Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism
Abstract
:1. Introduction
2. Results
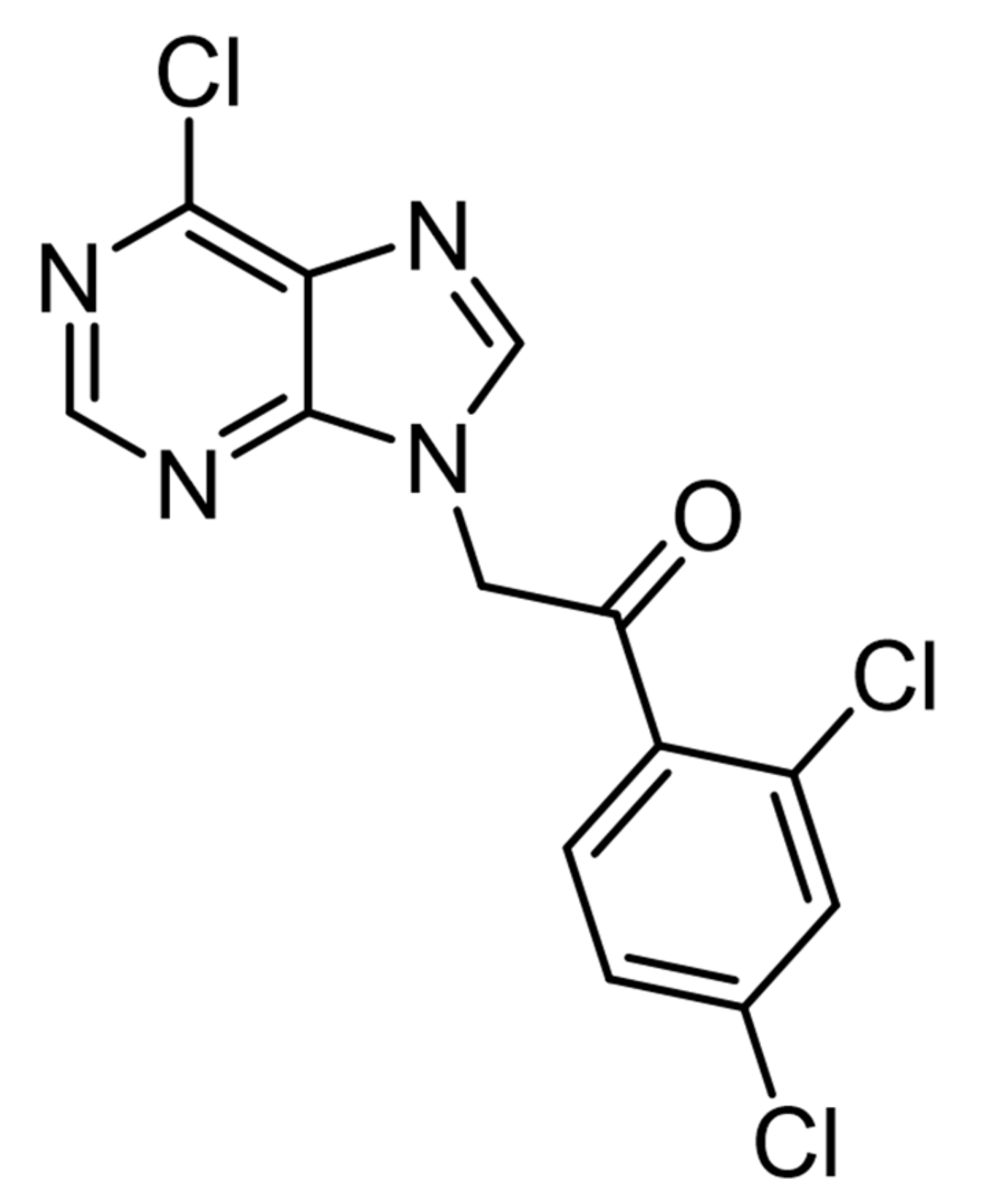
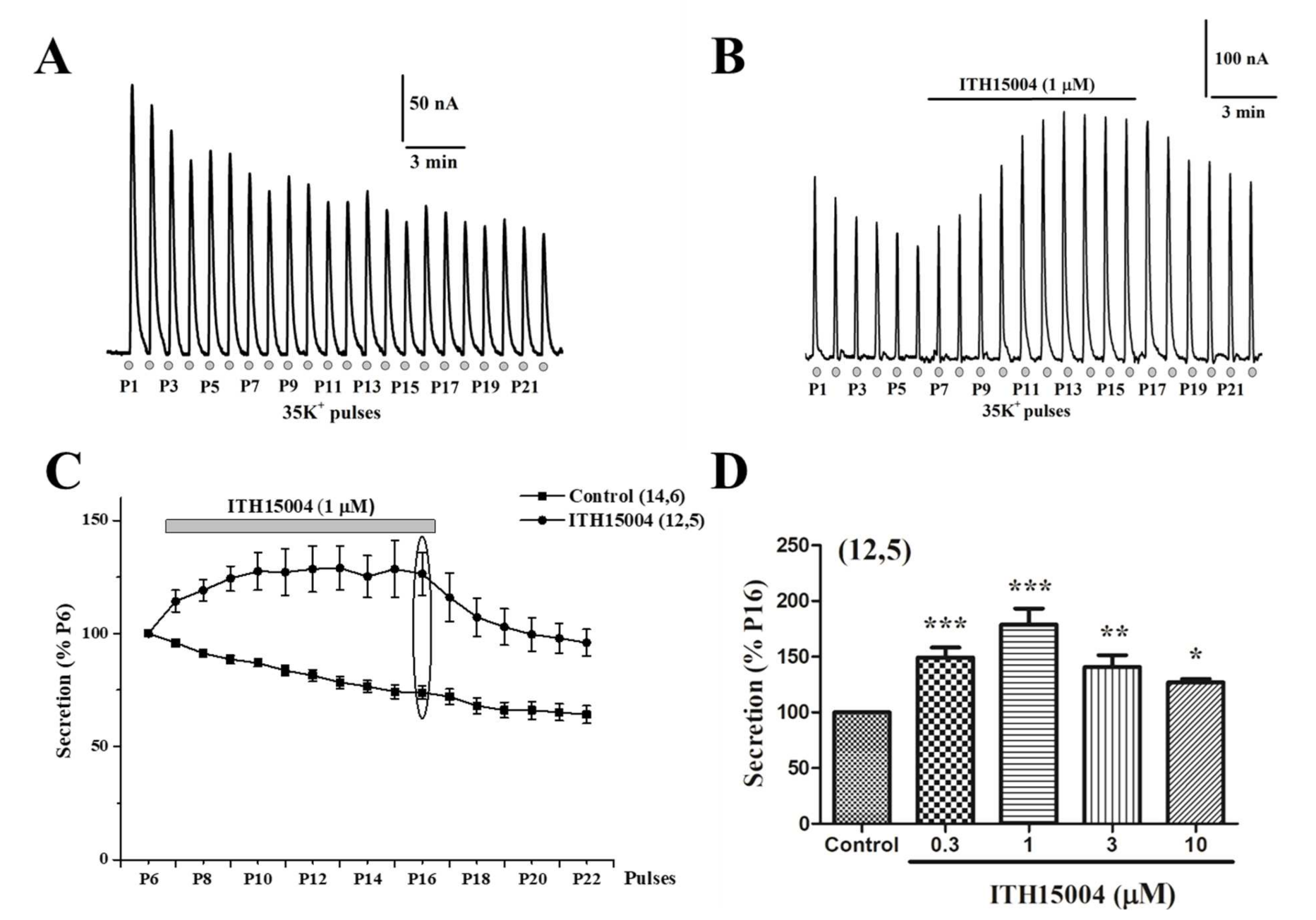
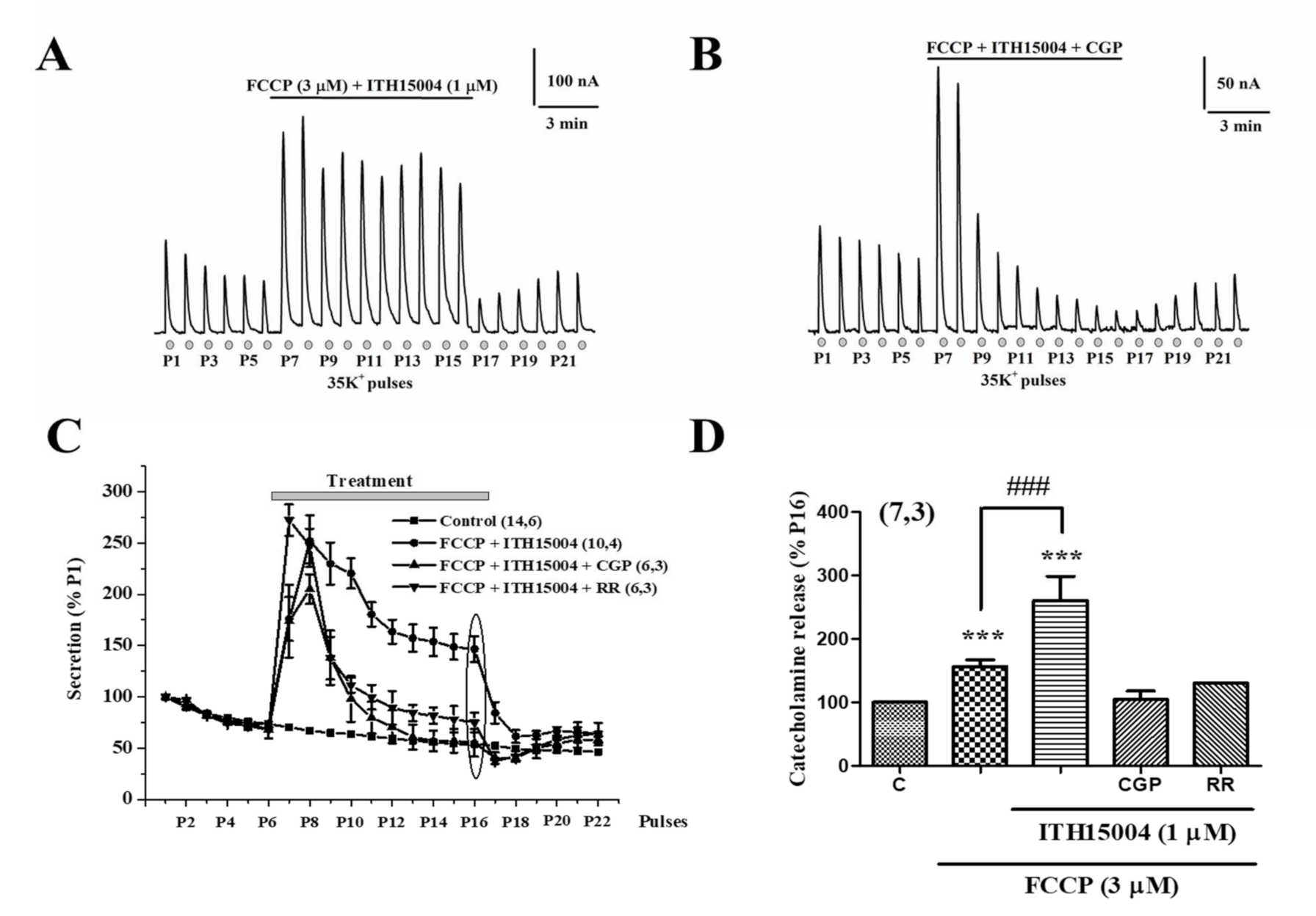
2.1. Effects of Compound ITH15004 on Catecholamine Release
2.2. Effects of ITH15004 on Secretion from Cells with Their Endoplasmic Reticulum Calcium Store Depleted
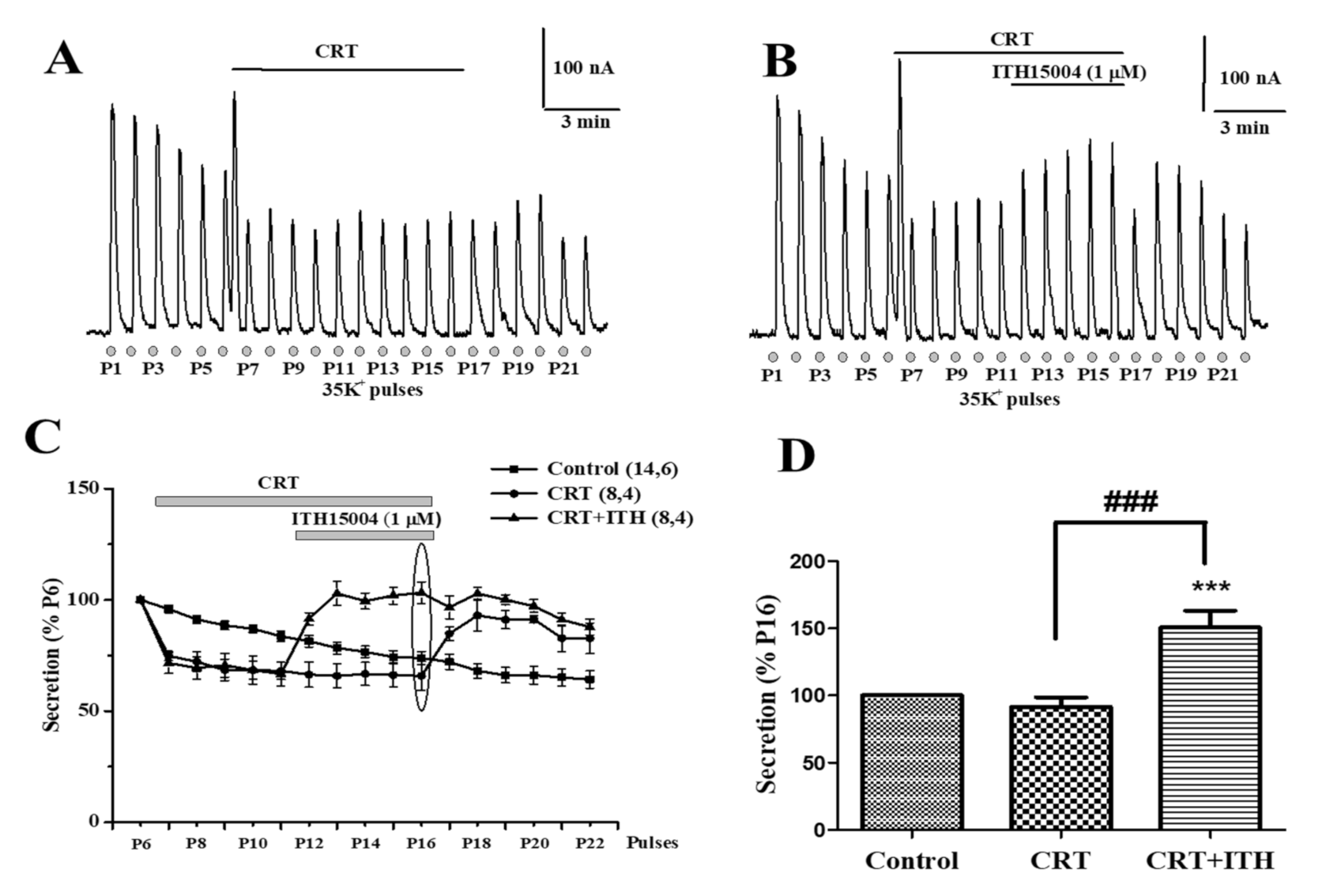
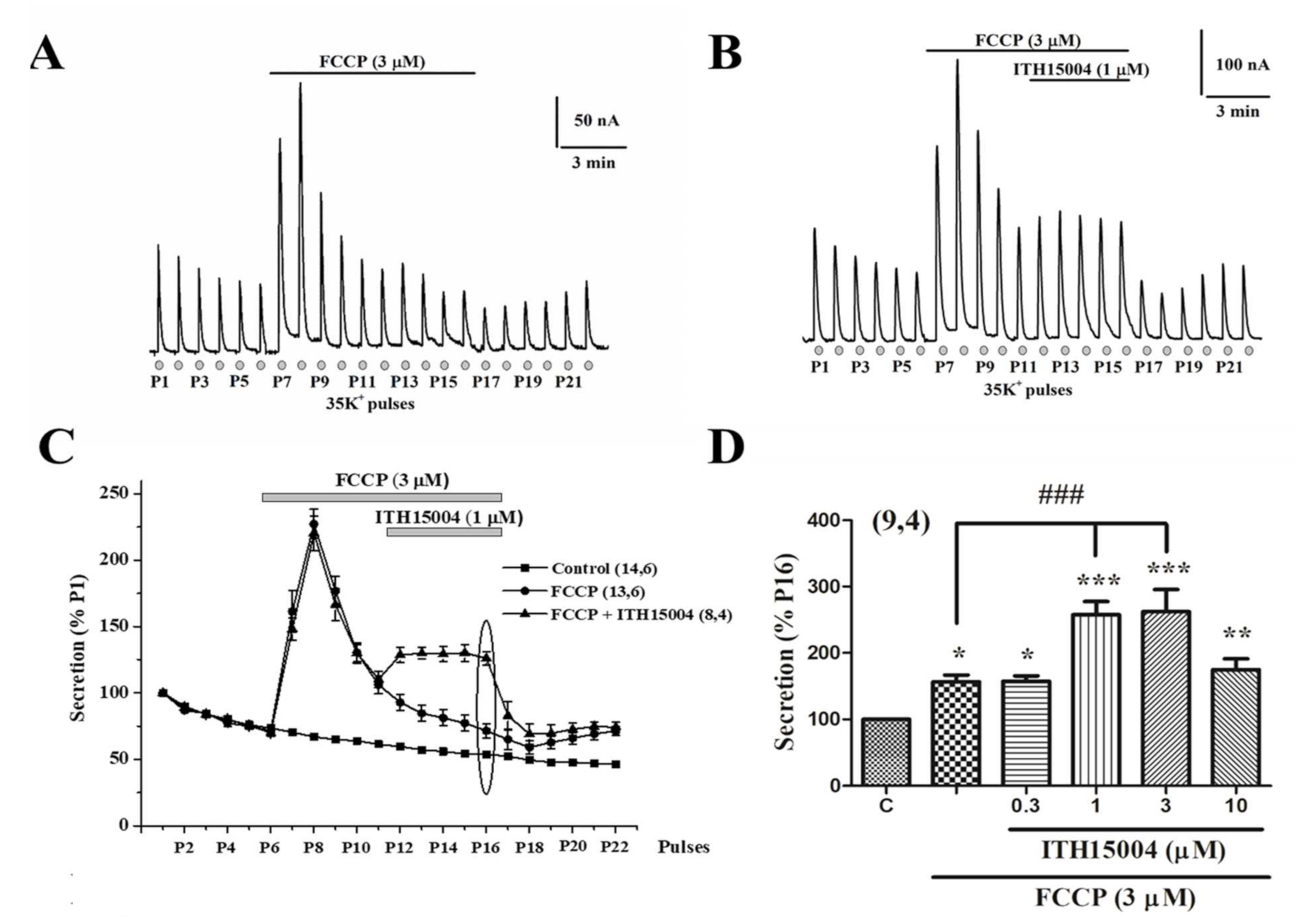
2.3. Effects of ITH15004 on Secretion upon the Pharmacological Interference with Mitocondrial Calcium Handling
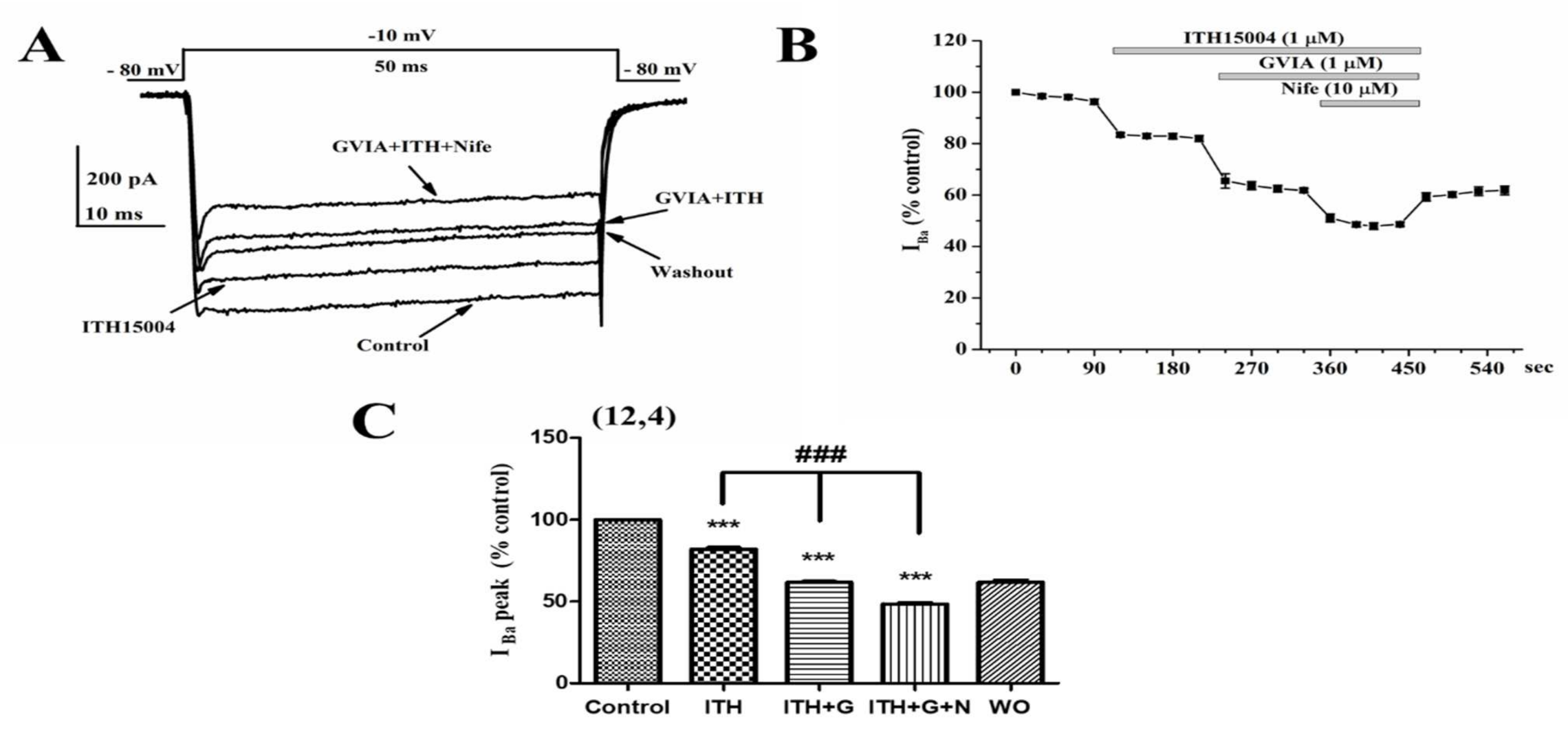
2.4. Effects of ITH15004 on Barium Currents
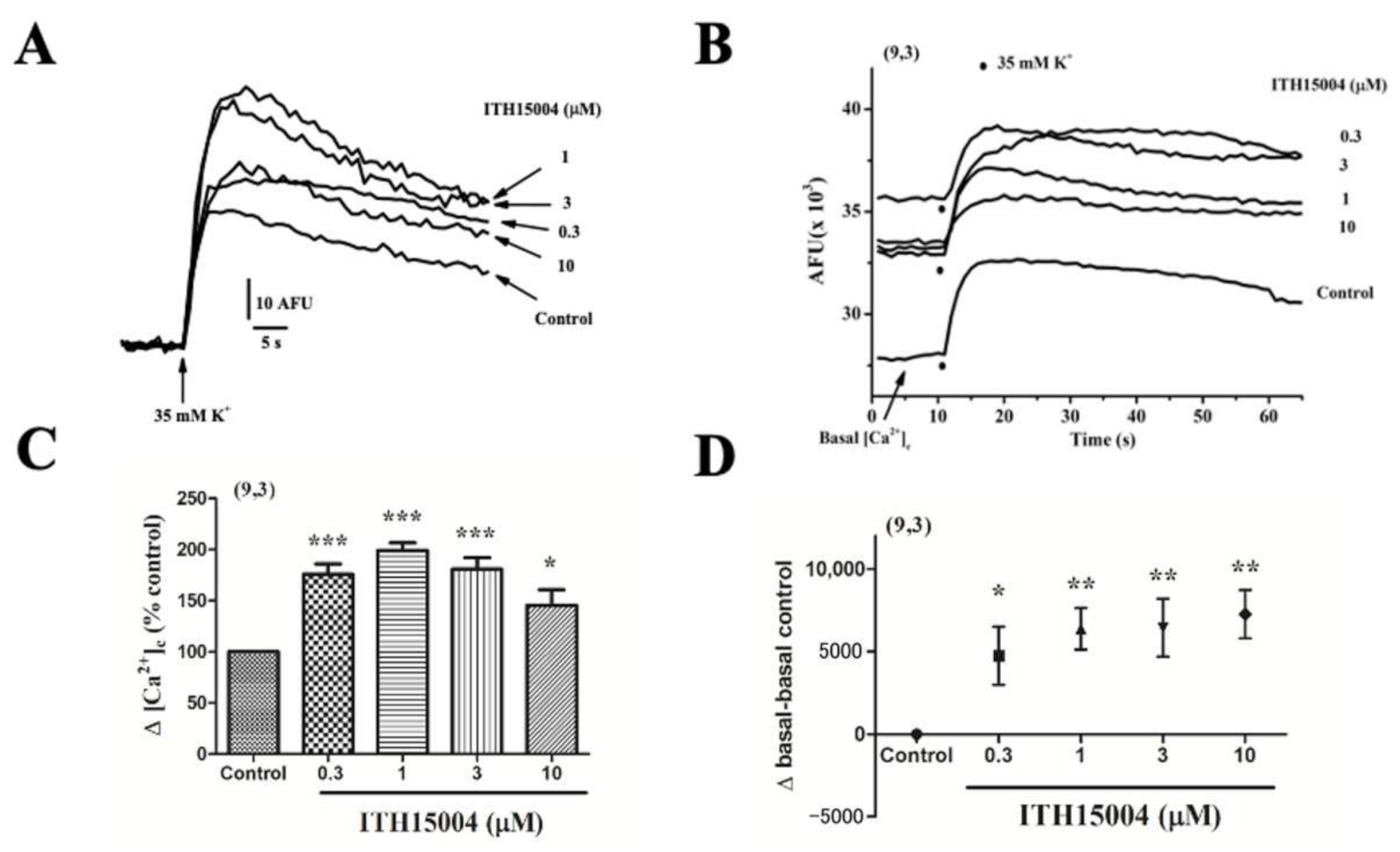
2.5. Effects of ITH15004 on the Cytosolic Calcium Transients Elicited by K+
2.6. Effects of ITH15004 on K+ Elicited [Ca2+]c Elevations in Cells with their ER Calcium Store Depleted
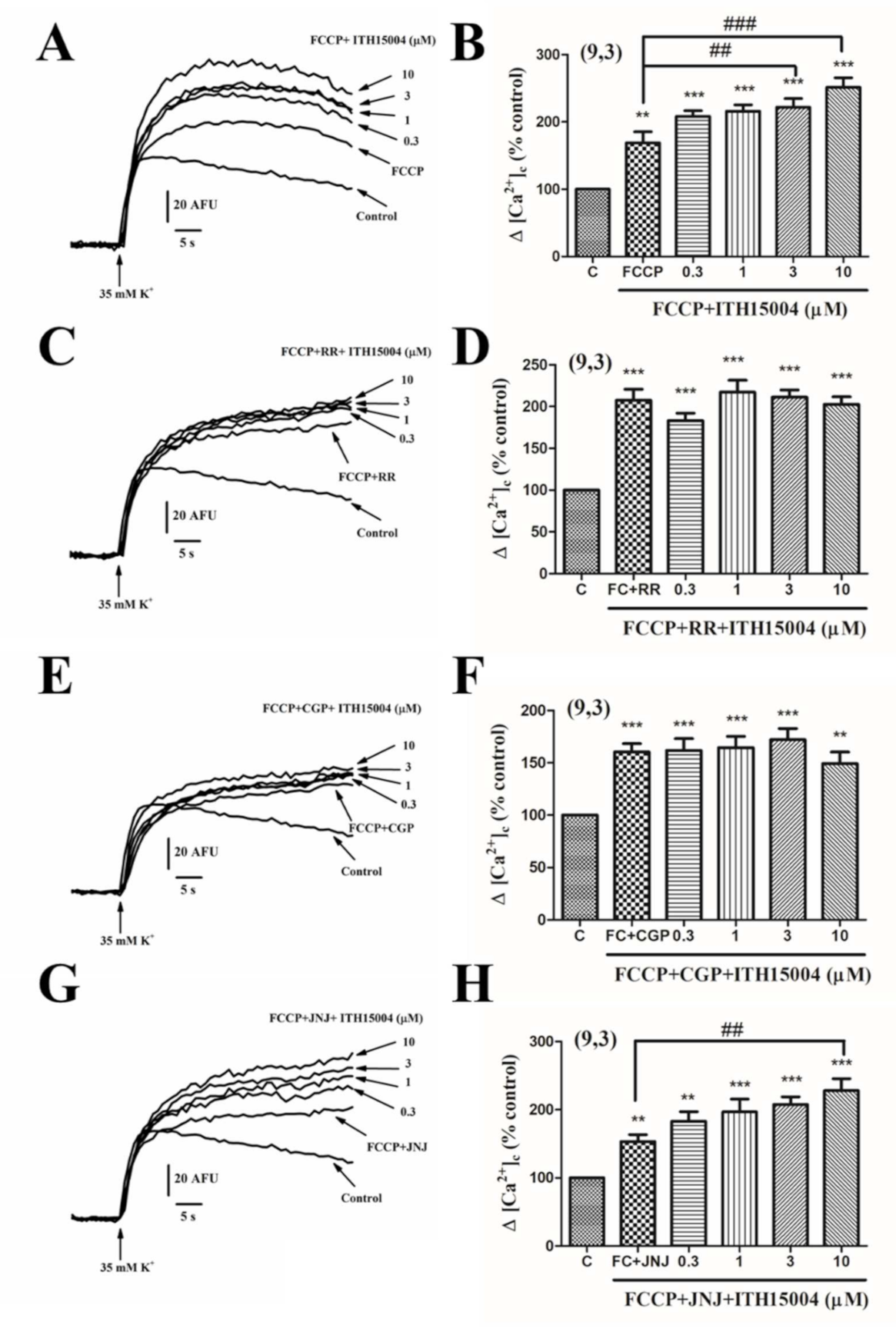
2.7. Effects of ITH15004 on 35K+ Elicited [Ca2+]c Elevations upon Interfering with Mitochondrial Calcium Handling
3. Discussion
4. Materials and Methods
4.1. Preparation of Bovine Chromaffin Cell Cultures
4.2. Real-Time Monitoring of Catecholamine Release Triggered by High-K+ Depolarizing Pulses
4.3. Electrophysiological Recording
4.4. Monitoring of Cytosolic Calcium Levels
4.5. Chemicals
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carbone, E.; Borges, R.; Eiden, L.E.; Garcia, A.G.; Hernandez-Cruz, A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019, 9, 1443–1502. [Google Scholar]
- Douglas, W.W. Stimulus-secretion coupling: The concept and clues from chromaffin and other cells. Br. J. Pharmacol. 1968, 34, 453–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neher, E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron 1998, 20, 389–399. [Google Scholar] [CrossRef] [Green Version]
- García, A.G.; García-De-Diego, A.M.; Gandía, L.; Borges, R.; García-Sancho, J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol. Rev. 2006, 86, 1093–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sancho, J.; de Diego, A.M.; García, A.G. Mitochondria and chromaffin cell function. Pflugers Arch. 2012, 464, 33–41. [Google Scholar] [CrossRef]
- Milla, J.; Montesinos, M.S.; Machado, J.D.; Borges, R.; Alonso, E.; Moreno-Ortega, A.J.; Cano-Abad, M.F.; Garcia, A.G.; Ruiz-Nuno, A. Ouabain enhances exocytosis through the regulation of calcium handling by the endoplasmic reticulum of chromaffin cells. Cell Calcium 2011, 50, 332–342. [Google Scholar] [CrossRef] [PubMed]
- de Pascual, R.; Alvarez-Ortego, N.; de Los Rios, C.; Jacob-Mazariego, G.; Garcia, A.G. Tetrabenazine Facilitates Exocytosis by Enhancing Calcium-Induced Calcium Release through Ryanodine Receptors. J. Pharmacol. Exp. Ther. 2019, 371, 219–230. [Google Scholar] [CrossRef]
- Cannon, W.B. Wisdom of the Body; W.W. Norton & Company: New York, NY, USA, 1932. [Google Scholar]
- Blum, K.; Chen, A.L.; Braverman, E.R.; Comings, D.E.; Chen, T.J.; Arcuri, V.; Blum, S.H.; Downs, B.W.; Waite, R.L.; Notaro, A.; et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr. Dis. Treat. 2008, 4, 893–918. [Google Scholar]
- Di Virgilio, F. P2X receptors and inflammation. Curr. Med. Chem. 2015, 22, 866–877. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, C.; Garcia-Magro, N.; Negredo, P.; Avendano, C.; Bhattacharya, A.; Ceusters, M.; Garcia, A.G. Chronic administration of P2X7 receptor antagonist JNJ-47965567 delays disease onset and progression, and improves motor performance in ALS SOD1(G93A) female mice. Dis. Model. Mech. 2020, 13, dmm045732. [Google Scholar] [CrossRef]
- Calzaferri, F.; Narros-Fernandez, P.; de Pascual, R.; de Diego, A.M.G.; Nicke, A.; Egea, J.; Garcia, A.G.; de Los Rios, C. Synthesis and Pharmacological Evaluation of Novel Non-nucleotide Purine Derivatives as P2X7 Antagonists for the Treatment of Neuroinflammation. J. Med. Chem. 2021, 64, 2272–2290. [Google Scholar] [CrossRef]
- Montero, M.; Alonso, M.T.; Carnicero, E.; Cuchillo-Ibáñez, I.; Albillos, A.; García, A.G.; García-Sancho, J.; Alvarez, J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2000, 2, 57–61. [Google Scholar] [CrossRef]
- Miranda-Ferreira, R.; de Pascual, R.; Caricati-Neto, A.; Gandia, L.; Jurkiewicz, A.; Garcia, A.G. Role of the endoplasmic reticulum and mitochondria on quantal catecholamine release from chromaffin cells of control and hypertensive rats. J. Pharmacol. Exp. Ther. 2009, 329, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, D.R.; Hlubek, M.D.; Stuenkel, E.L. Mitochondria regulate the Ca(2+)-exocytosis relationship of bovine adrenal chromaffin cells. J. Neurosci. 1999, 19, 9261–9270. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R.; Marchi, S.; Bonora, M.; Aguiari, P.; Bononi, A.; De Stefani, D.; Giorgi, C.; Leo, S.; Rimessi, A.; Siviero, R.; et al. Ca(2+) transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta 2009, 1787, 1342–1351. [Google Scholar] [CrossRef] [Green Version]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529 Pt 1, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium 2000, 28, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, C.; Nunez, L.; Montero, M.; Garcia, A.G.; Alonso, M.T.; Chamero, P.; Alvarez, J.; Garcia-Sancho, J. Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. Faseb J. 2002, 16, 343–353. [Google Scholar] [CrossRef]
- Garcia, A.G.; Sala, F.; Reig, J.A.; Viniegra, S.; Frias, J.; Fonteriz, R.; Gandia, L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature 1984, 309, 69–71. [Google Scholar] [CrossRef]
- Olivera, B.M.; Miljanich, G.P.; Ramachandran, J.; Adams, M.E. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu. Rev. Biochem. 1994, 63, 823–867. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.T.; Barrero, M.J.; Michelena, P.; Carnicero, E.; Cuchillo, I.; Garcia, A.G.; Garcia-Sancho, J.; Montero, M.; Alvarez, J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol. 1999, 144, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Douglas, W.W.; Rubin, R.P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J. Physiol. 1963, 167, 288–310. [Google Scholar] [CrossRef] [Green Version]
- von Ruden, L.; Neher, E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science 1993, 262, 1061–1065. [Google Scholar] [CrossRef]
- Lara, B.; Lopez, M.G.; Villarroya, M.; Gandia, L.; Cleeman, L.; Morad, M.; Garcia, A.G. A caffeine-sensitive Ca2+ store modulates K+-evoked secretion in chromaffin cells. Am. J. Physiol. 1997, 272, C1211–C1221. [Google Scholar] [CrossRef]
- Caricati-Neto, A.; Padin, J.F.; Silva-Junior, E.D.; Fernandez-Morales, J.C.; de Diego, A.M.; Jurkiewicz, A.; Garcia, A.G. Novel features on the regulation by mitochondria of calcium and secretion transients in chromaffin cells challenged with acetylcholine at 37 degrees C. Physiol. Rep. 2013, 1, e00182. [Google Scholar] [CrossRef] [PubMed]
- de Pascual, R.; Miranda-Ferreira, R.; Galvao, K.M.; Lameu, C.; Ulrich, H.; Smaili, S.S.; Jurkiewicz, A.; Garcia, A.G.; Gandia, L. Lower density of L-type and higher density of P/Q-type of calcium channels in chromaffin cells of hypertensive, compared with normotensive rats. Eur. J. Pharmacol. 2013, 706, 25–35. [Google Scholar] [CrossRef]
- Livett, B.G. Adrenal medullary chromaffin cells in vitro. Physiol. Rev. 1984, 64, 1103–1161. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.A.; López, M.G.; Gandía, L.; Michelena, P.; García, A.G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal. Biochem. 1990, 185, 243–248. [Google Scholar] [CrossRef]
- De Pascual, R.; Colmena, I.; Ruiz-Pascual, L.; Baraibar, A.M.; Egea, J.; Gandia, L.; Garcia, A.G. Regulation by L channels of Ca(2+)-evoked secretory responses in ouabain-treated chromaffin cells. Pflugers Arch. 2016, 468, 1779–1792. [Google Scholar] [CrossRef]
- Borges, R.; Sala, F.; García, A.G. Continuous monitoring of catecholamine release from perfused cat adrenals. J. Neurosci. Meth. 1986, 16, 289–300. [Google Scholar] [CrossRef]
- Green, D.J.; Perlman, R.L. On-line measurement of catecholamine secretion. Anal. Biochem. 1981, 110, 270–276. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guijo, J.M.; de Pascual, R.; García, A.G.; Gandía, L. Separation of calcium channel current components in mouse chromaffin cells superfused with low- and high-barium solutions. Pflugers Arch. 1998, 436, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.M.; Marty, A.; Neher, E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J. Physiol. 1982, 331, 577–597. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pascual, R.; Calzaferri, F.; Gonzalo, P.C.; Serrano-Nieto, R.; de los Ríos, C.; García, A.G.; Gandía, L. Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism. Int. J. Mol. Sci. 2022, 23, 440. https://doi.org/10.3390/ijms23010440
de Pascual R, Calzaferri F, Gonzalo PC, Serrano-Nieto R, de los Ríos C, García AG, Gandía L. Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism. International Journal of Molecular Sciences. 2022; 23(1):440. https://doi.org/10.3390/ijms23010440
Chicago/Turabian Stylede Pascual, Ricardo, Francesco Calzaferri, Paula C. Gonzalo, Rubén Serrano-Nieto, Cristóbal de los Ríos, Antonio G. García, and Luis Gandía. 2022. "Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism" International Journal of Molecular Sciences 23, no. 1: 440. https://doi.org/10.3390/ijms23010440
APA Stylede Pascual, R., Calzaferri, F., Gonzalo, P. C., Serrano-Nieto, R., de los Ríos, C., García, A. G., & Gandía, L. (2022). Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism. International Journal of Molecular Sciences, 23(1), 440. https://doi.org/10.3390/ijms23010440






