Alcohol in Psoriasis—From Bench to Bedside
Abstract
1. Introduction
2. Role of Alcohol in Psoriasis
2.1. Alcohol and Its Relation to Psoriasis Symptoms at the Cellular Level
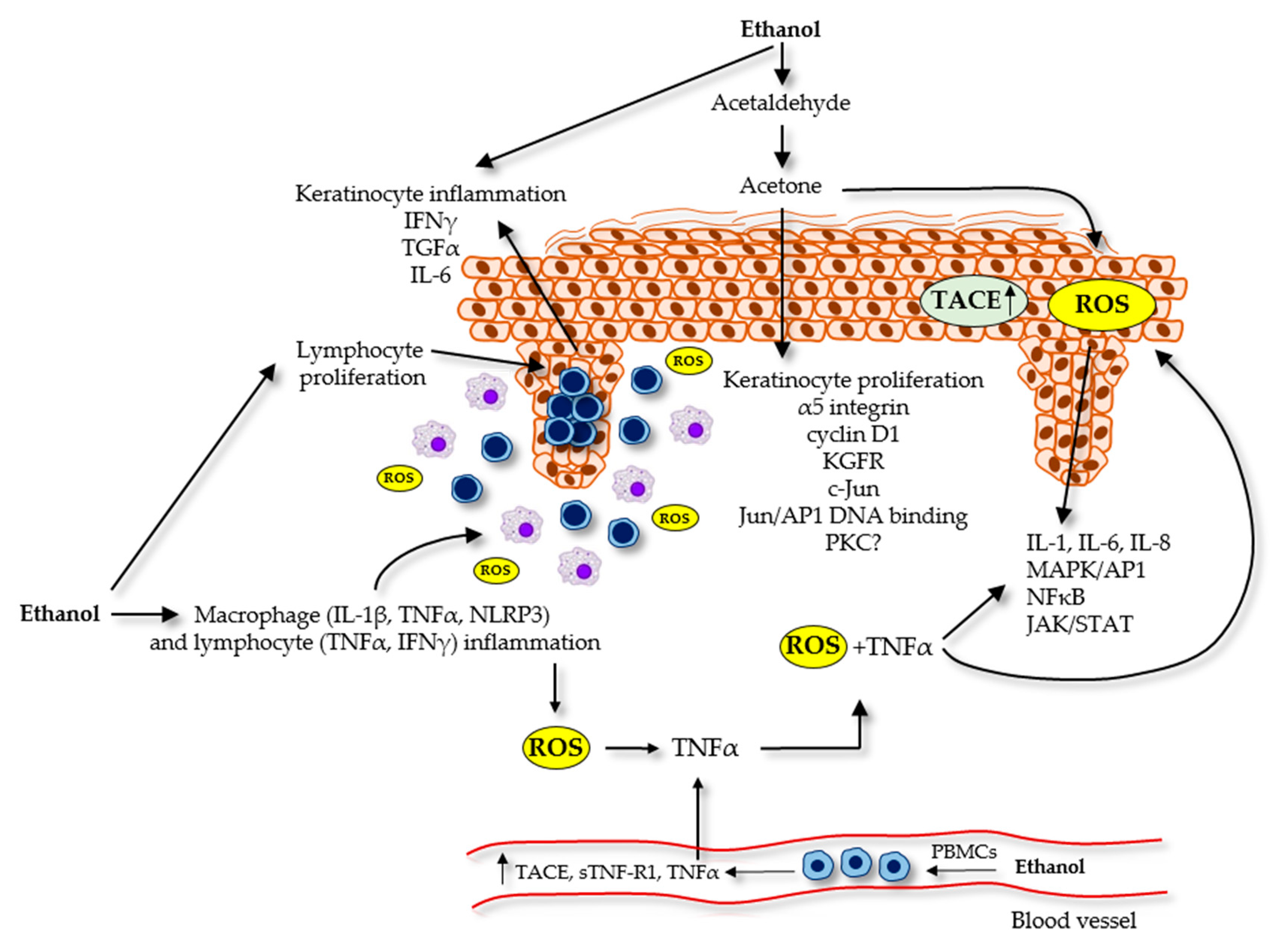
2.1.1. Alcohol and Keratinocytes
2.1.2. Alcohol and the Immune System
2.2. Alcohol and Patient Compliance
3. Role of Psoriasis in Alcohol Consumption
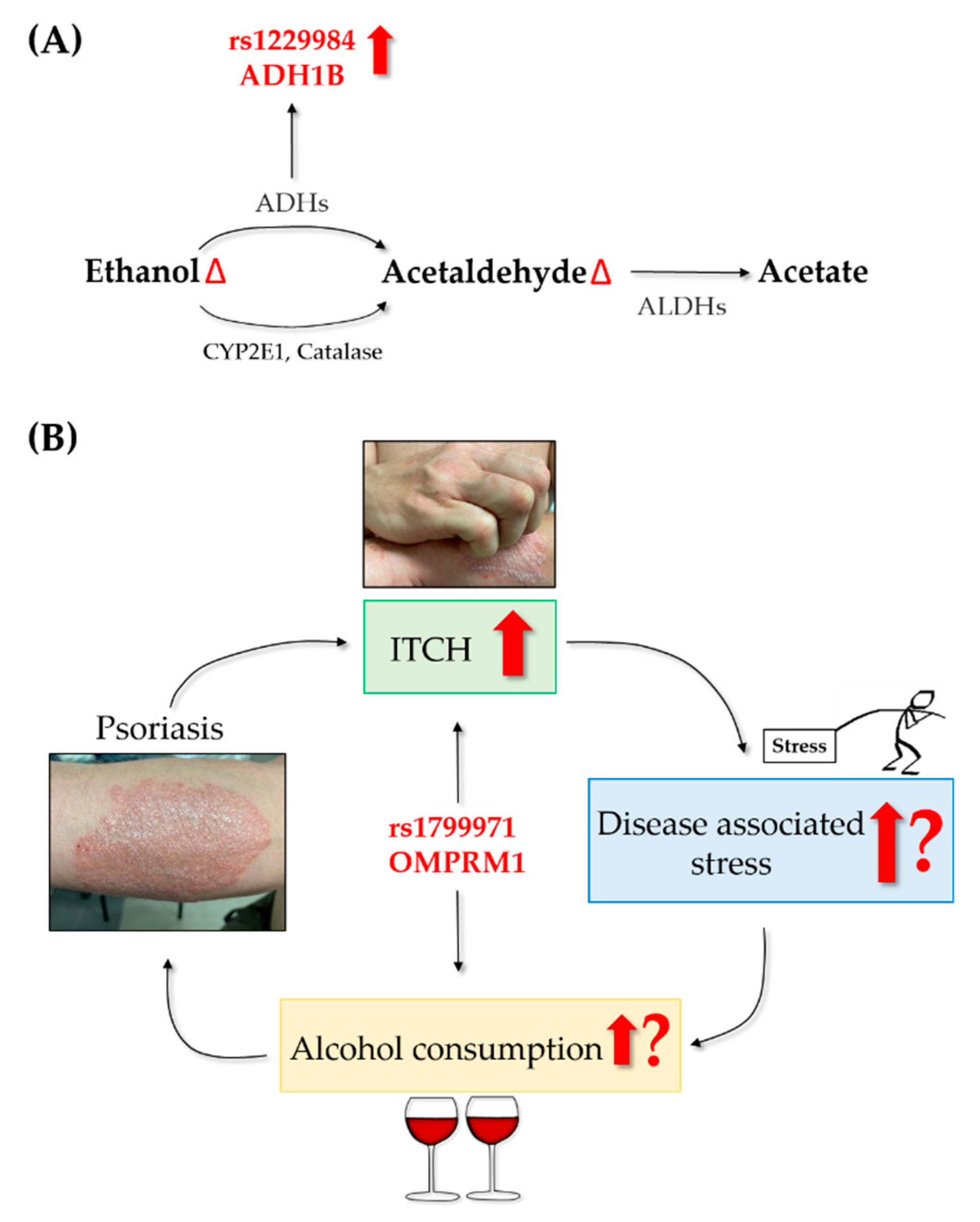
3.1. Stress and Mental Disorders in Psoriatic Patients
3.2. Alcohol Consumption and Addiction in Psoriatic Patients
4. Genetic Factors behind Alcohol Consumption in Psoriatic Patients
5. Alcohol Affecting Psoriasis Therapies
5.1. Acitretin
5.2. Methotrexate
6. Patient Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| 5-HT2R | 5-hydroxytryptamine receptor 2A |
| Acetyl-CoA | Acetyl coenzim A |
| ADH | Alcohol dehydrogenase |
| AhR | Aryl hydrocarbon receptor |
| ALDH | Aldehyde dehydrogenase |
| AP1 | Activator protein 1 |
| AUD | Alcohol use disorder |
| AUDIT | Alcohol use disorders identification test |
| CAGE | Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers |
| CCL20 | C-C Motif chemokine ligand 20 |
| CDT | Carbohydrate-deficient transferrin |
| c-Jun | Proto-oncogene c-Jun |
| CNS | Central nervous system |
| CYP2E1 DA | Cytochrome P450 isoform 2E1 Dopamine |
| DLQI | Dermatological life quality index |
| GABRA2 | Gamma-aminobutyric acid type A receptor subunit alpha2 |
| GABRG1 | Gamma-aminobutyric acid type A receptor subunit gamma1 |
| HPA-axis | Hypothalamic–pituitary–adrenal axis |
| IFNγ | Interferon-γ |
| IL | Interleukin |
| JAK | Janus kinase |
| KGFR | Keratinocyte growth factor receptor |
| MAPK | Mitogen-activated protein kinase |
| NAD | Nicotinamide adenine dinucleotide |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | NLR family pyrin domain containing 3 |
| OPRM1 | μ-opioid receptor 1 |
| PASI | Psoriasis Area Severity Index |
| PBMC | Peripheral blood mononuclear cell |
| Peth | Phosphatidylethanol |
| PKC | Protein kinase C |
| RALDH | Retinaldehyde dehydrogenase |
| RDH ROS | Retinol dehydrogenase Reactive oxygen species |
| SNP | Single nucleotide polymorphism |
| SSRI | Selective serotonin reuptake inhibitor |
| STAT | Signal transducers and activators of transcription |
| sTNF-R1 | Soluble TNF-receptor 1 |
| TACE | TNFα-converting enzyme |
| TGFα | Transforming growth factor-α |
| Th | T-helper |
| TNFR | Tumor necrosis factor receptor |
| TNFα | Tumor necrosis factor-α |
| γGT | γglutamyl-transferase |
References
- Al-Jefri, K.; Newbury-Birch, D.; Muirhead, C.R.; Gilvarry, E.; Araújo-Soares, V.; Reynolds, N.J.; Kaner, E.; Hampton, P.J. High prevalence of alcohol use disorders in patients with inflammatory skin diseases. Br. J. Dermatol. 2017, 177, 837–844. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Schmitt-Egenolf, M.; Eiermann, T.H.; Boehncke, W.H.; Ständer, M.; Sterry, W. Familial juvenile onset psoriasis is associated with the human leukocyte antigen (HLA) class I side of the extended haplotype Cw6-B57-DRB1*0701-DQA1*0201-DQB1*0303: A population- and family-based study. J. Investig. Dermatol. 1996, 106, 711–714. [Google Scholar] [CrossRef]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef]
- Strober, B.; Ryan, C.; van de Kerkhof, P.; van der Walt, J.; Kimball, A.B.; Barker, J.; Blauvelt, A. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J. Am. Acad. Dermatol. 2020, 82, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Brenaut, E.; Horreau, C.; Pouplard, C.; Barnetche, T.; Paul, C.; Richard, M.A.; Joly, P.; Le Maître, M.; Aractingi, S.; Aubin, F.; et al. Alcohol consumption and psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Zhu, C.Y.; Fan, Y.M. Alcohol consumption and psoriatic risk: A meta-analysis of case-control studies. J. Dermatol. 2012, 39, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Kemény, L. Alcohol, liver, systemic inflammation and skin: A focus on patients with psoriasis. Skin Pharmacol. Physiol. 2013, 26, 119–126. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Jacobi, U.; Bartoll, J.; Sterry, W.; Lademann, J. Orally administered ethanol: Transepidermal pathways and effects on the human skin barrier. Arch. Dermatol. Res. 2005, 296, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Kemény, L. Psoriasis and alcohol: Is cutaneous ethanol one of the missing links? Br. J. Dermatol. 2010, 162, 711–716. [Google Scholar] [CrossRef]
- Biscay, J.; Findlay, E.; Dennany, L. Electrochemical monitoring of alcohol in sweat. Talanta 2021, 224, 121815. [Google Scholar] [CrossRef]
- Farkas, A.; Kemény, L.; Széll, M.; Dobozy, A.; Bata-Csörgo, Z. Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: The possible role of alcohol in exacerbating psoriasis. Arch. Dermatol. Res. 2003, 295, 56–62. [Google Scholar] [CrossRef]
- Park, H.; Kim, K. Association of alcohol consumption with lipid profile in hypertensive men. Alcohol Alcohol. 2012, 47, 282–287. [Google Scholar] [CrossRef]
- Farkas, A.; Kemény, L. The alcohol metabolite acetaldehyde and psoriasis: Another trigger factor? Clin. Exp. Dermatol. 2010, 35, 923–925. [Google Scholar] [CrossRef]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Invest. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef]
- Mehic, D.; Bakiri, L.; Ghannadan, M.; Wagner, E.F.; Tschachler, E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J. Invest. Dermatol. 2005, 124, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Timmons, S.R.; Nwankwo, J.O.; Domann, F.E. Acetaldehyde activates Jun/AP-1 expression and DNA binding activity in human oral keratinocytes. Oral Oncol. 2002, 38, 281–290. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Braiman-Wiksman, L.; Daum, N.; Denning, M.F.; Tennenbaum, T. Protein kinase C family: On the crossroads of cell signaling in skin and tumor epithelium. J. Cancer Res. Clin. Oncol. 2007, 133, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Papp, H.; Czifra, G.; Lázár, J.; Gönczi, M.; Csernoch, L.; Kovács, L.; Bíro, T. Protein kinase C isozymes regulate proliferation and high cell density-mediated differentiation in HaCaT keratinocytes. Exp. Dermatol. 2003, 12, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Tavakkol, A.; Leach, K.; Burns, D.; Basta, P.; Loomis, C.; Griffiths, C.E.; Cooper, K.D.; Reynolds, N.J.; Elder, J.T.; et al. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: Reduced expression of protein kinase C-beta II in psoriasis. J. Invest. Dermatol. 1993, 101, 553–559. [Google Scholar] [CrossRef]
- Reynolds, N.J.; Yi, J.Y.; Fisher, G.J.; Cooper, K.D.; Voorhees, J.J.; Griffiths, C.E. Down-regulation of Langerhans cell protein kinase C-beta isoenzyme expression in inflammatory and hyperplastic dermatoses. Br. J. Dermatol. 1995, 133, 157–167. [Google Scholar] [CrossRef][Green Version]
- Tournier, S.; Gerbaud, P.; Anderson, W.B.; Lohmann, S.M.; Evain-Brion, D.; Raynaud, F. Post-translational abnormality of the type II cyclic AMP-dependent protein kinase in psoriasis: Modulation by retinoic acid. J. Cell Biochem. 1995, 57, 647–654. [Google Scholar] [CrossRef]
- Smith, C.P., Jr.; King, B.R.; Pennington, S.N. Cyclic AMP-dependent protein kinase activity in the brains of alcohol-preferring (P) and nonpreferring (NP) rats. Alcohol 1991, 8, 329–332. [Google Scholar] [CrossRef]
- Shibley, I.A., Jr.; Carver, F.M.; Pennington, S.N. Ethanol differentially affects metabolic and mitotic processes in chick embryonic cells. Alcohol. Clin. Exp. Res. 1997, 21, 460–466. [Google Scholar] [CrossRef]
- Steiner, J.; Kirsteins, L.; LaPaglia, N.; Lawrence, A.; Williams, D.; Emanuele, N.; Emanuele, M. The effect of acute ethanol (EtOH) exposure on protein kinase C (PKC) activity in anterior pituitary. Alcohol 1997, 14, 209–211. [Google Scholar] [CrossRef]
- Messing, R.O.; Petersen, P.J.; Henrich, C.J. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J. Biol. Chem. 1991, 266, 23428–23432. [Google Scholar] [CrossRef]
- Deitrich, R.A.; Bludeau, P.; Elk, M.E.; Baker, R.; Menez, J.F.; Gill, K. Effect of administered ethanol on protein kinase C in human platelets. Alcohol. Clin. Exp. Res. 1996, 20, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Bitrick, M.S. Ethanol enhances the in situ phosphorylation of MARCKS and protein kinase C activity in primary cultures of astrocytes. Life Sci. 1996, 58, 855–860. [Google Scholar] [CrossRef]
- Kim, S.K.; Choe, J.Y. Ethanol Augments Monosodium Urate-Induced NLRP3 Inflammasome Activation via Regulation of AhR and TXNIP in Human Macrophages. Yonsei Med. J. 2020, 61, 533–541. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Mitsuhashi, Y.; Kondo, S. Overexpression of tumour necrosis factor-alpha-converting enzyme in psoriasis. Br. J. Dermatol. 2005, 152, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Serwin, A.B.; Sokolowska, M.; Dylejko, E.; Chodynicka, B. Tumour necrosis factor (TNF-alpha) alpha converting enzyme and soluble TNF-alpha receptor type 1 in psoriasis patients in relation to the chronic alcohol consumption. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 712–717. [Google Scholar] [CrossRef]
- Ockenfels, H.M.; Keim-Maas, C.; Funk, R.; Nussbaum, G.; Goos, M. Ethanol enhances the IFN-gamma, TGF-alpha and IL-6 secretion in psoriatic co-cultures. Br. J. Dermatol. 1996, 135, 746–751. [Google Scholar] [CrossRef]
- Vasseur, P.; Pohin, M.; Gisclard, C.; Jégou, J.F.; Morel, F.; Silvain, C.; Lecron, J.C. Chronic Alcohol Consumption Exacerbates the Severity of Psoriasiform Dermatitis in Mice. Alcohol. Clin. Exp. Res. 2020, 44, 1728–1733. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramoto, K.; Koyama, M.; Ooi, K. Chronic liver injury in mice promotes impairment of skin barrier function via tumor necrosis factor-alpha. Cutan. Ocul. Toxicol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Parlet, C.P.; Waldschmidt, T.J.; Schlueter, A.J. Chronic ethanol feeding induces subset loss and hyporesponsiveness in skin T cells. Alcohol. Clin. Exp. Res. 2014, 38, 1356–1364. [Google Scholar] [CrossRef]
- Horváth, S.; Komlódi, R.; Perkecz, A.; Pintér, E.; Gyulai, R.; Kemény, Á. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef]
- Eissing, L.; Radtke, M.A.; Zander, N.; Augustin, M. Barriers to guideline-compliant psoriasis care: Analyses and concepts. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, S.S.; Goodfield, M.J. Objective assessment of compliance with psoriasis treatment. Arch. Dermatol. 2004, 140, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Feldman, S.R.; Camacho, F.T.; Balkrishnan, R. Better medication adherence results in greater improvement in severity of psoriasis. Br. J. Dermatol. 2004, 151, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Thorneloe, R.J.; Bundy, C.; Griffiths, C.E.; Ashcroft, D.M.; Cordingley, L. Adherence to medication in patients with psoriasis: A systematic literature review. Br. J. Dermatol. 2013, 168, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Koo, J. Psoriasis: Depression, anxiety, smoking, and drinking habits. Dermatol. Ther. 2010, 23, 174–180. [Google Scholar] [CrossRef]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef]
- Hunter, H.J.; Griffiths, C.E.; Kleyn, C.E. Does psychosocial stress play a role in the exacerbation of psoriasis? Br. J. Dermatol. 2013, 169, 965–974. [Google Scholar] [CrossRef]
- Ferreira, B.R.; Pio-Abreu, J.L.; Reis, J.P.; Figueiredo, A. Analysis of the Prevalence of Mental Disorders in Psoriasis: The Relevance of Psychiatric Assessment in Dermatology. Psychiatr. Danub. 2017, 29, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [CrossRef]
- Reich, A.; Mędrek, K.; Szepietowski, J.C. Interplay of Itch and Psyche in Psoriasis: An Update. Acta Derm. Venereol. 2016, 96, 55–57. [Google Scholar] [CrossRef]
- Hirotsu, C.; Nogueira, H.; Albuquerque, R.G.; Tomimori, J.; Tufik, S.; Andersen, M.L. The bidirectional interactions between psoriasis and obstructive sleep apnea. Int. J. Dermatol. 2015, 54, 1352–1358. [Google Scholar] [CrossRef]
- Rieder, E.; Tausk, F. Psoriasis, a model of dermatologic psychosomatic disease: Psychiatric implications and treatments. Int. J. Dermatol. 2012, 51, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.I.; Abreu, J.L.; Reis, J.P.; Figueiredo, A.M. Psoriasis and Associated Psychiatric Disorders: A Systematic Review on Etiopathogenesis and Clinical Correlation. J. Clin. Aesthet. Dermatol. 2016, 9, 36–43. [Google Scholar] [PubMed]
- Founta, O.; Adamzik, K.; Tobin, A.M.; Kirby, B.; Hevey, D. Psychological Distress, Alexithymia and Alcohol Misuse in Patients with Psoriasis: A Cross-Sectional Study. J. Clin. Psychol. Med. Settings 2019, 26, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.; Herrmann, M.; Fischer, T.; Lauffer, F.; Garzorz-Stark, N.; Böhner, A.; Spinner, C.D.; Biedermann, T.; Eyerich, K. Addiction: An underestimated problem in psoriasis health care. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.A.; Mason, D.L.; Cunningham, S.; O’Shea, S.J.; McCormick, P.A.; Stone, C.; Collins, P.; Rogers, S.; Kirby, B. Alcohol misuse in patients with psoriasis: Identification and relationship to disease severity and psychological distress. Br. J. Dermatol. 2011, 164, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.; Richards, H.L.; Mason, D.L.; Fortune, D.G.; Main, C.J.; Griffiths, C.E. Alcohol consumption and psychological distress in patients with psoriasis. Br. J. Dermatol. 2008, 158, 138–140. [Google Scholar] [CrossRef]
- Zou, L.; Lonne-Rahm, S.B.; Helander, A.; Stokkeland, K.; Franck, J.; Nordlind, K. Alcohol intake measured by phosphatidylethanol in blood and the lifetime drinking history interview are correlated with the extent of psoriasis. Dermatology 2015, 230, 375–380. [Google Scholar] [CrossRef]
- Ewing, J.A. Detecting alcoholism. The CAGE questionnaire. Jama 1984, 252, 1905–1907. [Google Scholar] [CrossRef]
- Mihailovic, N.; Szőllősi, G.J.; Rancic, N. Alcohol Consumption among the Elderly Citizens in Hungary and Serbia-Comparative Assessment. Int. J. Environ. Res. Public Health 2020, 17, 1289. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C.; Bucholz, K.K.; Madden, P.A.; Dinwiddie, S.H.; Slutske, W.S.; Bierut, L.J.; Statham, D.J.; Dunne, M.P.; Whitfield, J.B.; Martin, N.G. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol. Med. 1997, 27, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C. Genetic Influences on Alcoholism Risk: A Review of Adoption and Twin Studies. Alcohol Health Res. World 1995, 19, 166–171. [Google Scholar]
- Sigvardsson, S.; Bohman, M.; Cloninger, C.R. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch. Gen. Psychiatry 1996, 53, 681–687. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Li, T.K. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit. Rev. Neurobiol. 1998, 12, 339–369. [Google Scholar] [CrossRef]
- Irons, D.E.; Iacono, W.G.; Oetting, W.S.; Kirkpatrick, R.M.; Vrieze, S.I.; Miller, M.B.; McGue, M. Gamma-aminobutyric acid system genes--no evidence for a role in alcohol use and abuse in a community-based sample. Alcohol. Clin. Exp. Res. 2014, 38, 938–947. [Google Scholar] [CrossRef][Green Version]
- Dick, D.M.; Foroud, T. Candidate genes for alcohol dependence: A review of genetic evidence from human studies. Alcohol. Clin. Exp. Res. 2003, 27, 868–879. [Google Scholar] [CrossRef]
- Pfeifer, P.; Sariyar, M.; Eggermann, T.; Zerres, K.; Vernaleken, I.; Tüscher, O.; Fehr, C. Alcohol Consumption in Healthy OPRM1 G Allele Carriers and Its Association with Impulsive Behavior. Alcohol Alcohol. 2015, 50, 379–384. [Google Scholar] [CrossRef]
- Chamorro, A.J.; Marcos, M.; Mirón-Canelo, J.A.; Pastor, I.; González-Sarmiento, R.; Laso, F.J. Association of µ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: A systematic review and meta-analysis. Addict. Biol. 2012, 17, 505–512. [Google Scholar] [CrossRef]
- Bierut, L.J.; Goate, A.M.; Breslau, N.; Johnson, E.O.; Bertelsen, S.; Fox, L.; Agrawal, A.; Bucholz, K.K.; Grucza, R.; Hesselbrock, V.; et al. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol. Psychiatry 2012, 17, 445–450. [Google Scholar] [CrossRef]
- Polimanti, R.; Gelernter, J. ADH1B: From alcoholism, natural selection, and cancer to the human phenome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; Foroud, T. Genetics and alcoholism. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Luczak, S.E.; Glatt, S.J.; Wall, T.L. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol. Bull. 2006, 132, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Szentkereszty-Kovács, Z.; Fiatal, S.; Szegedi, A.; Kovács, D.; Janka, E.; Herszényi, K.; Holló, P.; Nikamo, P.; Ståhle, M.; Remenyik, É.; et al. The prevalence of ADH1B and OPRM1 alleles predisposing for alcohol consumption are increased in the Hungarian psoriasis population. Arch. Dermatol. Res. 2019, 311, 435–442. [Google Scholar] [CrossRef]
- Macgregor, S.; Lind, P.A.; Bucholz, K.K.; Hansell, N.K.; Madden, P.A.; Richter, M.M.; Montgomery, G.W.; Martin, N.G.; Heath, A.C.; Whitfield, J.B. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: An integrated analysis. Hum. Mol. Genet. 2009, 18, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Koller, G.; Zill, P.; Rujescu, D.; Ridinger, M.; Pogarell, O.; Fehr, C.; Wodarz, N.; Bondy, B.; Soyka, M.; Preuss, U.W. Possible association between OPRM1 genetic variance at the 118 locus and alcohol dependence in a large treatment sample: Relationship to alcohol dependence symptoms. Alcohol. Clin. Exp. Res. 2012, 36, 1230–1236. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Gelernter, J.; O’Malley, S.; Hernandez-Avila, C.A.; Kaufman, D. Association of alcohol or other drug dependence with alleles of the mu opioid receptor gene (OPRM1). Alcohol. Clin. Exp. Res. 1998, 22, 1359–1362. [Google Scholar] [CrossRef]
- Ray, L.A.; Hutchison, K.E. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol. Clin. Exp. Res. 2004, 28, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, P.; Reich, A.; Hołysz, M.; Gajda, M.; Wysokińska, E.; Kobuszewska, A.; Nevozhay, D.; Nowakowska, B.; Strzadała, L.; Jagodziński, P.P.; et al. Opioid Receptors in Psoriatic Skin: Relationship with Itch. Acta Derm. Venereol. 2017, 97, 564–570. [Google Scholar] [CrossRef]
- Jaworecka, K.; Muda-Urban, J.; Rzepko, M.; Reich, A. Molecular Aspects of Pruritus Pathogenesis in Psoriasis. Int. J. Mol. Sci. 2021, 22, 858. [Google Scholar] [CrossRef]
- Reich, A.; Szepietowski, J.C. Clinical Aspects of Itch: Psoriasis. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ramos-Casals, M.; Roberto Perez, A.; Diaz-Lagares, C.; Cuadrado, M.J.; Khamashta, M.A.; Group, B.S. Autoimmune diseases induced by biological agents: A double-edged sword? Autoimmun. Rev. 2010, 9, 188–193. [Google Scholar] [CrossRef]
- Duester, G. Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. J. Nutr. 1998, 128, 459s–462s. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, Y.; Bendelac, L.; Jubran, H.; Hirschberg, J.; Fainsod, A. Acetaldehyde inhibits retinoic acid biosynthesis to mediate alcohol teratogenicity. Sci. Rep. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Deltour, L.; Foglio, M.H.; Cuenca, A.E.; Duester, G. Distinct retinoid metabolic functions for alcohol dehydrogenase genes Adh1 and Adh4 in protection against vitamin A toxicity or deficiency revealed in double null mutant mice. J. Biol. Chem. 2002, 277, 13804–13811. [Google Scholar] [CrossRef]
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.G.; Vahlquist, C.; Andersson, E.; Törma, H.; Kragballe, K.; Vahlquist, A. Oral acitretin in psoriasis: Drug and vitamin A concentrations in plasma, skin and adipose tissue. Acta Derm. Venereol. 1992, 72, 84–88. [Google Scholar] [CrossRef]
- Larsen, F.G.; Jakobsen, P.; Eriksen, H.; Grønhøj, J.; Kragballe, K.; Nielsen-Kudsk, F. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J. Clin. Pharmacol. 1991, 31, 477–483. [Google Scholar] [CrossRef]
- Maier, H.; Hönigsmann, H. Concentration of etretinate in plasma and subcutaneous fat after long-term acitretin. Lancet 1996, 348, 1107. [Google Scholar] [CrossRef]
- Chou, R.C.; Wyss, R.; Huselton, C.A.; Wiegand, U.W. A newly discovered xenobiotic metabolic pathway: Ethyl ester formation. Life Sci. 1991, 49, Pl169–Pl172. [Google Scholar] [CrossRef]
- Larsen, F.G.; Jakobsen, P.; Knudsen, J.; Weismann, K.; Kragballe, K.; Nielsen-Kudsk, F. Conversion of acitretin to etretinate in psoriatic patients is influenced by ethanol. J. Invest. Dermatol. 1993, 100, 623–627. [Google Scholar] [CrossRef]
- Grønhøj Larsen, F.; Steinkjer, B.; Jakobsen, P.; Hjorter, A.; Brockhoff, P.B.; Nielsen-Kudsk, F. Acitretin is converted to etretinate only during concomitant alcohol intake. Br. J. Dermatol. 2000, 143, 1164–1169. [Google Scholar] [CrossRef]
- Laugier, J.P.; de Sousa, G.; Bun, H.; Geiger, J.M.; Surber, C.; Rahmani, R. Acitretin biotransformation into etretinate: Role of ethanol on in vitro hepatic metabolism. Dermatology 1994, 188, 122–125. [Google Scholar] [CrossRef]
- Gisondi, P.; Targher, G.; Zoppini, G.; Girolomoni, G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J. Hepatol. 2009, 51, 758–764. [Google Scholar] [CrossRef]
- Herédi, E.; Csordás, A.; Clemens, M.; Adám, B.; Gáspár, K.; Törőcsik, D.; Nagy, G.; Adány, R.; Gaál, J.; Remenyik, E.; et al. The prevalence of obesity is increased in patients with late compared with early onset psoriasis. Ann. Epidemiol. 2013, 23, 688–692. [Google Scholar] [CrossRef]
- Berends, M.A.; Snoek, J.; de Jong, E.M.; Van Krieken, J.H.; de Knegt, R.J.; van Oijen, M.G.; van de Kerkhof, P.C.; Drenth, J.P. Biochemical and biophysical assessment of MTX-induced liver fibrosis in psoriasis patients: Fibrotest predicts the presence and Fibroscan predicts the absence of significant liver fibrosis. Liver Int. 2007, 27, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.H.; Warner, A.; Costello, R. Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate. Ann. Rheum. Dis. 2017, 76, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Gyulai, R.; Bagot, M.; Griffiths, C.E.M.; Luger, T.; Naldi, L.; Paul, C.; Puig, L.; Kemény, L. Current practice of methotrexate use for psoriasis: Results of a worldwide survey among dermatologists. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; James, C.; Deighton, C. Methotrexate use and alcohol. Clin. Exp. Rheumatol. 2010, 28, S114–S116. [Google Scholar]
- Svanström, C.; Lonne-Rahm, S.B.; Nordlind, K. Psoriasis and alcohol. Psoriasis 2019, 9, 75–79. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szentkereszty-Kovács, Z.; Gáspár, K.; Szegedi, A.; Kemény, L.; Kovács, D.; Törőcsik, D. Alcohol in Psoriasis—From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 4987. https://doi.org/10.3390/ijms22094987
Szentkereszty-Kovács Z, Gáspár K, Szegedi A, Kemény L, Kovács D, Törőcsik D. Alcohol in Psoriasis—From Bench to Bedside. International Journal of Molecular Sciences. 2021; 22(9):4987. https://doi.org/10.3390/ijms22094987
Chicago/Turabian StyleSzentkereszty-Kovács, Zita, Krisztián Gáspár, Andrea Szegedi, Lajos Kemény, Dóra Kovács, and Dániel Törőcsik. 2021. "Alcohol in Psoriasis—From Bench to Bedside" International Journal of Molecular Sciences 22, no. 9: 4987. https://doi.org/10.3390/ijms22094987
APA StyleSzentkereszty-Kovács, Z., Gáspár, K., Szegedi, A., Kemény, L., Kovács, D., & Törőcsik, D. (2021). Alcohol in Psoriasis—From Bench to Bedside. International Journal of Molecular Sciences, 22(9), 4987. https://doi.org/10.3390/ijms22094987







