Is the Mitochondrial Membrane Potential (∆Ψ) Correctly Assessed? Intracellular and Intramitochondrial Modifications of the ∆Ψ Probe, Rhodamine 123
Abstract
:1. Introduction
- Binding of the fluorescent probe to cellular elements and structures of the mitochondrial matrix. Theoretically, positively charged hydrophobic/amphiphilic drugs can be bound to intracellular anionic constituents such as phospholipids and nucleic acids. In addition, the electrostatic interaction of cationic probes with the anionic moiety of proteins must also be considered and among those, mitochondrial proteins, so-called chargerins were proposed to play a role as binding partners of mitochondrial probes [14,15,16].
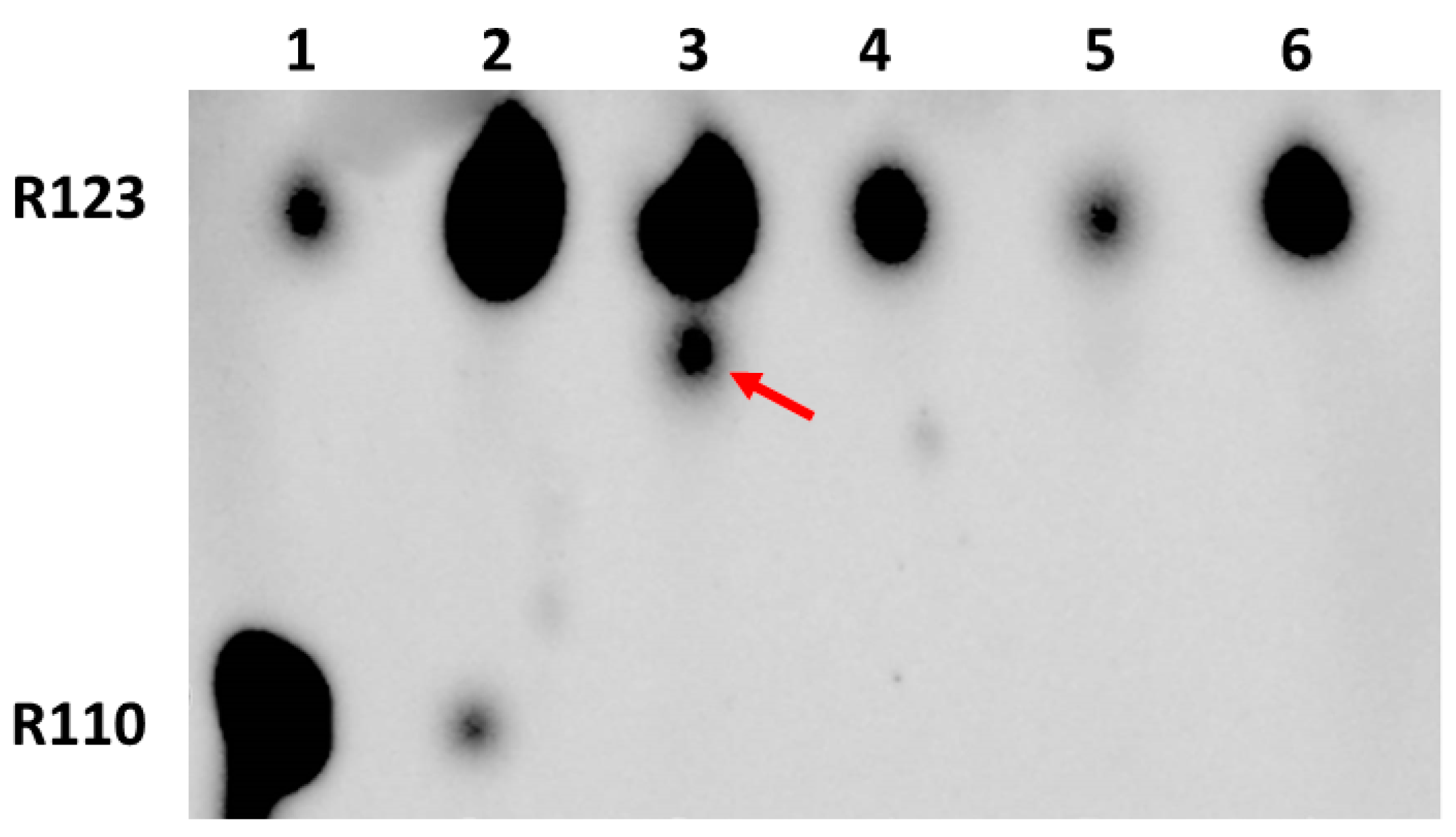
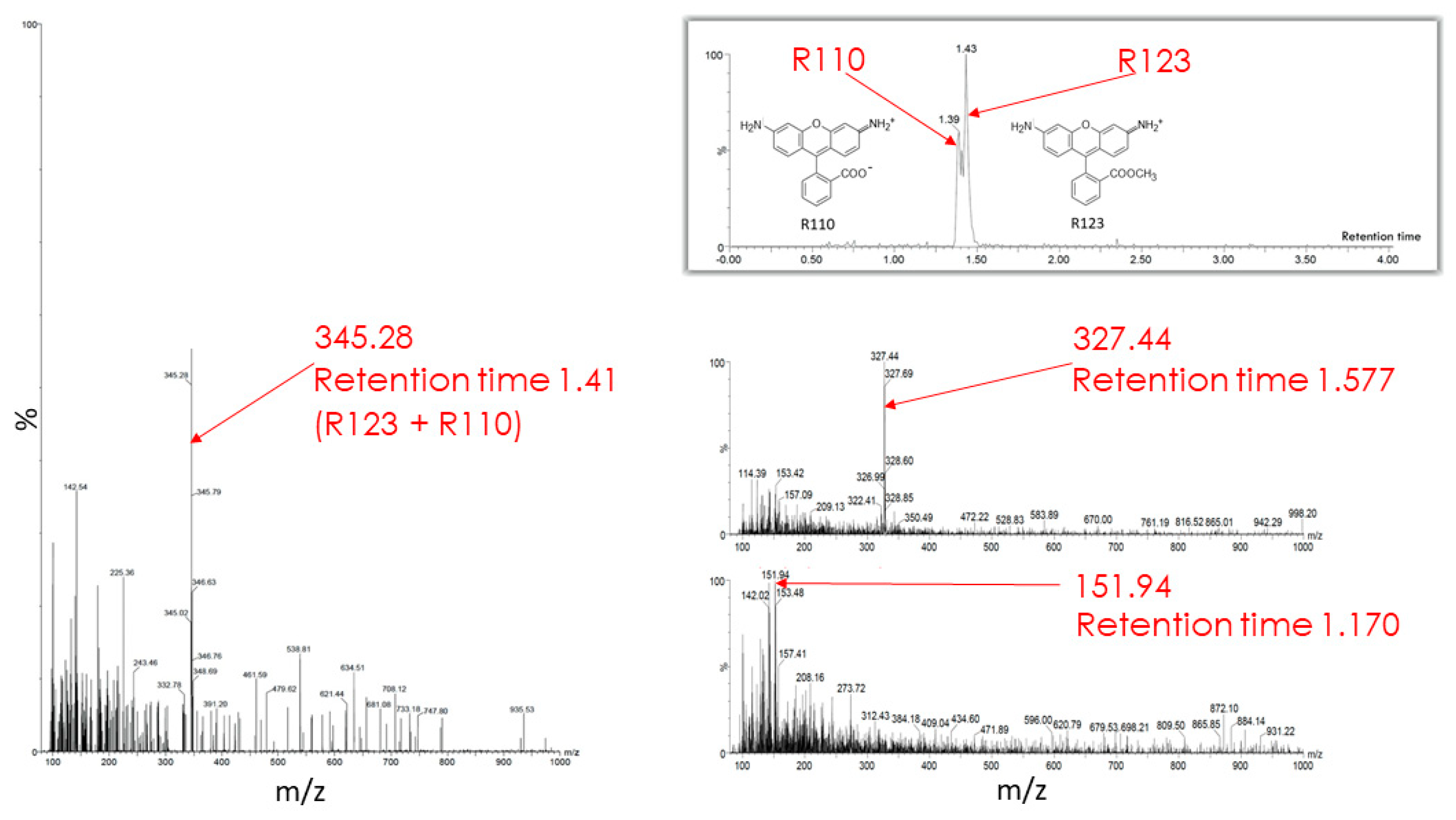
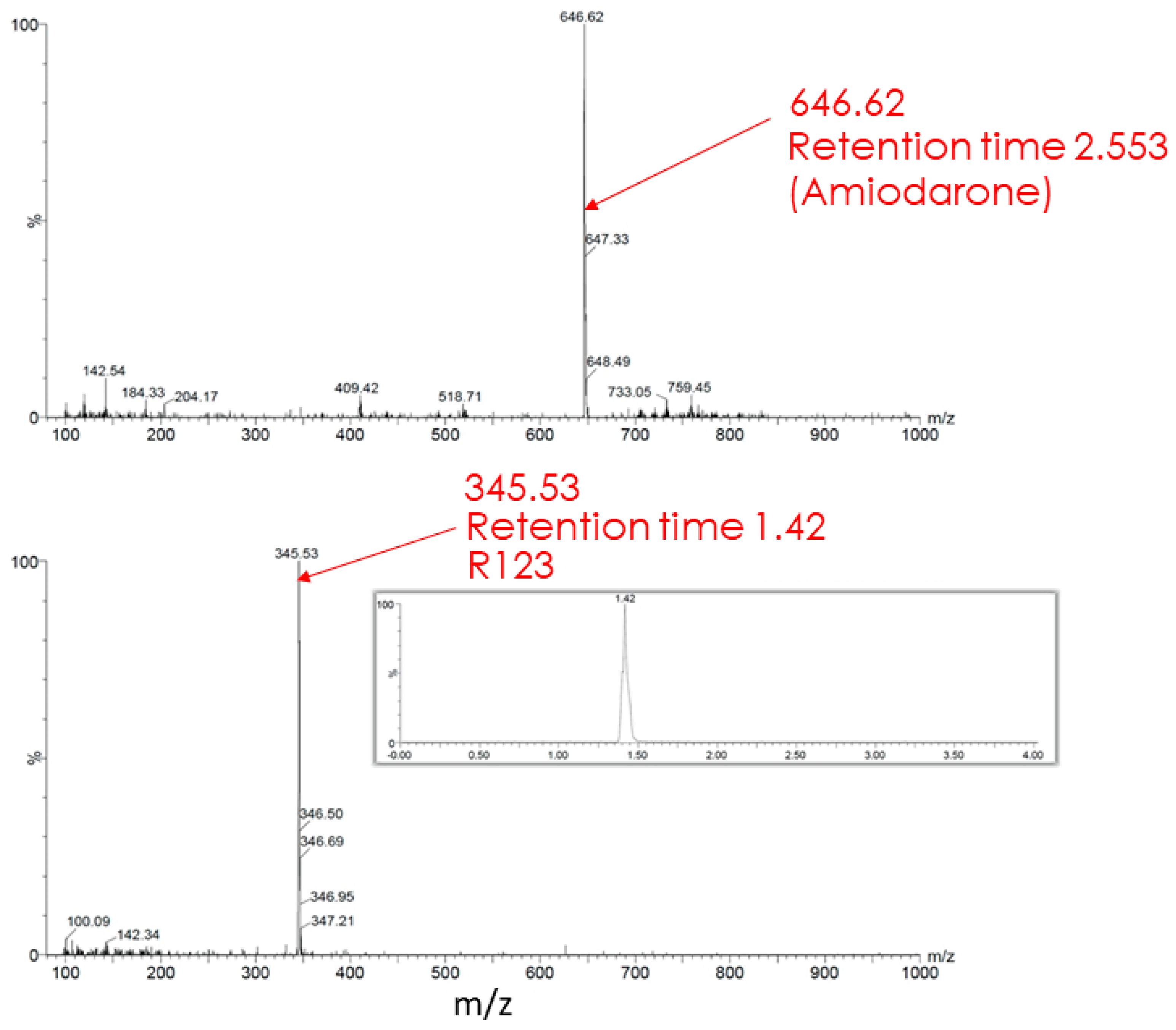
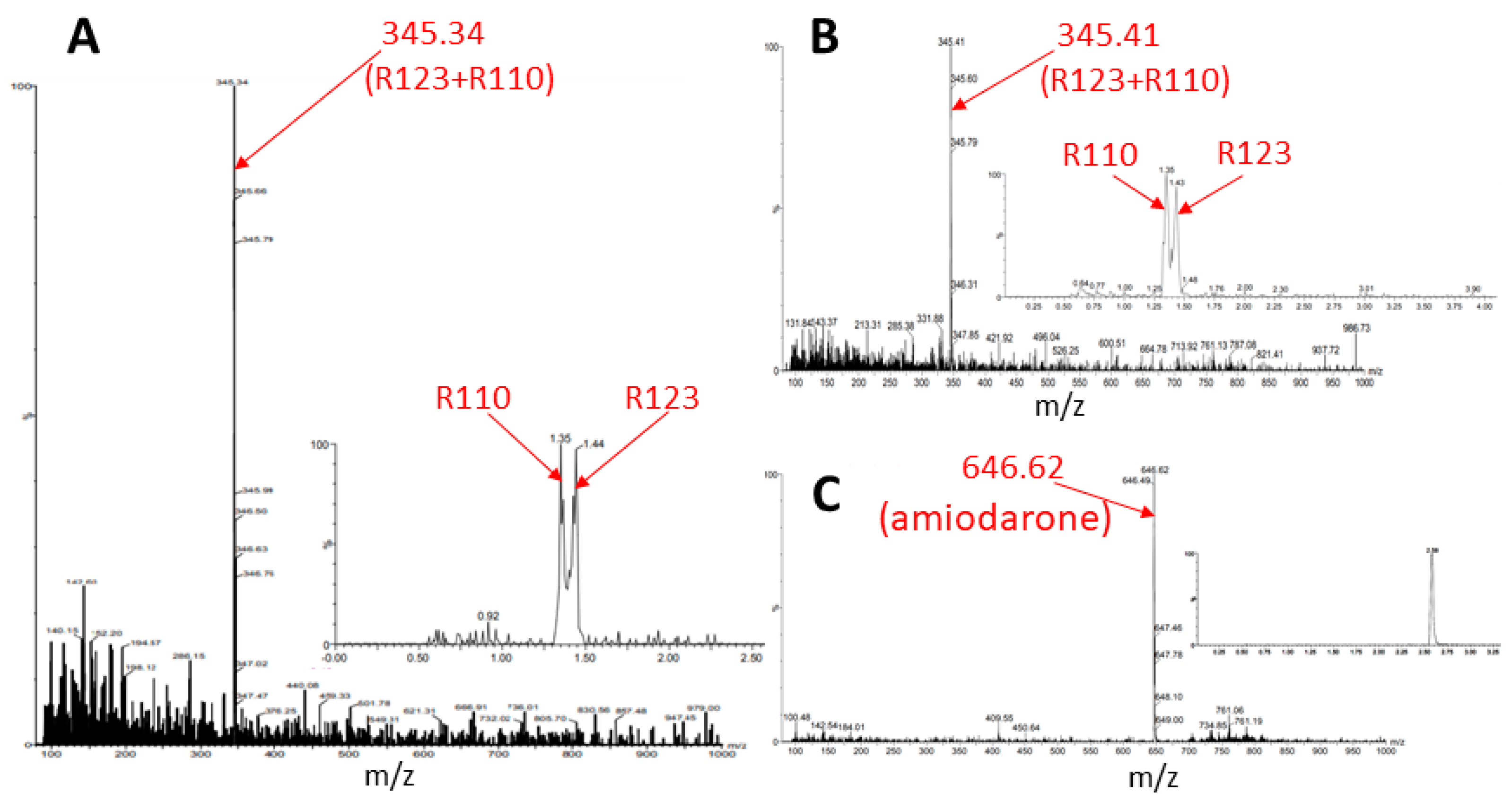
- Transformation of the permeable form of the probe into an impermeable form with the preservation of fluorescent properties. For example, as a result of deesterification by intracellular and intramitochondrial esterases, the highly membrane-permeable Rh123 (as well as tetramethyl rhodamines, TMRM, or TMRE) was proposed to be converted into the corresponding zwitterion (rhodamine 110, Rh110), in contrast to the parental cationic form, having a very low membrane permeability [17] thus possibly entrapping the modified probe in the compartment where the modification occurred.
- Intracellular/intramitochondrial degradation of the probe. We admit that not much data exists on this issue, but all probes introduced into the cell can be considered as xenobiotics, which obey the common cellular rule to be expelled from the cell either in intact form or after its modification, often with the involvement of cytochrome P450 [18,19]. There were attempts to make minimal analysis of the components being a result of catabolism of Rh123, but the exact identification of those has not been done [20,21].
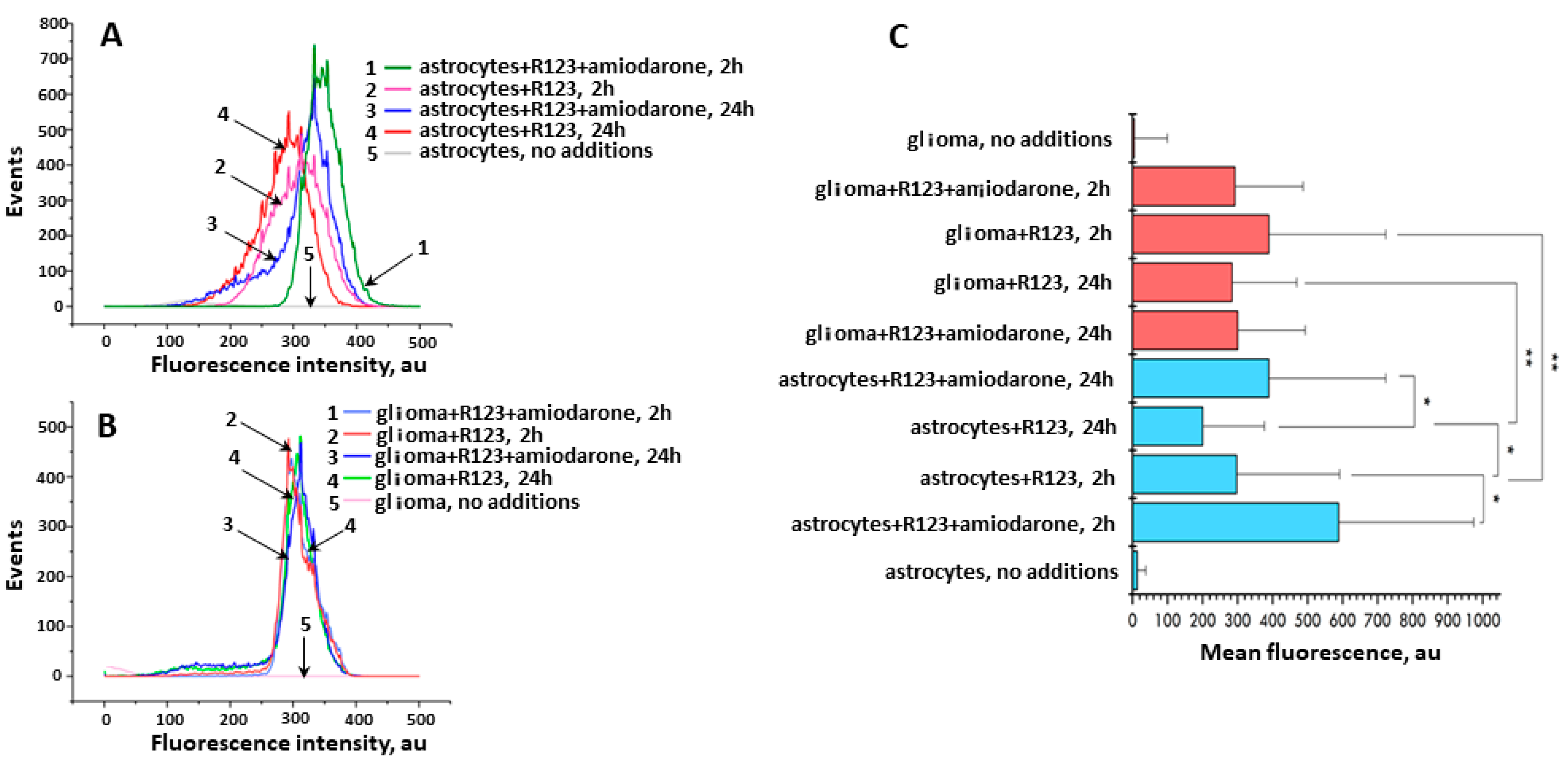
- The change in the contribution of the membrane potential on the cell membrane to the total fluorescence of the probe in the mitochondrial matrix [12].
- Activity of non-specific pumps in the cell and mitochondrial membrane efflux of xenobiotics in cells and mitochondria. The protein-meditated transport of xenobiotics (including mitochondrial cationic probes) across membranes is predominantly executed by the solute carrier (SLC) and ATP-binding cassette (ABC) superfamilies of proteins [22,23,24]. While SLC family members are responsible for both inward and outward transport, ABC transporters exclusively provide efflux mechanism in an energy-dependent fashion to overcome a concentration gradient. Of the latter, one of the most discussed mechanisms for expelling cationic dyes out of the cell is that they are very good substrates for a multidrug resistance protein (MDR) [25] highly involved in the occurrence of insensitivity of cells to a pharmacological therapy. Similar mechanisms of expelling drugs from mitochondria have been reported [26,27].
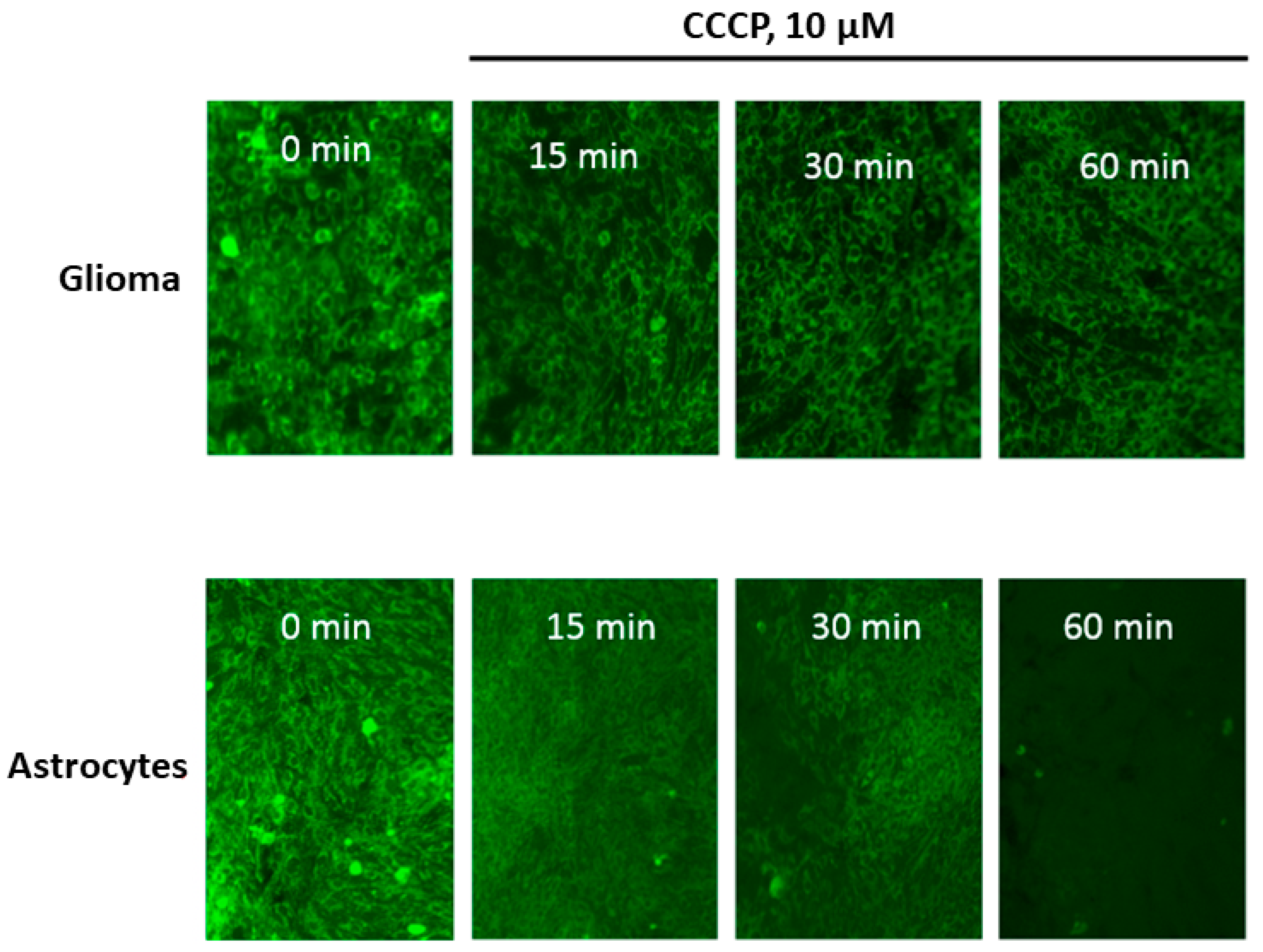
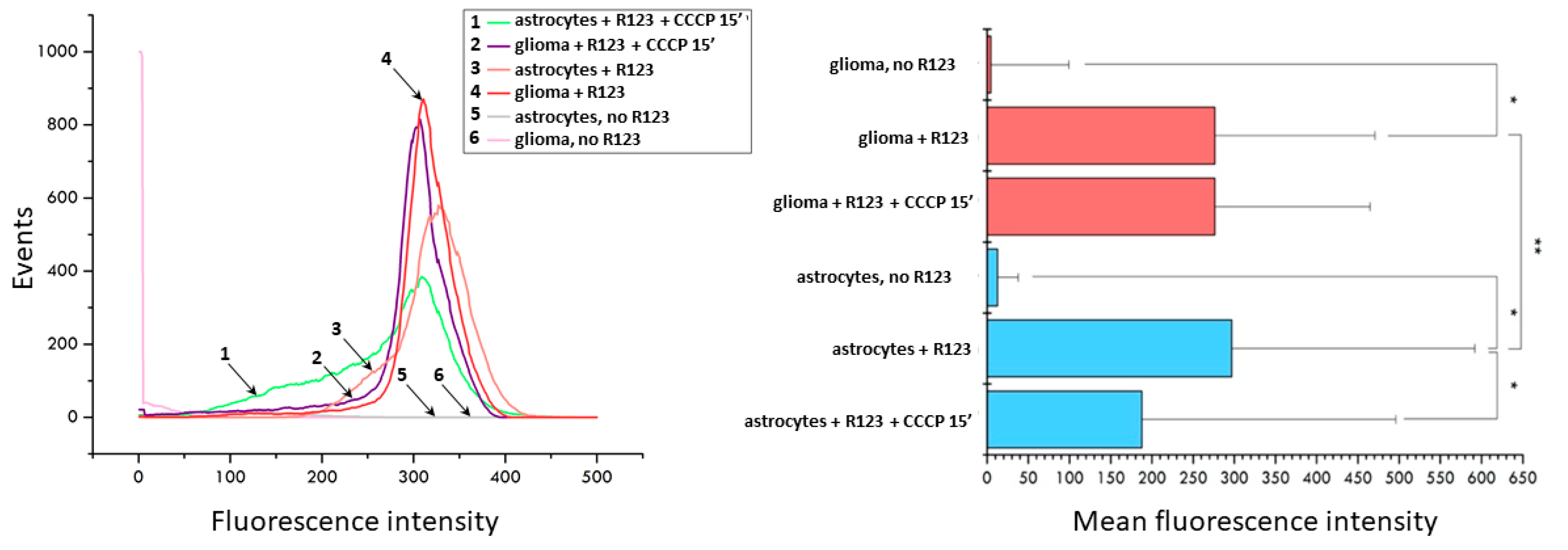
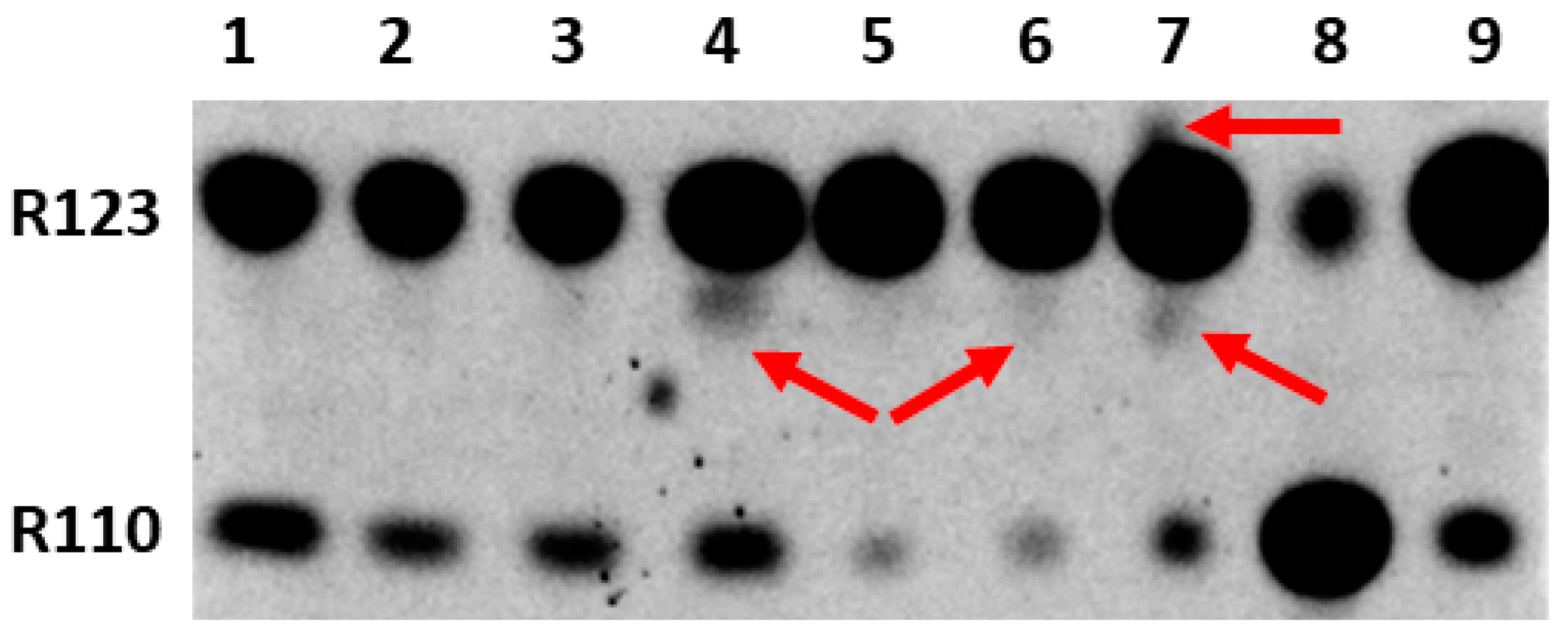
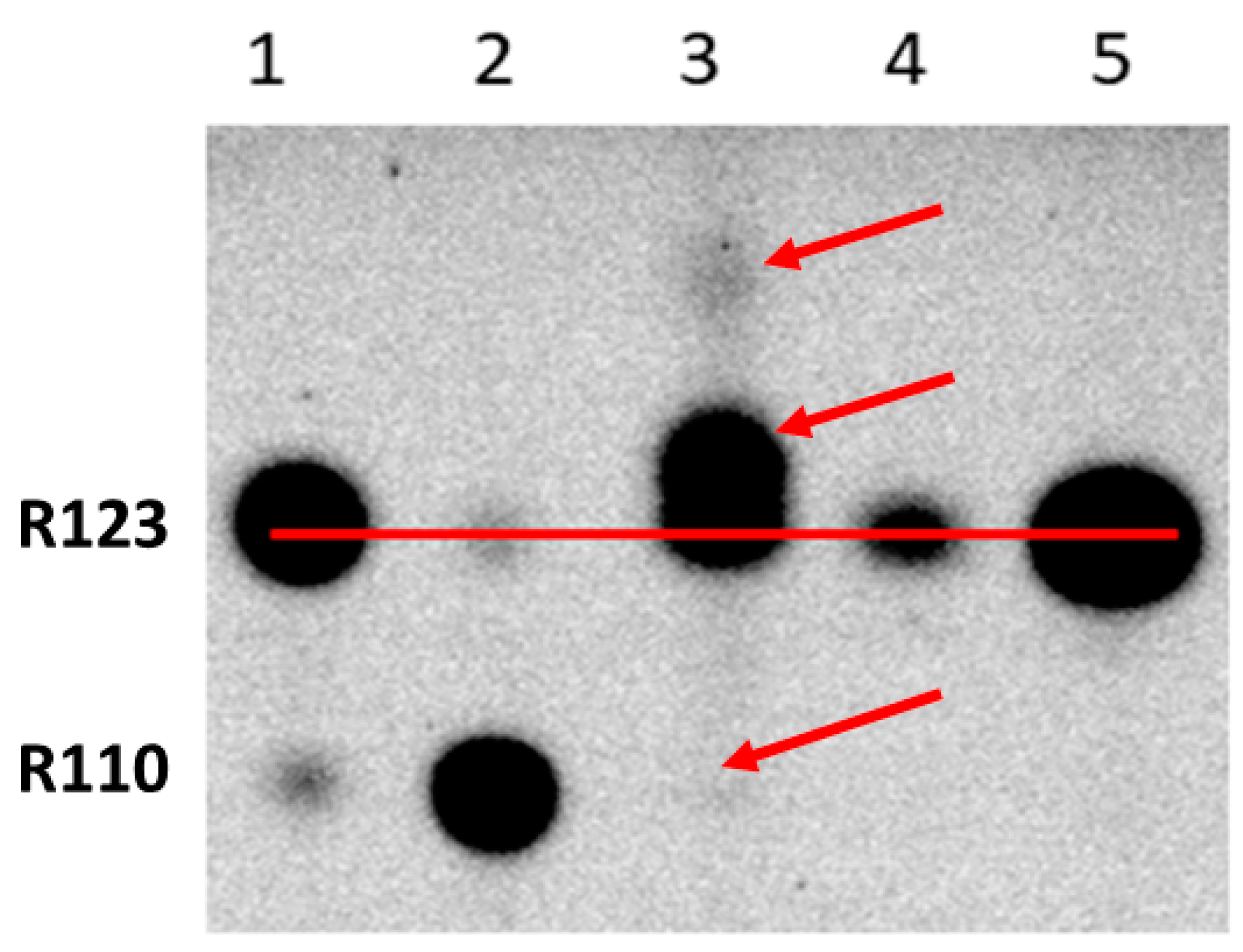
2. Results
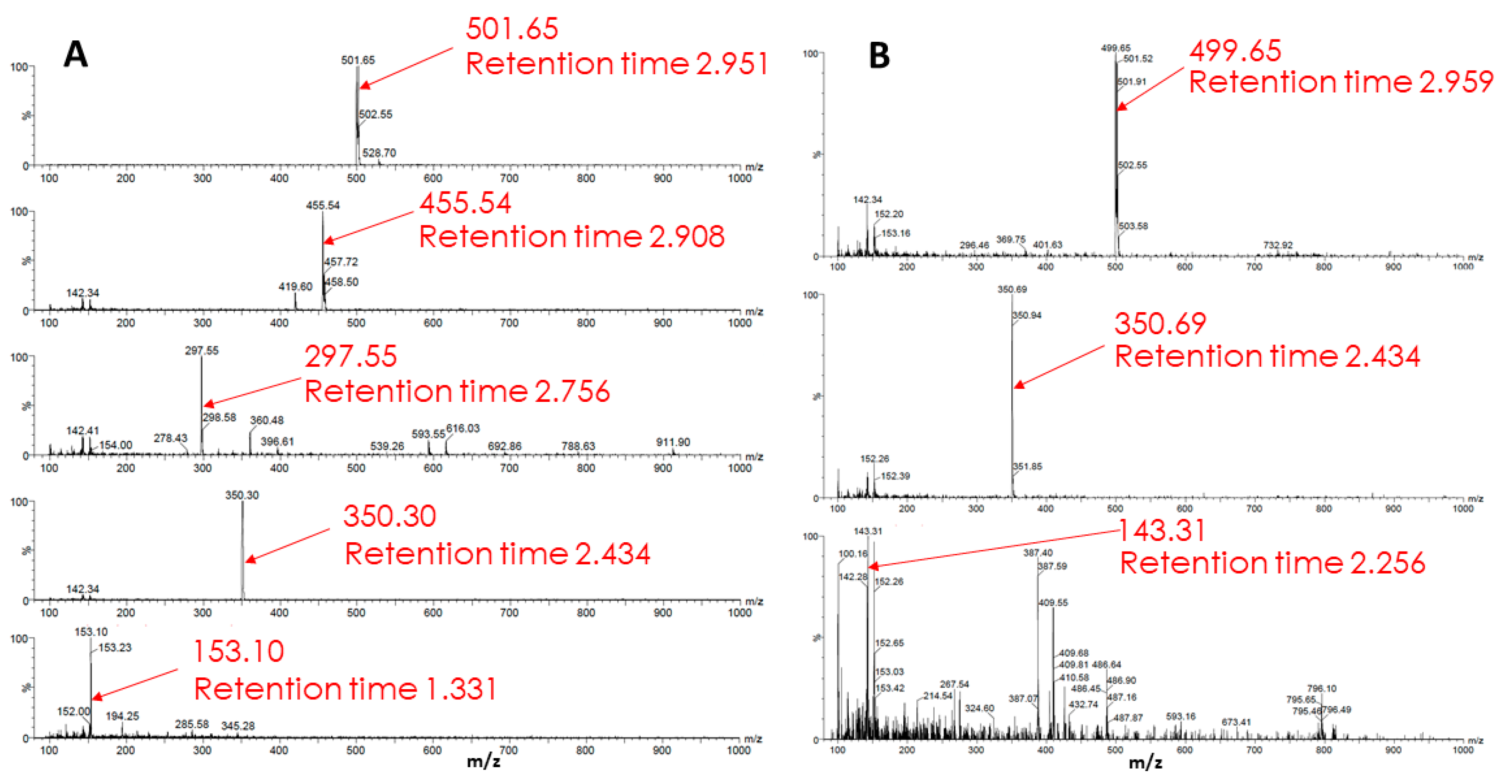
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. The Isolation of Mitochondria
4.3. Thin-Layer Chromatography
4.4. Fluorescent Microscopy
4.5. Flow Cytometry
4.6. High Performance Liquid Chromatography and Mass Spectrometry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 1966, 41, 445–502. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.E. ATP Synthesis by Rotary Catalysis (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1998, 37, 2308–2319. [Google Scholar] [CrossRef]
- Zorov, D.B.; Krasnikov, B.F.; Kuzminova, A.E.; Vysokikh, M.Y.; Zorova, L.D. Mitochondria revisited. Alternative functions of mitochondria. Biosci. Rep. 1997, 17, 507–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lisa, F.; Blank, P.S.; Colonna, R.; Gambassi, G.; Silverman, H.S.; Stern, M.D.; Hansford, R.G. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J. Physiol. 1995, 486, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [Green Version]
- Summerhayes, I.C.; Lampidis, T.J.; Bernal, S.D.; Nadakavukaren, J.J.; Nadakavukaren, K.K.; Shepherd, E.L.; Chen, L.B. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc. Natl. Acad. Sci. USA 1982, 79, 5292–5296. [Google Scholar] [CrossRef] [Green Version]
- Nadakavukaren, K.K.; Nadakavukaren, J.J.; Chen, L.B. Increased rhodamine 123 uptake by carcinoma cells. Cancer Res. 1985, 45, 6093–6099. [Google Scholar]
- Davis, S.; Weiss, M.J.; Wong, J.R.; Lampidis, T.J.; Chen, L.B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J. Biol. Chem. 1985, 260, 13844–13850. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell. Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A.; Zorov, D.B. Functional Significance of the Mitochondrial Membrane Potential. Biochem. Suppl. Ser. A Membr. Cell Biol. 2018, 12, 20–26. [Google Scholar] [CrossRef]
- Zorov, D.B.; Kobrinsky, E.; Juhaszova, M.; Sollott, S.J. Examining Intracellular Organelle Function Using Fluorescent Probes. Circ. Res. 2004, 95, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Higuti, T. Conformational coupling in H+-pumps and ATP synthesis? its analysis with anisotropic inhibitors of energy transduction in oxidative phosphorylation. Mol. Cell. Biochem. 1984, 61, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Higuti, T.; Takigawa, M.; Kotera, Y.; Oka, H.; Uchida, J.; Arakaki, R.; Fujita, T.; Ogawa, T. Purified hydrophobic proteins, chargerins, are essential for energy transduction in oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 1985, 82, 1331–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuti, T.; Negama, T.; Takigawa, M.; Uchida, J.; Yamane, T.; Asai, T.; Tani, I.; Oeda, K.; Shimizu, M.; Nakamura, K.; et al. A hydrophobic protein, chargerin, I.I.; purified from rat liver mitochondria is encoded in the unidentified reading frame A6L of mitochondrial DNA. J. Biol. Chem. 1988, 263, 6772–6776. [Google Scholar] [CrossRef]
- Fugit, K.D.; Anderson, B.D. The role of pH and ring-opening hydrolysis kinetics on liposomal release of topotecan. J. Control. Release 2013, 174, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, Bile Acid, and Cholesterol Transporters: Function and Regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef] [Green Version]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of Rhodamine-123 as a Tracer Dye for Use In In vitro Drug Transport Assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef] [Green Version]
- Sweatman, T.W.; Sehsadri, R.; Israel, M. Metabolism and elimination of rhodamine-123 in the rat. Cancer Chemother. Pharmacol. 1990, 27, 205–210. [Google Scholar] [CrossRef]
- Parasrampuria, R.; Mehvar, R. Effects of P-glycoprotein and Mrp2 inhibitors on the hepatobiliary disposition of Rhodamine 123 and its glucuronidated metabolite in isolated perfused rat livers. J. Pharm. Sci. 2010, 99, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Asp. Med. 2013, 34, 95–107. [Google Scholar] [CrossRef] [PubMed]
- DeGorter, M.K.; Xia, C.Q.; Yang, J.J.; Kim, R.B. Drug Transporters in Drug Efficacy and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 249–273. [Google Scholar] [CrossRef]
- Wigler, P.W. Cellular drug efflux and reversal therapy of cancer. J. Bioenerg. Biomembr. 1996, 28, 279–284. [Google Scholar] [CrossRef]
- Twentyman, P.; Rhodes, T.; Rayner, S. A comparison of rhodamine 123 accumulation and efflux in cells with P-glycoprotein-mediated and MRP-associated multidrug resistance phenotypes. Eur. J. Cancer 1994, 30, 1360–1369. [Google Scholar] [CrossRef]
- Munteanu, E.; Verdier, M.; Grandjean-Forestier, F.; Stenger, C.; Jayat-Vignoles, C.; Huet, S.; Robert, J.; Ratinaud, M.-H. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem. Pharmacol. 2006, 71, 1162–1174. [Google Scholar] [CrossRef]
- Roundhill, E.; Turnbull, D.; Burchill, S. Localization of MRP-1 to the outer mitochondrial membrane by the chaperone protein HSP90β. FASEB J. 2015, 30, 1712–1723. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.G.; Au, N.T.; Rettie, A.E. P450-Based Drug-Drug Interactions of Amiodarone and its Metabolites: Diversity of Inhibitory Mechanisms. Drug Metab. Dispos. 2015, 43, 1661–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, S.D.; Lampidis, T.J.; Summerhayes, I.C.; Chen, L.B. Rhodamine-123 Selectively Reduces Clonal Growth of Carcinoma Cells in Vitro. Science 1982, 218, 1117–1119. [Google Scholar] [CrossRef]
- Lampidis, T.J.; Bernal, S.D.; Summerhayes, I.C.; Chen, L.B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983, 43, 716–720. [Google Scholar] [PubMed]
- Bernal, S.D.; Lampidis, T.J.; McIsaac, R.M.; Chen, L.B. Anticarcinoma Activity in Vivo of Rhodamine 123, a Mitochondrial-Specific Dye. Science 1983, 222, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Modica-Napolitano, J.S.; Aprille, J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001, 49, 63–70. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Weissig, V. Treatment Strategies that Enhance the Efficacy and Selectivity of Mitochondria-Targeted Anticancer Agents. Int. J. Mol. Sci. 2015, 16, 17394–17421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; De Milito, A.; Olofsson, M.H.; Gullbo, J.; D’Arcy, P.; Linder, S. Targeting Mitochondrial Function to Treat Quiescent Tumor Cells in Solid Tumors. Int. J. Mol. Sci. 2015, 16, 27313–27326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Dong, L.; Neuzil, J. Mitochondria: An intriguing target for killing tumour-initiating cells. Mitochondrion 2016, 26, 86–93. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Feng, X.; Zhu, M.; Hoffmann, S.; Hsu, A.; Qian, K.; Huang, D.; Zhao, F.; Liu, W.; et al. Mitochondria-targeting fluorescent molecules for high efficiency cancer growth inhibition and imaging. Chem. Sci. 2019, 10, 7946–7951. [Google Scholar] [CrossRef]
- Kafkova, A.; Trnka, J. Mitochondria-targeted compounds in the treatment of cancer. Neoplasma 2020, 67, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Guengerich, F.P. Common and Uncommon Cytochrome P450 Reactions Related to Metabolism and Chemical Toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Weiss, M.J.; Chen, L.B.; Aprille, J.R. Rhodamine 123 inhibits bioenergetic function in isolated rat liver mitochondria. Biochem. Biophys. Res. Commun. 1984, 118, 717–723. [Google Scholar] [CrossRef]
- McCarthy, K.D.; de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell. Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorova, L.D.; Demchenko, E.A.; Korshunova, G.A.; Tashlitsky, V.N.; Zorov, S.D.; Andrianova, N.V.; Popkov, V.A.; Babenko, V.A.; Pevzner, I.B.; Silachev, D.N.; et al. Is the Mitochondrial Membrane Potential (∆Ψ) Correctly Assessed? Intracellular and Intramitochondrial Modifications of the ∆Ψ Probe, Rhodamine 123. Int. J. Mol. Sci. 2022, 23, 482. https://doi.org/10.3390/ijms23010482
Zorova LD, Demchenko EA, Korshunova GA, Tashlitsky VN, Zorov SD, Andrianova NV, Popkov VA, Babenko VA, Pevzner IB, Silachev DN, et al. Is the Mitochondrial Membrane Potential (∆Ψ) Correctly Assessed? Intracellular and Intramitochondrial Modifications of the ∆Ψ Probe, Rhodamine 123. International Journal of Molecular Sciences. 2022; 23(1):482. https://doi.org/10.3390/ijms23010482
Chicago/Turabian StyleZorova, Ljubava D., Evgeniya A. Demchenko, Galina A. Korshunova, Vadim N. Tashlitsky, Savva D. Zorov, Nadezda V. Andrianova, Vasily A. Popkov, Valentina A. Babenko, Irina B. Pevzner, Denis N. Silachev, and et al. 2022. "Is the Mitochondrial Membrane Potential (∆Ψ) Correctly Assessed? Intracellular and Intramitochondrial Modifications of the ∆Ψ Probe, Rhodamine 123" International Journal of Molecular Sciences 23, no. 1: 482. https://doi.org/10.3390/ijms23010482
APA StyleZorova, L. D., Demchenko, E. A., Korshunova, G. A., Tashlitsky, V. N., Zorov, S. D., Andrianova, N. V., Popkov, V. A., Babenko, V. A., Pevzner, I. B., Silachev, D. N., Plotnikov, E. Y., & Zorov, D. B. (2022). Is the Mitochondrial Membrane Potential (∆Ψ) Correctly Assessed? Intracellular and Intramitochondrial Modifications of the ∆Ψ Probe, Rhodamine 123. International Journal of Molecular Sciences, 23(1), 482. https://doi.org/10.3390/ijms23010482








