AU-Rich Element RNA Binding Proteins: At the Crossroads of Post-Transcriptional Regulation and Genome Integrity
Abstract
1. Introduction
2. The Eukaryotic DNA Damage Network
3. Multifunctional AU-RBPs Maintain Genome Integrity
4. Post-Transcriptional Regulation of DDR Genes by AU-RBPs
5. DNA Damage Induces Post-Translational Modifications of AU-RBPs
6. Subcellular Localisation of AU-RBPs in Response to DNA Damage
7. AU-RBPs at the Crossroads of DNA Repair and Genome Maintenance
8. AU-RBPs in Cancer
9. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindahl, T.; Barnes, D.E. Repair of Endogenous DNA Damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA Double-Strand Breaks: Signaling, Repair and the Cancer Connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; García-Muse, T. Causes of Genome Instability. Annu. Rev. Genet. 2013, 47, 1–32. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hoch, N.C.; Hanzlikova, H.; Rulten, S.L.; Tétreault, M.; Komulainen, E.; Ju, L.; Hornyak, P.; Zeng, Z.; Gittens, W.; Rey, S.A.; et al. XRCC1 Mutation Is Associated with PARP1 Hyperactivation and Cerebellar Ataxia. Nature 2017, 541, 87–91. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability an Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.S.; Elledge, S.J. The DNA Damage Response: Putting Checkpoints in Perspective. Nature 2000, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Smith, J.; Mun Tho, L.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Álvarez-Quilón, A.; Serrano-Benítez, A.; Lieberman, J.A.; Quintero, C.; Sánchez-Gutiérrez, D.; Escudero, L.M.; Cortés-Ledesma, F. ATM Specifically Mediates Repair of Double-Strand Breaks with Blocked DNA Ends. Nat. Commun. 2014, 5, 3347. [Google Scholar] [CrossRef]
- Haahr, P.; Hoffmann, S.; Tollenaere, M.A.X.; Ho, T.; Toledo, L.I.; Mann, M.; Bekker-Jensen, S.; Räschle, M.; Mailand, N. Activation of the ATR Kinase by the RPA-Binding Protein ETAA1. Nat. Cell Biol. 2016, 18, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. Sensing and Repairing DNA Double-Strand Breaks. Carcinogenesis 2002, 23, 687–696. [Google Scholar] [CrossRef]
- Kotsantis, P.; Petermann, E.; Boulton, S.J. Mechanisms of Oncogene-Induced Replication Stress: Jigsaw Falling into Place. Cancer Discov. 2018, 8, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-Cycle Checkpoints and Cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. DNA Damage Checkpoints: From Initiation to Recovery or Adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Huen, M.S.Y.; Chen, J. The DNA Damage Response Pathways: At the Crossroad of Protein Modifications. Cell Res. 2008, 18, 8–16. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR Substrate Analysis Reveals Extensive Protein Networks Responsive to DNA Damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef]
- Dietlein, F.; Thelen, L.; Reinhardt, H.C. Cancer-Specific Defects in DNA Repair Pathways as Targets for Personalized Therapeutic Approaches. Trends Genet. 2014, 30, 326–339. [Google Scholar] [CrossRef]
- Kleiman, F.E.; Manley, J.L. The BARD1-CstF-50 Interaction Links MRNA 3′ End Formation to DNA Damage and Tumor Suppression. Cell 2001, 104, 743–753. [Google Scholar] [CrossRef]
- Mirkin, N.; Fonseca, D.; Mohammed, S.; Cevher, M.A.; Manley, J.L.; Kleiman, F.E. The 3′ Processing Factor CstF Functions in the DNA Repair Response. Nucleic Acids Res. 2008, 36, 1792–1804. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Wang, W.; Wood, W.H.; Becker, K.G.; Gorospe, M. Global Analysis of Stress-Regulated MRNA Turnover by Using CDNA Arrays. Proc. Natl. Acad. Sci. USA 2002, 99, 10611–10616. [Google Scholar] [CrossRef]
- Braunstein, S.; Badura, M.L.; Xi, Q.; Formenti, S.C.; Schneider, R.J. Regulation of Protein Synthesis by Ionizing Radiation. Mol. Cell. Biol. 2009, 29, 5645–5656. [Google Scholar] [CrossRef] [PubMed]
- Kruiswijk, F.; Yuniati, L.; Magliozzi, R.; Low, T.Y.; Lim, R.; Bolder, R.; Mohammed, S.; Proud, C.G.; Heck, A.J.R.; Pagano, M.; et al. Coupled Activation and Degradation of EEF2K Regulates Protein Synthesis in Response to Genotoxic Stress. Sci. Signal. 2012, 5, ra40. [Google Scholar] [CrossRef]
- Dutertre, M.; Lambert, S.; Carreira, A.; Amor-Guéret, M.; Vagner, S. DNA Damage: RNA-Binding Proteins Protect from near and Far. Trends Biochem. Sci. 2014, 39, 141–149. [Google Scholar] [CrossRef]
- Powley, I.R.; Kondrashov, A.; Young, L.A.; Dobbyn, H.C.; Hill, K.; Cannell, I.G.; Stoneley, M.; Kong, Y.W.; Cotes, J.A.; Smith, G.C.M.; et al. Translational Reprogramming Following UVB Irradiation Is Mediated by DNA-PKcs and Allows Selective Recruitment to the Polysomes of MRNAs Encoding DNA Repair Enzymes. Genes Dev. 2009, 23, 1207–1220. [Google Scholar] [CrossRef]
- Badura, M.; Braunstein, S.; Zavadil, J.; Schneider, R.J. DNA Damage and EIF4G1 in Breast Cancer Cells Reprogram Translation for Survival and DNA Repair MRNAs. Proc. Natl. Acad. Sci. USA 2012, 109, 18767–18772. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, S.; Chinnaiyan, P.; Shankavaram, U.T.; Lü, X.; Camphausen, K.; Tofilon, P. Radiation-Induced Gene Translation Profiles Reveal Tumor Type and Cancer-Specific Components. Cancer Res. 2008, 68, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Audic, Y.; Hartley, R.S. Post-Transcriptional Regulation in Cancer. Biol. Cell 2004, 96, 479–498. [Google Scholar] [CrossRef]
- Pickering, B.M.; Willis, A.E. The Implications of Structured 5′ Untranslated Regions on Translation and Disease. Semin. Cell Dev. Biol. 2005, 16, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, T.; Williams, R.G.; Khabar, A. ARED 3.0: The Large and Diverse AU-Rich Transcriptome. Nucleic Acids Res. 2006, 34, D111–D114. [Google Scholar] [CrossRef]
- Baou, M.; Jewell, A.; Murphy, J.J. TIS11 Family Proteins and Their Roles in Posttranscriptional Gene Regulation. J. Biomed. Biotechnol. 2009, 2009, 1–11. [Google Scholar] [CrossRef]
- Lunde, B.M.; Moore, C.; Varani, G. RNA-Binding Proteins: Modular Design for Efficient Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Valverde, R.; Edwards, L.; Regan, L. Structure and Function of KH Domains. FEBS J. 2008, 275, 2712–2726. [Google Scholar] [CrossRef]
- Katahira, M.; Miyanoiri, Y.; Enokizono, Y.; Matsuda, G.; Nagata, T.; Ishikawa, F.; Uesugi, S. Structure of the C-Terminal RNA-Binding Domain of HnRNP D0 (AUF1), Its Interactions with RNA and DNA, and Change in Backbone Dynamics upon Complex Formation with DNA. J. Mol. Biol. 2001, 311, 973–988. [Google Scholar] [CrossRef]
- Castello, A.; Fischer, B.; Frese, C.K.; Horos, R.; Alleaume, A.M.; Foehr, S.; Curk, T.; Krijgsveld, J.; Hentze, M.W. Comprehensive Identification of RNA-Binding Domains in Human Cells. Mol. Cell 2016, 63, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2009, 38, D211–D222. [Google Scholar] [CrossRef]
- Wurth, L. Versatility of RNA-Binding Proteins in Cancer. Comp. Funct. Genom. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-Binding Proteins and Post-Transcriptional Gene Regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef]
- Conlon, E.G.; Manley, J.M. RNA-Binding Proteins in Neurodegeneration: Mechanisms in Aggregate. Genes Dev. 2017, 31, 1509–1528. [Google Scholar] [CrossRef]
- Lukong, K.E.; Chang, K.W.; Khandjian, E.W.; Richard, S. RNA-Binding Proteins in Human Genetic Disease. Trends Genet. 2008, 24, 416–425. [Google Scholar] [CrossRef]
- Pereira, B.; Billaud, M.; Almeida, R. RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer 2017, 3, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Mai, R.; Fang, W.; Lin, C.; Chiu, C. YB-1 Disrupts Mismatch Repair Complex Formation, Interferes with MutSα Recruitment on Mismatch and Inhibits Mismatch Repair through Interacting with PCNA. Oncogene 2013, 33, 5065–5077. [Google Scholar] [CrossRef] [PubMed]
- Gorthi, A.; Romero, J.C.; Loranc, E.; Cao, L.; Lawrence, L.A.; Goodale, E.; Iniguez, A.B.; Bernard, X.; Masamsetti, V.P.; Roston, S.; et al. EWS–FLI1 Increases Transcription to Cause R-Loops and Block BRCA1 Repair in Ewing Sarcoma. Nature 2018, 555, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Blackford, A.N.; Chapman, J.R.; Baskcomb, L.; Gravel, S.; Rusch, A.; Thomas, A.; Blundred, R.; Smith, P.; Kzhyshkowska, J.; et al. Regulation of DNA-End Resection by HnRNPU-like Proteins Promotes DNA Double-Strand Break Signaling and Repair. Mol. Cell 2012, 45, 505–516. [Google Scholar] [CrossRef]
- Alfano, L.; Caporaso, A.; Altieri, A.; Dell’Aquila, M.; Landi, C.; Bini, L.; Pentimalli, F.; Giordano, A. Depletion of the RNA Binding Protein HNRNPD Impairs Homologous Recombination by Inhibiting DNA-End Resection and Inducing R-Loop Accumulation. Nucleic Acids Res. 2019, 47, 4068–4085. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, W.; Cao, J.; Wang, F.; Geng, Y.; Cao, J.; Xu, X.; Zhou, J.; Liu, P.; Zhang, S. Upregulation of AUF1 Is Involved in the Proliferation of Esophageal Squamous Cell Carcinoma through GCH1. Int. J. Oncol. 2016, 49, 2001–2010. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Liu, T.; Li, D.; Wang, J.; Zhang, H.; Deng, Z.; Chen, F.; Cai, J. The Overexpression of AUF1 in Colorectal Cancer Predicts a Poor Prognosis and Promotes Cancer Progression by Activating ERK and AKT Pathways. Cancer Med. 2020, 9, 8612. [Google Scholar] [CrossRef]
- Andrade, D.; Mehta, M.; Griffith, J.; Oh, S.; Corbin, J.; Babu, A.; De, S.; Chen, A.; Zhao, Y.D.; Husain, S.; et al. HuR Reduces Radiation-Induced DNA Damage by Enhancing Expression of ARID1A. Cancers 2019, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.; Park, Y.; Seo, J.-W.; Park, O.H.; Ha, H.; Nam, J.-W.; Kim, Y.K. HuR Stabilizes a Polyadenylated Form of Replication-Dependent Histone MRNAs under Stress Conditions. FASEB J. 2019, 33, 2680–2693. [Google Scholar] [CrossRef]
- Cristini, A.; Groh, M.; Kristiansen, M.S.; Gromak, N. RNA/DNA Hybrid Interactome Identifies DXH9 as a Molecular Player in Transcriptional Termination and R-Loop-Associated DNA Damage. Cell Rep. 2018, 23, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, M.; Hong, J.; Kim, M.K.; Chung, I.K. Loss of RNA-Binding Protein HuR Facilitates Cellular Senescence through Posttranscriptional Regulation of TIN2 MRNA. Nucleic Acids Res. 2018, 46, 4271–4285. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J. HuR Stabilizes TFAM MRNA in an ATM/P38-Dependent Manner in Ionizing Irradiated Cancer Cells. Cancer Sci. 2018, 109, 2446–2457. [Google Scholar] [CrossRef]
- Chand, S.N.; Zarei, M.; Schiewer, M.J.; Kamath, A.R.; Romeo, C.; Lal, S.; Cozzitorto, J.A.; Nevler, A.; Scolaro, L.; Londin, E.; et al. Posttranscriptional Regulation of PARG MRNA by HuR Facilitates DNA Repair and Resistance to PARP Inhibitors. Cancer Res. 2017, 77, 5011–5025. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Abdelmohsen, K.; Kim, M.M.; Srikantan, S.; Lee, E.K.; Tominaga, K.; Selimyan, R.; Martindale, J.L.; Yang, X.; Lehrmann, E.; et al. Global Dissociation of HuR-MRNA Complexes Promotes Cell Survival after Ionizing Radiation. EMBO J. 2011, 30, 1040–1053. [Google Scholar] [CrossRef]
- Mazan-Mamczarz, K.; Hagner, P.R.; Zhang, Y.; Dai, B.; Lehrmann, E.; Becker, K.G.; Keene, J.D.; Gorospe, M.; Liu, Z.; Gartenhaus, R.B. ATM Regulates a DNA Damage Response Posttranscriptional RNA Operon in Lymphocytes. Blood 2011, 117, 2441. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Kuwano, Y.; Kim, H.H.; Gorospe, M. Posttranscriptional Gene Regulation by RNA-Binding Proteins during Oxidative Stress: Implications for Cellular Senescence. Biol. Chem. 2008, 389, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Saunus, J.M.; French, J.D.; Edwards, S.L.; Beveridge, D.J.; Hatchell, E.C.; Wagner, S.A.; Stein, S.R.; Davidson, A.; Simpson, K.J.; Francis, G.D.; et al. Posttranscriptional Regulation of the Breast Cancer Susceptibility Gene BRCA1 by the RNA Binding Protein HuR. Cancer Res. 2008, 68, 9469–9478. [Google Scholar] [CrossRef]
- Mazan-Mamczarz, K.; Galbán, S.; De Silanes, I.L.; Martindale, J.L.; Atasoy, U.; Keene, J.D.; Gorospe, M. RNA-Binding Protein HuR Enhances P53 Translation in Response to Ultraviolet Light Irradiation. Proc. Natl. Acad. Sci. USA 2003, 100, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Furneaux, H.; Cheng, H.; Caldwell, M.C.; Hutter, D.; Liu, Y.; Holbrook, N.; Gorospe, M. HuR Regulates P21 MRNA Stabilization by UV Light. Mol. Cell. Biol. 2000, 20, 760–769. [Google Scholar] [CrossRef]
- Wang, W.; Caldwell, M.C.; Lin, S.; Furneaux, H.; Gorospe, M. HuR Regulates Cyclin A and Cyclin B1 MRNA Stability during Cell Proliferation. EMBO J. 2000, 19, 2340–2350. [Google Scholar] [CrossRef]
- Heinonen, M.; Bono, P.; Narko, K.; Chang, S.H.; Lundin, J.; Joensuu, H.; Furneaux, H.; Hla, T.; Haglund, C.; Ristimäki, A. Cytoplasmic HuR Expression Is a Prognostic Factor in Invasive Ductal Breast Carcinoma. Cancer Res. 2005, 65, 2157–2161. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, X.; Lei, Y.; Liu, X.; Liu, Z.; Tong, T.; Wang, W. Loss of Repression of HuR Translation by MiR-16 May Be Responsible for the Elevation of HuR in Human Breast Carcinoma. J. Cell. Biochem. 2010, 111, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, B.; Wu, Y. Predictive and Prognostic Significance of Cytoplasmic Expression of ELAV-like Protein HuR in Invasive Breast Cancer Treated with Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2013, 141, 213–224. [Google Scholar] [CrossRef]
- Palomo-Irigoyen, M.; Pérez-Andrés, E.; Iruarrizaga-Lejarreta, M.; Barreira-Manrique, A.; Tamayo-Caro, M.; Vila-Vecilla, L.; Moreno-Cugnon, L.; Beitia, N.; Medrano, D.; Fernández-Ramos, D.; et al. HuR/ELAVL1 Drives Malignant Peripheral Nerve Sheath Tumor Growth and Metastasis. J. Clin. Investig. 2020, 130, 3848. [Google Scholar] [CrossRef] [PubMed]
- Trabucchi, M.; Briata, P.; Garcia-Mayoral, M.; Haase, A.D.; Filipowicz, W.; Ramos, A.; Gherzi, R.; Rosenfeld, M.G. The RNA-Binding Protein KSRP Promotes the Biogenesis of a Subset of MicroRNAs. Nature 2009, 459, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, G.; Berger, F.G.; He, X.; Lu, X. The ATM Kinase Induces MicroRNA Biogenesis in the DNA Damage Response. Mol. Cell 2011, 41, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chou, C.F.; Liu, S.; Crossman, D.; Yusuf, N.; Wu, Y.; Chen, C.Y. KSRP Modulates Melanoma Growth and Efficacy of Vemurafenib. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Bikkavilli, R.; Zerayesus, S.; Van Scoyk, M.; Wilson, L.; Wu, P.; Baskaran, A.; Tang, K.; Raheem, S.; Samuelson, B.; Reddy, N.; et al. K-Homology Splicing Regulatory Protein (KSRP) Promotes Post-Transcriptional Destabilization of Spry4 Transcripts in Non-Small Cell Lung Cancer. J. Biol. Chem. 2017, 292, 7423–7434. [Google Scholar] [CrossRef]
- Yan, M.; Sun, L.; Li, J.; Yu, H.; Lin, H.; Yu, T.; Zhao, F.; Zhu, M.; Liu, L.; Geng, Q.; et al. RNA-Binding Protein KHSRP Promotes Tumor Growth and Metastasis in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, F.; Oficjalska, K.; Tosetto, M.; Phelan, J.J.; Noonan, S.; Martin, P.; Killick, K.; Breen, L.; O’Neill, F.; Nolan, B.; et al. KH-Type Splicing Regulatory Protein Controls Colorectal Cancer Cell Growth and Modulates the Tumor Microenvironment. Am. J. Pathol. 2019, 189, 1916–1932. [Google Scholar] [CrossRef]
- Lafarga, V.; Sung, H.; Haneke, K.; Roessig, L.; Pauleau, A.; Bruer, M.; Rodriguez-Acebes, S.; Lopez-Contreras, A.J.; Gruss, O.J.; Erhardt, S.; et al. TIAR Marks Nuclear G2/M Transition Granules and Restricts CDK 1 Activity under Replication Stress. EMBO Rep. 2019, 20, e46224. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, C.; Ludeña, M.D.; Izquierdo, J.M. T-Cell Intracellular Antigens Function as Tumor Suppressor Genes. Cell Death Dis. 2015, 6, e1669. [Google Scholar] [CrossRef]
- Lee, T.-H.; Choi, J.Y.; Park, J.-M.; Kang, T.-H. Posttranscriptional Control of the Replication Stress Response via TTP-Mediated Claspin MRNA Stabilization. Oncogene 2020, 39, 3245–3257. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.E.; Kuwano, Y.; Alkharouf, N.; Blackshear, P.J.; Gorospe, M.; Wilson, G.M. The MRNA-Destabilizing Protein Tristetraprolin Is Suppressed in Many Cancers, Altering Tumorigenic Phenotypes and Patient Prognosis. Cancer Res. 2009, 69, 5168–5176. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 MRNA. Immunity 2017, 47, 1083–1099.e6. [Google Scholar] [CrossRef]
- Fallahi, M.; Amelio, A.L.; Cleveland, J.L.; Rounbehler, R.J. CREB Targets Define the Gene Expression Signature of Malignancies Having Reduced Levels of the Tumor Suppressor Tristetraprolin. PLoS ONE 2014, 9, e115517. [Google Scholar] [CrossRef]
- Kröhler, T.; Kessler, S.M.; Hosseini, K.; List, M.; Barghash, A.; Patial, S.; Laggai, S.; Gemperlein, K.; Haybaeck, J.; Müller, R.; et al. The MRNA-Binding Protein TTP/ZFP36 in Hepatocarcinogenesis and Hepatocellular Carcinoma. Cancers 2019, 11, 1754. [Google Scholar] [CrossRef]
- Vogel, K.U.; Bell, L.S.; Galloway, A.; Ahlfors, H.; Turner, M. The RNA-Binding Proteins Zfp36l1 and Zfp36l2 Enforce the Thymic β-Selection Checkpoint by Limiting DNA Damage Response Signaling and Cell Cycle Progression. J. Immunol. 2016, 197, 2673. [Google Scholar] [CrossRef]
- Hodson, D.; Janas, M.; Galloway, M.; Bell, S.E.; Andrews, S.; Li, C.M.; Pannell, R.; Siebel, C.W.; MacDonald, H.R.; Keersmaecker, K.D.; et al. Deletion of the RNA-Binding Proteins ZFP36L1 and ZFP36L2 Leads to Perturbed Thymic Development and T Lymphoblastic Leukemia. Nat. Immunol. 2010, 11, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of Somatic Mutations in 560 Breast Cancer Whole Genome Sequences. Nature 2016, 534, 47. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic Landscape and Chronological Reconstruction of Driver Events in Multiple Myeloma. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaehler, M.; Dworschak, M.; Rodin, J.P.; Ruemenapp, J.; Vater, I.; Penas, E.; Liu, C.; Cascorbi, I.; Nage, I. ZFP36L1 Plays an Ambiguous Role in the Regulation of Cell Expansion and Negatively Regulates CDKN1A in Chronic Myeloid Leukemia Cells. Exp. Hematol. 2021, 99, 54–64.e7. [Google Scholar] [CrossRef]
- Noguchi, A.; Adachi, S.; Yokota, N.; Hatta, T.; Natsume, T.; Kawahara, H. ZFP36L2 Is a Cell Cycle-Regulated CCCH Protein Necessary for DNA Lesion-Induced S-Phase Arrest. Biol. Open 2018, 7, bio031575. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Zhou, Y.; Yu, J.; Yu, Y.; Nie, Y.; Luo, W.; Yang, C.; Xiong, T.; Wu, W.K.K.; Li, Z.; et al. Whole-Genome Sequencing Reveals Novel Tandem-Duplication Hotspots and a Prognostic Mutational Signature in Gastric Cancer. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Seki, N.; Kurahara, H.; Osako, Y.; Idichi, T.; Arai, T.; Koshizuka, K.; Kita, Y.; Maemura, K.; Natsugoe, S. ZFP36L2 Promotes Cancer Cell Aggressiveness and Is Regulated by Antitumor MicroRNA-375 in Pancreatic Ductal Adenocarcinoma. Cancer Sci. 2017, 108, 124–135. [Google Scholar] [CrossRef]
- Priestley, P.; Baber, J.; Lolkema, M.P.; Steeghs, N.; de Bruijn, E.; Shale, C.; Duyvesteyn, K.; Haidari, S.; van Hoeck, A.; Onstenk, W.; et al. Pan-Cancer Whole-Genome Analyses of Metastatic Solid Tumours. Nature 2019, 575, 210–216. [Google Scholar] [CrossRef]
- Caldon, C.E.; Musgrove, E.A. Distinct and Redundant Functions of Cyclin E1 and Cyclin E2 in Development and Cancer. Cell Div. 2010, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Abdelmohsen, K.; Gorospe, M. Regulation of HuR by DNA Damage Response Kinases. J. Nucleic Acids 2010, 2010, 981487. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Ui, A.; Kanno, S.; Ogiwara, H.; Nagase, T.; Kohno, T.; Yasui, A. SWI/SNF Factors Required for Cellular Resistance to DNA Damage Include ARID1A and ARID1B and Show Interdependent Protein Stability. Cancer Res. 2014, 74, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, M.D.; Kiselev, V.Y.; Novère, N.L.; Curk, T.; Ule, J.; Turner, M. Tia1 Dependent Regulation of MRNA Subcellular Location and Translation Controls P53 Expression in B Cells. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, C.; Izquierdo, J.M. T-Cell Intracellular Antigens in Health and Disease. Cell Cycle 2015, 14, 2033–2043. [Google Scholar] [CrossRef]
- Lal, A.; Abdelmohsen, K.; Pullmann, R.; Kawai, T.; Galban, S.; Yang, X.; Brewer, G.; Gorospe, M. Posttranscriptional Derepression of GADD45α by Genotoxic Stress. Mol. Cell 2006, 22, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.; Turner, M. Cell Cycle RNA Regulons Coordinating Early Lymphocyte Development. Wiley Interdiscip. Rev. RNA 2017, 8, 1419. [Google Scholar] [CrossRef]
- Solaiman, N.S.; Sidali, A.; Kishore, K.; Norton, J.D.; Radhakrishnan, K.; Surendranath, K.; Murphy, J.J. RNA-Binding Protein ZFP36L1 Supresses Replication Stress-Induced DNA Damage. (manuscript in preparation; to be submitted).
- Suk, F.M.; Chang, C.C.; Lin, R.J.; Lin, S.Y.; Liu, S.C.; Jau, C.F.; Liang, Y.C. ZFP36L1 and ZFP36L2 Inhibit Cell Proliferation in a Cyclin D-Dependent and P53-Independent Manner. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Lebedeva, S.; Jens, M.; Theil, K.; Schwanhäusser, B.; Selbach, M.; Landthaler, M.; Rajewsky, N. Transcriptome-Wide Analysis of Regulatory Interactions of the RNA-Binding Protein HuR. Mol. Cell 2011, 43, 340–352. [Google Scholar] [CrossRef]
- Sedlyarov, V.; Fallman, J.; Ebner, F.; Huemer, J.; Sneezum, L.; Ivin, M.; Kreiner, K.; Tanzer, A.; Vogl, C.; Hofacker, I.; et al. Tristetraprolin Binding Site Atlas in the Macrophage Transcriptome Reveals a Switch for Inflammation Resolution. Mol. Syst. Biol. 2016, 12, 868. [Google Scholar] [CrossRef]
- Mukherjee, N.; Corcoran, D.L.; Nusbaum, J.D.; Reid, D.W.; Georgiev, S.; Hafner, M.; Ascano, M., Jr.; Tuschl, T.; Ohler, U.; Keene, J.D. Integrative Regulatory Mapping Indicates That the RNA-Binding Protein HuR (ELAVL1) Couples Pre-MRNA Processing and MRNA Stability. Mol. Cell 2011, 43, 327. [Google Scholar] [CrossRef]
- Mukherjee, N.; Jacobs, N.C.; Hafner, M.; Kennington, E.A.; Nusbaum, J.D.; Tuschl, T.; Blackshear, P.J.; Ohler, U. Global Target MRNA Specification and Regulation by the RNA-Binding Protein ZFP36. Genome Biol. 2014, 15, 1–16. [Google Scholar] [CrossRef]
- Bakheet, T.; Hitti, E.; Al-Saif, M.; Moghrabi, W.N.; Khabar, K.S.A. The AU-Rich Element Landscape across Human Transcriptome Reveals a Large Proportion in Introns and Regulation by ELAVL1/HuR. Biochim. Biophys. Acta-Gene Regul. Mech. 2018, 1861, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Jungmichel, S.; Rosenthal, F.; Altmeyer, M.; Lukas, J.; Hottiger, M.O.; Nielsen, M.L. Proteome-Wide Identification of Poly(ADP-Ribosyl)Ation Targets in Different Genotoxic Stress Responses. Mol. Cell 2013, 52, 272–285. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Pullmann, R.; Lal, A.; Kim, H.H.; Galban, S.; Yang, X.; Blethrow, J.D.; Walker, M.; Shubert, J.; Gillespie, D.A.; et al. Phosphorylation of HuR by Chk2 Regulates SIRT1 Expression. Mol. Cell 2007, 25, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Hasskamp, P.; Schmedding, I.; Morandell, S.; van Vugt, M.A.T.M.; Wang, X.Z.; Linding, R.; Ong, S.E.; Weaver, D.; Carr, S.A.; et al. DNA Damage Activates a Spatially Distinct Late Cytoplasmic Cell-Cycle Checkpoint Network Controlled by MK2-Mediated RNA Stabilization. Mol. Cell 2010, 40, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wan, G.; Langley, R.R.; Zhang, X.; Lu, X. Crosstalk between the DNA Damage Response Pathway and MicroRNAs. Cell. Mol. Life Sci. 2012, 69, 2895–2906. [Google Scholar] [CrossRef]
- Moskwa, P.; Buffa, F.M.; Pan, Y.; Panchakshari, R.; Gottipati, P.; Muschel, R.J.; Beech, J.; Kulshrestha, R.; Abdelmohsen, K.; Weinstock, D.M.; et al. MiR-182-Mediated Downregulation of BRCA1 Impacts DNA Repair and Sensitivity to PARP Inhibitors. Mol. Cell 2011, 41, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Yoshino, Y.; Morita, N.; Kaneda, N. Identification of Nuclear Import and Export Signals within the Structure of the Zinc Finger Protein TIS11. Biochem. Biophys. Res. Commun. 2002, 293, 1242–1247. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N. RNA Granules: Post-Transcriptional and Epigenetic Modulators of Gene Expression. Nat. Rev. Mol. Cell Biol. 2009, 10, 430–436. [Google Scholar] [CrossRef]
- Phillips, R.S.; Ramos, S.B.V.; Blackshear, P.J. Members of the Tristetraprolin Family of Tandem CCCH Zinc Finger Proteins Exhibit CRM1-Dependent Nucleocytoplasmic Shuttling. J. Biol. Chem. 2002, 277, 11606–11613. [Google Scholar] [CrossRef]
- Liang, J.; Lei, T.; Song, Y.; Yanes, N.; Qi, Y.; Fu, M. RNA-Destabilizing Factor Tristetraprolin Negatively Regulates NF-κB Signaling. J. Biol. Chem. 2009, 284, 29383. [Google Scholar] [CrossRef]
- Twizere, J.-C.; Kruys, V.; Lefeèbvre, L.; Vanderplasschen, A.; Collete, D.; Debacq, C.; Lai, W.S.; Jauniaux, J.-C.; Bernstein, L.R.; Semmes, O.J.; et al. Interaction of Retroviral Tax Oncoproteins With Tristetraprolin and Regulation of Tumor Necrosis Factor-α Expression. JNCI J. Natl. Cancer Inst. 2003, 95, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Noguchi, A.; Sakai, S.; Yokota, N.; Kawahara, H. Nuclear Accumulation of ZFP36L1 Is Cell Cycle-Dependent and Determined by a C-Terminal Serine-Rich Cluster. J. Biochem. 2020, 168, 477–489. [Google Scholar] [CrossRef]
- Kim, H.H.; Gorospe, M. Phosphorylated HuR Shuttles in Cycles. Cell Cycle 2008, 7, 3124. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaf, H.H.; Aboussekhra, A. ATR Controls the UV-Related Upregulation of the CDKN1A MRNA in a Cdk1/HuR-Dependent Manner. Mol. Carcinog. 2014, 53, 979–987. [Google Scholar] [CrossRef]
- Barkauskaite, E.; Brassington, A.; Tan, E.S.; Warwicker, J.; Dunstan, M.S.; Banos, B.; Lafite, P.; Ahel, M.; Mitchison, T.J.; Ahel, I.; et al. Visualization of Poly(ADP-Ribose) Bound to PARG Reveals Inherent Balance between Exo- and Endo-Glycohydrolase Activities. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.E.; Hochegger, H.; Takeda, S.; Caldecott, K.W. Poly(ADP-Ribose) Polymerase 1 Accelerates Single-Strand Break Repair in Concert with Poly(ADP-Ribose) Glycohydrolase. Mol. Cell. Biol. 2007, 27, 5597–5605. [Google Scholar] [CrossRef] [PubMed]
- Förch, P.; Puig, O.; Kedersha, N.; Martínez, C.; Granneman, S.; Séraphin, B.; Anderson, P.; Valcárcel, J. The Apoptosis-Promoting Factor TIA-1 Is a Regulator of Alternative Pre-MRNA Splicing. Mol. Cell 2000, 6, 1089–1098. [Google Scholar] [CrossRef]
- Taupin, J.; Tian, Q.; Kedersha, N.; Robertson, M.; Anderson, P. The RNA-Binding Protein TIAR Is Translocated from the Nucleus to the Cytoplasm during Fas-Mediated Apoptotic Cell Death. Proc. Natl. Acad. Sci. USA 1995, 92, 1629–1633. [Google Scholar] [CrossRef]
- Suswam, E.A.; Li, Y.Y.; Mahtani, H.; King, P.H. Novel DNA-Binding Properties of the RNA-Binding Protein TIAR. Nucleic Acids Res. 2005, 33, 4507–4518. [Google Scholar] [CrossRef] [PubMed]
- Huertas, P. DNA Resection in Eukaryotes: Deciding How to Fix the Break. Nat. Struct. Mol. Biol. 2010, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Iacovoni, J.S.; Caron, P.; Lassadi, I.; Nicolas, E.; Massip, L.; Trouche, D.; Legube, G. High-Resolution Profiling of GammaH2AX around DNA Double Strand Breaks in the Mammalian Genome. EMBO J. 2010, 29, 1446–1457. [Google Scholar] [CrossRef]
- Spagnolo, L.; Rivera-Calzada, A.; Pearl, L.H.; Llorca, O. Three-Dimensional Structure of the Human DNA-PKcs/Ku70/Ku80 Complex Assembled on DNA and Its Implications for DNA DSB Repair. Mol. Cell 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Matta, A.; Masui, O.; Srivastava, G.; Kaur, J.; Thakar, A.; Shukla, N.K.; RoyChoudhury, A.; Sharma, M.; Walfish, P.G.; et al. Nuclear Heterogeneous Nuclear Ribonucleoprotein D Is Associated with Poor Prognosis and Interactome Analysis Reveals Its Novel Binding Partners in Oral Cancer. J. Transl. Med. 2015, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.W.; Crothers, D.M. Stability and Properties of Double and Triple Helices: Dramatic Effects of RNA or DNA Backbone Composition. Science 1992, 258, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Westover, K.D.; Bushnell, D.A.; Kornberg, R.D. Structural Basis of Transcription: Nucleotide Selection by Rotation in the RNA Polymerase II Active Center. Cell 2004, 119, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, J.M.; Aguilera, A. R Loops: New Modulators of Genome Dynamics and Function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef]
- Gan, W.; Guan, Z.; Liu, J.; Gui, T.; Shen, K.; Manley, J.L.; Li, X. R-Loop-Mediated Genomic Instability Is Caused by Impairment of Replication Fork Progression. Genes Dev. 2011, 25, 2041–2056. [Google Scholar] [CrossRef]
- Gómez-González, B.; Aguilera, A. Activation-Induced Cytidine Deaminase Action Is Strongly Stimulated by Mutations of the THO Complex. Proc. Natl. Acad. Sci. USA 2007, 104, 8409–8414. [Google Scholar] [CrossRef]
- Hamperl, S.; Bocek, M.J.; Saldivar, J.C.; Swigut, T.; Cimprich, K.A. Transcription-Replication Conflict Orientation Modulates R-Loop Levels and Activates Distinct DNA Damage Responses. Cell 2017, 170, 774–786.e19. [Google Scholar] [CrossRef]
- Stirling, P.C.; Hieter, P. Canonical DNA Repair Pathways Influence R-Loop-Driven Genome Instability. J. Mol. Biol. 2017, 429, 3132–3138. [Google Scholar] [CrossRef]
- Stirling, P.C.; Chan, Y.A.; Minaker, S.W.; Aristizabal, M.J.; Barrett, I.; Sipahimalani, P.; Kobor, M.S.; Hieter, P. R-Loop-Mediated Genome Instability in MRNA Cleavage and Polyadenylation Mutants. Genes Dev. 2012, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wahba, L.; Amon, J.D.; Koshland, D.; Vuica-Ross, M. RNase H and Multiple RNA Biogenesis Factors Cooperate to Prevent RNA:DNA Hybrids from Generating Genome Instability. Mol. Cell 2011, 44, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Huertas, P.; Aguilera, A. Cotranscriptionally Formed DNA: RNA Hybrids Mediate Transcription Elongation Impairment and Transcription-Associated Recombination. Mol. Cell 2003, 12, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Inactivation of the SR Protein Splicing Factor ASF/SF2 Results in Genomic Instability. Cell 2005, 122, 365–378. [Google Scholar] [CrossRef]
- Tsantoulis, P.K.; Kotsinas, A.; Sfikakis, P.P.; Evangelou, K.; Sideridou, M.; Levy, B.; Mo, L.; Kittas, C.; Wu, X.-R.; Papavassiliou, A.G.; et al. Oncogene-Induced Replication Stress Preferentially Targets Common Fragile Sites in Preneoplastic Lesions. A Genome-Wide Study. Oncogene 2007, 27, 3256–3264. [Google Scholar] [CrossRef]
- Arlt, M.F.; Durkin, S.G.; Ragland, R.L.; Glover, T.W. Common Fragile Sites as Targets for Chromosome Rearrangements. DNA Repair 2006, 5, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Bignell, G.R.; Greenman, C.D.; Davies, H.; Butler, A.P.; Edkins, S.; Andrews, J.M.; Buck, G.; Chen, L.; Beare, D.; Latimer, C.; et al. Signatures of Mutation and Selection in the Cancer Genome. Nature 2010, 463, 893–898. [Google Scholar] [CrossRef]
- Hellman, A.; Zlotorynski, E.; Scherer, S.W.; Cheung, J.; Vincent, J.B.; Smith, D.I.; Trakhtenbrot, L.; Kerem, B. A Role for Common Fragile Site Induction in Amplification of Human Oncogenes. Cancer Cell 2002, 1, 89–97. [Google Scholar] [CrossRef]
- Zlotorynski, E.; Rahat, A.; Skaug, J.; Ben-Porat, N.; Ozeri, E.; Hershberg, R.; Levi, A.; Scherer, S.W.; Margalit, H.; Kerem, B. Molecular Basis for Expression of Common and Rare Fragile Sites. Mol. Cell. Biol. 2003, 23, 7143. [Google Scholar] [CrossRef]
- Helmrich, A.; Ballarino, B.; Tora, L. Collisions between Replication and Transcription Complexes Cause Common Fragile Site Instability at the Longest Human Genes. Mol. Cell 2011, 44, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nagpal, G.; Kumar, V.; Usmani, S.S.; Agrawal, P.; Raghava, G.P.S. HumCFS: A Database of Fragile Sites in Human Chromosomes. BMC Genom. 2019, 19, 985. [Google Scholar] [CrossRef]
- Pospisilova, H.; Baens, M.; Michaux, L.; Stul, M.; Van Hummelen, P.; Van Loo, P.; Vermeesch, J.; Jarosova, M.; Zemanova, Z.; Michalova, K.; et al. Interstitial Del(14)(q) Involving IGH: A Novel Recurrent Aberration in B-NHL. Leukemia 2007, 21, 2079–2083. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Perez, A.; Perez-Llamas, C.; Deu-Pons, J.; Tamborero, D.; Schroeder, M.P.; Jene-Sanz, A.; Santos, A.; Lopez-Bigas, N. IntOGen-Mutations Identifies Cancer Drivers across Tumor Types. Nat. Methods 2013, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Rounbehler, R.J.; Fallahi, M.; Yang, C.; Steeves, M.A.; Li, W.; Doherty, J.R.; Schaub, F.K.; Sanduja, S.; Dixon, D.A.; Blackshear, P.J.; et al. Tristetraprolin Impairs Myc-Induced Lymphoma and Abolishes the Malignant State. Cell 2012, 150, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Planel, S.; Salomon, A.; Jalinot, P.; Feige, J.J.; Cherradi, N. A Novel Concept in Antiangiogenic and Antitumoral Therapy: Multitarget Destabilization of Short-Lived MRNAs by the Zinc Finger Protein ZFP36L1. Oncogene 2010, 29, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Ichikawa, H.; Ohki, M. Potential Involvement of the AML1-MTG8 Fusion Protein in the Granulocytic Maturation Characteristic of the t(8;21) Acute Myelogenous Leukemia Revealed by Microarray Analysis. Leukemia 2002, 16, 874–885. [Google Scholar] [CrossRef][Green Version]
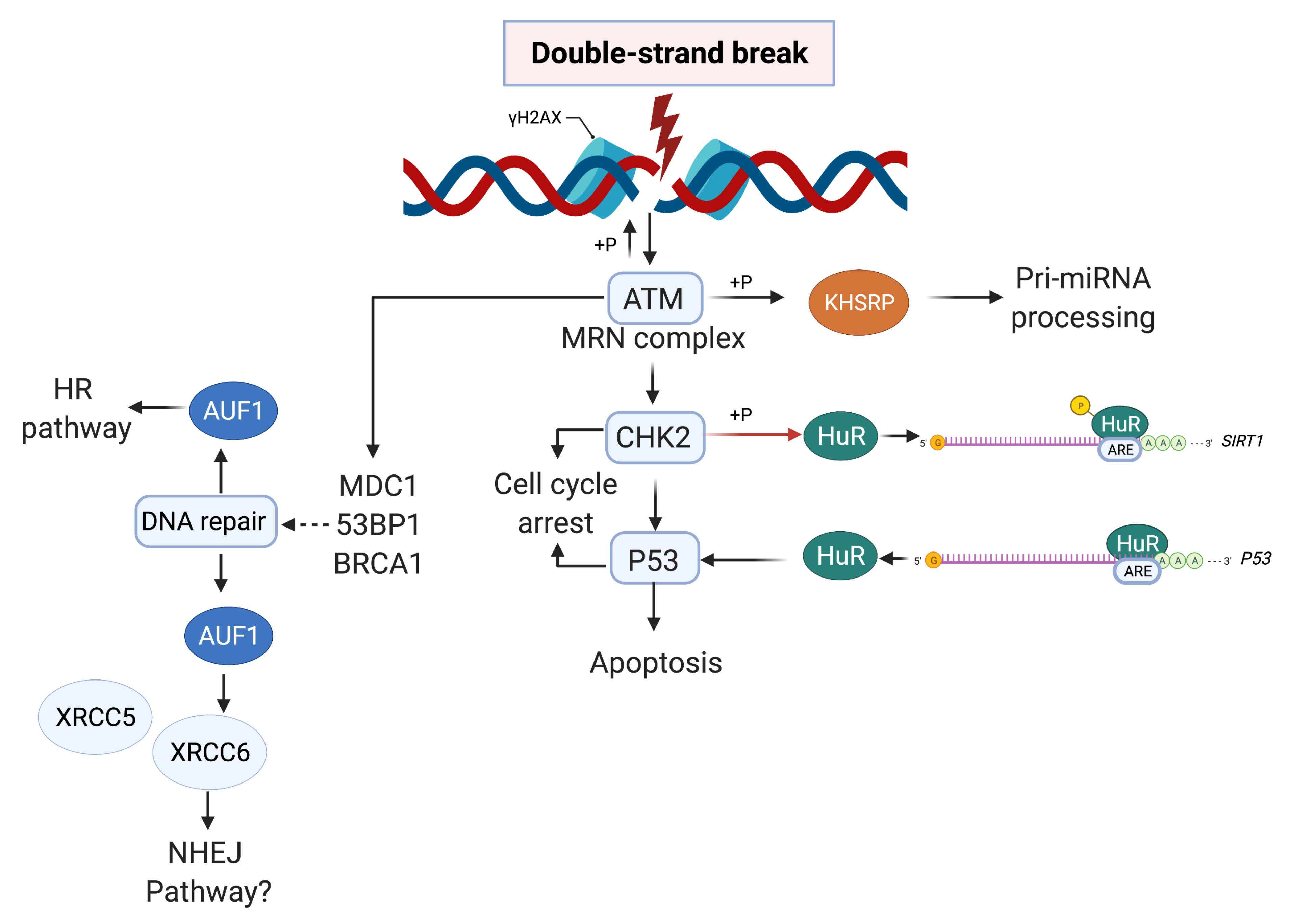
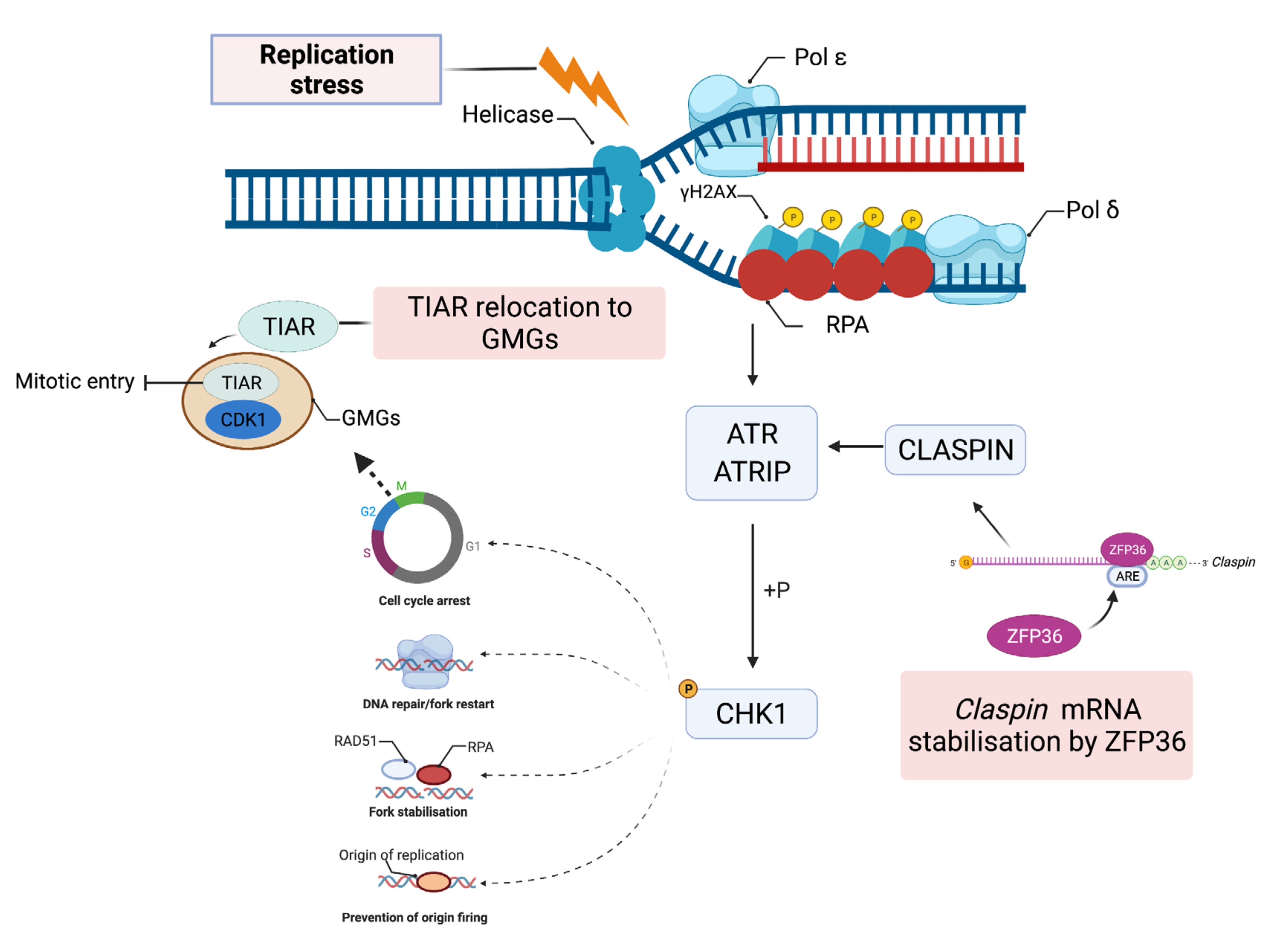
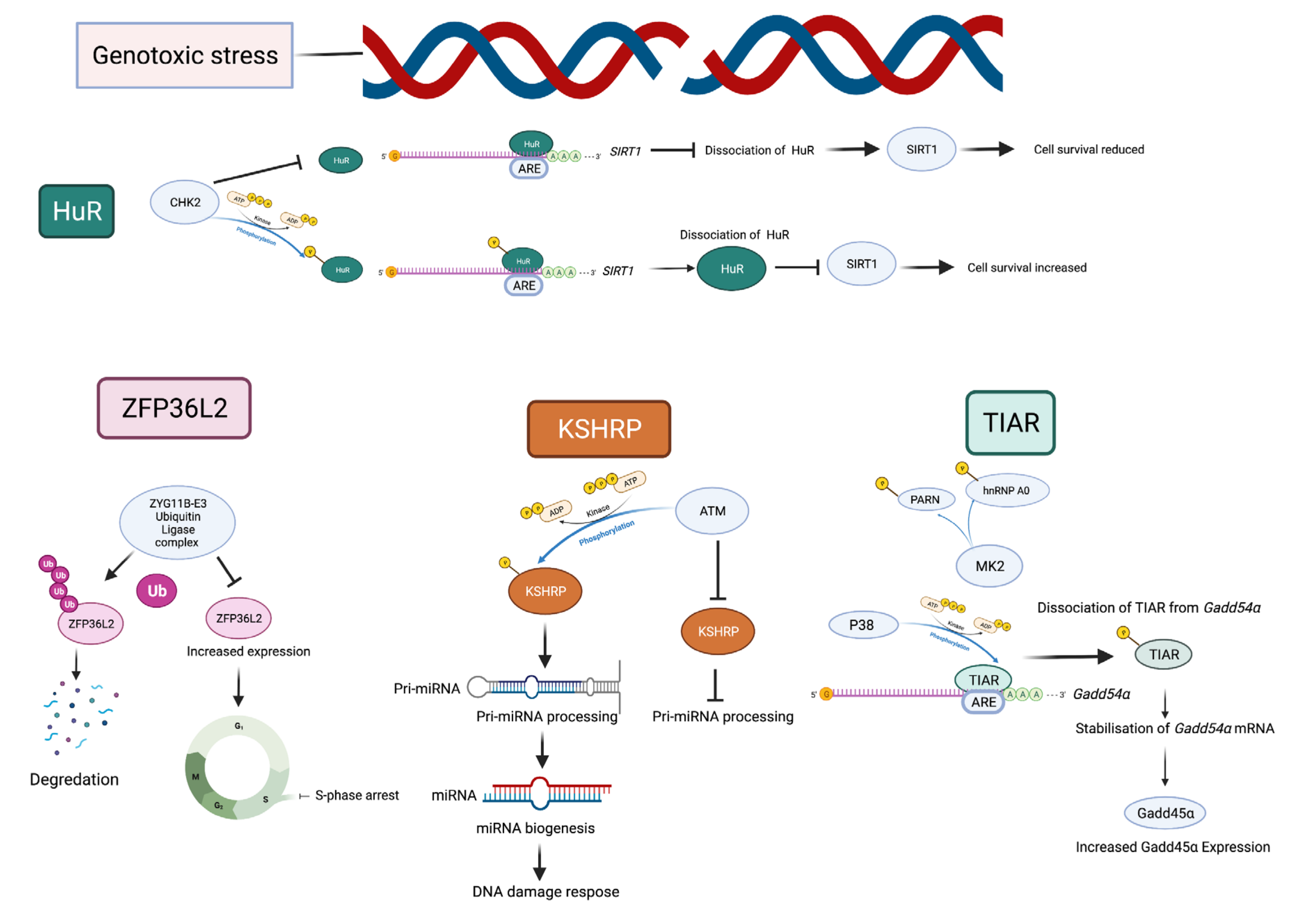
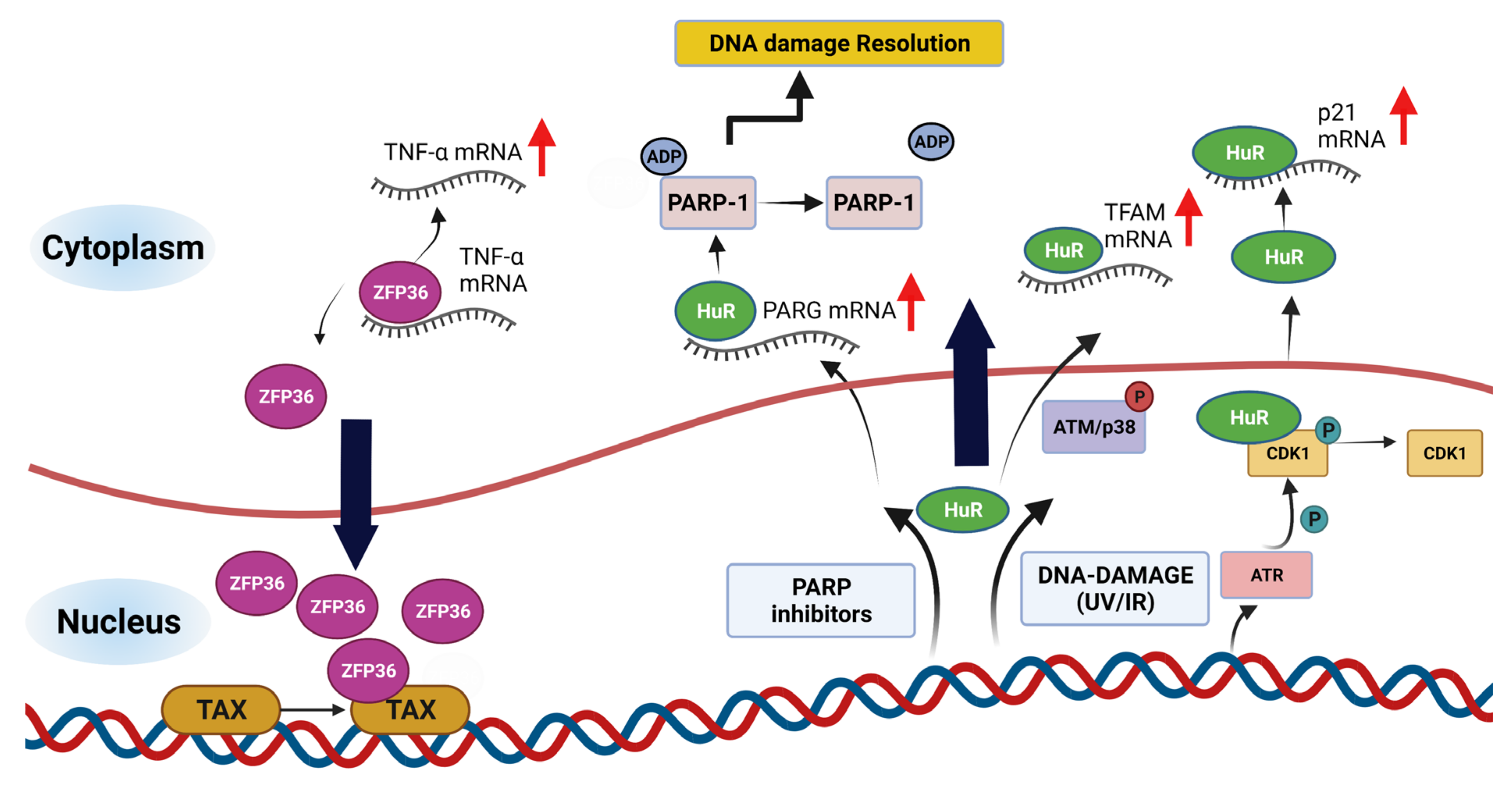
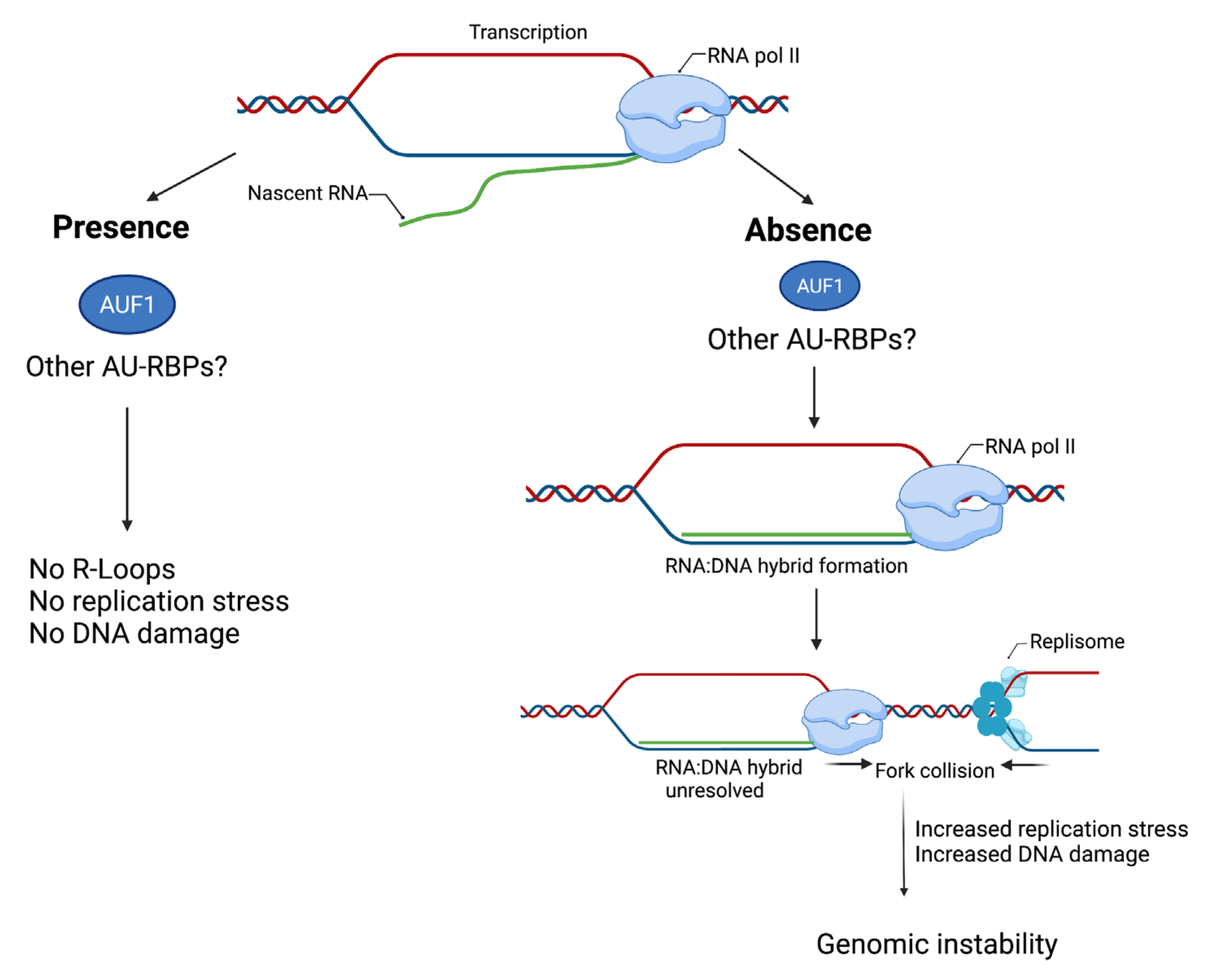
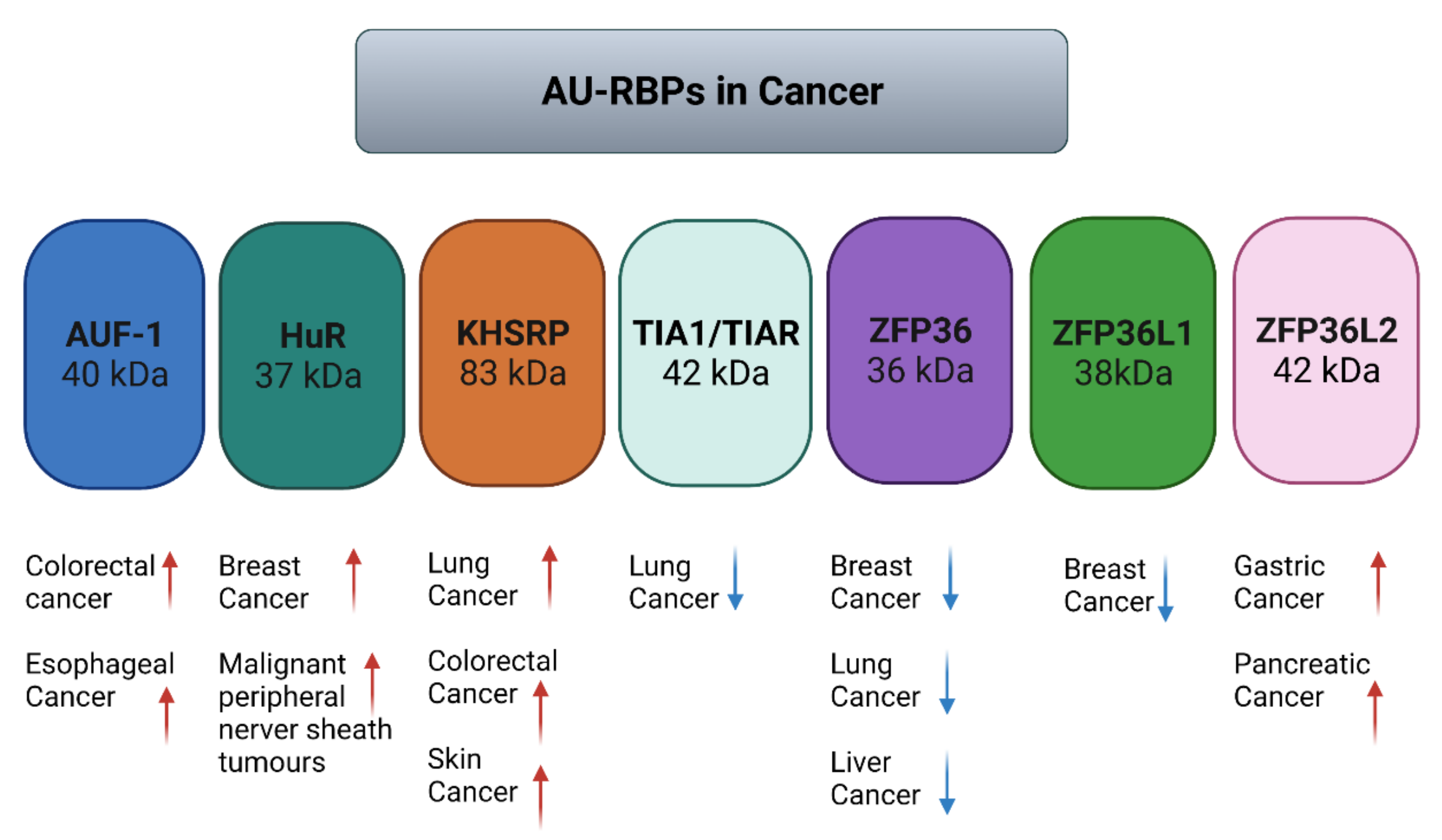
| Name (Aliases) AU-RBPs | Chromosome Location | Cell Replication Stress/DNA Damage Role | Role in Cancer—Selected Publications |
|---|---|---|---|
| AUF1 (HNRNBP, HNRPD) | 4q21.22 | Depletion of HNRNPD impairs homologous recombination by inhibiting DNA-end resection and inducing R-loop accumulation [48] | AUF1 is overexpressed in esophageal squamous cell carcinoma (ESCC) patient tissue samples [49] AUF1 overexpression promotes colorectal cancer (CRC) progression [50] |
| HuR (ELAVL1) | 19p13.2 | HuR reduces radiation-induced DNA Damage by enhancing the expression of ARID1A [51] poly(A)+ RDH mRNAs are post-transcriptionally regulated by HuR in cell stress conditions [52] Component of R loop interactome [53] HuR facilitates cellular senescence through posttranscriptional regulation of TIN2 mRNA [54] HuR stabilizes TFAM mRNA in an ATM/p38-dependent manner in irradiated cells [55] PARG mRNA post-transcriptional regulation by HuR facilitates DNA Repair [56] Chk2 phosphorylates HuR following ionising radiation [57] Double-stranded breaks induce CHK2-mediated phosphorylation of HuR in B lymphocytes [58] HuR functions in oxidative stress pathways [59] HuR post-transcriptionally regulates BRCA1 [60] HuR enhances p53 translation in response to ultraviolet light irradiation [61] HuR regulates p21 mRNA stabilisation by UV light [62] HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation [63] | HuR is elevated in clinical ductal invasive carcinoma (DIC) and ductal carcinoma in situ (DCIS) breast cancer samples [64,65] Elevated HuR cytoplasmic expression is a possible indicator of poor prognosis in breast cancer patients that received paclitaxel- and anthracycline-based neoadjuvant chemotherapy (NACT) [66] HuR is an oncogenic driver for malignant peripheral nerve sheath tumours (MPNSTs) [67] |
| KHSRP (KSRP) | 19p13.3 | DNA damage response phosphorylates KHSRP leading to direct binding to pre-miRNAs promoting miRNA biogenesis [68,69] | KHSRP critical for the growth of melanoma cells [70] KHSRP upregulated in lung cancers [71,72] KHSRP upregulated in colorectal cancer [73] |
| TIA1 (TIA-1) TIAR (TIAL1) | 2p13.3 10q26.11 | TIAR restricts G2/M transition under conditions of replication stress [74] | TIA1 and TIAR function as tumour suppressors and low expression correlates with poor prognosis in patients with lung squamous cell carcinoma [75] |
| ZFP36 (TTP, TIS11) | 19q13.2 | Modulation of ATR-CHK1 activation upon DNA damage through stabilisation of claspin mRNA [76] | ZFP36 is suppressed in many cancers [77] ZFP36 underexpressed in cancers of the breast, lung and liver [78,79,80] |
| ZFP36L1 (TIS11b) | 14q24.1 | Early developing T cells in zfp36pl1 zfp36l2 double knockout showed increased levels of DNA double-stranded breaks marker γH2AX [81] | Deletion of zfp36pl1 and zfp36l2 in mice leads to T lymphoblastic leukaemia [82] ZFP36L1 identified as a breast cancer driver gene [83] ZFP36L1 identified as a multiple myeloma driver gene [84] CRISPR-KO of ZFP36L1 reduces cell growth in chronic myeloid leukaemia cells [85] |
| ZFP36L2 (TIS11d) | 2p21 | ZFP36L2 is essential for cisplatin-induced DNA damage-induced S-phase arrest [86] Early developing T cells in zfp36pl1 zfp36l2 double knockout showed increased levels of DNA double-stranded breaks marker γH2AX [81] | ZFP36L2 is overexpressed in gastric cancer [87] ZFP36L2 is overexpressed pancreatic cancer [88] ZFP36L2 is a prevalent driver gene in metastatic solid tumours [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidali, A.; Teotia, V.; Solaiman, N.S.; Bashir, N.; Kanagaraj, R.; Murphy, J.J.; Surendranath, K. AU-Rich Element RNA Binding Proteins: At the Crossroads of Post-Transcriptional Regulation and Genome Integrity. Int. J. Mol. Sci. 2022, 23, 96. https://doi.org/10.3390/ijms23010096
Sidali A, Teotia V, Solaiman NS, Bashir N, Kanagaraj R, Murphy JJ, Surendranath K. AU-Rich Element RNA Binding Proteins: At the Crossroads of Post-Transcriptional Regulation and Genome Integrity. International Journal of Molecular Sciences. 2022; 23(1):96. https://doi.org/10.3390/ijms23010096
Chicago/Turabian StyleSidali, Ahmed, Varsha Teotia, Nadeen Shaikh Solaiman, Nahida Bashir, Radhakrishnan Kanagaraj, John J. Murphy, and Kalpana Surendranath. 2022. "AU-Rich Element RNA Binding Proteins: At the Crossroads of Post-Transcriptional Regulation and Genome Integrity" International Journal of Molecular Sciences 23, no. 1: 96. https://doi.org/10.3390/ijms23010096
APA StyleSidali, A., Teotia, V., Solaiman, N. S., Bashir, N., Kanagaraj, R., Murphy, J. J., & Surendranath, K. (2022). AU-Rich Element RNA Binding Proteins: At the Crossroads of Post-Transcriptional Regulation and Genome Integrity. International Journal of Molecular Sciences, 23(1), 96. https://doi.org/10.3390/ijms23010096






