Single-Chain Fragment Variables Targeting Leukocidin ED Can Alleviate the Inflammation of Staphylococcus aureus-Induced Mastitis in Mice
Abstract
:1. Introduction
2. Results
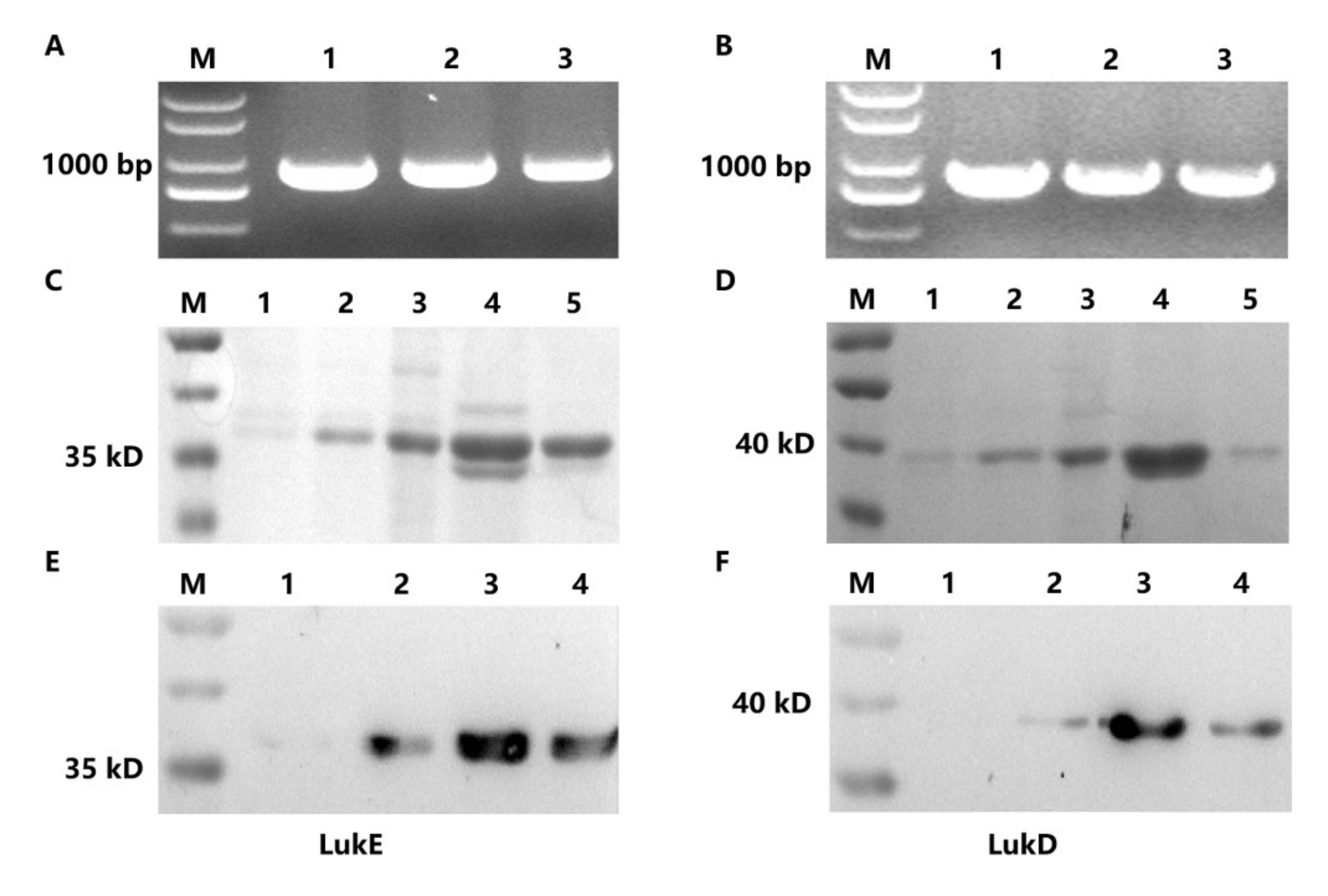
2.1. Expression and Purification of LukE and LukD
2.2. Screening of ScFvs Targeting LukE and LukD
2.3. Characteristics, Expression and Purification of ScFvs
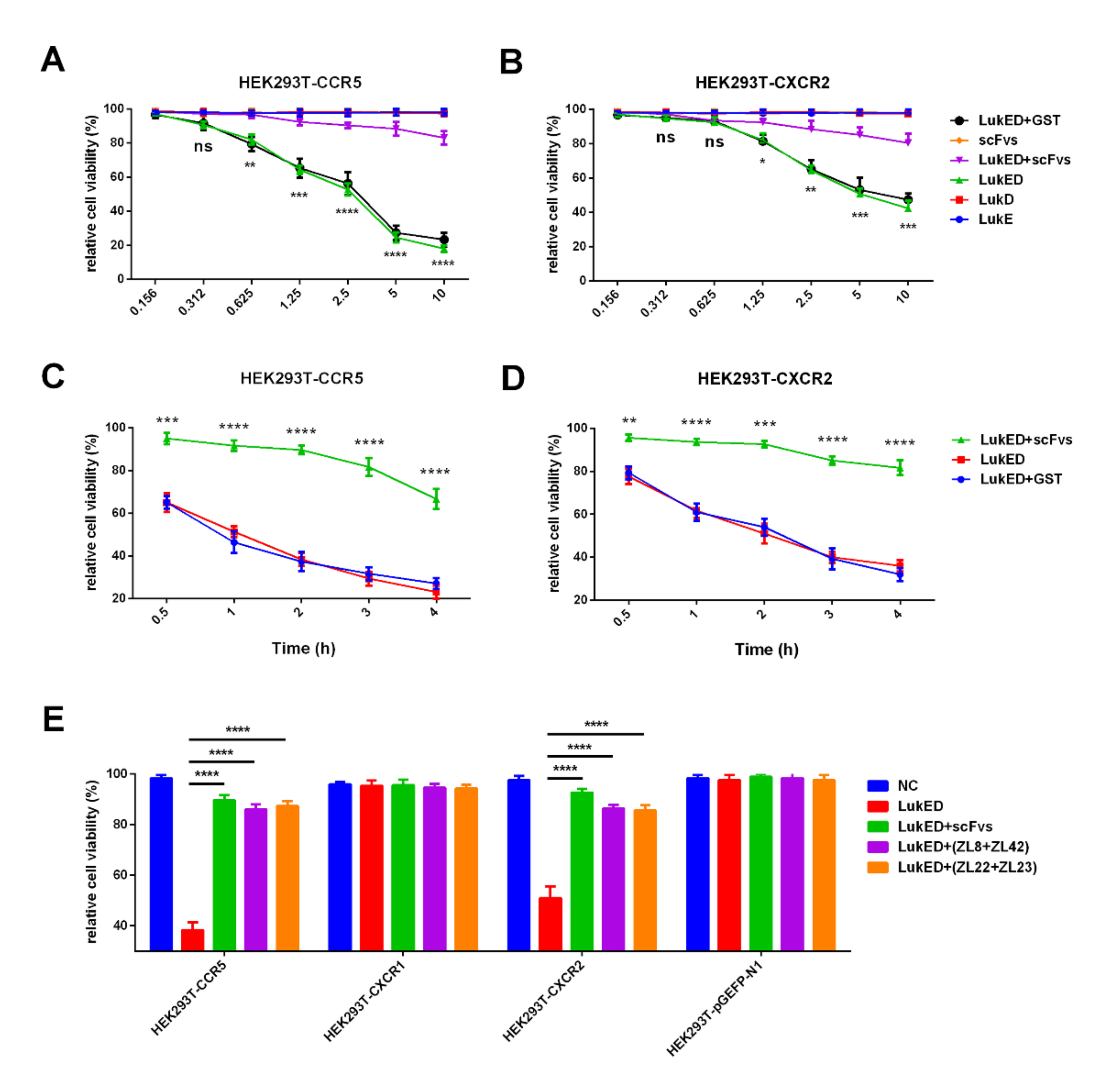
2.4. ScFvs Can Inhibit the Binding of LukED to CCR5 and CXCR2 In Vitro
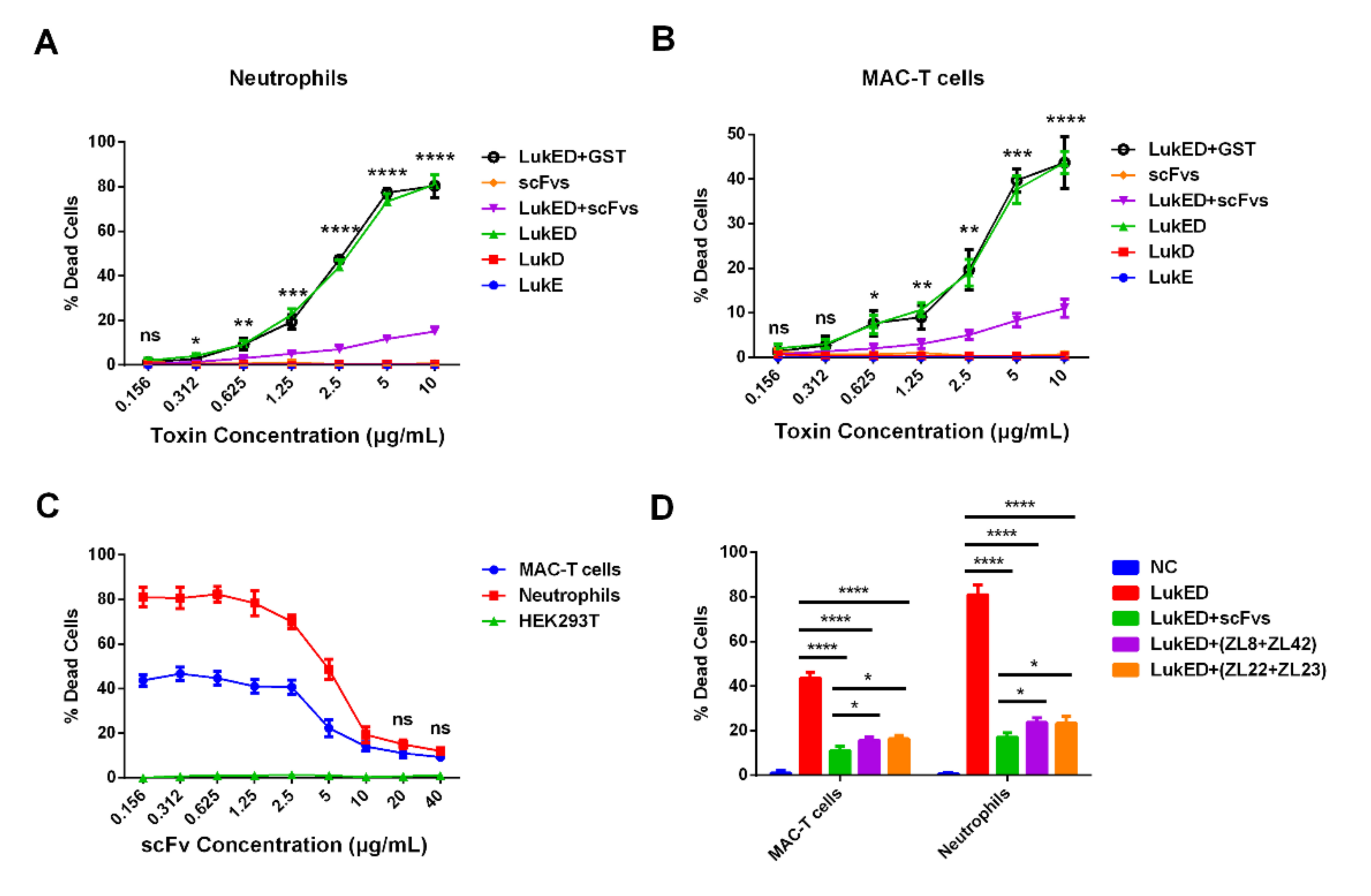
2.5. ScFvs Can Effectively Weaken Cell Killing Caused by LukED
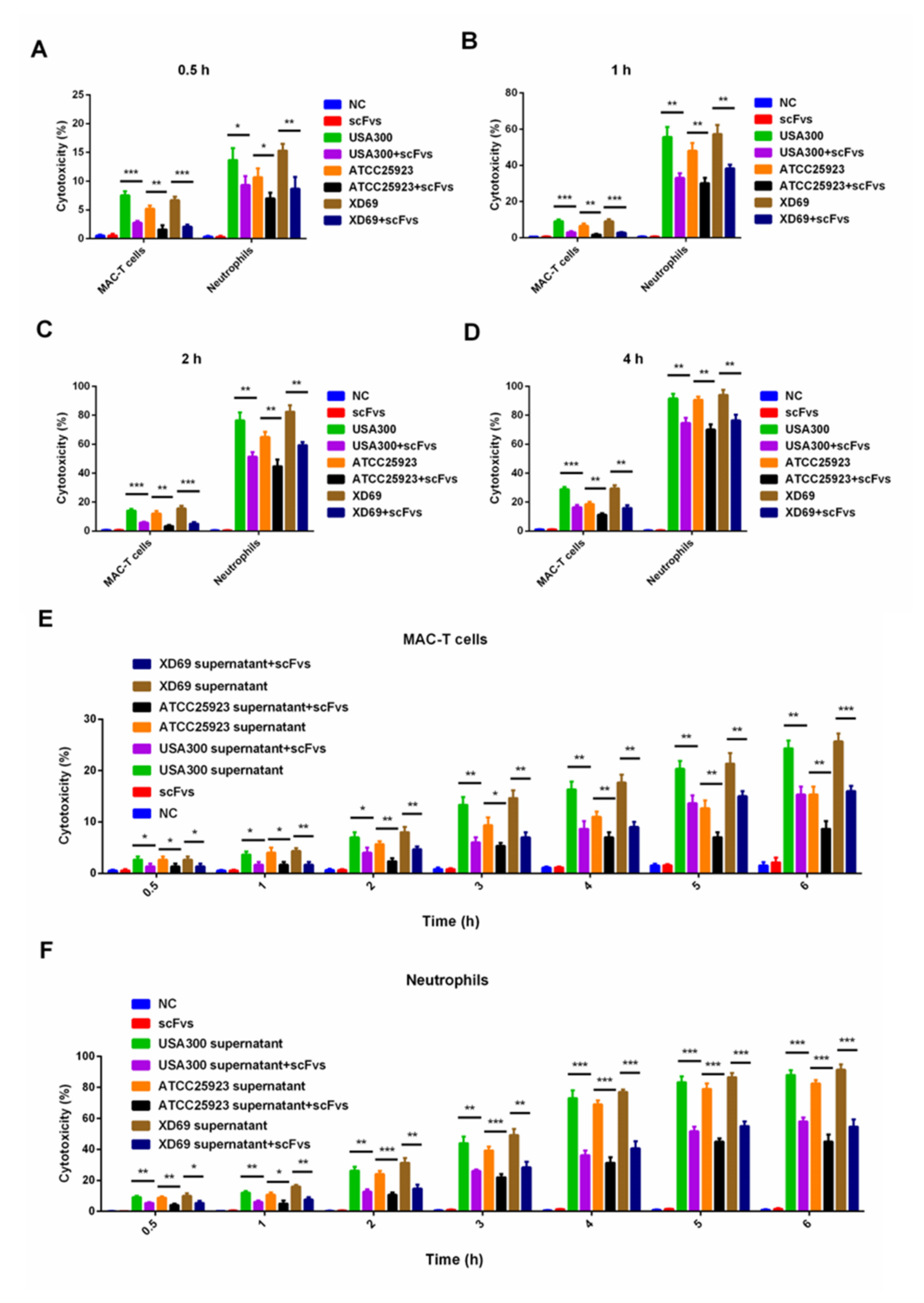
2.6. ScFv Can Weaken the Cytotoxicity of MAC-T Cells and Bovine Neutrophils Caused by S. aureus
2.7. ScFv Can Inhibit the S. aureus Proliferation In Vivo
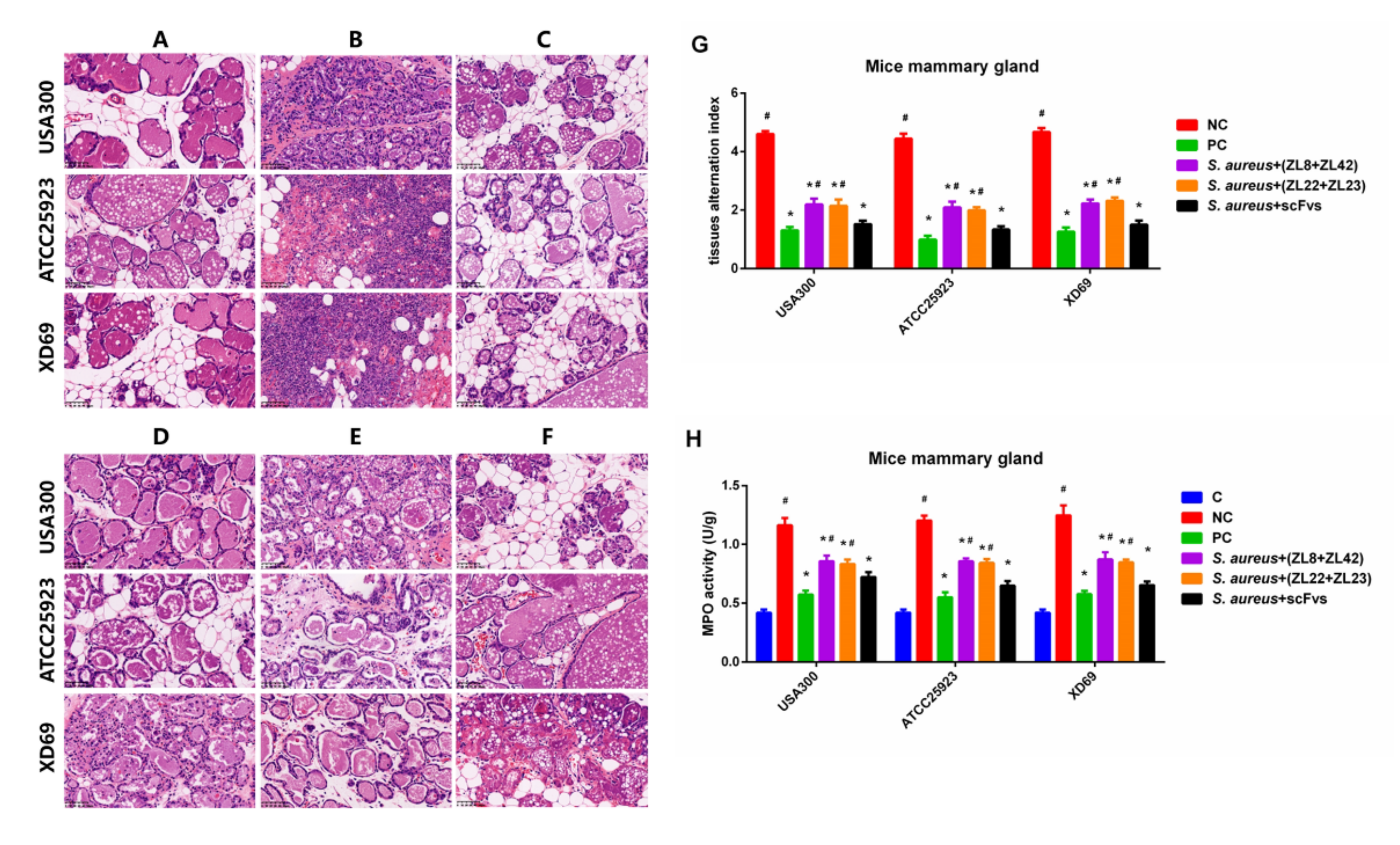
2.8. Histopathologic Changes and MPO Activity in Mammary Gland Tissues
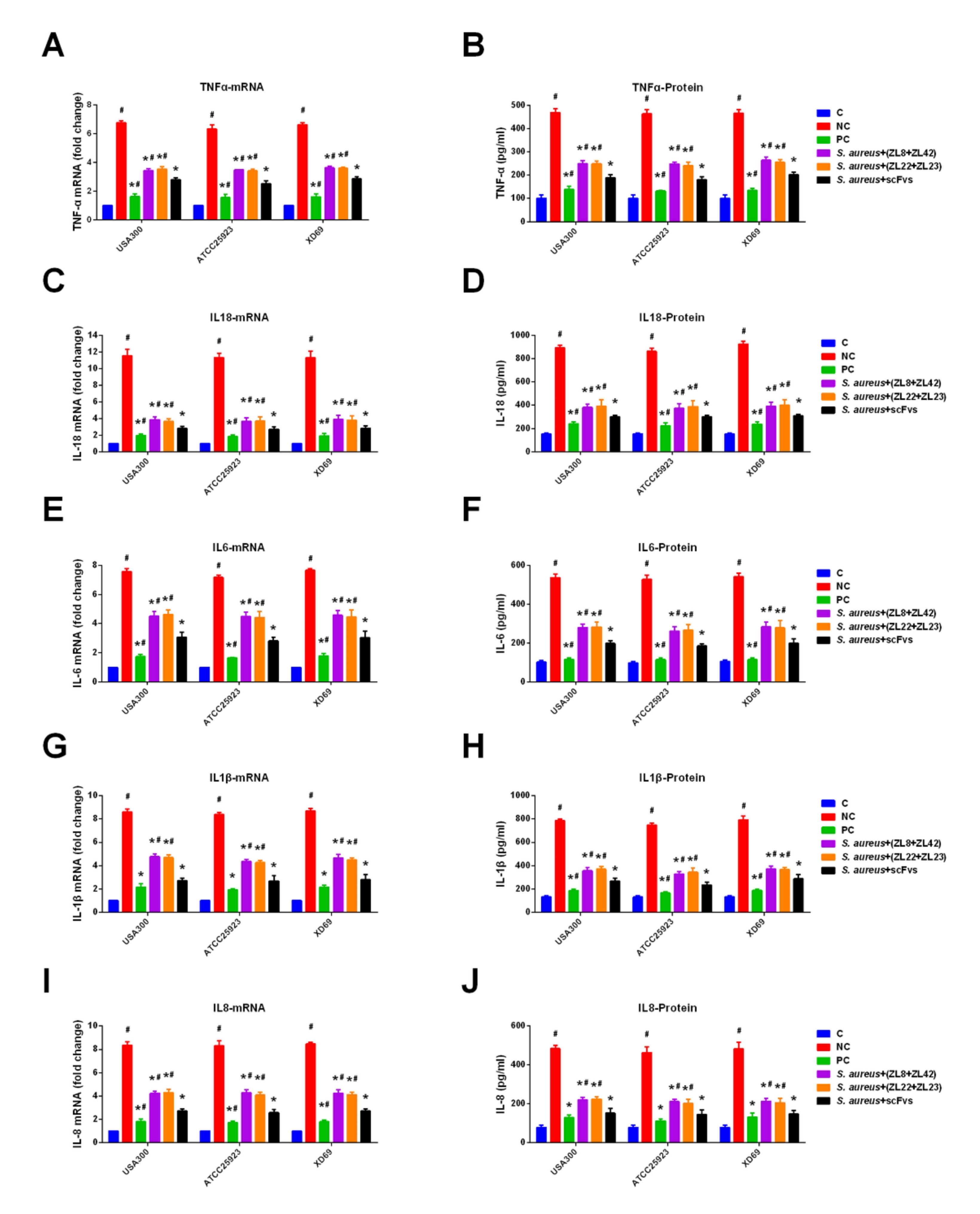
2.9. ScFvs Can Decrease the Production of Inflammatory Cytokine
3. Discussion
4. Materials and Methods
4.1. S. aureus Strains, Plasmid and Cell Culture
4.2. Protein Preparation of LukED
4.3. Affinity Selection of ScFvs against LukE and LukD
4.4. Protein Purification of ScFvs Targeting LukE and LukD
4.5. Plasmid Construction of Bovine Chemokine Receptor
4.6. Cell Transfection
4.7. Cell Viability Assay
4.8. Leukocyte Isolation
4.9. LDH Cytotoxicity Assay
4.10. Animal Experiment
4.11. Bacterial Proliferation of Mammary Gland Tissues
4.12. Histopathological Evaluation of Mammary Gland Tissues
4.13. MPO Activity Assay
4.14. ELISA Assay
4.15. qPCR Assay
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited Review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef] [Green Version]
- Burović, J. Isolation of bovine clinical mastitis bacterial pathogens and their antimicrobial susceptibility in the Zenica region in 2017. godini. Vet. Stanica 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Cvetni, L.; Samardija, M.; Duvnjak, S.; Habrun, B.; Beni, M. Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms 2021, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Maei, N.; Mazi, M.; Cvetni, M.; Butkovi, I.; Kneevi, K. Use of somatic cell count in the diagnosis of mastitis and its impacts on milk quality. Vet. Stanica 2021, 52, 751–764. [Google Scholar] [CrossRef]
- Alonzo, F., 3rd; Benson, M.A.; Chen, J.; Novick, R.P.; Shopsin, B.; Torres, V.J. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol. Microbiol. 2012, 83, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Abril, A.G.; Villa, T.G.; Barros-Velazquez, J.; Canas, B.; Sanchez-Perez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Saidi, R.; Kaidi, R.; Khelef, D.; Solmaz, H.; Ergun, Y.; Mimoune, N.; Cantekin, Z. Investigation of the presence of slime production, VanA gene and antiseptic resistance genes in Staphylococci isolated from bovine mastitis in Algeria. Vet. Stanica 2020, 52, 57–63. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Zhang, Y.; Wang, F.; Zhang, F.; Jia, Y.; Wu, D.; Tohti, K.; Cheng, M.; Zhu, J. Anti-Staphylococcus aureus Single-Chain Fragment Variables Play a Protective Anti-Inflammatory Role In Vitro and In Vivo. Vaccines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Herman-Bausier, P.; El-Kirat-Chatel, S.; Foster, T.J.; Geoghegan, J.A.; Dufrene, Y.F. Staphylococcus aureus Fibronectin-Binding Protein A Mediates Cell-Cell Adhesion through Low-Affinity Homophilic Bonds. mBio 2015, 6, e00413–e00415. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Benic, M.; Macesic, N.; Cvetnic, L.; Habrun, B.; Cvetnic, Z.; Turk, R.; Duricic, D.; Lojkic, M.; Dobranic, V.; Valpotic, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Vrieling, M.; Boerhout, E.M.; van Wigcheren, G.F.; Koymans, K.J.; Mols-Vorstermans, T.G.; de Haas, C.J.; Aerts, P.C.; Daemen, I.J.; van Kessel, K.P.; Koets, A.P.; et al. LukMF’ is the major secreted leukocidin of bovine Staphylococcus aureus and is produced in vivo during bovine mastitis. Sci. Rep. 2016, 6, 37759. [Google Scholar] [CrossRef]
- Hoekstra, J.; Rutten, V.; Sommeling, L.; van Werven, T.; Spaninks, M.; Duim, B.; Benedictus, L.; Koop, G. High Production of LukMF’ in Staphylococcus aureus Field Strains Is Associated with Clinical Bovine Mastitis. Toxins 2018, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Xu, S.; Huang, K.; Xu, X.; Hu, F.; He, C.; Shu, W.; Wang, Z.; Gong, F.; Zhang, C.; et al. Anti-staphylococcus Antibiotics Interfere with the Transcription of Leucocidin ED Gene in Staphylococcus aureus Strain Newman. Front. Microbiol. 2020, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Vrieling, M.; Koymans, K.J.; Heesterbeek, D.A.; Aerts, P.C.; Rutten, V.P.; de Haas, C.J.; van Kessel, K.P.; Koets, A.P.; Nijland, R.; van Strijp, J.A. Bovine Staphylococcus aureus Secretes the Leukocidin LukMF’ To Kill Migrating Neutrophils through CCR1. mBio 2015, 6, e00335. [Google Scholar] [CrossRef] [Green Version]
- Alonzo Iii, F.; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2012, 493, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Barrio, M.B.; Rainard, P.; Prevost, G. LukM/LukF’-PV is the most active Staphylococcus aureus leukotoxin on bovine neutrophils. Microbes Infect. 2006, 8, 2068–2074. [Google Scholar] [CrossRef]
- Lubkin, A.; Lee, W.L.; Alonzo, F.; Wang, C.; Aligo, J.; Keller, M.; Girgis, N.M.; Reyes-Robles, T.; Chan, R.; O’Malley, A.; et al. Staphylococcus aureus Leukocidins Target Endothelial DARC to Cause Lethality in Mice. Cell Host Microbe 2019, 25, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Naushad, S.; Nobrega, D.B.; Naqvi, S.A.; Barkema, H.W.; De Buck, J. Genomic Analysis of Bovine Staphylococcus aureus Isolates from Milk To Elucidate Diversity and Determine the Distributions of Antimicrobial and Virulence Genes and Their Association with Mastitis. mSystems 2020, 5, e00063-20. [Google Scholar] [CrossRef]
- Kreausukon, K.; Fetsch, A.; Kraushaar, B.; Alt, K.; Muller, K.; Kromker, V.; Zessin, K.H.; Kasbohrer, A.; Tenhagen, B.A. Prevalence, antimicrobial resistance, and molecular characterization of methicillin-resistant Staphylococcus aureus from bulk tank milk of dairy herds. J. Dairy Sci. 2012, 95, 4382–4388. [Google Scholar] [CrossRef] [Green Version]
- Lamari, I.; Mimoune, N.; Khelef, D. Effect of feed additive supplementation on bovine subclinical mastitis. Vet. Stanica 2021, 52, 445–460. [Google Scholar] [CrossRef]
- Mimoune, N.; Saidi, R.; Benadjel, O.; Khelef, D.; Kaidi, R. Alternative treatment of bovine mastitis. Vet. Stanica 2021, 52, 751–764. [Google Scholar] [CrossRef]
- Pereira, U.P.; Oliveira, D.G.; Mesquita, L.R.; Costa, G.M.; Pereira, L.J. Efficacy of Staphylococcus aureus vaccines for bovine mastitis: A systematic review. Vet. Microbiol. 2011, 148, 117–124. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shah, A.M.; Shah, A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine mastitis: An appraisal of its alternative herbal cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhu, J. Anti-Staphylococcus aureus single-chain variable region fragments provide protection against mastitis in mice. Appl. Microbiol. Biotechnol. 2016, 100, 2153–2162. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, X.; Zhang, M.; Yin, H.; Jiang, K.; Wu, H.; Dai, A.; Yang, S. Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-kappaB signaling pathway. Inflamm. Res. 2018, 67, 903–911. [Google Scholar] [CrossRef]
- Ascione, A.; Capecchi, B.; Campitelli, L.; Imperiale, V.; Flego, M.; Zamboni, S.; Gellini, M.; Alberini, I.; Pittiglio, E.; Donatelli, I.; et al. Human monoclonal antibodies in single chain fragment variable format with potent neutralization activity against influenza virus H5N1. Antivir. Res. 2009, 83, 238–244. [Google Scholar] [CrossRef]
- Guo, X.; Cao, H.; Wang, Y.; Liu, Y.; Chen, Y.; Wang, N.; Jiang, S.; Zhang, S.; Wu, Q.; Li, T.; et al. Screening scFv antibodies against infectious bursal disease virus by co-expression of antigen and antibody in the bacteria display system. Vet. Immunol. Immunopathol. 2016, 180, 45–52. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Ke, Y.; Zhang, L.; Zhang, B.; Yang, L.; Zhu, J. Single Chain Fragment Variable (scFv) Antibodies Targeting the Spike Protein of Porcine Epidemic Diarrhea Virus Provide Protection against Viral Infection in Piglets. Viruses 2019, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Hammers, C.M.; Stanley, J.R. Antibody phage display: Technique and applications. J. Invest. Derm. 2014, 134, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef]
- Sompunga, P.; Pruksametanan, N.; Rangnoi, K.; Choowongkomon, K.; Yamabhai, M. Generation of human and rabbit recombinant antibodies for the detection of Zearalenone by phage display antibody technology. Talanta 2019, 201, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Y.; He, J.; Wang, H.; Luo, Y.; Li, Y.; Liu, J.; Zhong, L.; Zhao, Y. PEGylated immunoliposome-loaded endoglin single-chain antibody enhances anti-tumor capacity of porcine alpha1,3GT gene. Biomaterials 2019, 217, 119231. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.; O’Reilly, A.; Charleton, M.; Kane, M. Development of a high-affinity anti-domoic acid sheep scFv and its use in detection of the toxin in shellfish. Anal. Chem. 2008, 80, 3205–3212. [Google Scholar] [CrossRef]
- Ge, S.; Xu, L.; Li, B.; Zhong, F.; Liu, X.; Zhang, X. Canine Parvovirus is diagnosed and neutralized by chicken IgY-scFv generated against the virus capsid protein. Vet. Res. 2020, 51, 110. [Google Scholar] [CrossRef]
- Kasprzak, A.; Szmyt, M.; Malkowski, W.; Przybyszewska, W.; Helak-Lapaj, C.; Seraszek-Jaros, A.; Surdacka, A.; Malkowska-Lanzafame, A.; Kaczmarek, E. Analysis of immunohistochemical expression of proinflammatory cytokines (IL-1alpha, IL-6, and TNF-alpha) in gallbladder mucosa: Comparative study in acute and chronic calculous cholecystitis. Folia Morphol. 2015, 74, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, D.; Brouillette, E.; Jaspar, F.; Malouin, F.; Mainil, J.; Bureau, F.; Lekeux, P. Helenalin reduces Staphylococcus aureus infection in vitro and in vivo. Vet. Microbiol. 2007, 119, 330–338. [Google Scholar] [CrossRef]
- Schmiedl, A.; Breitling, F.; Dubel, S. Expression of a bispecific dsFv-dsFv’ antibody fragment in Escherichia coli. Protein Eng. 2000, 13, 725–734. [Google Scholar] [CrossRef]
- Koti, M.; Farrugia, W.; Nagy, E.; Ramsland, P.A.; Kaushik, A.K. Construction of single-chain Fv with two possible CDR3H conformations but similar inter-molecular forces that neutralize bovine herpesvirus 1. Mol. Immunol. 2010, 47, 953–960. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Guan, Y.; Wang, F.; Zhu, J. The preparation and therapeutic roles of scFv-Fc antibody against Staphylococcus aureus infection to control bovine mastitis. Appl. Microbiol. Biotechnol. 2019, 103, 1703–1712. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Guo, S.; Xue, X.; Wang, Y.; Qiu, S.; Cui, J.; Ma, L.; Zhang, X.; Wang, J. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 2020, 20, 587. [Google Scholar] [CrossRef]
- De Haas, C.J.; Veldkamp, K.E.; Peschel, A.; Weerkamp, F.; Van Wamel, W.J.; Heezius, E.C.; Poppelier, M.J.; Van Kessel, K.P.; van Strijp, J.A. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004, 199, 687–695. [Google Scholar] [CrossRef]
- Gao, W.; Yang, J.; Liu, W.; Wang, Y.; Shao, F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E4857–E4866. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, B.; Wang, D.; Wang, L.; Deng, X.; Bi, C.; Xiong, Y.; Wu, Q.; Cui, Y.; Zhang, Y.; et al. Role of sortase A in the pathogenesis of Staphylococcus aureus-induced mastitis in mice. FEMS Microbiol. Lett. 2014, 351, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Tuchscherr, L.P.; Buzzola, F.R.; Alvarez, L.P.; Caccuri, R.L.; Lee, J.C.; Sordelli, D.O. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 2005, 73, 7932–7937. [Google Scholar] [CrossRef] [Green Version]
- Ge, B.J.; Zhao, P.; Li, H.T.; Sang, R.; Wang, M.; Zhou, H.Y.; Zhang, X.M. Taraxacum mongolicum protects against Staphylococcus aureus-infected mastitis by exerting anti-inflammatory role via TLR2-NF-kappaB/MAPKs pathways in mice. J. Ethnopharmacol. 2021, 268, 113595. [Google Scholar] [CrossRef]
- Shao, G.; Tian, Y.; Wang, H.; Liu, F.; Xie, G. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2015, 29, 263–268. [Google Scholar] [CrossRef]
- Guo, M.Y.; Li, W.Y.; Zhang, Z.; Qiu, C.; Li, C.; Deng, G. Betulin suppresses S. aureus-induced mammary gland inflammatory injury by regulating PPAR-gamma in mice. Int. Immunopharmacol. 2015, 29, 824–831. [Google Scholar] [CrossRef]
- Li, M.; Guo, X.; Qi, W.; Wu, Z.; de Bruijn, J.D.; Xiao, Y.; Bao, C.; Yuan, H. Macrophage polarization plays roles in bone formation instructed by calcium phosphate ceramics. J. Mater. Chem. B 2020, 8, 1863–1877. [Google Scholar] [CrossRef]
- Liu, H.; French, B.A.; Nelson, T.J.; Li, J.; Tillman, B.; French, S.W. IL-8 signaling is up-regulated in alcoholic hepatitis and DDC fed mice with Mallory Denk Bodies (MDBs) present. Exp. Mol. Pathol. 2015, 99, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yin, Y.; Wang, M.; Fan, T.; Zhu, Y.; Shen, L.; Peng, S.; Gao, J.; Deng, G.; Meng, X.; et al. Disrupting phosphatase SHP2 in macrophages protects mice from high-fat diet-induced hepatic steatosis and insulin resistance by elevating IL-18 levels. J. Biol. Chem. 2020, 295, 10842–10856. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Sakwinska, O.; Giddey, M.; Moreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus host range and human-bovine host shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Robles, T.; Alonzo, F., 3rd; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Pena, J.L.; Goncalves Schwarz, D.G.; Willian de Lima Brasil, A.; Licursi de Oliveira, L.; Albuquerque Caldeira, J.L.; Scatamburlo Moreira, M.A. Differences in the coinfective process of Staphylococcus aureus and Streptococcus agalactiae in bovine mammary epithelial cells infected by Mycobacterium avium subsp. paratuberculosis. Microb. Pathog. 2020, 149, 104476. [Google Scholar] [CrossRef]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-alpha expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Jiang, A.; Zhang, Y.; Zhang, X.; Wu, D.; Liu, Z.; Li, S.; Liu, X.; Han, Z.; Wang, C.; Wang, J.; et al. Morin alleviates LPS-induced mastitis by inhibiting the PI3K/AKT, MAPK, NF-kappaB and NLRP3 signaling pathway and protecting the integrity of blood-milk barrier. Int. Immunopharmacol. 2020, 78, 105972. [Google Scholar] [CrossRef]
- Kaplanski, G.; Marin, V.; Montero-Julian, F.; Mantovani, A.; Farnarier, C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003, 24, 25–29. [Google Scholar] [CrossRef]
- Verdrengh, M.; Thomas, J.A.; Hultgren, O.H. IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect. 2004, 6, 1268–1272. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-alpha, IL-1beta and IL-6) via TLRs following NF-kappaB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Kumar, A.; Chakraborty, S.; Verma, A.K.; Tiwari, R.; Dhama, K.; Singh, U.; Kumar, S. Trends in diagnosis and control of bovine mastitis: A review. Pak. J. Biol. Sci. 2013, 16, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.N.; Valand, P.; Nauriyal, D.S.; Joshi, C.G. Immunomodulation of IL-1, IL-6 and IL-8 cytokines by Prosopis juliflora alkaloids during bovine sub-clinical mastitis. 3 Biotech. 2018, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, X.; Xu, J.; Li, S.; Wang, S.; Zhu, Y.; Wang, J. Antimicrobial Effect of Zophobas morio Hemolymph against Bovine Mastitis Pathogens. Microorganisms 2020, 8, 1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Song, X.; Zhang, Z.; Guo, M.; Jiang, H.; Wang, W.; Cao, Y.; Zhu, L.; Zhang, N. Stevioside inhibits inflammation and apoptosis by regulating TLR2 and TLR2-related proteins in S. aureus-infected mouse mammary epithelial cells. Int. Immunopharmacol. 2014, 22, 192–199. [Google Scholar] [CrossRef]


| Round of Screening | Input (cfu/mL) | Output (cfu/mL) | Output /Input | Enrichment Fold | Total Enrichment Fold |
|---|---|---|---|---|---|
| 1 | 1.00 × 1013 | 1.65 × 106 | 1.65 × 10−7 | 1 | |
| 2 | 1.00 × 1013 | 2.77 × 106 | 2.77 × 10−7 | 1.68 | |
| 3 | 1.00 × 1013 | 8.67 × 106 | 8.67 × 10−7 | 3.13 | |
| 4 | 1.00 × 1013 | 6.25 × 107 | 6.25 × 10−6 | 7.21 | |
| 5 | 1.00 × 1013 | 2.14 × 108 | 2.14 × 10−5 | 3.42 | 129.70 |
| Round of Screening | Input (cfu/mL) | Output (cfu/mL) | Output /Input | Enrichment Fold | Total Enrichment Fold |
|---|---|---|---|---|---|
| 1 | 1.00 × 1013 | 4.25 × 105 | 4.25 × 10−8 | 1 | |
| 2 | 1.00 × 1013 | 1.32 × 106 | 1.32 × 10−7 | 3.11 | |
| 3 | 1.00 × 1013 | 4.24 × 106 | 4.24 × 10−7 | 3.21 | |
| 4 | 1.00 × 1013 | 6.06 × 107 | 6.06 × 10−6 | 14.29 | |
| 5 | 1.00 × 1013 | 1.25 × 108 | 1.25 × 10−5 | 2.06 | 294.12 |
| Primer Name | Sequence (5′-3′) | Size (bp) | Reference |
|---|---|---|---|
| LukE-F | CCCGGATCCAATACTAATATTGAAAATATTGGTGATG | 849 | This study |
| LukE-R | CCGCTCGAGTTAATTATGTCCTTTCACTTTAATTTCG | ||
| LukD-F | CGCGGATCCGCTCAACATATCACACCTGTAAGC | 906 | This study |
| LukD-R | CCGCTCGAGTTATACTCCAGGATTAGTTTC |
| Primer | Sequence (5′-3′) |
|---|---|
| CCR5-F | CCGCTCGAGGCCACCATGGATTATCAAACATCAACTCCCCTCTA |
| CCR5-R | CCCAAGCTTCAAGCCAACAGAGATTTCCTGTTCTC |
| CXCR1-F | CCGCTCGAGGCCACCATGGTTGGTGACTCAGTCTTTCAACC |
| CXCR1-R | CCCAAGCTTGAGGGTAGTAGACGTGTTCCCTGAA |
| CXCR2-F | CCGCTCGAGGCCACCATGGCTGAAACAAAATTTACTTCAAATATAGAAGGA |
| CXCR2-R | CCCAAGCTTGAGGGTAGTAGACGTGTTCCCTGA |
| Primer Name | Sequence (5′-3′) | Size (bp) | Reference |
|---|---|---|---|
| IL-18-F | TGGTTCCATGCTTTCTGGACTCCT | 132 | [8] |
| IL-18-R | TTCCTGGGCCAAGAGGAAGTGATT | ||
| TNF-α-F | GCCTCCCTCTCATCAGTCTA | 223 | [8] |
| TNF-α-R | GGCAGCCTTGTCCCTG | ||
| IL-6-F | AGTTGTGCATGGCAATTCTGA | 213 | [8] |
| IL-6-R | AGGACTCTGGCTTGTCTTTCT | ||
| IL-1β-F | ACCTGTGTCTTCCCGTGG | 171 | [8] |
| IL-1β-R | TCATCTCGAGCCTGTAGTG | ||
| IL-8-F | CGGCAATGAAGCTTCTGTAT | 224 | [8] |
| IL-8-R | CCTTGAAACTCTTTGCCTCA | ||
| GAPDH-F | CAATGTGTCCGTCGTGGATCT | 124 | [8] |
| GAPDH-R | GTCCTCAGTGTAGCCCAAGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ye, X.; Jia, Y.; Cheng, M.; Wu, D.; Tohti, K.; Zhu, J. Single-Chain Fragment Variables Targeting Leukocidin ED Can Alleviate the Inflammation of Staphylococcus aureus-Induced Mastitis in Mice. Int. J. Mol. Sci. 2022, 23, 334. https://doi.org/10.3390/ijms23010334
Zhang L, Ye X, Jia Y, Cheng M, Wu D, Tohti K, Zhu J. Single-Chain Fragment Variables Targeting Leukocidin ED Can Alleviate the Inflammation of Staphylococcus aureus-Induced Mastitis in Mice. International Journal of Molecular Sciences. 2022; 23(1):334. https://doi.org/10.3390/ijms23010334
Chicago/Turabian StyleZhang, Lei, Xin Ye, Yan Jia, Manling Cheng, Dangjin Wu, Kalbinur Tohti, and Jianguo Zhu. 2022. "Single-Chain Fragment Variables Targeting Leukocidin ED Can Alleviate the Inflammation of Staphylococcus aureus-Induced Mastitis in Mice" International Journal of Molecular Sciences 23, no. 1: 334. https://doi.org/10.3390/ijms23010334
APA StyleZhang, L., Ye, X., Jia, Y., Cheng, M., Wu, D., Tohti, K., & Zhu, J. (2022). Single-Chain Fragment Variables Targeting Leukocidin ED Can Alleviate the Inflammation of Staphylococcus aureus-Induced Mastitis in Mice. International Journal of Molecular Sciences, 23(1), 334. https://doi.org/10.3390/ijms23010334






