TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII Using Electroacupuncture Treatment for Chronic Pain and Depression Comorbidity in a Murine Model
Abstract
1. Introduction
2. Results
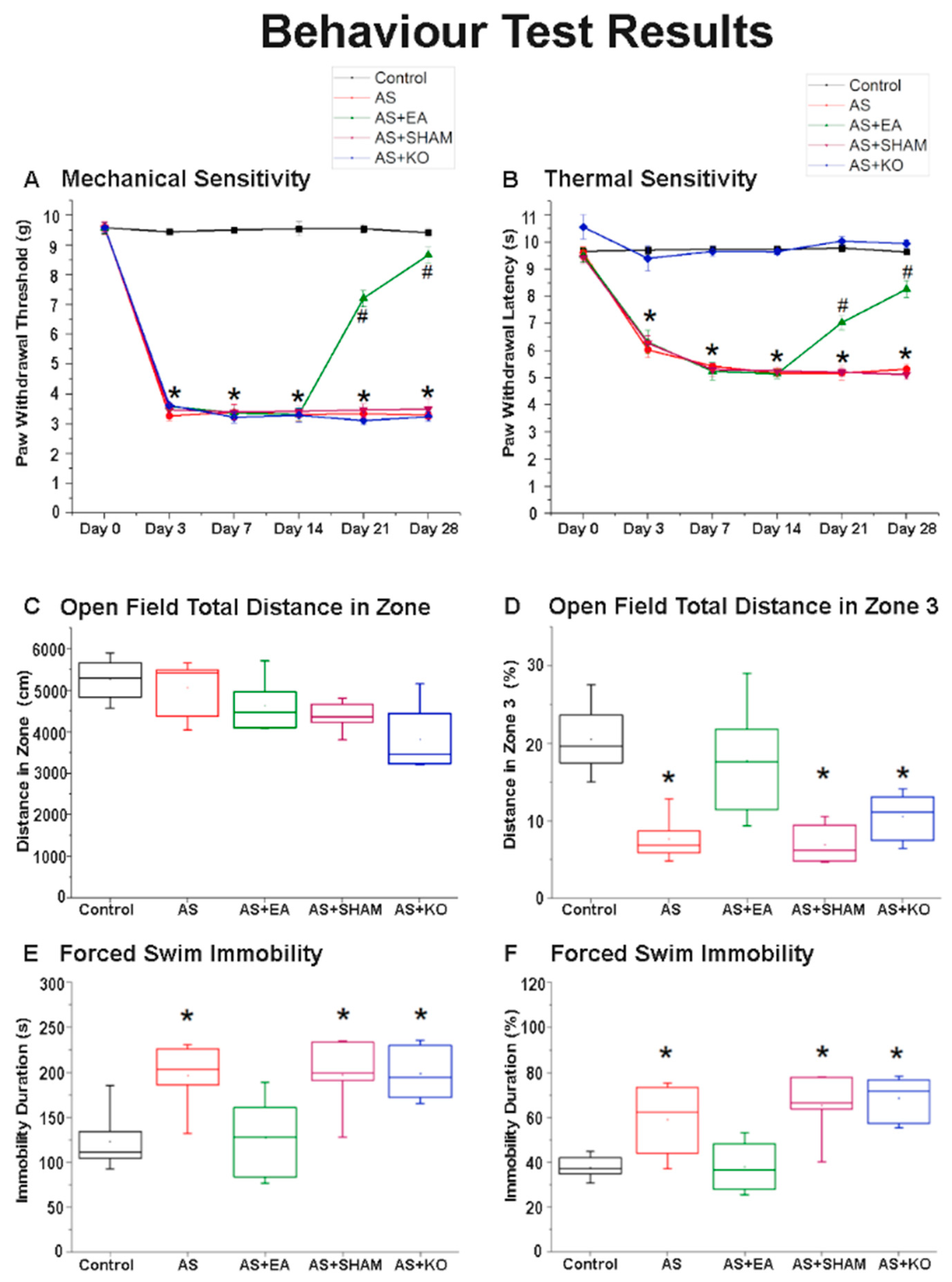
2.1. The Effect of EA Treatment on Mechanical and Thermal Nociceptive Behavioral Responses
2.2. The Effect of EA Treatment on Depression-Like Behavior in Open Field Testing (OFT) and Forced Swimming Test (FST) Behavioral Responses
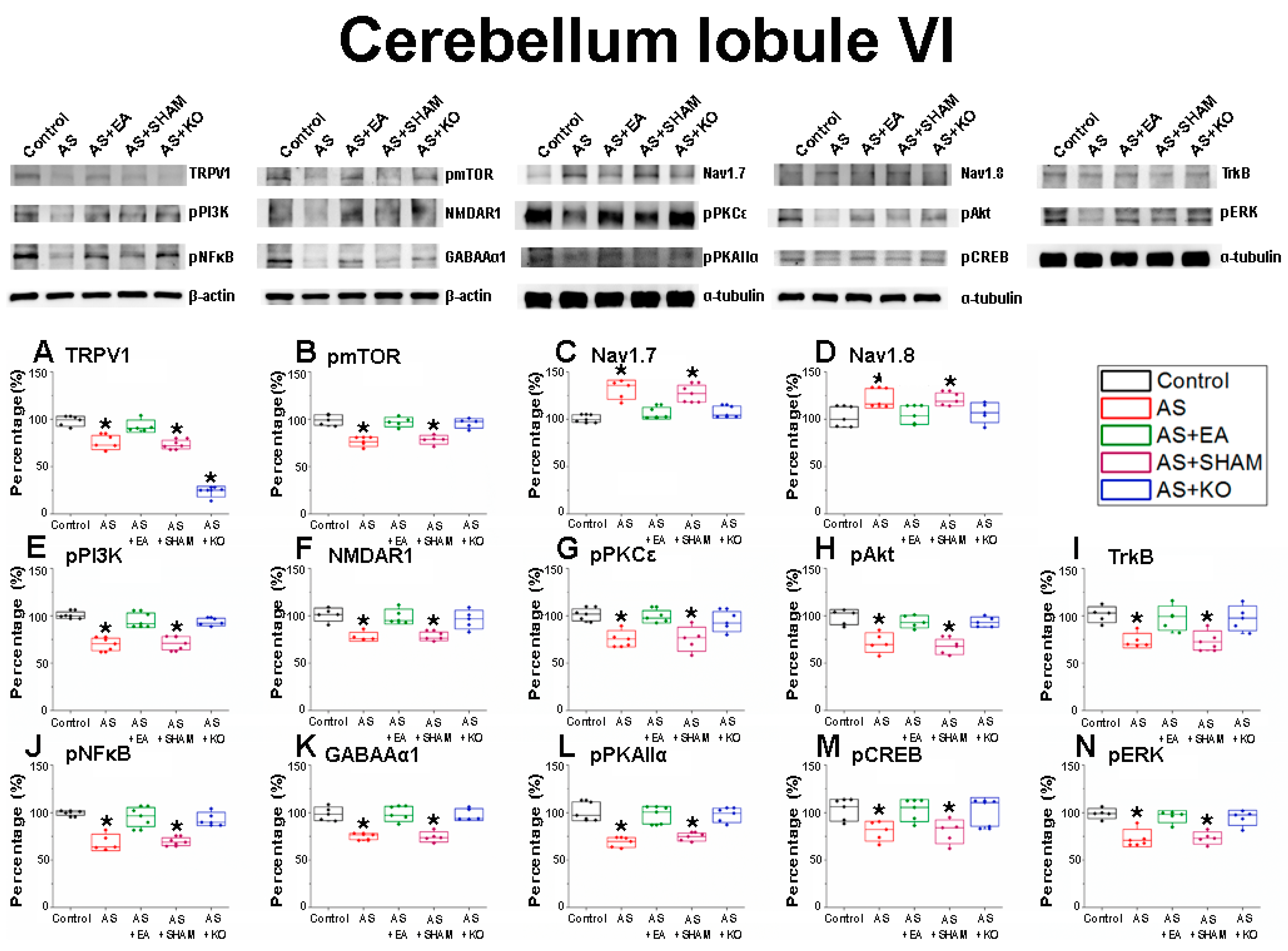
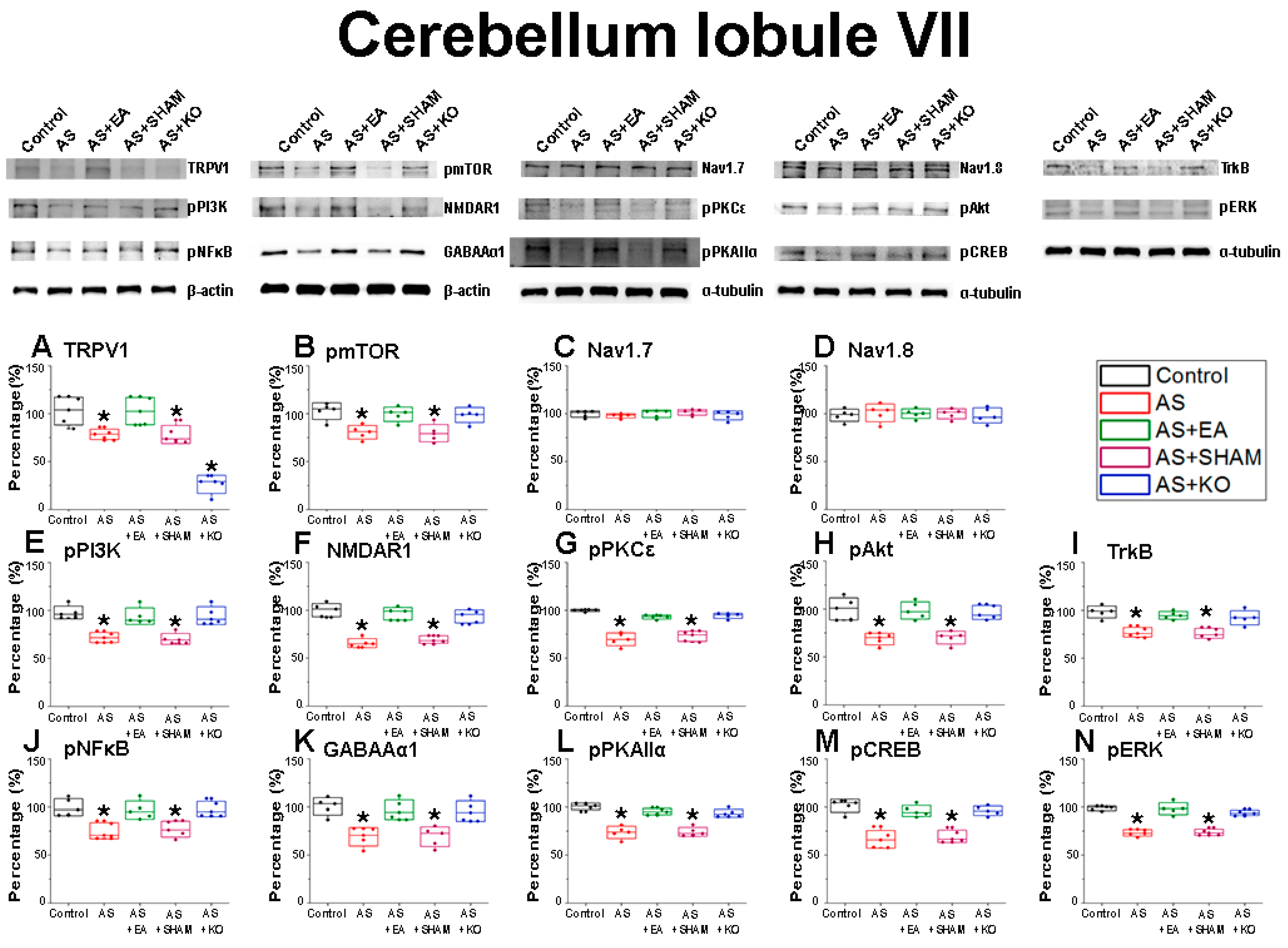
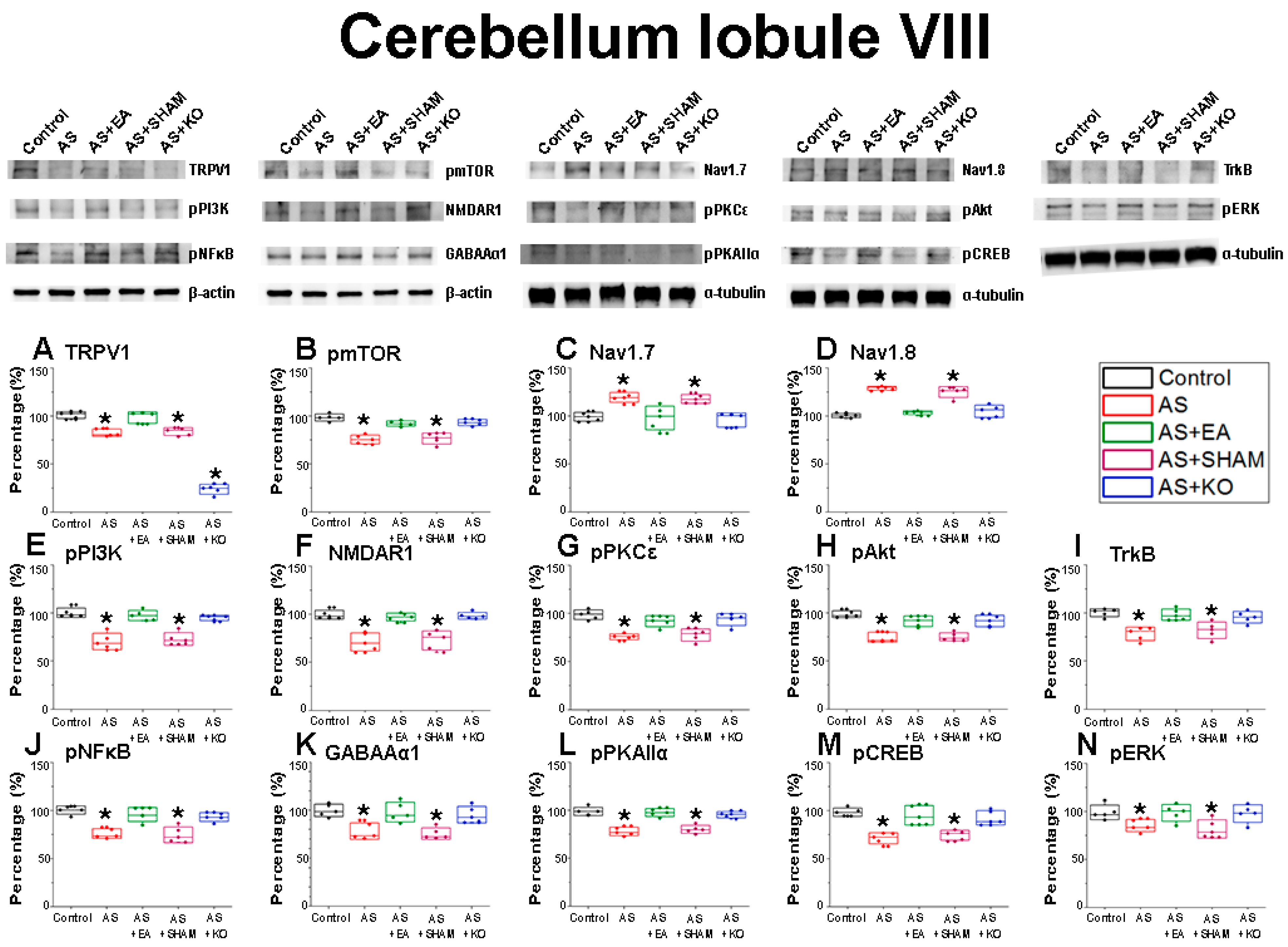
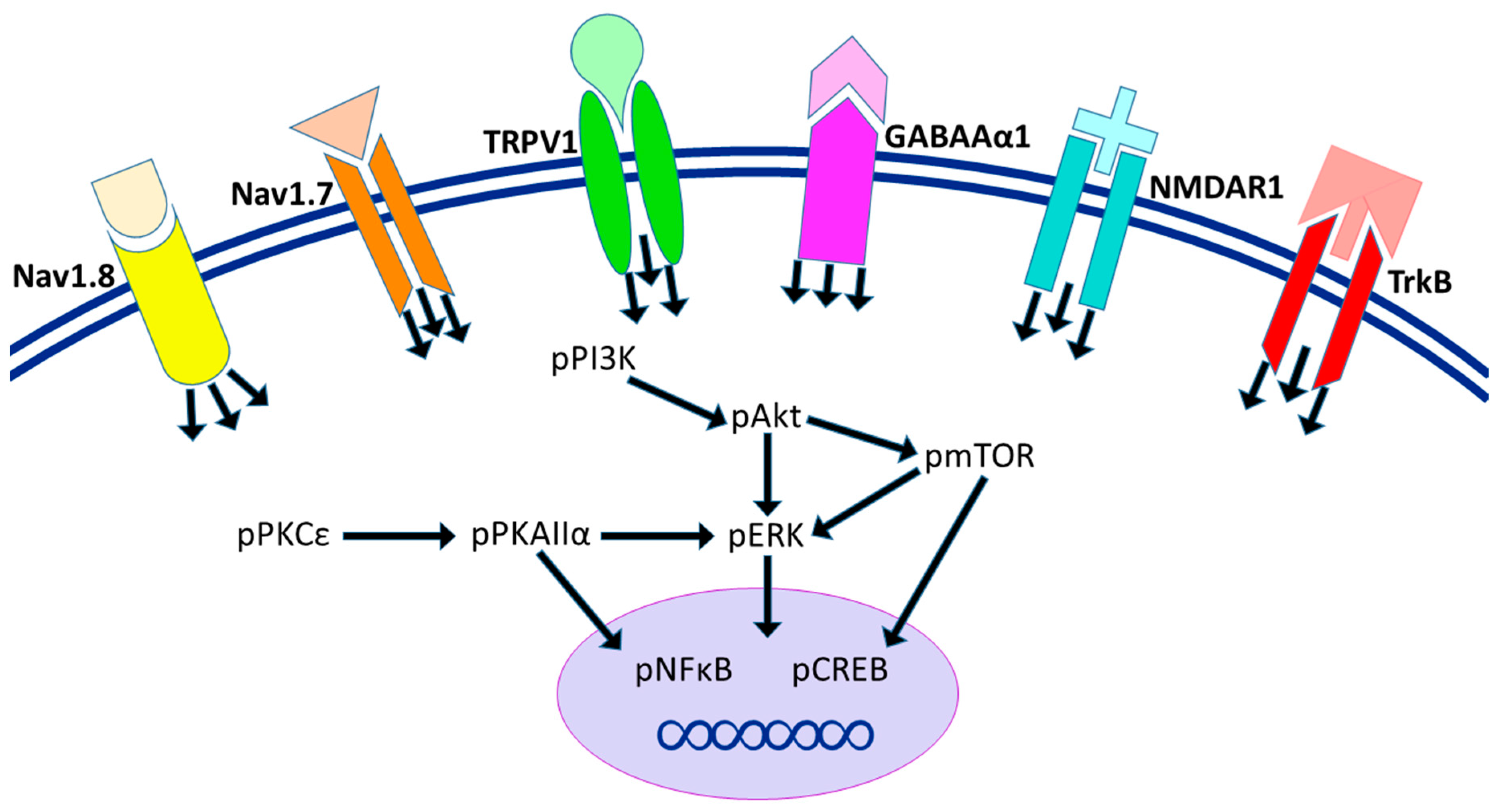
2.3. The Effect of EA Treatment on CPDC Associated Pathways and Related Molecules in the Cerebellum Lobules VI, VII, and VIII
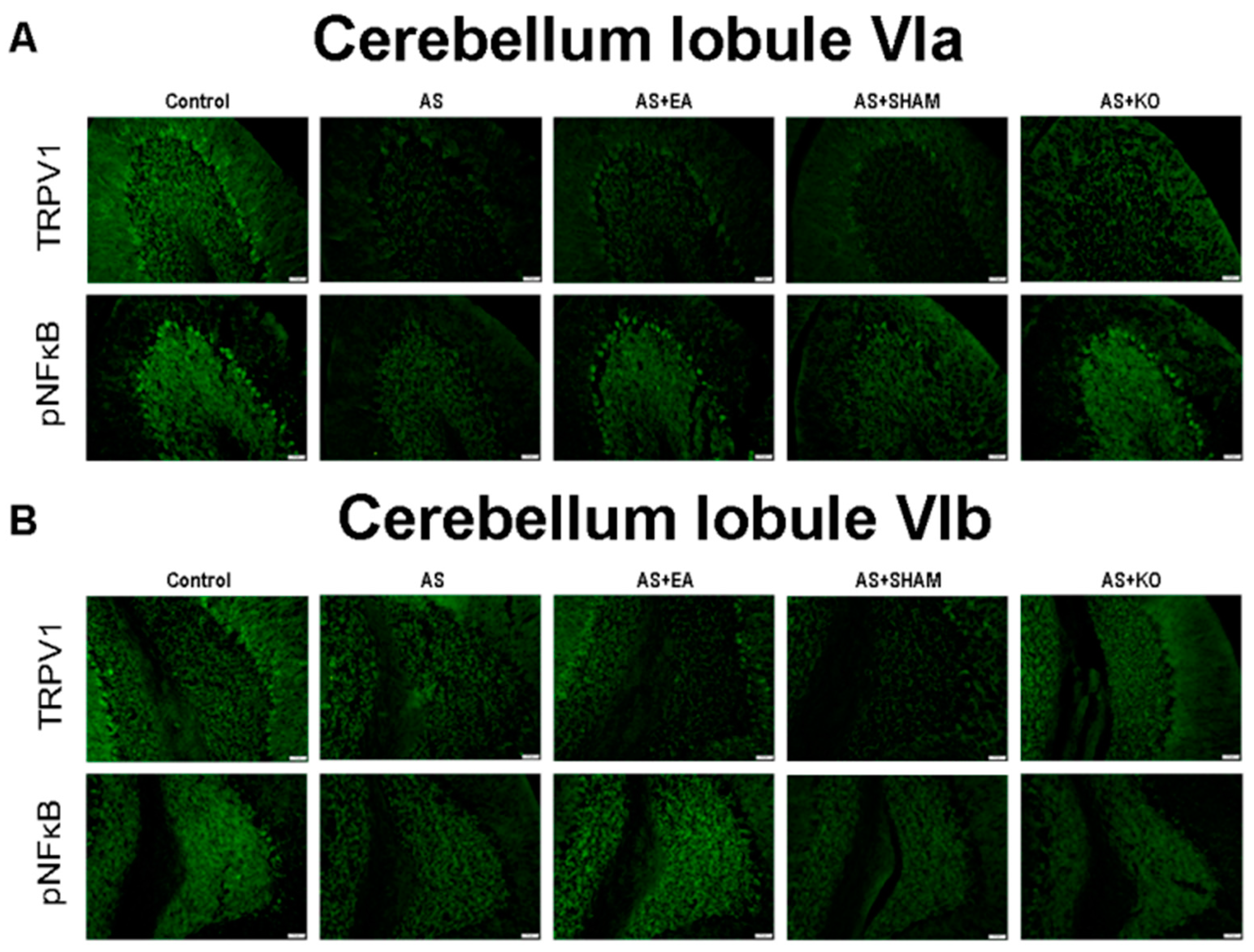
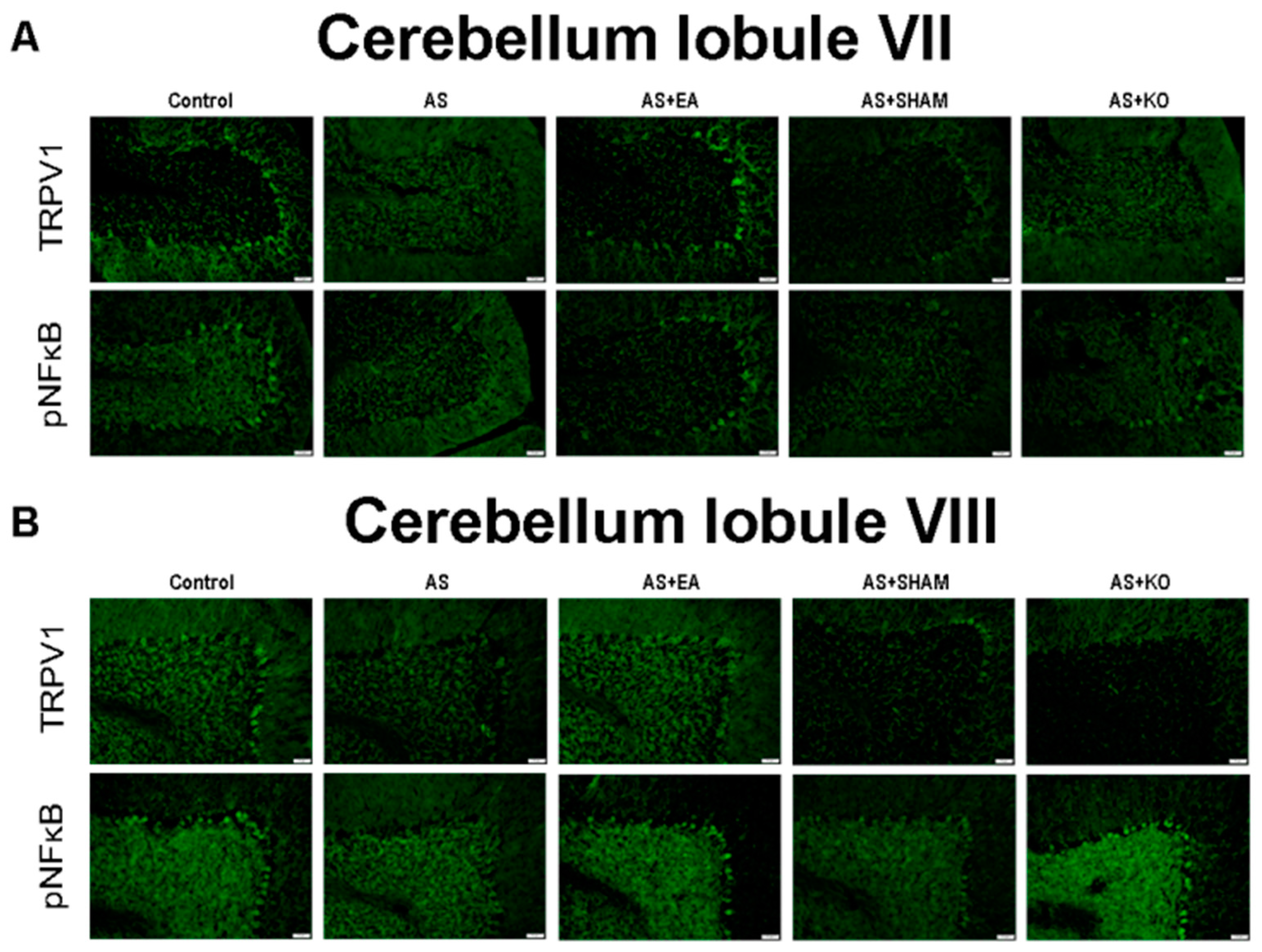
2.4. The Effect of EA Treatment on Protein Expression Alteration in Cerebellum Lobules VI, VII, and VIII as Observed by Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. AS Model
4.3. EA Treatment
4.4. Behavioral Examination
4.4.1. Nociceptive Behavioral Examination
4.4.2. Depression Behavioral Examination
4.5. Immunoblotting
4.6. Immunofluorescence
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, C.; Pae, C.-U. Pain and Depression: A Neurobiological Perspective of Their Relationship. Psychiatry Investig. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and inju-ries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Stein, D.J.; Phillips, K.A.; Bolton, D.; Fulford, K.W.M.; Sadler, J.Z.; Kendler, K.S. What is a mental/psychiatric disorder? From DSM-IV to DSM-V. Psychol. Med. 2010, 40, 1759–1765. [Google Scholar] [CrossRef]
- Satyanarayanan, S.K.; Shih, Y.-H.; Wen, Y.-R.; Palani, M.; Lin, Y.-W.; Su, H.; Gałecki, P.; Su, K.-P. miR-200a-3p modulates gene expression in comorbid pain and depression: Molecular implication for central sensitization. Brain Behav. Immun. 2019, 82, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; DeSmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, M.V.; Moroz, O.F.; Zholos, A.V. Multifunctional TRPV1 Ion Channels in Physiology and Pathology with Focus on the Brain, Vasculature, and Some Visceral Systems. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Peng, Y.-Y.; Peng, B.-W. Modulation of neuroinflammation: Role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav. Immun. 2017, 64, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Chou, A.I.W.; Su, H.; Su, K.P. Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and de-pression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids. Brain Behav. Immun. 2020, 89, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Riediger, C.; Schuster, T.; Barlinn, K.; Maier, S.; Weitz, J.; Siepmann, T. Adverse Effects of Antidepressants for Chronic Pain: A Systematic Review and Meta-analysis. Front. Neurol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.S. Medication Overuse in Chronic Pain. Curr. Pain Headache Rep. 2017, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Predictable, S.; Laurencic, G.; Malone, D. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351. [Google Scholar]
- Read, J.; Williams, J. Adverse effects of antidepressants reported by a large international cohort: Emotional blunting, sui-cidality, and withdrawal effects. Curr. Drug Saf. 2018, 13, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Cronin, A.M.; Maschino, A.C.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Witt, C.M.; Linde, K. Acupuncture for chronic pain: Individual patient data meta-analysis. Arch. Intern. Med. 2012, 172, 1444–1453. [Google Scholar] [CrossRef]
- Tu, C.-H.; Macdonald, I.; Chen, Y.-H. The Effects of Acupuncture on Glutamatergic Neurotransmission in Depression, Anxiety, Schizophrenia, and Alzheimer’s Disease: A Review of the Literature. Front. Psychiatry 2019, 10, 14. [Google Scholar] [CrossRef]
- Smith, C.A.; Hay, P.P.; MacPherson, H. Acupuncture for depression. Cochrane Database Syst. Rev. 2010, 2018, CD004046. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Kavelaars, A.; Heijnen, C.J.; Dantzer, R. Neuroinflammation and Comorbidity of Pain and Depression. Pharmacol. Rev. 2014, 66, 80–101. [Google Scholar] [CrossRef]
- Coutaux, A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Jt. Bone Spine 2017, 84, 657–661. [Google Scholar] [CrossRef]
- Napadow, V.; Makris, N.; Liu, J.; Kettner, N.W.; Kwong, K.K.; Hui, K.K. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 2004, 24, 193–205. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, H.; Wu, Z.; He, Q.; Liu, J.; Xu, Y.; Yao, S.; He, X.; Chen, Y.; Liang, Y.; et al. Electroacupuncture alleviates chronic pain-induced anxiety disorders by regulating the rACC-thalamus circuitry. Front. Neurosci. 2020. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, S.; Shao, F.; Fang, J.; Xu, Y.; Wang, W.; Sun, H.; Liu, X.; Du, J.; Fang, J. Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression. Int. J. Mol. Sci. 2019, 20, 3248. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, W.H.; Hsieh, C.L.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechano-sensitive TRPV1 as an “acupuncture-responding channel”. BMC Complementary Altern. Med. 2014, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moulton, E.A.; Schmahmann, J.D.; Becerra, L.; Borsook, D. The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev. 2010, 65, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Bingel, U.; Quante, M.; Knab, R.; Bromm, B.; Weiller, C.; Büchel, C. Subcortical structures involved in pain processing: Evidence from single-trial fMRI. Pain 2002, 99, 313–321. [Google Scholar] [CrossRef]
- Gary, Z.Y.; Ly, M.; Karim, H.T.; Muppidi, N.; Aizenstein, H.J.; Ibinson, J.W. Accelerated brain aging in chronic low back pain. Brain Res. 2021, 1755, 147263. [Google Scholar]
- Meier, S.K.; Ray, K.L.; Waller, N.C.; Gendron, B.C.; Aytur, S.A.; Robin, D.A. Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain. Brain Sci 2020, 11, 10. [Google Scholar] [CrossRef]
- Buckner, R.L. The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Depping, M.S.; Schmitgen, M.M.; Bach, C.; Listunova, L.; Kienzle, J.; Kubera, K.M.; Roesch-Ely, D.; Wolf, R.C. Abnormal cerebellar volume in patients with remitted major depression with persistent cognitive deficits. Cerebellum 2020, 19, 762–770. [Google Scholar] [CrossRef]
- Sluka, K.; Kalra, A.; Moore, S. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyper-algesia. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2001, 24, 37–46. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Z.; Wu, L.; Zhou, J. Co-occurrence of chronic pain, depressive symptoms, and poor sleep quality in a health check-up population in China:A multicenter survey. J. Affect. Disord. 2021, 281, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Trøstheim, M.; Eikemo, M.; Ernst, G.; Leknes, S. Anhedonia in chronic pain and prescription opioid misuse. Psychol. Med. 2020, 50, 1977–1988. [Google Scholar] [CrossRef]
- Patil, S.; Sen, S.; Bral, M.; Reddy, S.; Bradley, K.K.; Cornett, E.M.; Fox, C.J.; Kaye, A.D. The Role of Acupuncture in Pain Management. Curr. Pain Headache Rep. 2016, 20, 22. [Google Scholar] [CrossRef]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.N.; Sintsova, O.V.; Leychenko, E.V.; Kozlov, S.A. TRPV1 Ion Channel: Structural Features, Activity Modulators, and Therapeutic Potential. Biochemistry (Moscow) 2021, 86, S50–S70. [Google Scholar] [CrossRef]
- Lin, J.; Song, Z.; Chen, X.; Zhao, R.; Chen, J.; Chen, H.; Yang, X.; Wu, Z. Trans-cinnamaldehyde shows anti-depression effect in the forced swimming test and possible involvement of the endocannabinoid system. Biochem. Biophys. Res. Commun. 2019, 518, 351–356. [Google Scholar] [CrossRef]
- Clausi, S.; Iacobacci, C.; Lupo, M.; Olivito, G.; Molinari, M.; Leggio, M. The Role of the Cerebellum in Unconscious and Conscious Processing of Emotions: A Review. Appl. Sci. 2017, 7, 521. [Google Scholar] [CrossRef]
- Coombes, S.A.; Misra, G. Pain and motor processing in the human cerebellum. Pain 2016, 157, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ge, H.; Sun, M.; Gao, Y. Selecting an Appropriate Animal Model of Depression. Int. J. Mol. Sci. 2019, 20, 4827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Li, Y.; Sun, X.; Chang, X.; Zheng, H.; Gong, B.; Huang, Y.; Yang, M.; Wu, X.; et al. The long-term effect of acupuncture for migraine prophylaxis: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, L.; Luo, X.; Wang, M.; Chen, G.; Zhang, Q.; Liu, W.; Zhou, Z.; Song, J.; Jing, H.; et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: Multicentre, randomised clinical trial. BMJ 2020, 368, m697. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Humo, M.; Lu, H.; Yalcin, I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019, 377, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-G.; Chen, J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: Challenges and perspectives. Prog. Neurobiol. 2014, 116, 13–32. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, B.; Ly, D.V.; Vo, T.B.T.; Yoo, Y.-M.; Jung, E.-M.; Jeung, E.-B. Effects of steroid hormone on Calbindin-D9k expression in rat cerebellum. Endocr. Abstr. 2018. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchański, T.; Martin, I.S.; Laverty, D.; Karia, D.; Malinauskas, A.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABA A receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Campos, A.C.P.; Antunes, G.F.; Matsumoto, M.; Pagano, R.L.; Martinez, R.C.R. Neuroinflammation, Pain and Depression: An Overview of the Main Findings. Front. Psychol. 2020, 11, 1825. [Google Scholar] [CrossRef]
- Liang, M.; Zhong, H.; Rong, J.; Li, Y.; Zhu, C.; Zhou, L.; Zhou, R. Postnatal Lipopolysaccharide Exposure Impairs Adult Neurogenesis and Causes Depression-like Behaviors Through Astrocytes Activation Triggering GABAA Receptor Downregulation. Neuroscience 2019, 422, 21–31. [Google Scholar] [CrossRef]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Russo, C.D. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, C.F.; Chen, S.J.; Liang, W.N.; Wang, M.; Wang, S.S.; Dong, S.Q.; Yi, L.-T.; Li, C.-D. The antidepressant-like effects of Chaihu Shugan San: Dependent on the hippocampal BDNF-TrkB-ERK/Akt signaling activation in perimenopausal depression-like rats. Biomed. Pharmacother. 2018, 105, 45–52. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflügers Arch. Eur. J. Physiol. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Elman, I.; Borsook, D. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev. 2014, 39, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Krishnakumar, A.; Paulose, C. Decreased glutamate receptor binding and NMDA R1 gene expression in hip-pocampus of pilocarpine-induced epileptic rats: Neuroprotective role of Bacopa monnieri extract. Epilepsy Behav. 2008, 12, 54–60. [Google Scholar] [CrossRef]
- Zhuang, Z.-Y.; Xu, H.; Clapham, D.E.; Ji, R.-R. Phosphatidylinositol 3-Kinase Activates ERK in Primary Sensory Neurons and Mediates Inflammatory Heat Hyperalgesia through TRPV1 Sensitization. J. Neurosci. 2004, 24, 8300–8309. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E. Noradrenergic inhibition of presynaptic TRPV1 channels: A new pathway of pain control. J. Physiol. 2017, 595, 2413–2414. [Google Scholar] [CrossRef] [PubMed]
- Inprasit, C.; Huang, Y.C.; Lin, Y.W. Evidence for acupoint catgut embedding treatment and TRPV1 gene deletion in-creasing weight control in murine model. Int. J. Mol. Med. 2020, 45, 779–792. [Google Scholar]
- Yasuda, S.; Yoshida, M.; Yamagata, H.; Iwanaga, Y.; Suenaga, H.; Ishikawa, K.; Nakano, M.; Okuyama, S.; Furukawa, Y.; Furukawa, S.; et al. Imipramine Ameliorates Pain-related Negative Emotion via Induction of Brain-derived Neurotrophic Factor. Cell. Mol. Neurobiol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef]
- Motaghinejad, O.; Motaghinejad, M.; Motevalian, M.; Rahimi-Sharbaf, F.; Beiranvand, T. The effect of maternal forced exercise on offspring pain perception, motor activity and anxiety dis-order: The role of 5-HT2 and D2 receptors and CREB gene expression. J. Exerc. Rehabil. 2017, 13, 514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inprasit, C.; Lin, Y.-W. TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model. Int. J. Mol. Sci. 2020, 21, 3312. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.M.; Alemán, F.; Catroli, G.F.; Hunt, M.; Hu, M.; Dailamy, A.; Pla, A.; Woller, S.A.; Palmer, N.; Parekh, U.; et al. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci. Transl. Med. 2021, 13, eaay9056. [Google Scholar] [CrossRef]
- Alvarez, P.; Bogen, O.; Green, P.G.; Levine, J.D. Nociceptor Overexpression of NaV1.7 Contributes to Chronic Muscle Pain Induced by Early-Life Stress. J. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.A.; Souslova, V.; Wood, J.N.; Cervero, F. Deficits in Visceral Pain and Referred Hyperalgesia in Nav1.8 (SNS/PN3)-Null Mice. J. Neurosci. 2002, 22, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.M.; Hsieh, C.L.; Lin, Y.W. Electroacupuncture reduces chronic fibromyalgia pain through attenuation of tran-sient receptor potential vanilloid 1 signaling pathway in mouse brains. Iran. J. Basic Med. Sci. 2020, 23, 894. [Google Scholar] [PubMed]
- Huang, H.-Y.; Liao, H.-Y.; Lin, Y.-W. Effects and Mechanisms of Electroacupuncture on Chronic Inflammatory Pain and Depression Comorbidity in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.T.; Kim, S.K.; Kim, E.H.; Kim, J.H.; Youn, D.H.; Lee, B.H.; Chae, Y.-B.; Choi, S.-M. Acupuncture point locations for experimental animal studies in rats and mice. Korean J. Acupunct. 2010, 27, 67–78. [Google Scholar]
| Statistical Analysis Depicting the Expression Levels of TRPV1-Associated Proteins in the 5 Comparative Groups of an Acid-Saline Induced CPDC Model | |||||
| Cerebellum Lobule VI | |||||
| Protein | Control | AS | AS + EA | AS + SHAM | AS + KO |
| TRPV1 | 99.96 ± 2.86 | 71.75 ± 4.58 * | 92.37 ± 2.38 | 71.22 ± 2.63 * | 44.39 ± 8.59 * |
| pmTOR | 99.96 ± 2.16 | 75.76 ± 1.86 * | 96.01 ± 2.13 | 77.53 ± 1.88 * | 96.48 ± 1.96 |
| Nav1.7 | 100.04 ± 1.65 | 132.43 ± 3.9 * | 105.05 ± 2.64 | 128.22 ± 3.46 * | 106.36 ± 2.62 |
| Nav1.8 | 100.01 ± 4.63 | 118.93 ± 4.93 * | 102.86 ± 3.93 | 120.76 ± 2.51 * | 105.03 ± 4.35 |
| pPI3K | 100.04 ± 1.4 | 68.04 ± 3 * | 96.12 ± 2.95 | 69.77 ± 2.9 * | 94.13 ± 1.58 |
| pNMDAR1 | 100.04 ± 2.85 | 76.99 ± 1.95 * | 97.51 ± 3.35 | 76.32 ± 2.59 * | 95.11 ± 3.73 |
| pPKCε | 100.01 ± 2.52 | 73.34 ± 4.02 * | 96.37 ± 3.59 | 74.24 ± 4.77 * | 92.69 ± 4.04 |
| pAkt | 99.97 ± 3.21 | 71.76 ± 3.78 * | 93.72 ± 2.39 | 68.46 ± 3.21 * | 94.95 ± 2.43 |
| TrkB | 100.05 ± 3.28 | 73.17 ± 2.87 * | 96.98 ± 4.69 | 73 ± 3.84 * | 96.5 ± 4.84 |
| pNFκB | 99.98 ± 0.92 | 69.43 ± 3.3 * | 96.33 ± 3.76 | 70.31 ± 1.75 * | 93.33 ± 2.58 |
| GABAAα1 | 100.01 ± 2.86 | 74.93 ± 1.22 * | 98.87 ± 2.91 | 73 ± 2.45 * | 98.2 ± 2.29 |
| pPKAIIα | 100.03 ± 3.92 | 69.15 ± 2.02 * | 97.7 ± 3.39 | 72.12 ± 2.63 * | 95.42 ± 3.3 |
| pCREB | 99.95 ± 5.02 | 78.85 ± 4.16 * | 98.8 ± 5.55 | 78.28 ± 4.87 * | 97.68 ± 6.27 |
| pERK | 100.03 ± 2.13 | 73.25 ± 3.36 * | 95.53 ± 2.38 | 73.1 ± 2.36 * | 93.86 ± 3.02 |
| Cerebellum Lobule VII | |||||
| Protein | Control | AS | AS + EA | AS + SHAM | AS + KO |
| TRPV1 | 100.04 ± 5.91 | 78.04 ± 1.99 * | 100.59 ± 5.58 | 77.51 ± 3.51 * | 44.22 ± 1.53 * |
| pmTOR | 99.96 ± 4.15 | 79.23 ± 2.91 * | 96.77 ± 3.81 | 78.04 ± 3.71 * | 95.58 ± 4.23 |
| Nav1.7 | 99.95 ± 1.25 | 98.27 ± 0.99 | 98.46 ± 1.99 | 101.35 ± 1.12 | 102.36 ± 1.26 |
| Nav1.8 | 100.01 ± 2.84 | 101.91 ± 3.58 | 101.34 ± 2.21 | 101.15 ± 2.33 | 99.59 ± 3.21 |
| pPI3K | 100.17 ± 3.32 | 72.9 ± 2.22 * | 96.08 ± 3.77 | 71.46 ± 2.44 * | 96.28 ± 3.82 |
| pNMDAR1 | 100.04 ± 2.55 | 67.86 ± 2.67 * | 96.75 ± 2.42 | 70.98 ± 2.08 * | 94.87 ± 2.81 |
| pPKCε | 99.95 ± 0.43 | 71.91 ± 3.28 * | 93.68 ± 0.96 | 72.67 ± 1.96 * | 93.64 ± 1.16 |
| pAkt | 100.06 ± 4.19 | 69.61 ± 2.38 * | 96.84 ± 3.67 | 69.53 ± 2.52 * | 96.59 ± 2.68 |
| TrkB | 100.04 ± 2.93 | 74.01 ± 3.66 * | 96.19 ± 2.08 | 73.79 ± 2.87 * | 94.1 ± 3.3 |
| pNFκB | 100.02 ± 3.23 | 76.58 ± 3.3 * | 97.37 ± 3.47 | 76.73 ± 3.05 * | 99.53 ± 3.29 |
| GABAAα1 | 99.97 ± 3.36 | 69.57 ± 3.54 * | 97.55 ± 3.74 | 69.4 ± 3.76 * | 95.07 ± 3.94 |
| pPKAIIα | 99.99 ± 1.37 | 73.36 ± 2.36 * | 95.17 ± 1.22 | 74.34 ± 1.69 * | 93.61 ± 1.49 |
| pCREB | 100.02 ± 2.8 | 65.82 ± 3.44 * | 96.72 ± 2.22 | 69.09 ± 2.41 * | 95.18 ± 2.03 |
| pERK | 100.05 ± 1.66 | 75.59 ± 2.62 * | 99.18 ± 2.44 | 73.56 ± 1.22 * | 92.92 ± 1.49 |
| Cerebellum Lobule VIII | |||||
| Protein | Control | AS | AS + EA | AS + SHAM | AS + KO |
| TRPV1 | 100.03 ± 1.6 | 81.71 ± 1.69 * | 97.31 ± 2.4 | 82.58 ± 1.88 * | 24.38 ± 2.00 * |
| pmTOR | 100.04 ± 2.14 | 76.94 ± 2.26 * | 93.65 ± 1.75 | 78.75 ± 3.12 * | 94.93 ± 2.15 |
| Nav1.7 | 99.99 ± 1.87 | 121.07 ± 2.59 * | 99.53 ± 4.81 | 119.73 ± 2.7 * | 98.12 ± 3.58 |
| Nav1.8 | 100.04 ± 0.82 | 129.31 ± 0.92 * | 103.25 ± 0.74 | 124.2 ± 2.16 * | 104.73 ± 2.34 |
| pPI3K | 99.97 ± 1.78 | 70.8 ± 3.19 * | 96.86 ± 1.98 | 73.66 ± 2.55 * | 94.66 ± 1.04 |
| pNMDAR1 | 100.01 ± 1.77 | 72.77 ± 3.87 * | 98.06 ± 1.85 | 74.93 ± 4.32 * | 99.39 ± 1.63 |
| pPKCε | 99.96 ± 2.3 | 74.36 ± 1.67 * | 93.14 ± 2.44 | 78.35 ± 2.51 * | 94.14 ± 2.36 |
| pAkt | 99.97 ± 1.81 | 73.82 ± 2.07 * | 90.77 ± 2.08 | 74.55 ± 1.62 * | 91.36 ± 2.19 |
| TrkB | 99.99 ± 1.52 | 77.3 ± 2.71 * | 98.11 ± 2.14 | 80.68 ± 3.37 * | 95.19 ± 2.25 |
| pNFκB | 100.04 ± 1.64 | 76.11 ± 1.87 * | 96.68 ± 2.78 | 75.38 ± 3.06 * | 93.51 ± 1.69 |
| GABAAα1 | 99.99 ± 2.37 | 78.08 ± 3.12 * | 97.47 ± 3.78 | 75.95 ± 2.22 * | 95.13 ± 3.16 |
| pPKAIIα | 100 ± 2.07 | 77.19 ± 1.79 * | 97.39 ± 1.53 | 79.51 ± 1.62 * | 95.58 ± 1.23 |
| pCREB | 100.01 ± 2.29 | 73.12 ± 2.83 * | 96.31 ± 3.88 | 75.73 ± 2.37 * | 93.61 ± 2.91 |
| pERK | 100.03 ± 2.94 | 85.53 ± 2.33 * | 99.62 ± 3.37 | 82.59 ± 3.61 * | 99.62 ± 3.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lottering, B.; Lin, Y.-W. TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII Using Electroacupuncture Treatment for Chronic Pain and Depression Comorbidity in a Murine Model. Int. J. Mol. Sci. 2021, 22, 5028. https://doi.org/10.3390/ijms22095028
Lottering B, Lin Y-W. TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII Using Electroacupuncture Treatment for Chronic Pain and Depression Comorbidity in a Murine Model. International Journal of Molecular Sciences. 2021; 22(9):5028. https://doi.org/10.3390/ijms22095028
Chicago/Turabian StyleLottering, Bernice, and Yi-Wen Lin. 2021. "TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII Using Electroacupuncture Treatment for Chronic Pain and Depression Comorbidity in a Murine Model" International Journal of Molecular Sciences 22, no. 9: 5028. https://doi.org/10.3390/ijms22095028
APA StyleLottering, B., & Lin, Y.-W. (2021). TRPV1 Responses in the Cerebellum Lobules VI, VII, VIII Using Electroacupuncture Treatment for Chronic Pain and Depression Comorbidity in a Murine Model. International Journal of Molecular Sciences, 22(9), 5028. https://doi.org/10.3390/ijms22095028







