Depletion of Retinal Dopaminergic Activity in a Mouse Model of Rod Dysfunction Exacerbates Experimental Autoimmune Uveoretinitis: A Role for the Gateway Reflex
Abstract
1. Introduction
2. Results
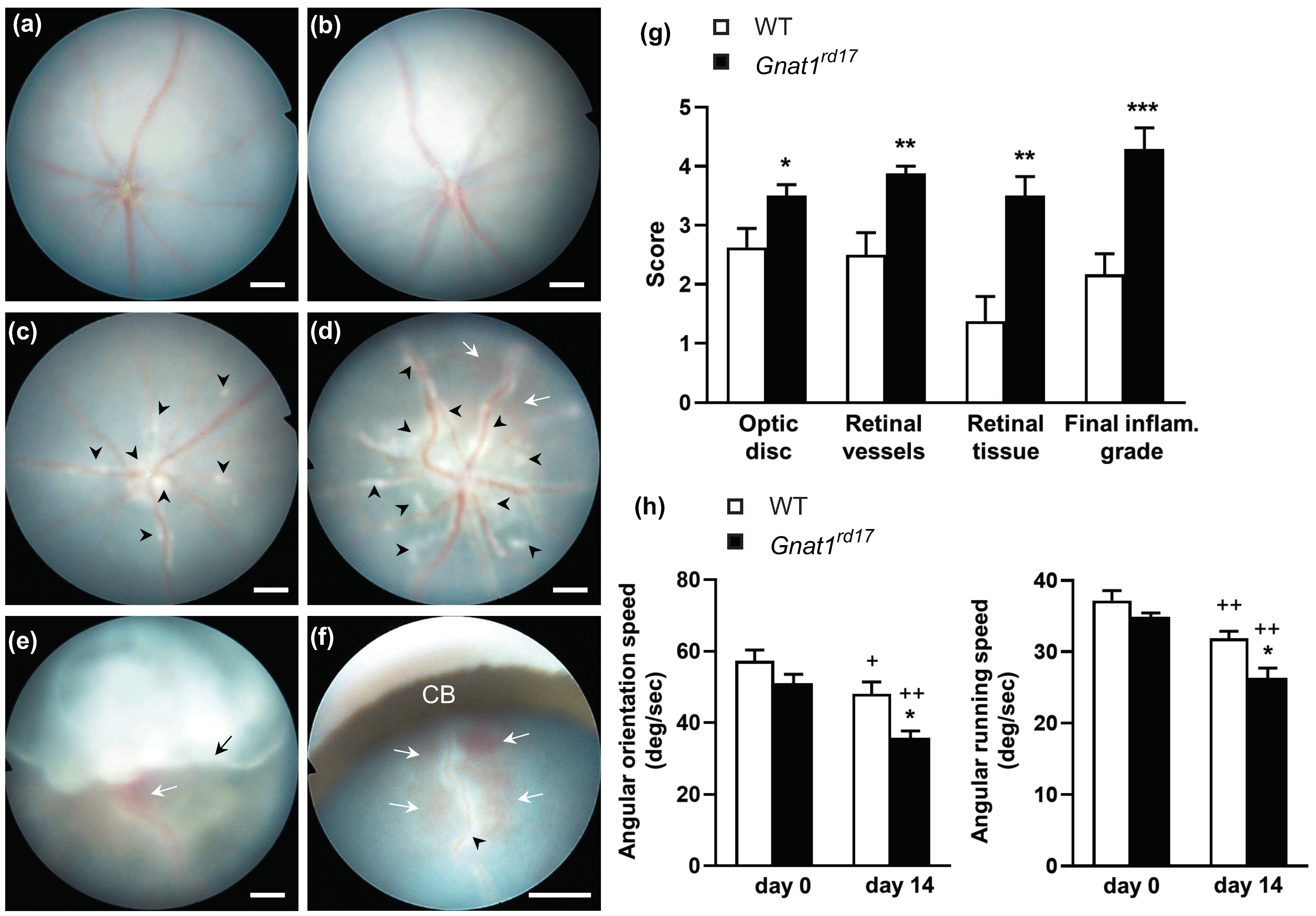
2.1. Gnat1rd17 Mice Exhibit Aggravated EAU Severity
2.2. Exacerbation of EAU in Gnat1rd17 Mice Is Associated with Abundant Immune-Cell Infiltrates and Retinal Neuroinflammatory Response
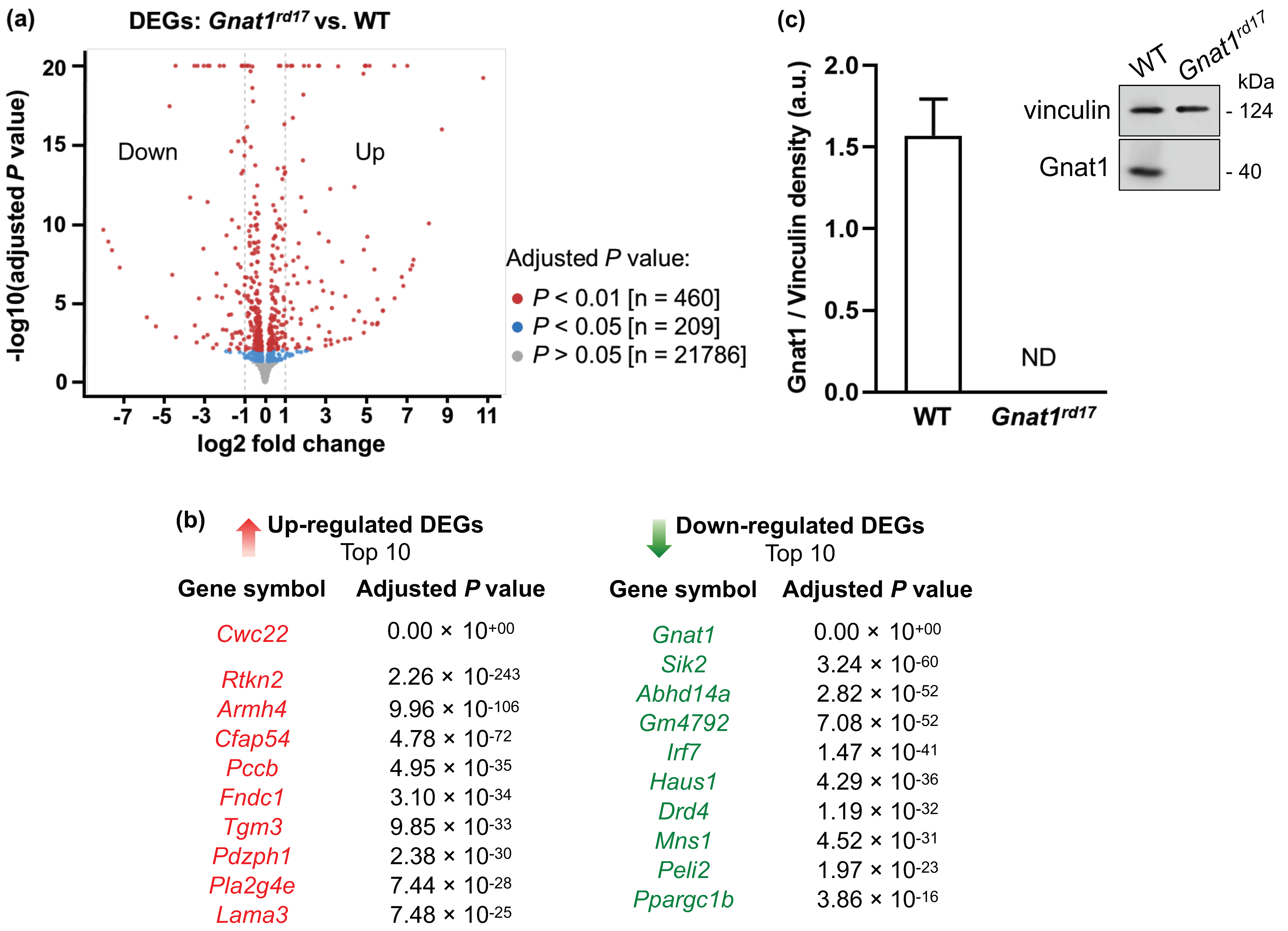
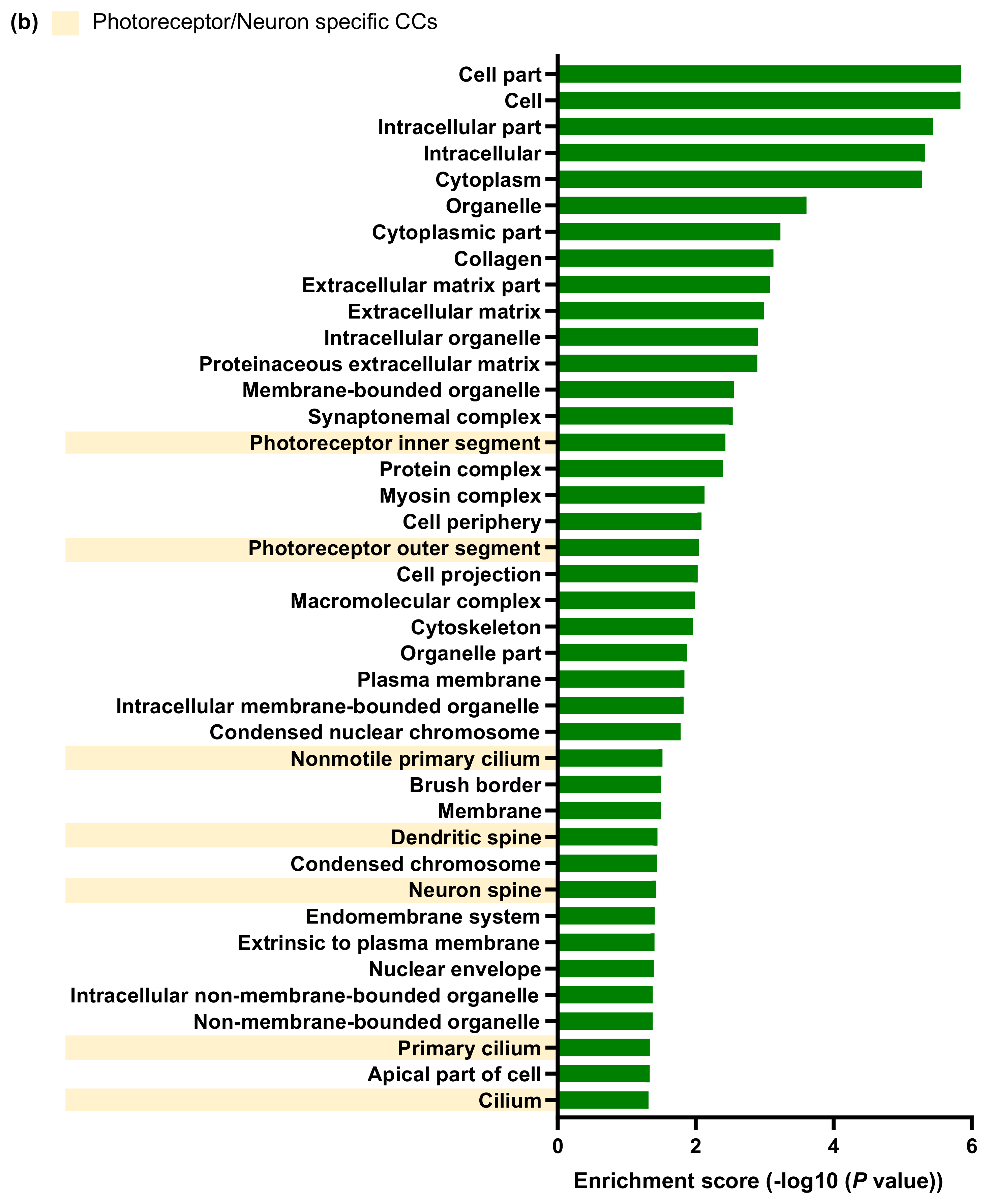
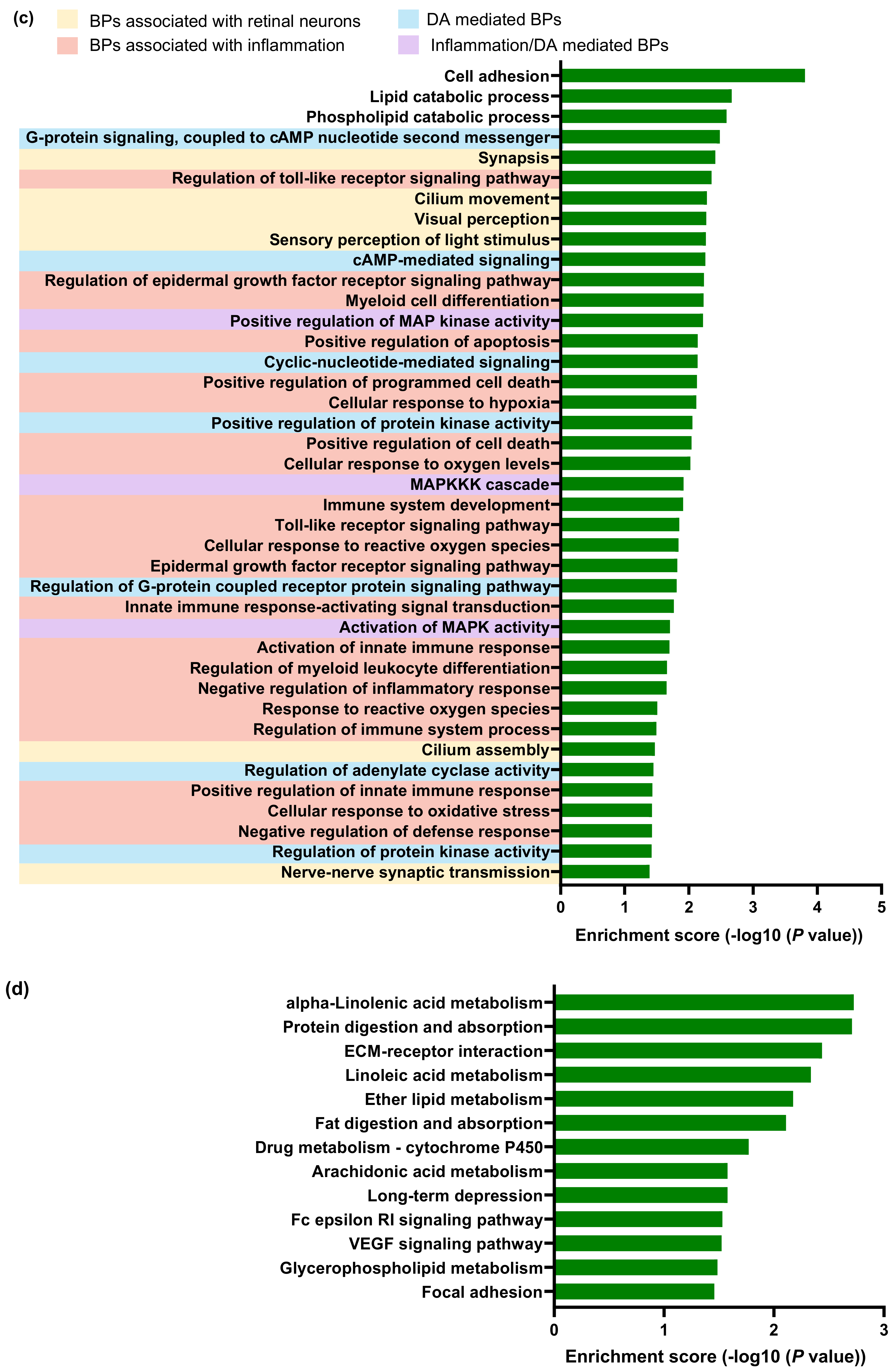
2.3. Transcriptomic Changes in the Neuroretina of Gnat1rd17 Mice Revealed Deregulation of DA- and Inflammation-Associated Pathways
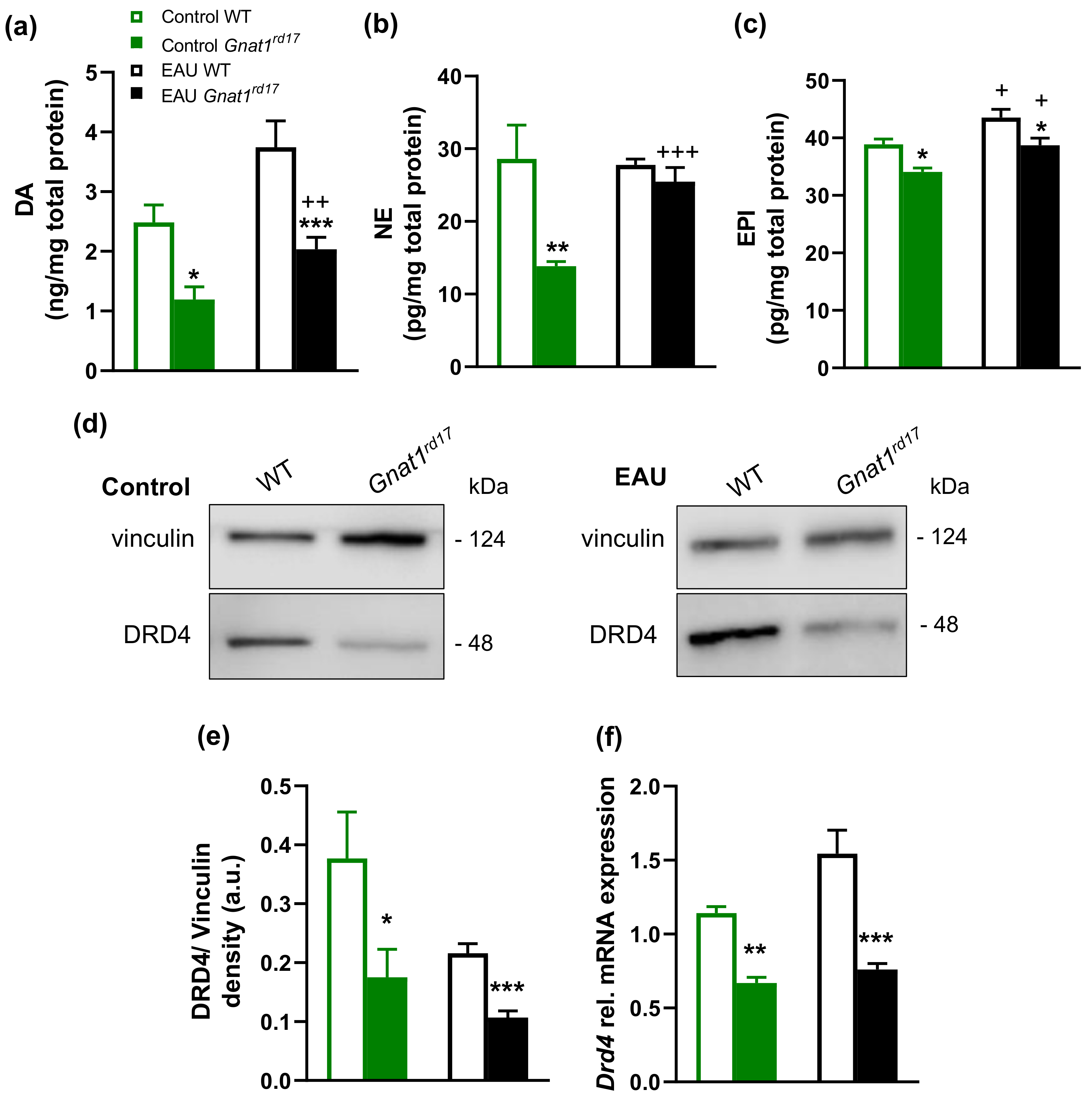
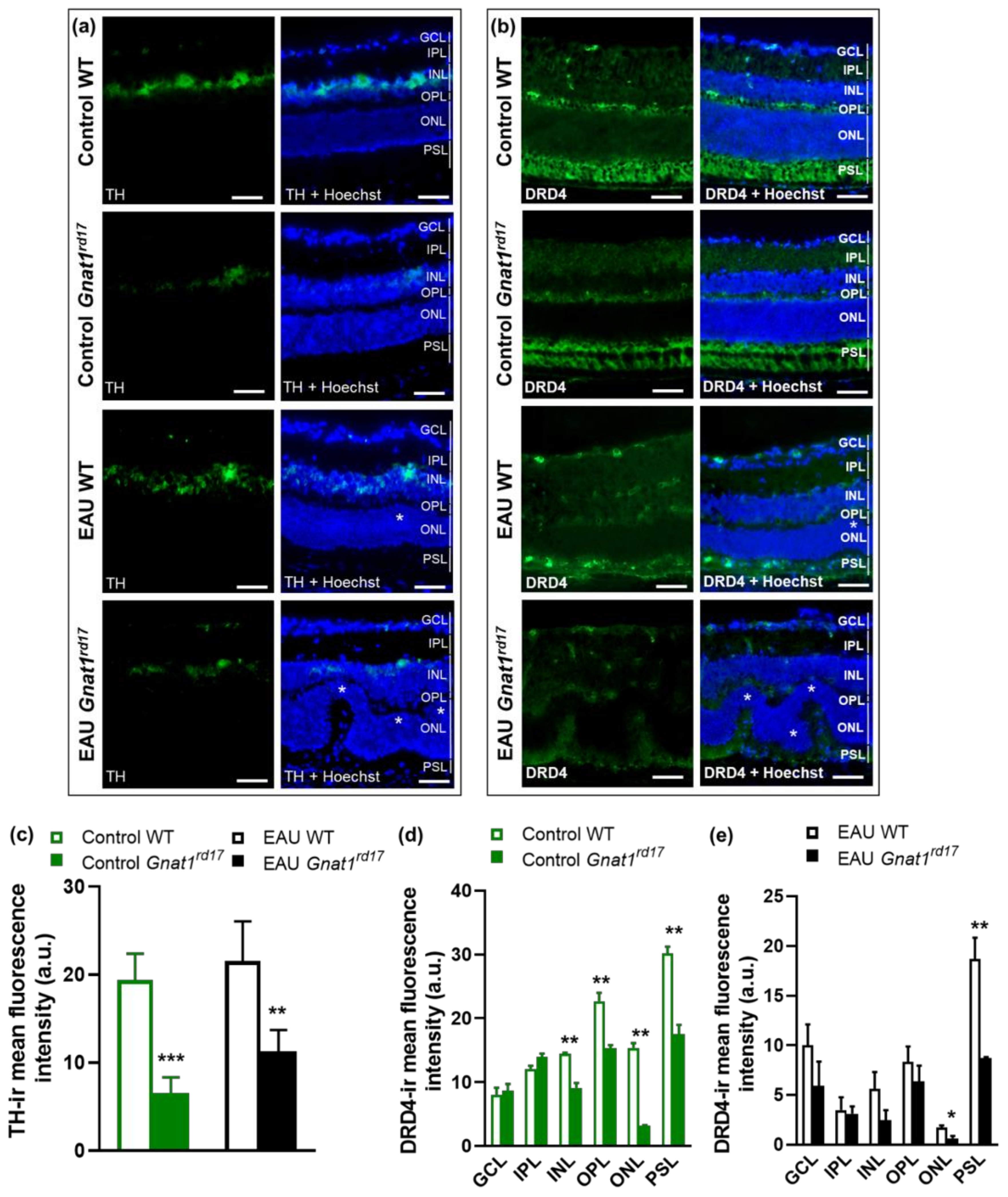
2.4. Gnat1rd17 Mice Show Low Retinal DA Levels and DRD4 Expression, Independent of EAU
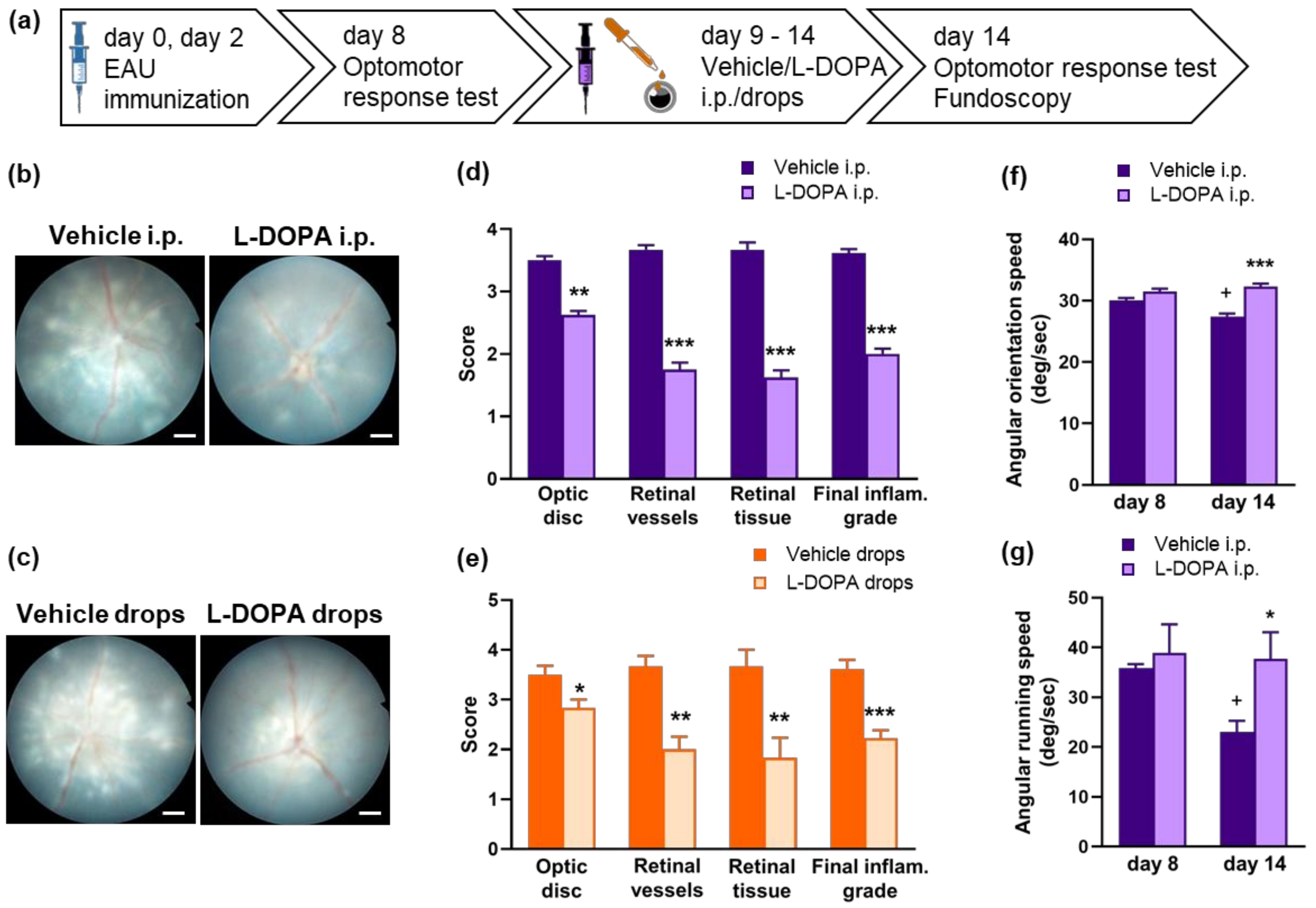
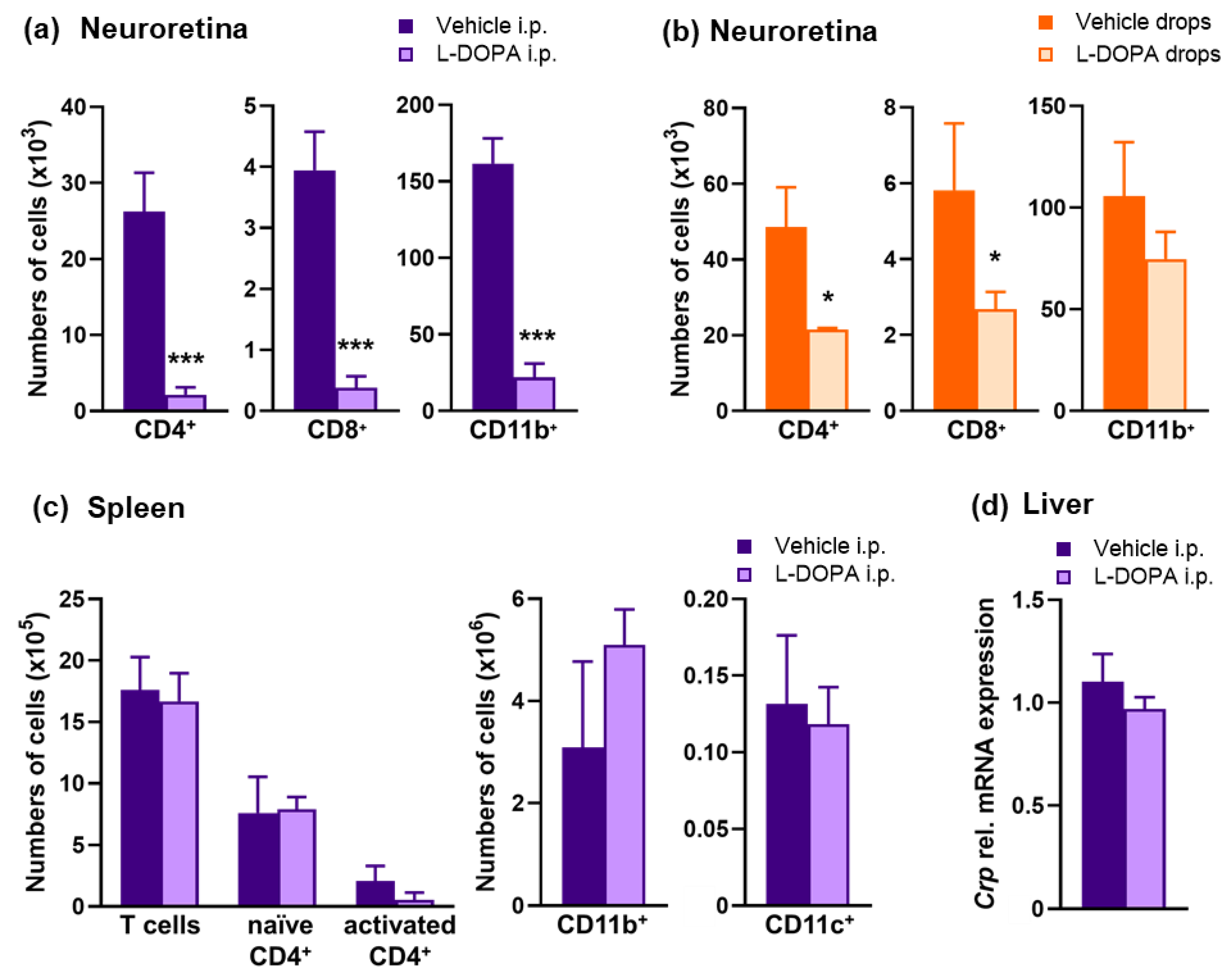
2.5. L-DOPA Treatment Attenuates EAU Severity in Gnat1rd17 Mice
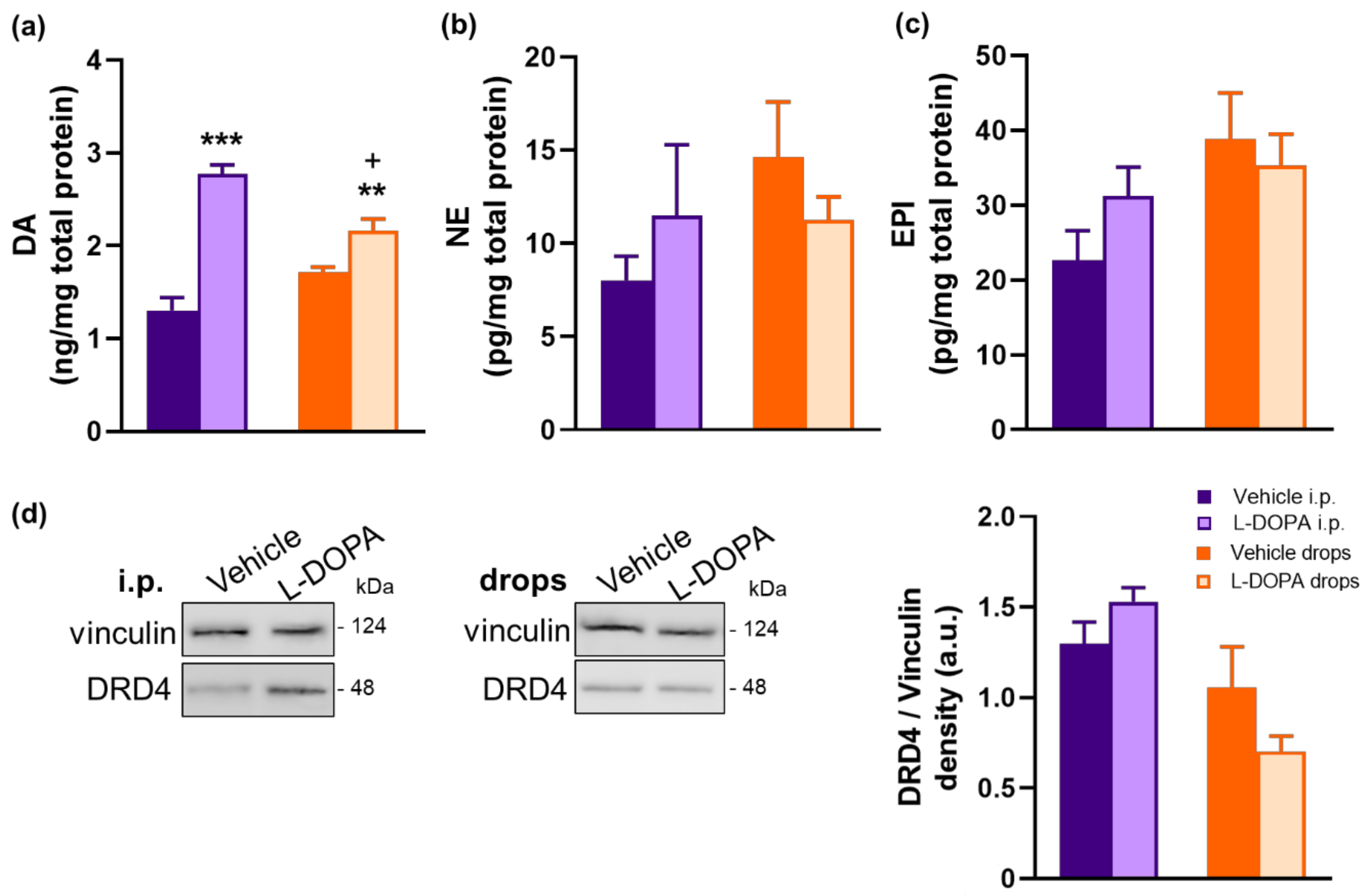
2.6. L-DOPA Treatment Rerestored Reduced Retinal DA Levels in EAU Gnat1rd17 Mice
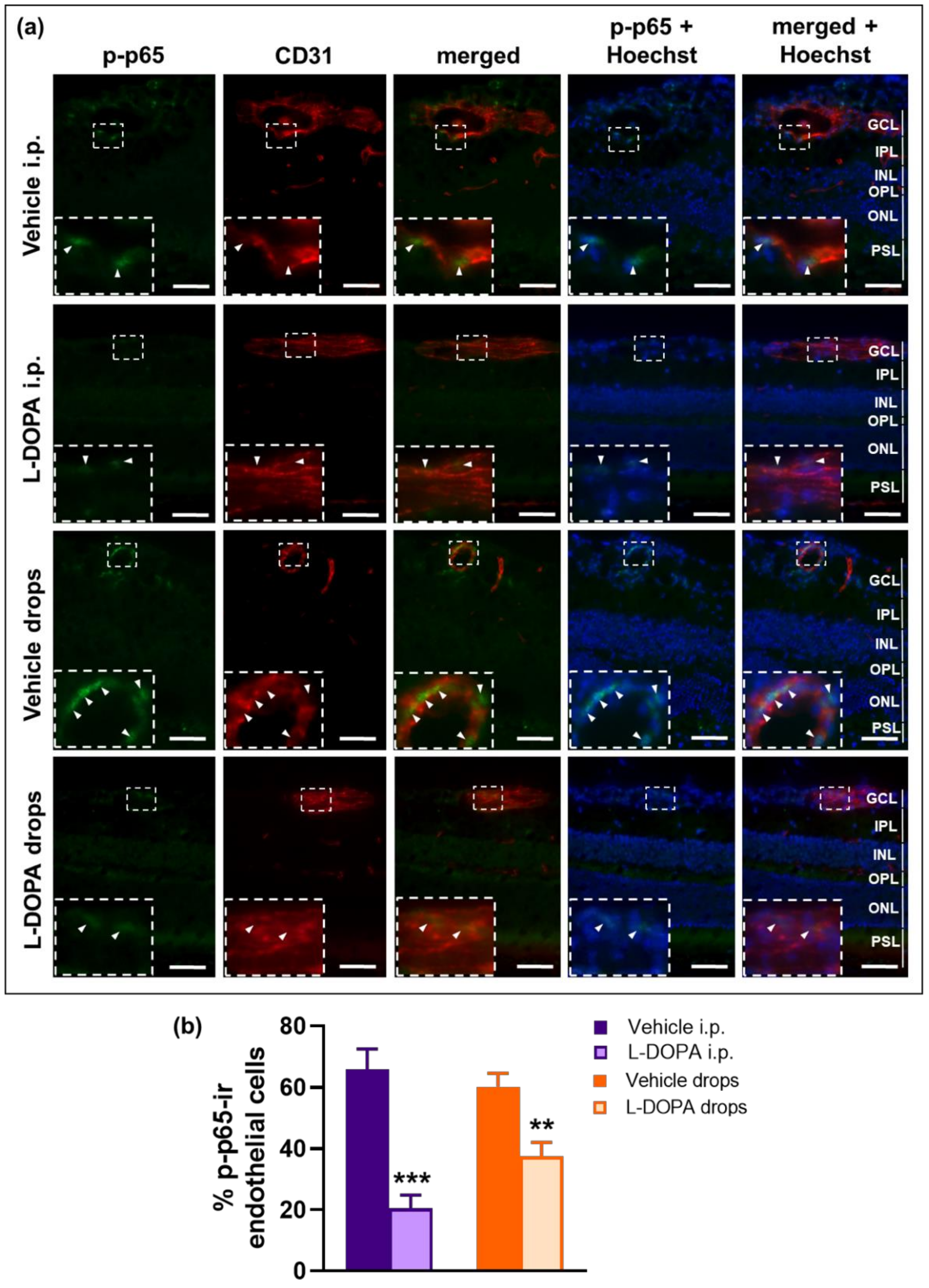
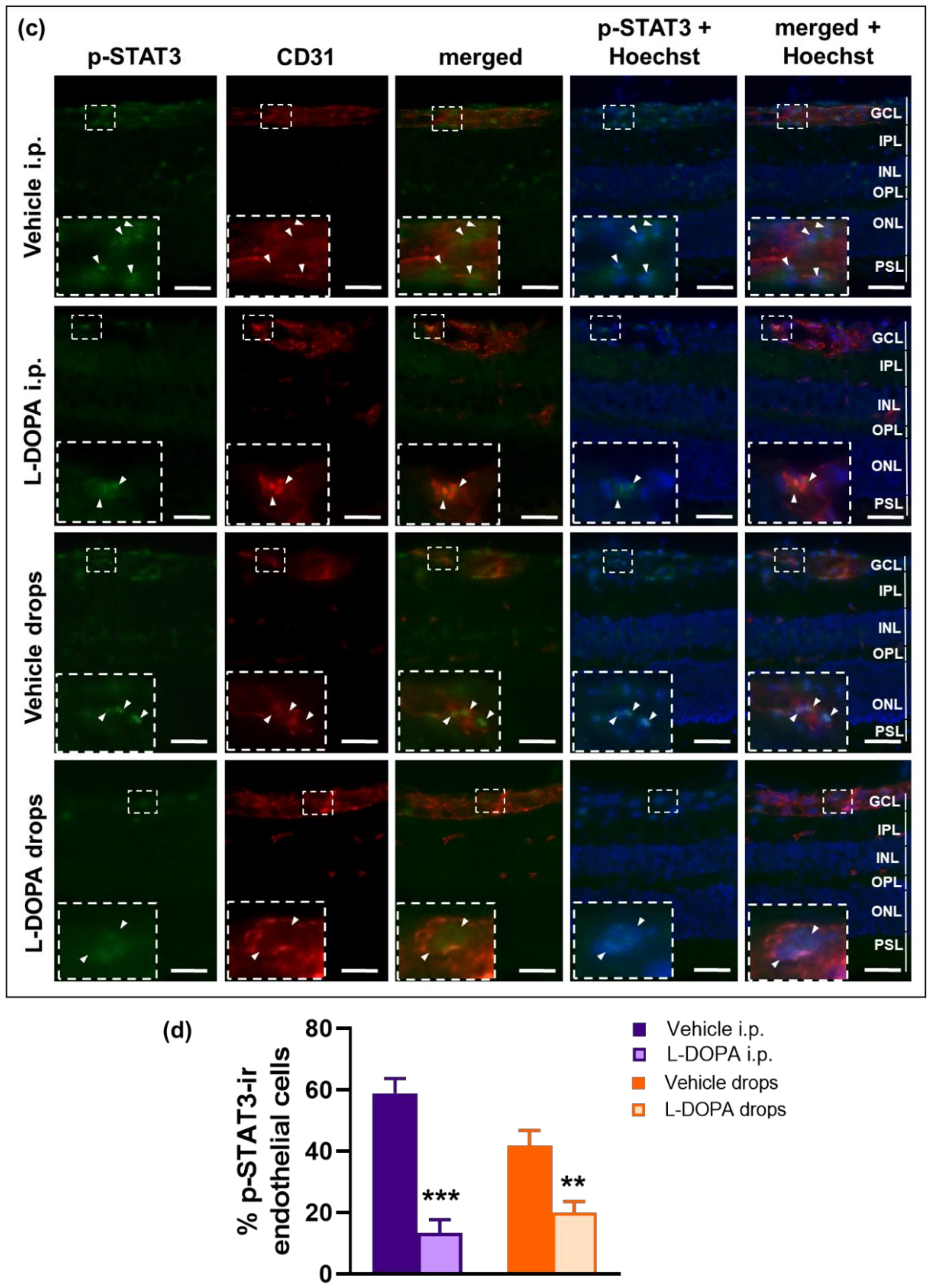
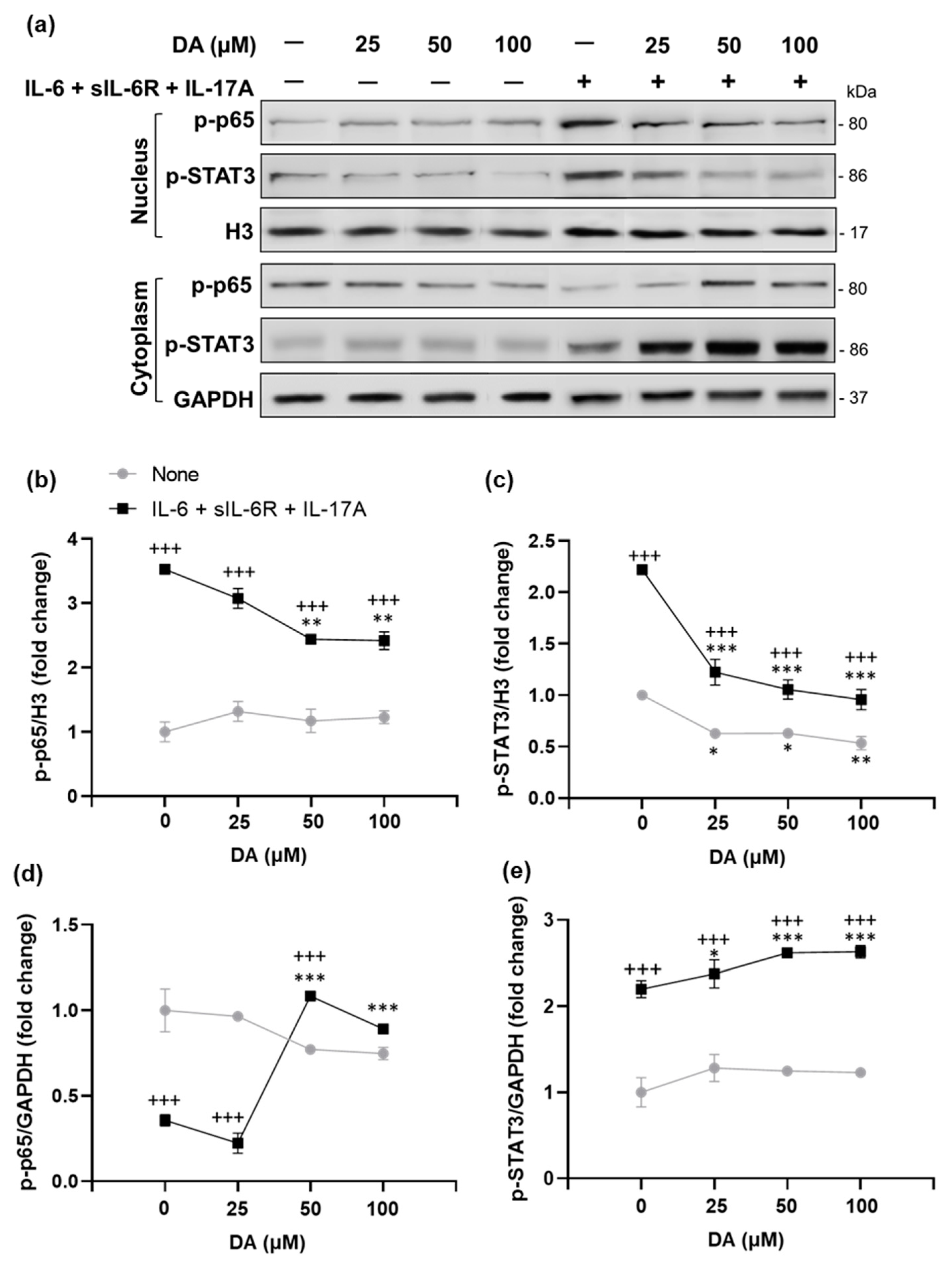
2.7. The Anti-Inflammatory Effects of L-DOPA/DA in EAU Gnat1rd17 Mice Are Mediated by the Suppression of NF-κB (p65) and STAT3 Activity in Retinal Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. EAU Induction
4.4. L-DOPA Treatment
4.5. Optomotor Response
4.6. Eye Fundus Imaging and EAU Clinical Score
4.7. Flow Cytometry
4.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.9. RNA Sequencing (RNA-seq) and Bioinformatics Analysis
4.10. Immunohistochemistry
4.11. Western Blot and ELISA Analyses
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suttorp-Schulten, M.S.; Rothova, A. The possible impact of uveitis in blindness: A literature survey. Br. J. Ophthalmol. 1996, 80, 844–848. [Google Scholar] [CrossRef]
- Prete, M.; Dammacco, R.; Fatone, M.C.; Racanelli, V. Autoimmune uveitis: Clinical, pathogenetic, and therapeutic features. Clin. Exp. Med. 2016, 16, 125–136. [Google Scholar] [CrossRef]
- Lee, R.W.; Nicholson, L.B.; Sen, H.N.; Chan, C.C.; Wei, L.; Nussenblatt, R.B.; Dick, A.D. Autoimmune and autoinflammatory mechanisms in uveitis. Semin. Immunopathol. 2014, 36, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Rothova, A.; Suttorp-van Schulten, M.S.; Frits Treffers, W.; Kijlstra, A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br. J. Ophthalmol. 1996, 80, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.D.; Tundia, N.; Sorg, R.; Zhao, C.; Chao, J.; Joshi, A.; Skup, M. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology 2016, 123, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Katsumi, O.; Pruett, R.C.; Sakaue, H.; Mehta, M. Retinal function in birdshot retinochoroidopathy. Acta Ophthalmol. 1991, 69, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Gouliopoulos, N.S.; Kalogeropoulos, C. Electrophysiological examination in uveitis: A review of the literature. Clin. Ophthalmol. 2014, 8, 199–214. [Google Scholar] [CrossRef]
- Koestinger, A.; Guex-Crosier, Y.; Borruat, F.X. Autoimmune retinal dysfunction in sarcoid chorioretinopathy. Klin. Mon. Augenheilkd. 2006, 223, 428–430. [Google Scholar] [CrossRef]
- Sevgi, D.D.; Davoudi, S.; Comander, J.; Sobrin, L. Retinal pigmentary changes in chronic uveitis mimicking retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1801–1810. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Dutta Majumder, P.; Menia, N.; Roy, R.; Sen, P.; George, A.E.; Ganesh, S.K.; Biswas, J. Uveitis in Patients with Retinitis Pigmentosa: 30 Years’ Consecutive Data. Ocul. Immunol. Inflamm. 2018, 26, 1283–1288. [Google Scholar] [CrossRef]
- Lichtinger, A.; Chowers, I.; Amer, R. Usher syndrome associated with Fuchs’ heterochromic uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1481–1485. [Google Scholar] [CrossRef]
- Turan-Vural, E.; Torun-Acar, B.; Tükenmez, N.; Sevim, M.Ş.; Buttanri, B.; Acar, S. Usher syndrome associated with Fuchs’ heterochromic uveitis: A case report. Clin. Ophthalmol. 2011, 5, 557–559. [Google Scholar] [CrossRef]
- Benson, M.D.; MacDonald, I.M. Bilateral uveitis and Usher syndrome: A case report. J. Med. Case Rep. 2015, 9, 60. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Appukuttan, B.; Wilmarth, P.A.; Pan, Y.; Stempel, A.J.; Chipps, T.J.; Benedetti, E.E.; Zamora, D.O.; Choi, D.; David, L.L.; et al. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res. 2013, 32, 102–180. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A.; Küchle, M.; Derevjanik, N.L.; Henderer, J.D.; Mahlow, J.; Green, W.R.; Campochiaro, P.A. Blood-retinal barrier breakdown in retinitis pigmentosa: Light and electron microscopic immunolocalization. Histol. Histopathol. 1995, 10, 913–923. [Google Scholar] [PubMed]
- McCarron, J.G.; Wilson, C.; Heathcote, H.R.; Zhang, X.; Buckley, C.; Lee, M.D. Heterogeneity and emergent behaviour in the vascular endothelium. Curr. Opin. Pharmacol. 2019, 45, 23–32. [Google Scholar] [CrossRef]
- Arima, Y.; Harada, M.; Kamimura, D.; Park, J.H.; Kawano, F.; Yull, F.E.; Kawamoto, T.; Iwakura, Y.; Betz, U.A.; Márquez, G.; et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 2012, 148, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Kamimura, D.; Atsumi, T.; Harada, M.; Kawamoto, T.; Nishikawa, N.; Stofkova, A.; Ohki, T.; Higuchi, K.; Morimoto, Y.; et al. A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model. Elife 2015, 4, e08733. [Google Scholar] [CrossRef]
- Arima, Y.; Ohki, T.; Nishikawa, N.; Higuchi, K.; Ota, M.; Tanaka, Y.; Nio-Kobayashi, J.; Elfeky, M.; Sakai, R.; Mori, Y.; et al. Brain micro-inflammation at specific vessels dysregulates organ-homeostasis via the activation of a new neural circuit. Elife 2017, 6, e25517. [Google Scholar] [CrossRef]
- Stofkova, A.; Kamimura, D.; Ohki, T.; Ota, M.; Arima, Y.; Murakami, M. Photopic light-mediated down-regulation of local α1A-adrenergic signaling protects blood-retina barrier in experimental autoimmune uveoretinitis. Sci. Rep. 2019, 9, 2353. [Google Scholar] [CrossRef]
- Nakagawa, I.; Kamimura, D.; Atsumi, T.; Arima, Y.; Murakami, M. Role of Inflammation Amplifier-Induced Growth Factor Expression in the Development of Inflammatory Diseases. Crit. Rev. Immunol. 2015, 35, 365–378. [Google Scholar] [CrossRef]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef]
- Stofkova, A.; Murakami, M. Neural activity regulates autoimmune diseases through the gateway reflex. Bioelectron. Med. 2019, 5, 14. [Google Scholar] [CrossRef]
- Kamimura, D.; Tanaka, Y.; Hasebe, R.; Murakami, M. Bidirectional communication between neural and immune systems. Int. Immunol. 2020, 32, 693–701. [Google Scholar] [CrossRef]
- Uchida, M.; Yamamoto, R.; Matsuyama, S.; Murakami, K.; Hasebe, R.; Hojyo, S.; Tanaka, Y.; Murakami, M. Gateway reflexes, neuronal circuits that regulate the gateways for autoreactive T cells in organs that have blood barriers. Int. Immunol. 2021, dxab022, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Tanaka, Y.; Murakami, M. The gateway reflex: Breaking through the blood barriers. Int. Immunol. 2021, 33, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Koch, P.; Chen, M.; Lau, A.; Reid, D.M.; Forrester, J.V. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp. Eye. Res. 2008, 87, 319–326. [Google Scholar] [CrossRef]
- Bansal, S.; Barathi, V.A.; Iwata, D.; Agrawal, R. Experimental autoimmune uveitis and other animal models of uveitis: An update. Indian J. Ophthalmol. 2015, 63, 211–218. [Google Scholar] [CrossRef] [PubMed]
- The Jackson Laboratory. Available online: https://www.jax.org/strain/008811 (accessed on 25 November 2021).
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Wang, J.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. A new mouse model of retinal degeneration (rd17). In Proceedings of the ARVO Annual Meeting Abstract, Fort Lauderdale, FL, USA, 6–10 May 2007. [Google Scholar]
- Yao, K.; Qiu, S.; Wang, Y.V.; Park, S.; Mohns, E.J.; Mehta, B.; Liu, X.; Chang, B.; Zenisek, D.; Crair, M.C.; et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 2018, 560, 484–488. [Google Scholar] [CrossRef]
- Collin, G.B.; Gogna, N.; Chang, B.; Damkham, N.; Pinkney, J.; Hyde, L.F.; Stone, L.; Naggert, J.K.; Nishina, P.M.; Krebs, M.P. Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss. Cells 2020, 9, 931. [Google Scholar] [CrossRef]
- Miyamoto, M.; Imai, R.; Sugimoto, S.; Aoki, M.; Nagai, H.; Ando, T. Visual electrophysiological features of two naturally occurring mouse models with retinal dysfunction. Curr. Eye Res. 2006, 31, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Aoki, M.; Hirai, K.; Sugimoto, S.; Kawasaki, K.; Imai, R. A nonsense mutation in Gnat1, encoding the alpha subunit of rod transducin, in spontaneous mouse models of retinal dysfunction. Exp. Eye Res. 2010, 90, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Aoki, M.; Sugimoto, S.; Kawasaki, K.; Imai, R. IRD1 and IRD2 mice, naturally occurring models of hereditary retinal dysfunction, show late-onset and progressive retinal degeneration. Curr. Eye Res. 2010, 35, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.; Duignan, E.; Humphries, P.; Palfi, A.; Kenna, P.F.; Farrar, G.J. A novel homozygous truncating GNAT1 mutation implicated in retinal degeneration. Br. J. Ophthalmol. 2016, 100, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Méjécase, C.; Laurent-Coriat, C.; Mayer, C.; Poch, O.; Mohand-Saïd, S.; Prévot, C.; Antonio, A.; Boyard, F.; Condroyer, C.; Michiels, C.; et al. Identification of a Novel Homozygous Nonsense Mutation Confirms the Implication of GNAT1 in Rod-Cone Dystrophy. PLoS ONE 2016, 11, e0168271. [Google Scholar] [CrossRef] [PubMed]
- Abdeljalil, J.; Hamid, M.; Abdel-Mouttalib, O.; Stéphane, R.; Raymond, R.; Johan, A.; José, S.; Pierre, C.; Serge, P. The optomotor response: A robust first-line visual screening method for mice. Vis. Res. 2005, 45, 1439–1446. [Google Scholar] [CrossRef]
- Schmucker, C.; Seeliger, M.; Humphries, P.; Biel, M.; Schaeffel, F. Grating acuity at different luminances in wild-type mice and in mice lacking rod or cone function. Investig. Ophthalmol. Vis. Sci. 2005, 46, 398–407. [Google Scholar] [CrossRef]
- Hohki, S.; Ohguro, N.; Haruta, H.; Nakai, K.; Terabe, F.; Serada, S.; Fujimoto, M.; Nomura, S.; Kawahata, H.; Kishimoto, T.; et al. Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp. Eye Res. 2010, 91, 162–170. [Google Scholar] [CrossRef]
- Şahin, E.; Karaman, G.; Uslu, M.; Karul, A.; Şendur, N.; Şavk, E. Adiponectin levels, insulin resistance and their relationship with serum levels of inflammatory cytokines in patients with Behçet’s disease. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1498–1502. [Google Scholar] [CrossRef]
- Murakami, Y.; Ikeda, Y.; Nakatake, S.; Fujiwara, K.; Tachibana, T.; Yoshida, N.; Notomi, S.; Hisatomi, T.; Yoshida, S.; Ishibashi, T.; et al. C-Reactive protein and progression of vision loss in retinitis pigmentosa. Acta Ophthalmol. 2018, 96, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Busetto, V.; Barbosa, I.; Basquin, J.; Marquenet, É.; Hocq, R.; Hennion, M.; Paternina, J.A.; Namane, A.; Conti, E.; Bensaude, O.; et al. Structural and functional insights into CWC27/CWC22 heterodimer linking the exon junction complex to spliceosomes. Nucleic Acids Res. 2020, 48, 5670–5683. [Google Scholar] [CrossRef]
- Bertrand, R.E.; Wang, J.; Li, Y.; Cheng, X.; Wang, K.; Stoilov, P.; Chen, R. Cwc27, associated with retinal degeneration, functions as a splicing factor in vivo. Hum. Mol. Genet. 2021, ddab319, Advance online publication. [Google Scholar] [CrossRef]
- Vial, D.; Piomelli, D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J. Neurochem. 1995, 64, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Lokhandwala, M.F. Dopamine-1 receptor G-protein coupling and the involvement of phospholipase A2 in dopamine-1 receptor mediated cellular signaling mechanisms in the proximal tubules of SHR. Clin. Exp. Hypertens. 1997, 19, 131–140. [Google Scholar] [CrossRef]
- Gnegy, M.E. Catecholamines. In Basic Neurochemistry: Principles of Molecular, Cellular and Medical Neurobiology, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2012; pp. 283–299. [Google Scholar]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Commodaro, A.G.; Bombardieri, C.R.; Peron, J.P.; Saito, K.C.; Guedes, P.M.; Hamassaki, D.E.; Belfort, R.N.; Rizzo, L.V.; Belfort, R., Jr.; de Camargo, M.M. p38{alpha} MAP kinase controls IL-17 synthesis in vogt-koyanagi-harada syndrome and experimental autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3567–3574. [Google Scholar] [CrossRef]
- Wei, R.; Dong, L.; Xiao, Q.; Sun, D.; Li, X.; Nian, H. Engagement of Toll-like receptor 2 enhances interleukin (IL)-17(+) autoreactive T cell responses via p38 mitogen-activated protein kinase signalling in dendritic cells. Clin. Exp. Immunol. 2014, 178, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Oldenhof, J.; Vickery, R.; Anafi, M.; Oak, J.; Ray, A.; Schoots, O.; Pawson, T.; von Zastrow, M.; Van Tol, H.H. SH3 binding domains in the dopamine D4 receptor. Biochemistry 1998, 37, 15726–15736. [Google Scholar] [CrossRef][Green Version]
- Oak, J.N.; Lavine, N.; Van Tol, H.H. Dopamine D(4) and D(2L) Receptor Stimulation of the Mitogen-Activated Protein Kinase Pathway Is Dependent on trans-Activation of the Platelet-Derived Growth Factor Receptor. Mol. Pharmacol. 2001, 60, 92–103. [Google Scholar] [CrossRef]
- Zhen, X.; Zhang, J.; Johnson, G.P.; Friedman, E. D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol. Pharmacol. 2001, 60, 857–864. [Google Scholar]
- Sarkar, C.; Das, S.; Chakroborty, D.; Chowdhury, U.R.; Basu, B.; Dasgupta, P.S.; Basu, S. Cutting Edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J. Immunol. 2006, 177, 7525–7529. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Yang, Y.; Qi, C.; Xiong, T.; Chen, Y.; Wu, G.; Zeng, C.; Wang, D. DRD4 (Dopamine D4 Receptor) Mitigate Abdominal Aortic Aneurysm via Decreasing P38 MAPK (mitogen-activated protein kinase)/NOX4 (NADPH Oxidase 4) Axis-Associated Oxidative Stress. Hypertension 2021, 78, 294–307. [Google Scholar] [CrossRef]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001, 276, 36059–36062. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Klemm, S.; Ruland, J. Inflammatory signal transduction from the Fc epsilon RI to NF-kappa B. Immunobiology 2006, 211, 815–820. [Google Scholar] [CrossRef]
- Wheeler-Jones, C.; Abu-Ghazaleh, R.; Cospedal, R.; Houliston, R.A.; Martin, J.; Zachary, I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997, 420, 28–32. [Google Scholar] [CrossRef]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Lorton, D.; Bellinger, D.L. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int. J. Mol. Sci. 2015, 16, 5635–5665. [Google Scholar] [CrossRef] [PubMed]
- Klitten, L.L.; Rath, M.F.; Coon, S.L.; Kim, J.S.; Klein, D.C.; Møller, M. Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Exp. Eye Res. 2008, 87, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lei, S. Cross interaction of dopaminergic and adrenergic systems in neural modulation. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 137–142. [Google Scholar]
- Wu, L.; Tai, Y.; Hu, S.; Zhang, M.; Wang, R.; Zhou, W.; Tao, J.; Han, Y.; Wang, Q.; Wei, W. Bidirectional Role of β2-Adrenergic Receptor in Autoimmune Diseases. Front. Pharmacol. 2018, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar]
- Zhong, H.; Voll, R.E.; Ghosh, S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1998, 1, 661–671. [Google Scholar] [CrossRef]
- Okazaki, T.; Sakon, S.; Sasazuki, T.; Sakurai, H.; Doi, T.; Yagita, H.; Okumura, K.; Nakano, H. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem. Biophys. Res. Commun. 2003, 300, 807–812. [Google Scholar] [CrossRef]
- Kurdi, M.; Booz, G.W. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J. Cardiovasc. Pharmacol. 2007, 50, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.D.; Krasnoperova, N.V.; Lyubarsky, A.L.; Isayama, T.; Nicoló, M.; Kosaras, B.; Wong, G.; Gannon, K.S.; Margolskee, R.F.; Sidman, R.L.; et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl. Acad. Sci. USA 2000, 97, 13913–13918. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.C.; Hippert, C.; Duran, Y.; West, E.L.; Bainbridge, J.W.; Warre-Cornish, K.; Luhmann, U.F.; Lakowski, J.; Sowden, J.C.; Ali, R.R.; et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 354–359. [Google Scholar] [CrossRef]
- Cameron, M.A.; Pozdeyev, N.; Vugler, A.A.; Cooper, H.; Iuvone, P.M.; Lucas, R.J. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur. J. Neurosci. 2009, 29, 761–767. [Google Scholar] [CrossRef]
- Munteanu, T.; Noronha, K.J.; Leung, A.C.; Pan, S.; Lucas, J.A.; Schmidt, T.M. Light-dependent pathways for dopaminergic amacrine cell development and function. Elife 2018, 7, e39866. [Google Scholar] [CrossRef]
- Park, H.n.; Jabbar, S.B.; Tan, C.C.; Sidhu, C.S.; Abey, J.; Aseem, F.; Schmid, G.; Iuvone, P.M.; Pardue, M.T. Visually-driven ocular growth in mice requires functional rod photoreceptors. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6272–6279. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Milosavljevic, N.; Allen, A.E.; Vessey, K.A.; Jobling, A.I.; Fletcher, E.L.; Breen, P.P.; Morley, J.W.; Cameron, M.A. Rod Photoreceptor Activation Alone Defines the Release of Dopamine in the Retina. Curr. Biol. 2019, 29, 763–774.e5. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, T.; Madigan, M.C.; Fernando, N.; Aggio-Bruce, R.; Zhou, F.; Pierce, M.; Chen, Y.; Huang, L.; Natoli, R.; et al. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell. Neurosci. 2020, 14, 577935. [Google Scholar] [CrossRef]
- Luger, D.; Caspi, R.R. New perspectives on effector mechanisms in uveitis. Semin. Immunopathol. 2008, 30, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E.; Alhakeem, S.A.; Mbanefo, E.C. Uveitis: Molecular Pathogenesis and Emerging Therapies. Front. Immunol. 2021, 12, 623725. [Google Scholar] [CrossRef] [PubMed]
- Laties, A.M. Central retinal artery innervation. Absence of adrenergic innervation to the intraocular branches. Arch. Ophthalmol. 1967, 77, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Huber-Lang, M.; Sarma, J.V.; Ward, P.A. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol. Med. 2008, 14, 195–204. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis. Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef]
- Tufford, A.R.; Onyak, J.R.; Sondereker, K.B.; Lucas, J.A.; Earley, A.M.; Mattar, P.; Hattar, S.; Schmidt, T.M.; Renna, J.M.; Cayouette, M. Melanopsin retinal ganglion cells regulate cone photoreceptor lamination in the mouse retina. Cell Rep. 2018, 23, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.; Hankins, M.W.; Hirano, J.; Archer, S.N. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res. 1997, 37, 3509–3529. [Google Scholar] [CrossRef]
- Ogilvie, J.M.; Speck, J.D. Dopamine has a critical role in photoreceptor degeneration in the rd mouse. Neurobiol. Dis. 2002, 10, 33–40. [Google Scholar] [CrossRef]
- Ogilvie, J.M.; Hakenewerth, A.M.; Gardner, R.R.; Martak, J.G.; Maggio, V.M. Dopamine receptor loss of function is not protective of rd1 rod photoreceptors in vivo. Mol. Vis. 2009, 15, 2868–2878. [Google Scholar]
- Chen, Y.W.; Huang, Y.P.; Wu, P.C.; Chiang, W.Y.; Wang, P.H.; Chen, B.Y. The functional vision protection effect of danshensu via dopamine D1 receptors: In vivo study. Nutrients 2021, 13, 978. [Google Scholar] [CrossRef]
- Nir, I.; Harrison, J.M.; Haque, R.; Low, M.J.; Grandy, D.K.; Rubinstein, M.; Iuvone, P.M. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J. Neurosci. 2002, 22, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Chaurasia, S.S.; Hwang, C.K.; Iuvone, P.M. Dopamine D₄ receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur. J. Neurosci. 2011, 34, 57–64. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Aung, M.H.; Mees, L.; Olson, D.E.; Pozdeyev, N.; Iuvone, P.M.; Thule, P.M.; Pardue, M.T. Dopamine Deficiency Mediates Early Rod-Driven Inner Retinal Dysfunction in Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 572–581. [Google Scholar] [CrossRef]
- Motz, C.T.; Chesler, K.C.; Allen, R.S.; Bales, K.L.; Mees, L.M.; Feola, A.J.; Maa, A.Y.; Olson, D.E.; Thule, P.M.; Iuvone, P.M.; et al. Novel Detection and Restorative Levodopa Treatment for Preclinical Diabetic Retinopathy. Diabetes 2020, 69, 1518–1527. [Google Scholar] [CrossRef]
- Brilliant, M.H.; Vaziri, K.; Connor, T.B., Jr.; Schwartz, S.G.; Carroll, J.J.; McCarty, C.A.; Schrodi, S.J.; Hebbring, S.J.; Kishor, K.S.; Flynn, H.W., Jr.; et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am. J. Med. 2016, 129, 292–298. [Google Scholar] [CrossRef]
- Laengle, U.W.; Court, M.; Markstein, R.; Germann, P.G.; Nogues, V.; Roman, D. Effects of anti-glaucoma drugs timolol and GLC756, a novel mixed dopamine D2 receptor agonist and D1 receptor antagonist, on endotoxin-induced-uveitis and -arthritis in rats. Exp. Toxicol. Pathol. 2005, 57, 127–134. [Google Scholar] [CrossRef]
- Lu, W.; Ding, Z.; Liu, F.; Shan, W.; Cheng, C.; Xu, J.; He, W.; Huang, W.; Ma, J.; Yin, Z. Dopamine delays articular cartilage degradation in osteoarthritis by negative regulation of the NF-κB and JAK2/STAT3 signaling pathways. Biomed. Pharmacother. 2019, 119, 109419. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Wang, X.; Xing, J.S.; Yu, Z.L.; Lou, J.C.; Ma, X.C.; Zhang, B. Anti-cancer effects of dopamine in human glioma: Involvement of mitochondrial apoptotic and anti-inflammatory pathways. Oncotarget 2017, 8, 88488–88500. [Google Scholar] [CrossRef]
- Sun, C.K.; Kao, Y.H.; Lee, P.H.; Wu, M.C.; Chen, K.C.; Lin, Y.C.; Tsai, M.S.; Chen, P.H. Dopamine impairs functional integrity of rat hepatocytes through nuclear factor kappa B activity modulation: An in vivo, ex vivo, and in vitro study. Liver Transpl. 2015, 21, 1520–1532. [Google Scholar] [CrossRef]
- Niewiarowska-Sendo, A.; Kozik, A.; Guevara-Lora, I. Influence of bradykinin B2 receptor and dopamine D2 receptor on the oxidative stress, inflammatory response, and apoptotic process in human endothelial cells. PLoS ONE 2018, 13, e0206443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, Y.; Liu, J.; Cao, Y.; Si, C.; Zheng, R.; Zeng, C.; Guan, H.; Li, L. Dopamine D4 receptor protected against hyperglycemia-induced endothelial dysfunction via PI3K /eNOS pathway. Biochem. Biophys. Res. Commun. 2019, 518, 554–559. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am. J. Physiol. Heart. Circ. Physiol. 2004, 287, H1554–H1560. [Google Scholar] [CrossRef]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Zallocchi, M. Usher protein functions in hair cells and photoreceptors. Int. J. Biochem. Cell. Biol. 2014, 46, 80–89. [Google Scholar] [CrossRef]
- Kobayashi, M.; Chandrasekhar, A.; Cheng, C.; Martinez, J.A.; Ng, H.; de la Hoz, C.; Zochodne, D.W. Diabetic polyneuropathy, sensory neurons, nuclear structure and spliceosome alterations: A role for CWC22. Dis. Model. Mech. 2017, 10, 215–224. [Google Scholar] [CrossRef]
- Furrer, P.; Mayer, J.M.; Plazonnet, B.; Gurny, R. Ocular tolerance of absorption enhancers in ophthalmic preparations. AAPS PharmSci. 2002, 4, E2. [Google Scholar] [CrossRef]
- Ewels, P.; Hammarén, R.; Peltzer, A.; Moreno, D.; Garcia, M.; Panneerselvam, S.; Sven, F.; Alneberg, J.; Di Tommaso, P.; Jemt, A.; et al. nf-core/rnaseq: Nf-core/rnaseq Version 1.4.2. Zenodo. 2019. Available online: https://doi.org/10.5281/zenodo.3503887 (accessed on 25 November 2021).
- Krueger, F. Trim Galore; Babraham Bioinformatics. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 25 November 2021).
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2019, 47, D745–D751. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- MGI Gene Ontology Term Finder. Available online: http://www.informatics.jax.org/gotools/MGI_Term_Finder.html (accessed on 25 November 2021).
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamoto’s film method (2020). Methods Mol. Biol. 2021, 2230, 259–281. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stofkova, A.; Zloh, M.; Andreanska, D.; Fiserova, I.; Kubovciak, J.; Hejda, J.; Kutilek, P.; Murakami, M. Depletion of Retinal Dopaminergic Activity in a Mouse Model of Rod Dysfunction Exacerbates Experimental Autoimmune Uveoretinitis: A Role for the Gateway Reflex. Int. J. Mol. Sci. 2022, 23, 453. https://doi.org/10.3390/ijms23010453
Stofkova A, Zloh M, Andreanska D, Fiserova I, Kubovciak J, Hejda J, Kutilek P, Murakami M. Depletion of Retinal Dopaminergic Activity in a Mouse Model of Rod Dysfunction Exacerbates Experimental Autoimmune Uveoretinitis: A Role for the Gateway Reflex. International Journal of Molecular Sciences. 2022; 23(1):453. https://doi.org/10.3390/ijms23010453
Chicago/Turabian StyleStofkova, Andrea, Miloslav Zloh, Dominika Andreanska, Ivana Fiserova, Jan Kubovciak, Jan Hejda, Patrik Kutilek, and Masaaki Murakami. 2022. "Depletion of Retinal Dopaminergic Activity in a Mouse Model of Rod Dysfunction Exacerbates Experimental Autoimmune Uveoretinitis: A Role for the Gateway Reflex" International Journal of Molecular Sciences 23, no. 1: 453. https://doi.org/10.3390/ijms23010453
APA StyleStofkova, A., Zloh, M., Andreanska, D., Fiserova, I., Kubovciak, J., Hejda, J., Kutilek, P., & Murakami, M. (2022). Depletion of Retinal Dopaminergic Activity in a Mouse Model of Rod Dysfunction Exacerbates Experimental Autoimmune Uveoretinitis: A Role for the Gateway Reflex. International Journal of Molecular Sciences, 23(1), 453. https://doi.org/10.3390/ijms23010453






