The Role of Neutrophils in the Pathogenesis of Chronic Lymphocytic Leukemia
Abstract
1. Introduction
2. Neutrophils Recruitment and Phenotype in CLL
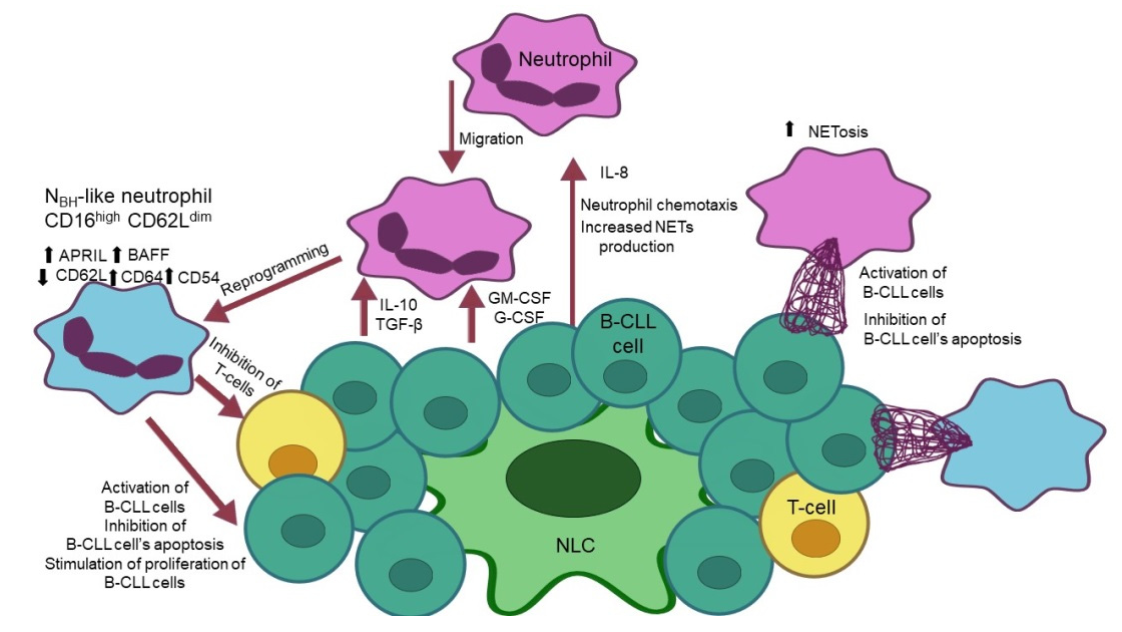
3. Interactions of Neutrophils with Malignant B Cells
4. The Mechanisms of Immunosuppression Derived by Neutrophils in CLL
5. Defects in Neutrophils’ Functions and Their Influence on Immune Response Development in CLL and DLBCL
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Burdick, M.D.; Londhe, V.; Xue, Y.Y.; Li, K.; Phillips, R.J.; Strieter, R.M. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J. Clin. Investig. 2002, 110, 1703–1716. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef]
- Demkow, U. Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis. Cancers 2021, 13, 4495. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Wu, M.; Ma, M.; Tan, Z.; Zheng, H.; Liu, X. Neutrophil: A New Player in Metastatic Cancers. Front. Immunol. 2020, 11, 565165. [Google Scholar] [CrossRef]
- SenGupta, S.; Hein, L.E.; Parent, C.A. The Recruitment of Neutrophils to the Tumor Microenvironment Is Regulated by Multiple Mediators. Front. Immunol. 2021, 12, 734188. [Google Scholar] [CrossRef]
- Zou, J.M.; Qin, J.; Li, Y.C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.S.; Chi, G.; Guo, F.; et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef]
- Shojaei, F.; Singh, M.; Thompson, J.D.; Ferrara, N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc. Natl. Acad. Sci. USA 2008, 105, 2640–2645. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831. [Google Scholar] [CrossRef]
- Yui, S.; Osawa, Y.; Ichisugi, T.; Morimoto-Kamata, R. Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism. Mediat. Inflamm. 2014, 2014, 971409. [Google Scholar] [CrossRef]
- Bodogai, M.; Moritoh, K.; Lee-Chang, C.; Hollander, C.M.; Sherman-Baust, C.A.; Wersto, R.P.; Araki, Y.; Miyoshi, I.; Yang, L.; Trinchieri, G.; et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015, 75, 3456–3465. [Google Scholar] [CrossRef]
- Seifert, M.; Sellmann, L.; Bloehdorn, J.; Wein, F.; Stilgenbauer, S.; Dürig, J.; Küppers, R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J. Exp. Med. 2012, 209, 2183–2198. [Google Scholar] [CrossRef]
- Rosenwald, A.; Alizadeh, A.A.; Widhopf, G.; Simon, R.; Davis, R.E.; Yu, X.; Yang, L.; Pickeral, O.K.; Rassenti, L.Z.; Powell, J.; et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J. Exp. Med. 2001, 194, 1639–1647. [Google Scholar] [CrossRef]
- Herishanu, Y.; Pérez-Galán, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef]
- Wiestner, A. The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015, 100, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.; Bottek, J.; Thiebes, S.; Zec, K.; Kudla, M.; Soun, C.; de Dios Panal, E.; Lill, J.K.; Pfennig, A.; Herrmann, R.; et al. Proteomic and bioinformatic profiling of neutrophils in CLL reveals functional defects that predispose to bacterial infections. Blood Adv. 2021, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Robrecht, S.; Bahlo, J.; Fink, A.M.; Cramer, P.; Tresckow, J.V.; Lange, E.; Kiehl, M.; Dreyling, M.; Ritgen, M.; et al. Richter transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia 2021, 35, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Auge, H.; Notarantonio, A.B.; Morizot, R.; Quinquenel, A.; Fornecker, L.M.; Hergalant, S.; Feugier, P.; Broséus, J. Microenvironment Remodeling and Subsequent Clinical Implications in Diffuse Large B-Cell Histologic Variant of Richter Syndrome. Front. Immunol. 2020, 11, 594841. [Google Scholar] [CrossRef]
- Federmann, B.; Mueller, M.R.; Steinhilber, J.; Horger, M.S.; Fend, F. Diagnosis of Richter transformation in chronic lymphocytic leukemia: Histology tips the scales. Ann. Hematol. 2018, 97, 1859–1868. [Google Scholar] [CrossRef]
- Rossi, D.; Spina, V.; Gaidano, G. Biology and treatment of Richter syndrome. Blood 2018, 131, 2761–2772. [Google Scholar] [CrossRef]
- Roessner, P.M.; Seiffert, M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020, 34, 2012–2024. [Google Scholar] [CrossRef]
- Jurado-Camino, T.; Córdoba, R.; Esteban-Burgos, L.; Hernández-Jiménez, E.; Toledano, V.; Hernandez-Rivas, J.A.; Ruiz-Sainz, E.; Cobo, T.; Siliceo, M.; Perez de Diego, R.; et al. Chronic lymphocytic leukemia: A paradigm of innate immune cross-tolerance. J. Immunol. 2015, 194, 719–727. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Schmitt, M.; Własiuk, P.; Chen, J.; Bojarska-Junak, A.; Kowal, M.; Roliñski, J.; Dmoszyñska, A. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia 2008, 22, 222–224. [Google Scholar] [CrossRef]
- Middleton, O.; Cosimo, E.; Dobbin, E.; McCaig, A.M.; Clarke, C.; Brant, A.M.; Leach, M.T.; Michie, A.M.; Wheadon, H. Complement deficiencies limit CD20 monoclonal antibody treatment efficacy in CLL. Leukemia 2015, 29, 107–114. [Google Scholar] [CrossRef]
- Parikh, S.A.; Vojdeman, F.J.; Andersen, M.K.; Brown Pde, N.; Geisler, C.H.; Weis Bjerrum, O.; Niemann, C.U. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer 2015, 121, 2883–2891. [Google Scholar] [CrossRef]
- Royle, J.A.; Baade, P.D.; Joske, D.; Girschik, J.; Fritschi, L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: A population-based study. Br. J. Cancer 2011, 105, 1076–1081. [Google Scholar] [CrossRef]
- Podaza, E.; Risnik, D.; Colado, A.; Elías, E.; Almejún, M.B.; Fernandez Grecco, H.; Bezares, R.F.; Borge, M.; Gamberale, R.; Giordano, M. Chronic lymphocytic leukemia cells increase neutrophils survival and promote their differentiation into CD16(high) CD62L(dim) immunosuppressive subset. Int. J. Cancer 2019, 144, 1128–1134. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Georgiadou, S.P.; Wierda, W.G.; Wright, S.; Albert, N.D.; Ferrajoli, A.; Keating, M.; Lewis, R.E. Impaired bactericidal but not fungicidal activity of polymorphonuclear neutrophils in patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2013, 54, 1730–1733. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Mishalian, I.; Singhal, S.; Cheng, G.; Kapoor, V.; Horng, W.; Fridlender, G.; Bayuh, R.; Worthen, G.S.; et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS ONE 2012, 7, e31524. [Google Scholar] [CrossRef]
- Itala, M.; Vainio, O.; Remes, K. Functional abnormalities in granulocytes predict susceptibility to bacterial infections in chronic lymphocytic leukaemia. Eur. J. Haematol. 1996, 57, 46–53. [Google Scholar] [CrossRef]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Ngo, V.L.; Geem, D.; Harusato, A.; Hirota, S.A.; Parkos, C.A.; Lukacs, N.W.; Nusrat, A.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N.; et al. IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria. Mucosal Immunol. 2017, 10, 673–684. [Google Scholar] [CrossRef]
- Vogt, K.L.; Summers, C.; Chilvers, E.R.; Condliffe, A.M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Investig. 2018, 48, e12967. [Google Scholar] [CrossRef]
- Gatjen, M.; Brand, F.; Grau, M.; Gerlach, K.; Kettritz, R.; Westermann, J.; Anagnostopoulos, I.; Lenz, P.; Lenz, G.; Höpken, U.E.; et al. Splenic Marginal Zone Granulocytes Acquire an Accentuated Neutrophil B-Cell Helper Phenotype in Chronic Lymphocytic Leukemia. Cancer Res. 2016, 76, 5253–5265. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Singer, M.K.; Assem, M.; Abdel Ghaffar, A.B.; Morcos, N.Y. Role of TNF-alpha as a survival prognostic marker in chronic lymphocytic leukemia patients. Egypt J. Immunol. 2011, 18, 51–60. [Google Scholar]
- Wierda, W.G.; Johnson, M.M.; Do, K.A.; Manshouri, T.; Dey, A.; O’Brien, S.; Giles, F.J.; Kantarjian, H.; Thomas, D.; Faderl, S.; et al. Plasma interleukin 8 level predicts for survival in chronic lymphocytic leukaemia. Br. J. Haematol. 2003, 120, 452–456. [Google Scholar] [CrossRef]
- Molica, S.; Vitelli, G.; Levato, D.; Levato, L.; Dattilo, A.; Gandolfo, G.M. Clinico-biological implications of increased serum levels of interleukin-8 in B-cell chronic lymphocytic leukemia. Haematologica 1999, 84, 208–211. [Google Scholar]
- Risnik, D.; Podaza, E.; Almejún, M.B.; Colado, A.; Elías, E.E.; Bezares, R.F.; Fernández-Grecco, H.; Cranco, S.; Sánchez-Ávalos, J.C.; Borge, M.; et al. Revisiting the role of interleukin-8 in chronic lymphocytic leukemia. Sci. Rep. 2017, 7, 15714. [Google Scholar] [CrossRef]
- Gregoire, M.; Guilloton, F.; Pangault, C.; Mourcin, F.; Sok, P.; Latour, M.; Amé-Thomas, P.; Flecher, E.; Fest, T.; Tarte, K. Neutrophils trigger a NF-kappaB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 2015, 6, 16471–16487. [Google Scholar] [CrossRef]
- Miyata-Takata, T.; Takata, K.; Toji, T.; Goto, N.; Kasahara, S.; Takahashi, T.; Tari, A.; Noujima-Harada, M.; Miyata, T.; Sato, Y.; et al. Elevation of serum interleukins 8, 4, and 1beta levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci. Rep. 2015, 5, 18434. [Google Scholar] [CrossRef]
- Manfroi, B.; McKee, T.; Mayol, J.F.; Tabruyn, S.; Moret, S.; Villiers, C.; Righini, C.; Dyer, M.; Callanan, M.; Schneider, P.; et al. CXCL-8/IL8 Produced by Diffuse Large B-cell Lymphomas Recruits Neutrophils Expressing a Proliferation-Inducing Ligand APRIL. Cancer Res. 2017, 77, 1097–1107. [Google Scholar] [CrossRef]
- Bankir, M.; Acik, D.Y. IL-17 and IL-23 levels in patients with early-stage chronic lymphocytic leukemia. North Clin. Istanb. 2021, 8, 24–30. [Google Scholar] [CrossRef]
- Garley, M.; Jabłońska, E.; Sawicka-Powierza, J.; Ratajczak-Wrona, W.; Kłoczko, J.; Piszcz, J. Expression of subtypes of interleukin-17 ligands and receptors in patients with B-cell chronic lymphocytic leukemia. Clin. Lab 2014, 60, 1677–1683. [Google Scholar] [CrossRef]
- Zhong, W.; Xu, X.; Zhu, Z.; Yang, L.; Du, H.; Xia, Z.; Yuan, Z.; Xiong, H.; Du, Q.; Wei, Y.; et al. Increased interleukin-17A levels promote rituximab resistance by suppressing p53 expression and predict an unfavorable prognosis in patients with diffuse large B cell lymphoma. Int. J. Oncol. 2018, 52, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, G.; Papajik, T.; Gajdos, P.; Mikulkova, Z.; Urbanova, R.; Gabcova, G.; Kudelka, M.; Turcsányi, P.; Ryznerova, P.; Prochazka, V.; et al. Neutrophils in chronic lymphocytic leukemia are permanently activated and have functional defects. Oncotarget 2017, 8, 84889–84901. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Buschle, M.; Campana, D.; Carding, S.R.; Richard, C.; Hoffbrand, A.V.; Brenner, M.K. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J. Exp. Med. 1993, 177, 213–218. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, Y.L.; Peng, L.S.; Chen, N.; Chen, W.; Lv, Y.P.; Mao, F.Y.; Zhang, J.Y.; Cheng, P.; Teng, Y.S.; et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef]
- Shan, Z.G.; Yan, Z.B.; Peng, L.S.; Cheng, P.; Teng, Y.S.; Mao, F.Y.; Fan, K.; Zhuang, Y.; Zhao, Y.L. Granulocyte-Macrophage Colony-Stimulating Factor-Activated Neutrophils Express B7-H4 That Correlates with Gastric Cancer Progression and Poor Patient Survival. J. Immunol. Res. 2021, 2021, 6613247. [Google Scholar] [CrossRef]
- Wislez, M.; Fleury-Feith, J.; Rabbe, N.; Moreau, J.; Cesari, D.; Milleron, B.; Mayaud, C.; Antoine, M.; Soler, P.; Cadranel, J. Tumor-derived granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor prolong the survival of neutrophils infiltrating bronchoalveolar subtype pulmonary adenocarcinoma. Am. J. Pathol. 2001, 159, 1423–1433. [Google Scholar] [CrossRef][Green Version]
- Su, X.; Xu, Y.; Fox, G.C.; Xiang, J.; Kwakwa, K.A.; Davis, J.L.; Belle, J.I.; Lee, W.C.; Wong, W.H.; Fontana, F.; et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J. Clin. Investig. 2021, 131, 20. [Google Scholar] [CrossRef]
- Pistoia, V.; Corcione, A.; Baldi, L.; Zupo, S.; Dono, M.; Ferrarini, M. Production of hematopoietic growth factors by human B lymphocytes: Mechanisms and possible implications. Stem. Cells 1993, 11 (Suppl. S2), 150–155. [Google Scholar] [CrossRef]
- Chow, K.U.; Schneider, B.; Mitrou, P.S.; Weidmann, E. Influence of various cytokines on the expression of CD20 on the surface of CLL-cells in vitro. Leuk Res. 2001, 25, 99–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, J.; Sheng, X.; Zhu, N.; Deng, S.; Chen, J.; Yu, L.; Zhou, Y.; Lin, C.; Shen, J. GM-CSF enhanced the effect of CHOP and R-CHOP on inhibiting diffuse large B-cell lymphoma progression via influencing the macrophage polarization. Cancer Cell Int. 2021, 21, 141. [Google Scholar] [CrossRef]
- Balazs, M.; Martin, F.; Zhou, T.; Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 2002, 17, 341–352. [Google Scholar] [CrossRef]
- Scapini, P.; Nardelli, B.; Nadali, G.; Calzetti, F.; Pizzolo, G.; Montecucco, C.; Cassatella, M.A. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 2003, 197, 297–302. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef]
- Schwaller, J.; Schneider, P.; Mhawech-Fauceglia, P.; McKee, T.; Myit, S.; Matthes, T.; Tschopp, J.; Donze, O.; Le Gal, F.A.; Huard, B. Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B-cell lymphoma aggressiveness. Blood 2007, 109, 331–338. [Google Scholar] [CrossRef]
- Manfroi, B.; Moreaux, J.; Righini, C.; Ghiringhelli, F.; Sturm, N.; Huard, B. Tumor-associated neutrophils correlate with poor prognosis in diffuse large B-cell lymphoma patients. Blood Cancer J. 2018, 8, 66. [Google Scholar] [CrossRef]
- Podaza, E.; Sabbione, F.; Risnik, D.; Borge, M.; Almejún, M.B.; Colado, A.; Fernández-Grecco, H.; Cabrejo, M.; Bezares, R.F.; Trevani, A.; et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol. Immunother. 2017, 66, 77–89. [Google Scholar] [CrossRef]
- Sangaletti, S.; Tripodo, C.; Vitali, C.; Portararo, P.; Guarnotta, C.; Casalini, P.; Cappetti, B.; Miotti, S.; Pinciroli, P.; Fuligni, F.; et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Discov. 2014, 4, 110–129. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Hardisty, G.; Rossi, A.G.; Gwyer Findlay, E. The Outcome of Neutrophil-T Cell Contact Differs Depending on Activation Status of Both Cell Types. Front. Immunol. 2021, 12, 633486. [Google Scholar] [CrossRef]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Sivanandham, R.; Brocca-Cofano, E.; Krampe, N.; Falwell, E.; Venkatraman, S.M.K.; Ribeiro, R.M.; Apetrei, C.; Pandrea, I. Neutrophil extracellular trap production contributes to pathogenesis in SIV-infected nonhuman primates. J. Clin. Investig. 2018, 128, 5178–5183. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.O.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol. Med. 2020, 12, e10681. [Google Scholar] [CrossRef]
- Rotondo, R.; Bertolotto, M.; Barisione, G.; Astigiano, S.; Mandruzzato, S.; Ottonello, L.; Dallegri, F.; Bronte, V.; Ferrini, S.; Barbieri, O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J. Leukoc. Biol. 2011, 89, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Stachura, J.; Tonecka, K.; Fidyt, K.; Braniewska, A.; Sas, Z.; Kotula, I.; Rygiel, T.P.; Boon, L.; Golab, J.; et al. Inhibition of IDO leads to IL-6-dependent systemic inflammation in mice when combined with photodynamic therapy. Cancer Immunol. Immunother. 2020, 69, 1101–1112. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Findlay, E.G. Orchestration of Adaptive T Cell Responses by Neutrophil Granule Contents. Mediat. Inflamm. 2019, 2019, 8968943. [Google Scholar] [CrossRef]
- Millrud, C.R.; Kågedal, Å.; Kumlien Georén, S.; Winqvist, O.; Uddman, R.; Razavi, R.; Munck-Wikland, E.; Cardell, L.O. NET-producing CD16(high) CD62L[1] neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int. J. Cancer 2017, 140, 2557–2567. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Tak, T.; Wijten, P.; Heeres, M.; Pickkers, P.; Scholten, A.; Heck, A.J.R.; Vrisekoop, N.; Leenen, L.P.; Borghans, J.A.M.; Tesselaar, K.; et al. Human CD62L neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood 2017, 129, 3476–3485. [Google Scholar] [CrossRef]
- Golay, J.; Da Roit, F.; Bologna, L.; Ferrara, C.; Leusen, J.H.; Rambaldi, A.; Klein, C.; Introna, M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013, 122, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhu, S.; Li, T.; Wu, J.; Zang, G.; Lv, X.; Qiao, Y.; Huang, J.; Shao, Y.; Cui, J.; et al. A Dual TLR7/TLR9 Inhibitor HJ901 Inhibits ABC-DLBCL Expressing the MyD88 L265P Mutation. Front. Cell Dev. Biol. 2020, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ilizaliturri, F.J.; Jupudy, V.; Ostberg, J.; Oflazoglu, E.; Huberman, A.; Repasky, E.; Czuczman, M.S. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin. Cancer Res. 2003, 9, 5866–5873. [Google Scholar]
| Cytokine | Role in Neutrophils Recruitment | Refs. |
|---|---|---|
| IL-8 | In CLL IL-8 is secreted by leukemia-associated monocytes and macrophages. In DLBCL both malignant and stroma cells seem to be the source of this chemokine. The elevated secretion of IL-8 was described as a prognostic marker that correlates with Rai stage and beta-2 microglobulin factors and is associated with the high risk of patient death in CLL. Additionally, in DLBCL upregulation of IL-8 correlates with the level of proliferation inducing ligand (APRIL) in patients’ serum. | [43,44,45,46,47,48] |
| IL-17A | Elevated in CLL patients’ serum. Unfavorable prognosis marker that promotes the resistance to rituximab in DLBCL. However, IL-17A has not been yet linked directly with the neutrophils infiltration in CLL and DLBCL. | [49,50,51] |
| CXCR2 | Expression increased in bone marrow-derived neutrophils of Eμ-TCL1 murine model of CLL. | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachowska, M.; Wojciechowska, A.; Muchowicz, A. The Role of Neutrophils in the Pathogenesis of Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2022, 23, 365. https://doi.org/10.3390/ijms23010365
Wachowska M, Wojciechowska A, Muchowicz A. The Role of Neutrophils in the Pathogenesis of Chronic Lymphocytic Leukemia. International Journal of Molecular Sciences. 2022; 23(1):365. https://doi.org/10.3390/ijms23010365
Chicago/Turabian StyleWachowska, Malgorzata, Alicja Wojciechowska, and Angelika Muchowicz. 2022. "The Role of Neutrophils in the Pathogenesis of Chronic Lymphocytic Leukemia" International Journal of Molecular Sciences 23, no. 1: 365. https://doi.org/10.3390/ijms23010365
APA StyleWachowska, M., Wojciechowska, A., & Muchowicz, A. (2022). The Role of Neutrophils in the Pathogenesis of Chronic Lymphocytic Leukemia. International Journal of Molecular Sciences, 23(1), 365. https://doi.org/10.3390/ijms23010365






