Focal Ischemic Injury to the Early Neonatal Rat Brain Models Cognitive and Motor Deficits with Associated Histopathological Outcomes Relevant to Human Neonatal Brain Injury
Abstract
1. Introduction
2. Results
2.1. ET-1 Induced Neonatal Ischemia Results in Deficits in Motor Coordination
2.2. ET-1 Induced Neonatal Ischemia Impairs Associative Learning in the Pairwise Visual Discrimination Touchscreen Task
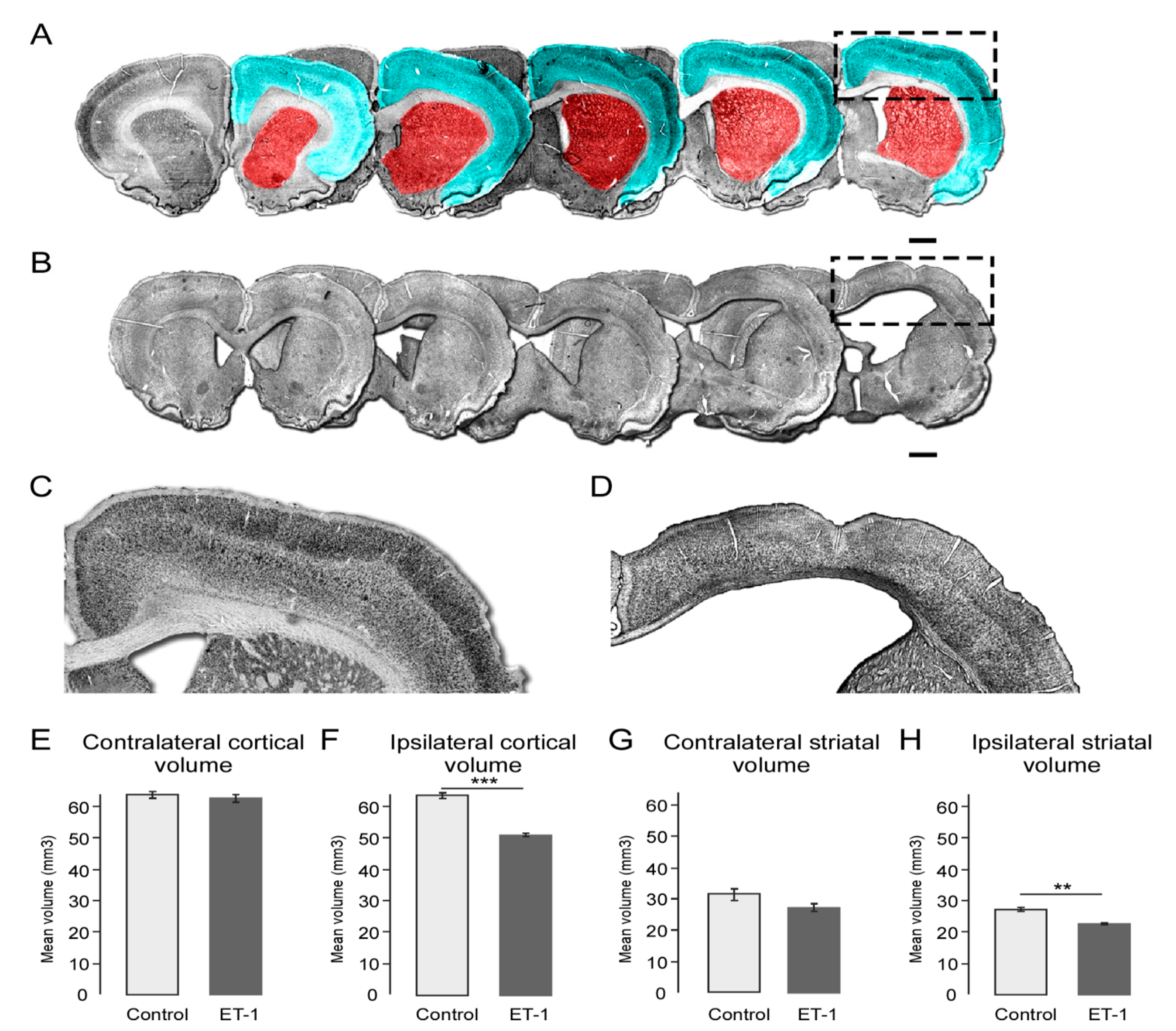
2.3. Early Neonatal Ischemia Results in Reduced Adult Cortical and Striatal Volume
2.4. ET-1 Induced Neonatal Ischemia Results in Specific Patterns of White Matter Damage
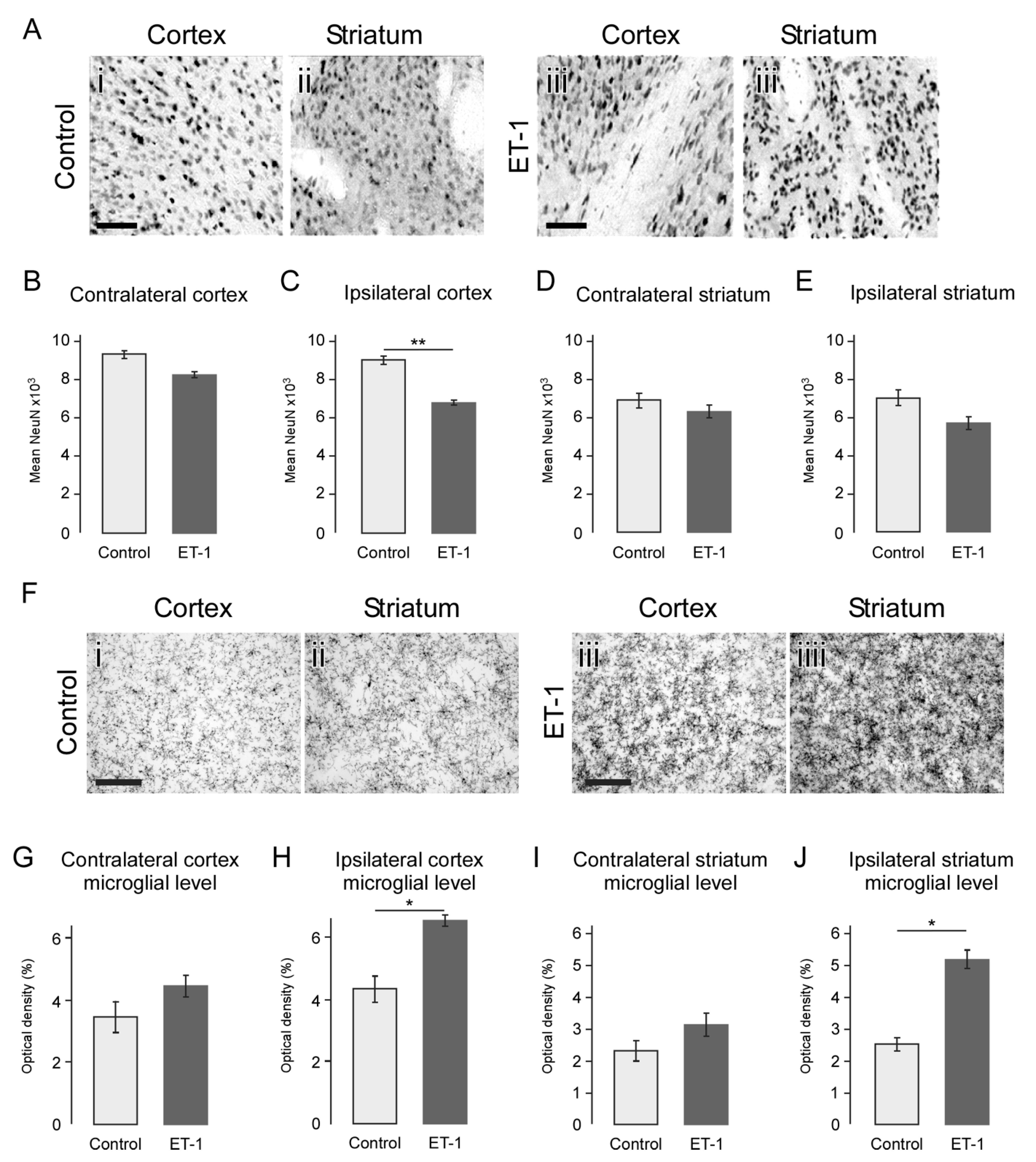
2.5. Atrophy Resulting from Neonatal Ischemia Is Associated with Neuronal Loss
2.6. Neonatal Ischemia Results in a Chronic Neuroinflammatory State Persisting up to 24 Weeks
2.7. Histopathological Features Correlate with Motor and Cognitive Deficits
2.8. White Matter Damage Is a Significant Predictor of Pairwise Discrimination Performance
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Surgical Procedures
4.3. Behavioural Testing
4.3.1. Accelerating Rotarod
4.3.2. Pairwise Discrimination Touchscreen Testing
4.4. Tissue Processing and Histological Assessment
4.5. Histological Quantification
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Cerebral palsy |
| ET-1 | Endothelin-1 |
| PD | Pairwise discrimination |
| ITI | Inter-trial interval |
| IHC | Immunohistochemical |
| DAB | diaminobenzidine |
| ABC | avidin-biotin complex |
| CC | Corpus callosum |
| PVWMB | periventricular white matter bundles |
| HI | Hypoxic ischemia |
References
- Tsai, A.J.; Lasky, R.E.; John, S.D.; Evans, P.W.; Kennedy, K.A. Predictors of Neurodevelopmental Outcomes in Preterm Infants with Intraparenchymal Hemorrhage. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2014, 34, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Garnier, Y.; Jensen, A. Journal of the Society for Gynecologic Investigation Perinatal Brain Damage: Underlying Mechanisms and Neuroprotective Strategies. J. Soc. Gynecol. Investig. 2002, 9, 319–328. [Google Scholar] [CrossRef]
- Rees, S.; Harding, R.; Walker, D. The Biological Basis of Injury and Neuroprotection in the Fetal. Int. J. Dev. Neurosci. 2012, 29, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.C. Neurodevelopmental Outcomes of Preterm Infants. Curr. Opin. Neurol. 2008, 21, 123–129. [Google Scholar] [CrossRef]
- Vincer, M.J.; Allen, A.C.; Joseph, K.S.; Stinson, D.A.; Scott, H.; Wood, E. Increasing Prevalence of Cerebral Palsy among Very Preterm Infants: A Population-Based Study. Pediatrics 2006, 118, e1621–e1626. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Luo, N.L.; Mallinson, R.A.; O’Malley, J.P.; Wallen, L.D.; Frei, B.; Morrow, J.D.; Petito, C.K.; Roberts, C.T.; Murdoch, G.H.; et al. Selective Vulnerability of Preterm White Matter to Oxidative Damage Defined by F2-Isoprostanes. Ann. Neurol. 2005, 58, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Faustino, J.; Derugin, N.; Vexler, Z.S. Acute and Chronic Vascular Responses to Experimental Focal Arterial Stroke in the Neonate Rat. Transl. Stroke Res. 2013, 4, 179–188. [Google Scholar] [CrossRef][Green Version]
- Chicha, L.; Smith, T.; Guzman, R. Stem Cells for Brain Repair in Neonatal Hypoxia-Ischemia. Childs Nerv. Syst. 2014, 30, 37–46. [Google Scholar] [CrossRef]
- Inder, T.E.; Anderson, N.J.; Spencer, C.; Wells, S.; Volpe, J.J. White Matter Injury in the Premature Infant: A Comparison between Serial Cranial Sonographic and MR Findings at Term. Am. J. Neuroradiol. 2003, 24, 805–809. [Google Scholar]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The Influence of Immaturity on Hypoxic-ischemic Brain Damage in the Rat. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Vannucci, S.J. A Model of Perinatal Hypoxic-Ischemic Brain Damage. Ann. N. Y. Acad. Sci. 1997, 835, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mallard, C.; Vexler, Z.S. Modeling Ischemia in the Immature Brain: How Translational Are Animal Models? Stroke 2015, 46, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Grafe, M.R. Developmental Changes in the Sensitivity of the Neonatal Rat Brain to Hypoxic/Ischemic Injury. Brain Res. 1994, 653, 161–166. [Google Scholar] [CrossRef]
- Back, S.A.; Han, B.H.; Luo, N.L.; Chricton, C.A.; Xanthoudakis, S.; Tam, J.; Arvin, K.L.; Holtzman, D.M. Selective Vulnerability of Late Oligodendrocyte Progenitors to Hypoxia–Ischemia. J. Neurosci. 2002, 22, 455–463. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.M.; Threlkeld, S.W.; Fitch, R.H. The Effects of Erythropoietin on Auditory Processing Following Neonatal Hypoxic–Ischemic Injury. Brain Res. 2006, 1087, 190–195. [Google Scholar] [CrossRef]
- Wright, J.L.; Chu, H.X.; Kagan, B.J.; Ermine, C.M.; Kauhausen, J.A.; Parish, C.L.; Sobey, C.G.; Thompson, L.H. Local Injection of Endothelin-1 in the Early Neonatal Rat Brain Models Ischemic Damage Associated with Motor Impairment and Diffuse Loss in Brain Volume. Neuroscience 2018. [Google Scholar] [CrossRef]
- Westmacott, R.; MacGregor, D.; Askalan, R.; deVeber, G. Late Emergence of Cognitive Deficits after Unilateral Neonatal Stroke. Stroke 2009, 40, 2012–2019. [Google Scholar] [CrossRef]
- Clowry, G.J.; Basuodan, R.; Chan, F. What Are the Best Animal Models for Testing Early Intervention in Cerebral Palsy? Front. Neurol. 2014, 5. [Google Scholar] [CrossRef]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Talpos, J.C.; Fletcher, A.C.; Circelli, C.; Tricklebank, M.D.; Dix, S.L. The Pharmacological Sensitivity of a Touchscreen-Based Visual Discrimination Task in the Rat Using Simple and Perceptually Challenging Stimuli. Psychopharmacology 2012, 221, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bryce, C.A.; Howland, J.G. Stress Facilitates Late Reversal Learning Using a Touchscreen-Based Visual Discrimination Procedure in Male Long Evans Rats. Behav. Brain Res. 2014, 278C, 21–28. [Google Scholar] [CrossRef][Green Version]
- Horner, A.E.; Heath, C.J.; Hvoslef-Eide, M.; Kent, B.A.; Kim, C.H.; Nilsson, S.R.O.; Alsiö, J.; Oomen, C.A.; Holmes, A.; Saksida, L.M.; et al. The Touchscreen Operant Platform for Testing Learning and Memory in Rats and Mice. Nat. Protoc. 2013, 8, 1961–1984. [Google Scholar] [CrossRef] [PubMed]
- Krigger, K.W. Cerebral Palsy: An Overview. Am. Fam. Physician 2006, 73, 91–100. [Google Scholar] [PubMed]
- Rennie, J.M.; Hagmann, C.F.; Robertson, N.J. Outcome after Intrapartum Hypoxic Ischaemia at Term. Semin. Fetal. Neonatal Med. 2007, 12, 398–407. [Google Scholar] [CrossRef]
- Alexander, M.; Garbus, H.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Behavioral and Histological Outcomes Following Neonatal HI Injury in a Preterm (P3) and Term (P7) Rodent Model. Behav. Brain Res. 2014, 49, 1841–1850. [Google Scholar] [CrossRef]
- Bona, E.; Johansson, B.; Hagberg, H. Sensorimotor Function and Neuropathology Five to Six Weeks after Hypoxia-Ischemia in Seven-Day-Old Rats. Pediatr. Res. 1997, 42, 678–683. [Google Scholar] [CrossRef]
- Fan, L.; Lin, S.; Pang, Y.; Lei, M.; Zhang, F.; Rhodes, P.; Cai, Z. Hypoxia-Ischemia Induced Neurological Dysfunction and Brain Injury in the Neonatal Rat. Behav. Brain Res. 2005, 165, 80–90. [Google Scholar] [CrossRef]
- Jansen, E.M.; Low, W.C. Long-Term Effects of Neonatal Ischemic-Hypoxic Brain Injury on Sensorimotor and Locomotor Tasks in Rats. Behav. Brain Res. 1996, 78, 189–194. [Google Scholar] [CrossRef]
- Lubics, A.; Reglodi, D.; Tamás, A.; Kiss, P.; Szalai, M.; Szalontay, L.; Lengvári, I. Neurological Reflexes and Early Motor Behavior in Rats Subjected to Neonatal Hypoxic-Ischemic Injury. Behav. Brain Res. 2005, 157, 157–165. [Google Scholar] [CrossRef]
- Westmacott, R.; Askalan, R.; Macgregor, D.; Anderson, P.; Deveber, G. Cognitive Outcome Following Unilateral Arterial Ischaemic Stroke in Childhood: Effects of Age at Stroke and Lesion Location. Dev. Med. Child. Neurol. 2010, 52, 386–393. [Google Scholar] [CrossRef]
- Fernández-López, D.; Natarajan, N.; Ashwal, S.; Vexler, Z.S. Mechanisms of Perinatal Arterial Ischemic Stroke. J. Cereb. Blood Flow Metab. 2014, 34, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Golomb, M.R.; Garg, B.P.; Edwards-Brown, M.; Williams, L.S. Very Early Arterial Ischemic Stroke in Premature Infants. Pediatr. Neurol. 2008, 38, 329–334. [Google Scholar] [CrossRef]
- Ikeda, T.; Mishima, K.; Yoshikawa, T.; Iwasaki, K.; Fujiwara, M.; Xia, Y.X.; Ikenoue, T. Selective and Long-Term Learning Impairment Following Neonatal Hypoxic-Ischemic Brain Insult in Rats. Behav. Brain Res. 2001, 118, 17–25. [Google Scholar] [CrossRef]
- Gonzalez, F.F.; Abel, R.; Almli, C.R.; Mu, D.; Wendland, M.; Ferriero, D.M. Erythropoietin Sustains Cognitive Function and Brain Volume after Neonatal Stroke. Dev. Neurosci. 2009, 31, 403–411. [Google Scholar] [CrossRef]
- Berger, S.; Wolfer, D.P.; Selbach, O.; Alter, H.; Erdmann, G.; Reichardt, H.M.; Chepkova, A.N.; Welzl, H.; Haas, H.L.; Lipp, H.-P.; et al. Loss of the Limbic Mineralocorticoid Receptor Impairs Behavioral Plasticity. Proc. Natl. Acad. Sci. USA 2006, 103, 195. [Google Scholar] [CrossRef]
- Roze, E.; Kerstjens, J.M.; Maathuis, C.G.B.; ter Horst, H.J.; Bos, A.F. Risk Factors for Adverse Outcome in Preterm Infants with Periventricular Hemorrhagic Infarction. Pediatrics 2008, 122, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.G. On the Morphogenesis of Ulegyria. Acta Neuropathol. 1981, 53, 331–332. [Google Scholar] [CrossRef]
- Mercuri, E.; Barnett, A.L. Neonatal Brain MRI and Motor Outcome at School Age in Children with Neonatal Encephalopathy: A Review of Personal Experience. Neural Plast. 2003, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Diez-Sebastian, J.; Kapellou, O.; Gindner, D.; Allsop, J.M.; Rutherford, M.A.; Cowan, F.M. Predicting Motor Outcome and Death in Term Hypoxic-Ischemic Encephalopathy. Neurology 2011, 76, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Ermine, C.M.; Somaa, F.; Wang, T.-Y.; Kagan, B.J.; Parish, C.L.; Thompson, L.H. Long-Term Motor Deficit and Diffuse Cortical Atrophy Following Focal Cortical Ischemia in Athymic Rats. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Grether, J.K. Causes of Cerebral Palsy. Curr. Opin. Pedaitr. 1999, 11, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.; Groenendaal, F.; Van Haastert, I.-L.; Rademaker, K.; Hanlo, P.; De Vries, L. Neurodevelopmental Outcome of Preterm Infants with Severe Intraventricular Hemorrhage and Therapy for Post-Hemorrhagic Ventricular Dilatation. J. Pediatr. 2008, 152, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Neshterova, M. The Diagnostic and Prognostic Implications of Brain Hyperechogenicity in Hypoxic-Ischaemic Encephalopathy Grade II. Eur. J. Ultrasound 1998, 8, 7–15. [Google Scholar] [CrossRef]
- Khwaja, O.; Volpe, J.J. Pathogenesis of Cerebral White Matter Injury of Prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 93, F153–F161. [Google Scholar] [CrossRef] [PubMed]
- Van Der Aa, N.E.; Northington, F.J.; Stone, B.S.; Groenendaal, F.; Benders, M.J.N.L.; Porro, G.; Yoshida, S.; Mori, S.; De Vries, L.S.; Zhang, J. Quantification of White Matter Injury Following Neonatal Stroke with Serial DTI. Pediatr. Res. 2013, 73, 756–762. [Google Scholar] [CrossRef]
- Uehara, H.; Yoshioka, H.; Kawase, S.; Nagai, H.; Ohmae, T.; Hasegawa, K.; Sawada, T. A New Model of White Matter Injury in Neonatal Rats with Bilateral Carotid Artery Occlusion. Brain Res. 1999, 837, 213–220. [Google Scholar] [CrossRef]
- Cai, Z.; Pang, Y.; Xiao, F.; Rhodes, P.G. Chronic Ischemia Preferentially Causes White Matter Injury in the Neonatal Rat Brain. Brain Res. 2001, 898, 126–135. [Google Scholar] [CrossRef]
- Fancy, S.P.J.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as Regulatory and Therapeutic Target in Newborn Brain Injury and Remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.J.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.M.; Rowitch, D.H. Myelin Regeneration: A Recapitulation of Development? Annu. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- Woodward, L.J.; Anderson, P.J.; Austin, N.C.; Howard, K.; Inder, T.E. Neonatal MRI to Predict Neurodevelopmental Outcomes in Preterm Infants. N. Engl. J. Med. 2006, 355, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Buser, J.R.; Maire, J.; Riddle, A.; Gong, X.; Nguyen, T.; Nelson, K.; Luo, N.L.; Ren, J.; Struve, J.; Sherman, L.S.; et al. Arrested Preoligodendrocyte Maturation Contributes to Myelination Failure in Premature Infants. Ann. Neurol. 2012, 71, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Faustino, J.V.; Wang, X.; Johnson, C.E.; Klibanov, A.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Microglial Cells Contribute to Endogenous Brain Defenses after Acute Neonatal Focal Stroke. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Ermine, C.M.; Wright, J.L.; Stanic, D.; Parish, C.L.; Thompson, L.H. Ischemic Injury Does Not Stimulate Striatal Neuron Replacement Even during Periods of Active Striatal Neurogenesis. iScience 2020, 23, 101175. [Google Scholar] [CrossRef] [PubMed]
- Altunkaynak, B.Z.; Altunkaynak, E.; Unal, D.; Unal, B. A Novel Application for the Cavalieri Principle: A Stereological and Methodological Study. Eurasian J. Med. 2009, 41, 99–101. [Google Scholar] [PubMed]



| Hemisphere | Measurement | Rotarod | Touchscreen |
|---|---|---|---|
| Ipsilateral | Cortical Volume | 0.40 * | 0.46 * |
| Striatal Volume | 0.35 | 0.39 | |
| NeuN density (cortex) | 0.55 ** | 0.48 * | |
| NeuN density (striatum) | 0.36 | 0.44 * | |
| Corpus Callosum area | 0.20 | 0.40 * | |
| PVWMB area | 0.32 | 0.04 | |
| Contralateral | Cortical Volume | 0.22 | −0.07 |
| Striatal Volume | 0.35 | 0.27 | |
| NeuN density (cortex) | 0.37 | 0.32 | |
| NeuN density (striatum) | 0.03 | 0.08 | |
| Corpus Callosum area | 0.21 | 0.33 | |
| PVWMB area | −0.14 | −0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagan, B.J.; Ermine, C.M.; Frausin, S.; Parish, C.L.; Nithianantharajah, J.; Thompson, L.H. Focal Ischemic Injury to the Early Neonatal Rat Brain Models Cognitive and Motor Deficits with Associated Histopathological Outcomes Relevant to Human Neonatal Brain Injury. Int. J. Mol. Sci. 2021, 22, 4740. https://doi.org/10.3390/ijms22094740
Kagan BJ, Ermine CM, Frausin S, Parish CL, Nithianantharajah J, Thompson LH. Focal Ischemic Injury to the Early Neonatal Rat Brain Models Cognitive and Motor Deficits with Associated Histopathological Outcomes Relevant to Human Neonatal Brain Injury. International Journal of Molecular Sciences. 2021; 22(9):4740. https://doi.org/10.3390/ijms22094740
Chicago/Turabian StyleKagan, Brett J., Charlotte M. Ermine, Stefano Frausin, Clare L. Parish, Jess Nithianantharajah, and Lachlan H. Thompson. 2021. "Focal Ischemic Injury to the Early Neonatal Rat Brain Models Cognitive and Motor Deficits with Associated Histopathological Outcomes Relevant to Human Neonatal Brain Injury" International Journal of Molecular Sciences 22, no. 9: 4740. https://doi.org/10.3390/ijms22094740
APA StyleKagan, B. J., Ermine, C. M., Frausin, S., Parish, C. L., Nithianantharajah, J., & Thompson, L. H. (2021). Focal Ischemic Injury to the Early Neonatal Rat Brain Models Cognitive and Motor Deficits with Associated Histopathological Outcomes Relevant to Human Neonatal Brain Injury. International Journal of Molecular Sciences, 22(9), 4740. https://doi.org/10.3390/ijms22094740






