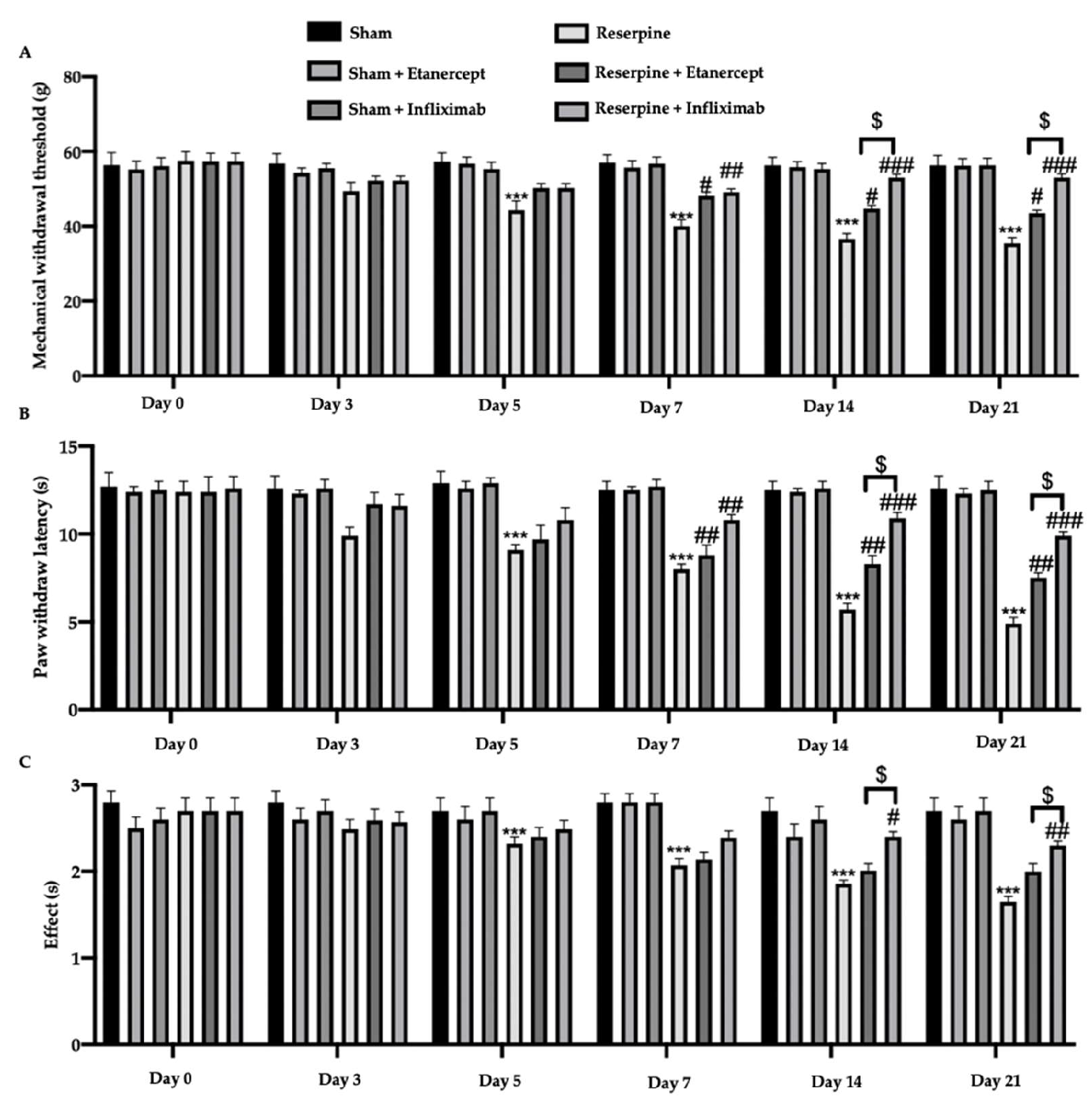
2.2. Effect of TNF-α Inhibitors on Pain Hypersensitivity Induced by Fibromyalgia
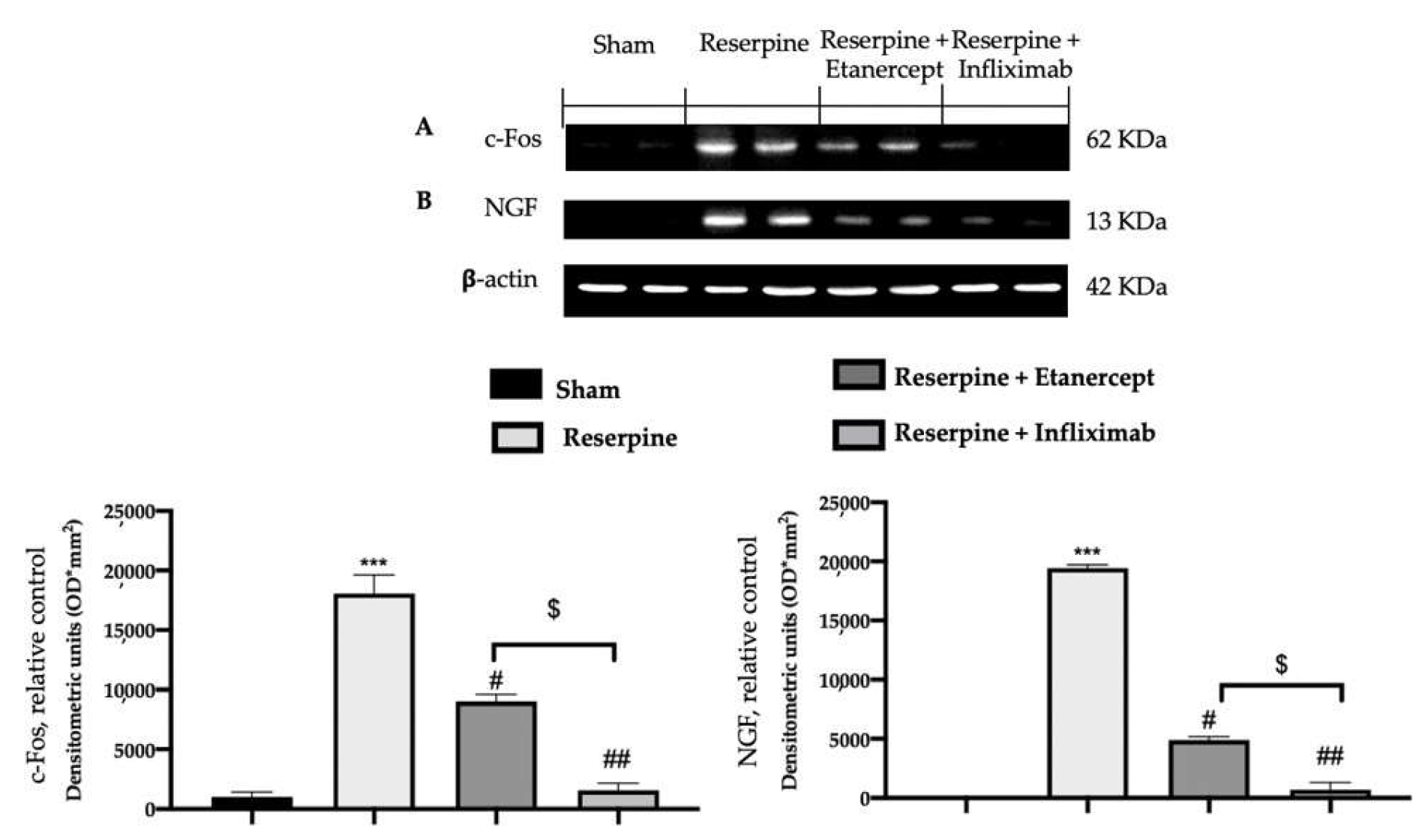
2.3. Effect of TNF-α Inhibitors on Pain-Related Mediators Induced by Fibromyalgia
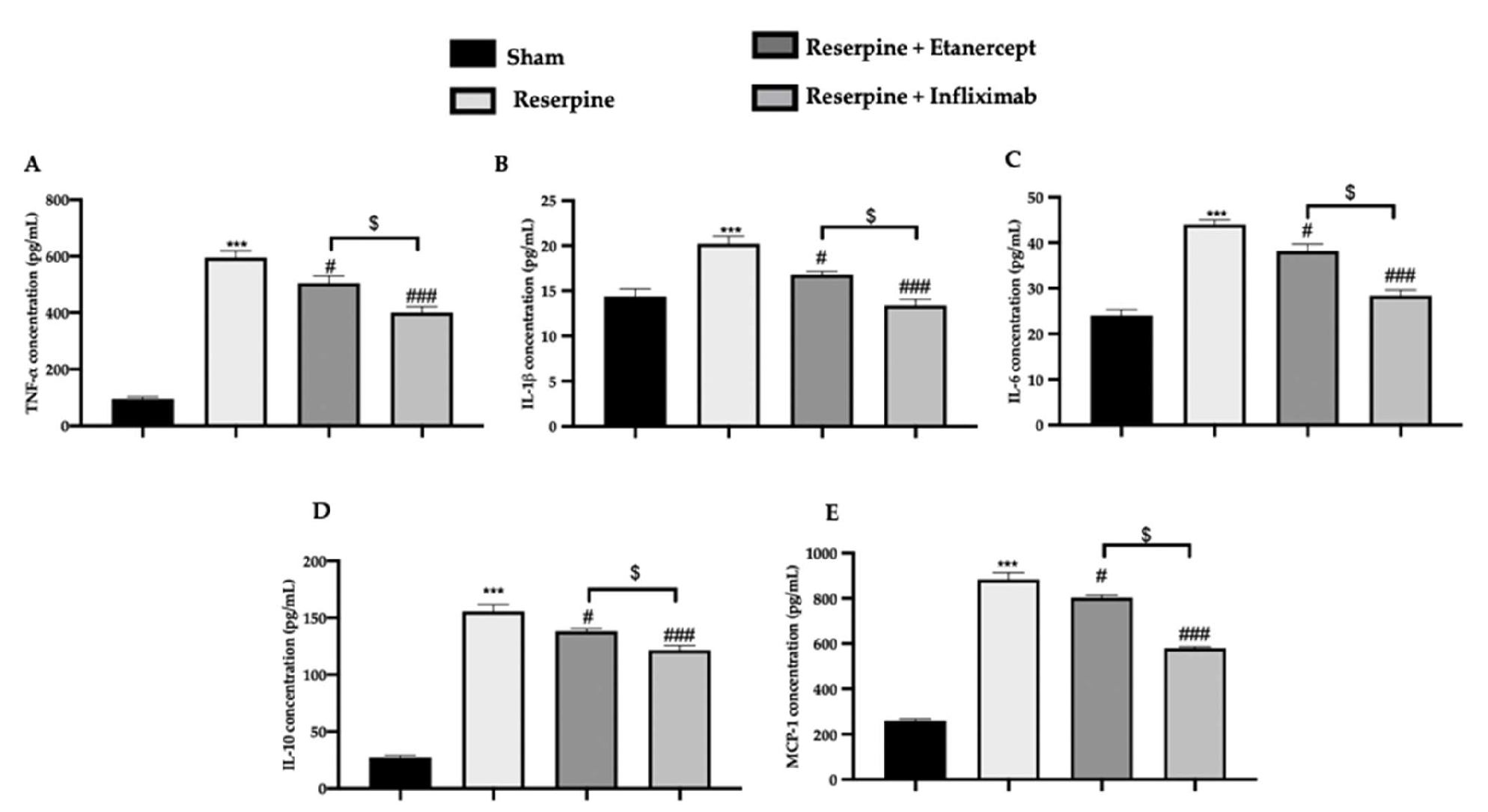
2.4. Effect of TNF-α Inhibitors on Pro-Inflammatory Cytokines’ Overexpression Induced by Fibromyalgia
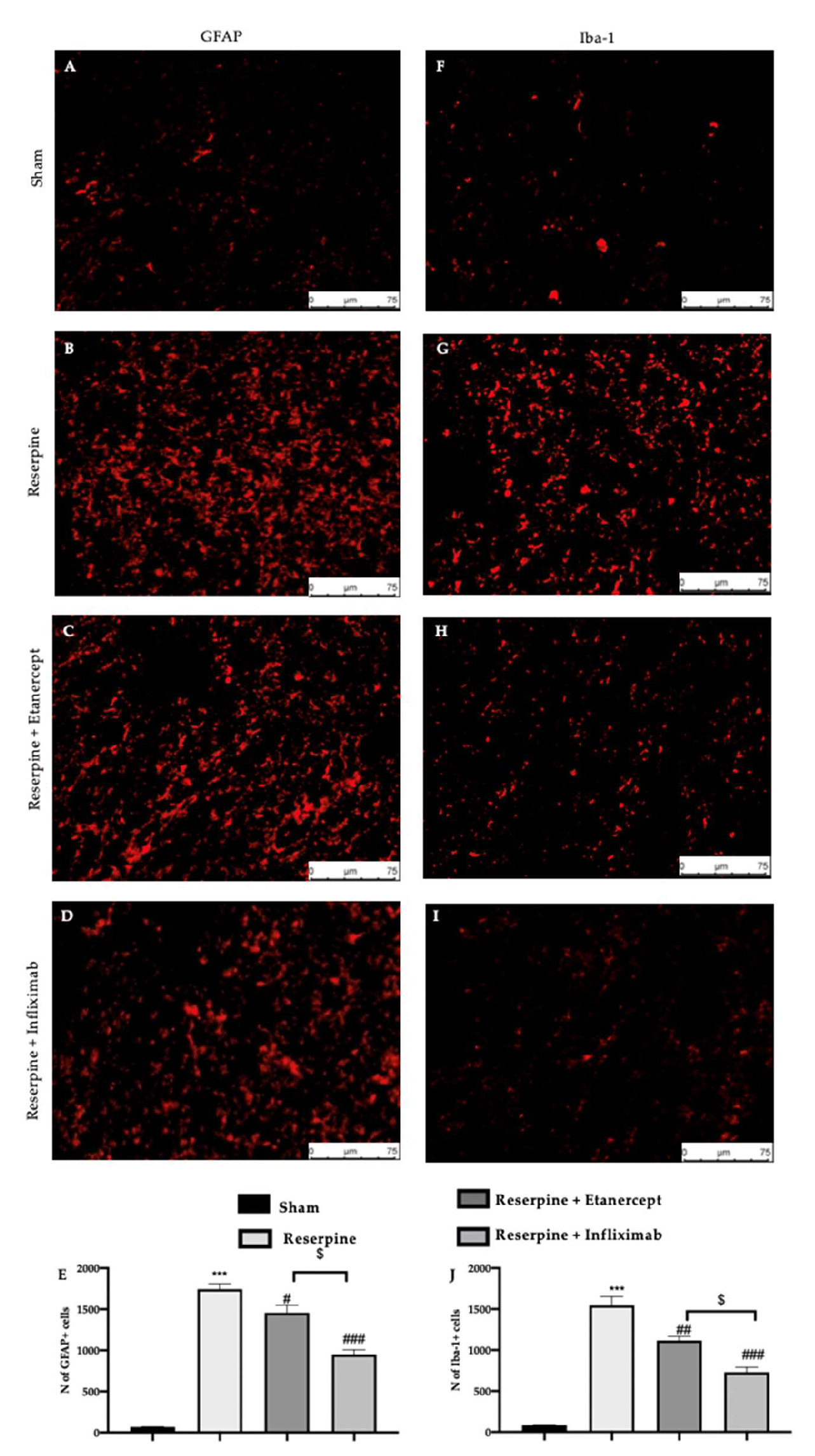
2.5. Effect of TNF-α Inhibitors on Glia Activation Induced by Fibromyalgia
2.6. Effect of TNF-α Inhibitors on Purinergic Receptor and p38-MAPK Expression Induced by Fibromyalgia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Model
4.3. Treatment
4.4. Experimental Froups
4.5. Behavioral Analysis
4.5.1. Von Frey Hair Test
4.5.2. Hot Plate Test
4.5.3. Tail Flick Warm Water Test
4.6. Western Blot Analysis
4.7. Immunofluorescence Analysis
4.8. ELISA Analysis
4.9. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maffei, M.E. Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Potini, B.R.; Azam, A.T. Diagnostic Challenges and Management of Fibromyalgia. Cureus 2021, 13, e18692. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.F.G.; Abud-Mendoza, C. Physiopathology of fibromyalgia. Reumatol. Clin. (Engl. Ed.) 2020, 16, 191–194. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain 2019, 15, 1744806918819944. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- Menzies, V.; Lyon, D.E.; Elswick, R.K., Jr.; Montpetit, A.J.; McCain, N.L. Psychoneuroimmunological Relationships in Women with Fibromyalgia. Biol. Res. Nurs. 2014, 15, 219–225. [Google Scholar] [CrossRef]
- Bradley, L.A. Pathophysiology of Fibromyalgia. Am. J. Med. 2009, 122, S22–S30. [Google Scholar] [CrossRef]
- Peritore, A.F.; Crupi, R.; Scuto, M.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; D’Amico, R.; Fusco, R.; Di Paola, R.; et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord.—Drug Targets 2020, 19, 27–43. [Google Scholar] [CrossRef]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered profile of chemokines in fibromyalgia patients. Ann. Clin. Biochem. 2013, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Behm, F.G.; Gavin, I.M.; Karpenko, O.; Lindgren, V.; Gaitonde, S.; Gashkoff, P.A.; Gillis, B.S. Unique immunologic patterns in fibromyalgia. BMC Clin. Pathol. 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pintó, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Benlidayi, I.C. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Üçeyler, N.; Rogausch, J.P.; Toyka, K.V.; Sommer, C. Differential expression of cytokines in painful and painless neuropathies. Neurology 2007, 69, 42–49. [Google Scholar] [CrossRef]
- Genevay, S.; Finckh, A.; Zufferey, P.; Viatte, S.; Balagué, F.; Gabay, C. Adalimumab in acute sciatica reduces the long-term need for surgery: A 3-year follow-up of a randomised double-blind placebo-controlled trial. Ann. Rheum. Dis. 2012, 71, 560–562. [Google Scholar] [CrossRef]
- Alexander, G.M.; Peterlin, B.L.; Perreault, M.J.; Grothusen, J.R.; Schwartzman, R.J. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J. Pain 2012, 13, 10–20. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Caraffa, A.; Ronconi, G.; Kritas, S.K. Impact of mast cells in fibromyalgia and low-grade chronic inflammation: Can IL-37 play a role? Dermatol. Ther. 2020, 33, e13191. [Google Scholar] [CrossRef]
- DeLeo, J.A.; Rutkowski, M.D.; Stalder, A.K.; Campbell, I.L. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. NeuroReport 2000, 11, 599–602. [Google Scholar] [CrossRef]
- Hatashita, S.; Sekiguchi, M.; Kobayashi, H.; Konno, S.-I.; Kikuchi, S.-I. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976) 2008, 33, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Galbraith, J.A.; Heckman, H.M.; Myers, R.R. Pathology of experimental compression neuropathy producing hyperesthesia. J. Neuropathol. Exp. Neurol. 1993, 52, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Frisén, J.; Risling, M.; Fried, K. Distribution and axonal relations of macrophages in a neuroma. Neuroscience 1993, 55, 1003–1013. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M.; Tozaki-Saitoh, H. Modification of neuropathic pain sensation through microglial ATP receptors. Purinergic Signal. 2007, 3, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.E.; Nico, L.; VandeVord, P.; Skoff, A.M. Modulation of neuropathic pain by a glial-derived factor. Pain Med. 2009, 10, 1229–1236. [Google Scholar] [CrossRef]
- Hansson, E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol. 2006, 187, 321–327. [Google Scholar] [CrossRef]
- Moss, A.; Beggs, S.; Vega-Avelaira, D.; Costigan, M.; Hathway, G.J.; Salter, M.W.; Fitzgerald, M. Spinal microglia and neuropathic pain in young rats. Pain 2007, 128, 215–224. [Google Scholar] [CrossRef]
- Thacker, M.A.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.; Thompson, S.W.N.; Marchand, F.; McMahon, S.B. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef]
- Bergsteinsdottir, K.; Kingston, A.; Jessen, K.R. Rat Schwann cells can be induced to express major histocompatibility complex class II moleculesin vivo. J. Neurocytol. 1992, 21, 382–390. [Google Scholar] [CrossRef]
- Constable, A.L.; Armati, P.J.; Toyka, K.V.; Hartung, H.-P. Production of prostanoids by Lewis rat Schwann cells in vitro. Brain Res. 1994, 635, 75–80. [Google Scholar] [CrossRef]
- Bolin, L.M.; Verity, A.N.; Silver, J.E.; Shooter, E.M.; Abrams, J.S. Interleukin-6 production by schwann cells and induction in sciatic nerve injury. J. Neurochem. 1995, 64, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sci. 1995, 57, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef]
- Empl, M.; Renaud, S.; Erne, B.; Fuhr, P.; Straube, A.; Schaeren-Wiemers, N.; Steck, A.J. TNF-alpha expression in painful and nonpainful neuropathies. Neurology 2001, 56, 1371–1377. [Google Scholar] [CrossRef]
- Covey, W.C.; Ignatowski, T.A.; Renauld, A.E.; Knight, P.R.; Nader, N.D.; Spengler, R.N. Expression of neuron-associated tumor necrosis factor alpha in the brain is increased during persistent pain. Reg. Anesth. Pain Med. 2002, 27, 357–366. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef]
- Marchand, F.; Tsantoulas, C.; Singh, D.; Grist, J.; Clark, A.K.; Bradbury, E.J.; McMahon, S.B. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur. J. Pain 2009, 13, 673–681. [Google Scholar] [CrossRef]
- Genevay, S.; Stingelin, S.; Gabay, C. Efficacy of etanercept in the treatment of acute, severe sciatica: A pilot study. Ann. Rheum. Dis. 2004, 63, 1120–1123. [Google Scholar] [CrossRef]
- Karppinen, J.; Korhonen, T.; Malmivaara, A.; Paimela, L.; Kyllonen, E.; Lindgren, K.A.; Rantanen, P.; Tervonen, O.; Niinimaki, J.; Seitsalo, S.; et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine (Phila Pa 1976) 2003, 28, 750–753, Discussion in 753–754. [Google Scholar] [CrossRef]
- Korhonen, T.; Karppinen, J.; Malmivaara, A.; Autio, R.; Niinimäki, J.; Paimela, L.; Kyllönen, E.; Lindgren, K.-A.; Tervonen, O.; Seitsalo, S.; et al. Efficacy of infliximab for disc herniation-induced sciatica: One-year follow-up. Spine (Phila Pa 1976) 2004, 29, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Karppinen, J.; Paimela, L.; Malmivaara, A.; Lindgren, K.-A.; Bowman, C.; Hammond, A.; Kirkham, B.; Järvinen, S.; Niinimäki, J.; et al. The Treatment of disc herniation-induced sciatica with infliximab: One-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976) 2006, 31, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Wenzell, D.; Hurley, R.W.; Kurihara, C.; Buckenmaier, C.C., 3rd; Griffith, S.; Larkin, T.M.; Dahl, E.; Morlando, B.J. A double-blind, placebo-controlled, dose–response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 2007, 107, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Bogduk, N.; Dragovich, A.; Buckenmaier, C.C., 3rd; Griffith, S.; Kurihara, C.; Raymond, J.; Richter, P.J.; Williams, N.; Yaksh, T.L. Randomized, Double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 2009, 110, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Katanosaka, K.; Yasui, M.; Hayashi, K.; Yamashita, M.; Wakatsuki, K.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain 2015, 156, 415–427. [Google Scholar] [CrossRef]
- Tamano, R.; Ishida, M.; Asaki, T.; Hasegawa, M.; Shinohara, S. Effect of spinal monoaminergic neuronal system dysfunction on pain threshold in rats, and the analgesic effect of serotonin and norepinephrine reuptake inhibitors. Neurosci. Lett. 2016, 615, 78–82. [Google Scholar] [CrossRef]
- Wells, J.A.; Shibata, S.; Fujikawa, A.; Takahashi, M.; Saga, T.; Aoki, I. Functional MRI of the Reserpine-Induced Putative Rat Model of Fibromyalgia Reveals Discriminatory Patterns of Functional Augmentation to Acute Nociceptive Stimuli. Sci. Rep. 2017, 7, 38325. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O.; Yoshida, M.; Miwa, M.; Miura, R.; Saito, H.; Nagakura, Y. Coexistence of Alterations of Gastrointestinal Function and Mechanical Allodynia in the Reserpine-Induced Animal Model of Fibromyalgia. Dig. Dis. Sci. 2019, 64, 2538–2547. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Inoue, K.; Nakajima, K.; Morimoto, T.; Kikuchi, Y.; Koizumi, S.; Illes, P.; Kohsaka, S.; Inoue, K.; Nakajima, K.; Morimoto, T.; et al. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. Br. J. Pharmacol. 1998, 123, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Ohsawa, K.; Inoue, K.; Kohsaka, S. Purinergic receptors in microglia: Functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 2013, 61, 47–54. [Google Scholar] [CrossRef]
- Suzuki, T.; Hide, I.; Ido, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Production and Release of Neuroprotective Tumor Necrosis Factor by P2X7 Receptor-Activated Microglia. J. Neurosci. 2004, 24, 1–7. [Google Scholar] [CrossRef]
- Lister, M.F.; Sharkey, J.; Sawatzky, D.A.; Hodgkiss, J.P.; Davidson, D.J.; Rossi, A.G.; Finlayson, K. The role of the purinergic P2X7 receptor in inflammation. J. Inflamm. 2007, 4, 5. [Google Scholar] [CrossRef]
- Crown, E.D.; Ye, Z.; Johnson, K.M.; Xu, G.-Y.; McAdoo, D.J.; Hulsebosch, C.E. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006, 199, 397–407. [Google Scholar] [CrossRef]
- Crown, E.D.; Gwak, Y.S.; Ye, Z.; Johnson, K.M.; Hulsebosch, C.E. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008, 213, 257–267. [Google Scholar] [CrossRef]
- Terayama, R.; Omura, S.; Fujisawa, N.; Yamaai, T.; Ichikawa, H.; Sugimoto, T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience 2008, 153, 1245–1255. [Google Scholar] [CrossRef]
- Wen, Y.-R.; Suter, M.R.; Ji, R.-R.; Yeh, G.-C.; Wu, Y.-S.; Wang, K.-C.; Kohno, T.; Sun, W.-Z.; Wang, C.-C. Activation of p38 Mitogen-activated Protein Kinase in Spinal Microglia Contributes to Incision-induced Mechanical Allodynia. Anesthesiology 2009, 110, 155–165. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol Against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In vitro and in vivo Study. Front Vet Sci 2020, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Zanella, J.M.; Burright, E.N.; Hildebrand, K.; Hobot, C.; Cox, M.; Christoferson, L.; McKay, W.F. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine (Phila Pa 1976) 2008, 33, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; DuBois, D.C.; Almon, R.R.; Jusko, W.J. Interrelationships between Infliximab and Recombinant Tumor Necrosis Factor-alpha in Plasma Using Minimal Physiologically Based Pharmacokinetic Models. Drug Metab Dispos 2017, 45, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Hoogland, G.; Del Rosario, J.S.; Steinbusch, H.W.; Visser-Vandewalle, V.; Daemen, M.A. Tumor necrosis factor-α inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J. Neurosci. Res. 2014, 92, 1490–1498. [Google Scholar] [CrossRef]
- Ferrier, J.; Marchand, F.; Balayssac, D. Assessment of mechanical allodynia in rats using the electronic von frey test. Bio-protocol 2016, 6, e1933. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants (Basel) 2020, 9, 660. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized Palmitoylethanolamide/Polydatin Treatment Causes Endometriotic Lesion Regression in a Rodent Model of Surgically Induced Endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium Occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO-1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Effect of a new formulation of micronized and ultramicronized N-palmitoylethanolamine in a tibia fracture mouse model of complex regional pain syndrome. PLoS ONE 2017, 12, e0178553. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; di Paola, R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front. Neurol. 2018, 9, 590. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflammation 2018, 15, 1–13. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef] [PubMed][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Franco, G.; Marino, Y.; Di Paola, D.; Cuzzocrea, S.; Impellizzeri, D.; Di Paola, R.; et al. Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. Int. J. Mol. Sci. 2022, 23, 6139. https://doi.org/10.3390/ijms23116139
Cordaro M, Siracusa R, D’Amico R, Genovese T, Franco G, Marino Y, Di Paola D, Cuzzocrea S, Impellizzeri D, Di Paola R, et al. Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. International Journal of Molecular Sciences. 2022; 23(11):6139. https://doi.org/10.3390/ijms23116139
Chicago/Turabian StyleCordaro, Marika, Rosalba Siracusa, Ramona D’Amico, Tiziana Genovese, Gianluca Franco, Ylenia Marino, Davide Di Paola, Salvatore Cuzzocrea, Daniela Impellizzeri, Rosanna Di Paola, and et al. 2022. "Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia" International Journal of Molecular Sciences 23, no. 11: 6139. https://doi.org/10.3390/ijms23116139
APA StyleCordaro, M., Siracusa, R., D’Amico, R., Genovese, T., Franco, G., Marino, Y., Di Paola, D., Cuzzocrea, S., Impellizzeri, D., Di Paola, R., & Fusco, R. (2022). Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. International Journal of Molecular Sciences, 23(11), 6139. https://doi.org/10.3390/ijms23116139










