Abstract
Calcium carbonate (CaCO3)-based materials have received notable attention for biomedical applications owing to their safety and beneficial characteristics, such as pH sensitivity, carbon dioxide (CO2) gas generation, and antacid properties. Herein, to additionally incorporate antioxidant and anti-inflammatory functions, we prepared tannylated CaCO3 (TA-CaCO3) materials using a simple reaction between tannic acid (TA), calcium (Ca2+), and carbonate (CO32−) ions. TA-CaCO3 synthesized at a molar ratio of 1:75 (TA:calcium chloride (CaCl2)/sodium carbonate (Na2CO3)) showed 3–6 μm particles, comprising small nanoparticles in a size range of 17–41 nm. The TA-CaCO3 materials could efficiently neutralize the acid solution and scavenge free radicals. In addition, these materials could significantly reduce the mRNA levels of pro-inflammatory factors and intracellular reactive oxygen species, and protect chondrocytes from toxic hydrogen peroxide conditions. Thus, in addition to their antacid property, the prepared TA-CaCO3 materials exert excellent antioxidant and anti-inflammatory effects through the introduction of TA molecules. Therefore, TA-CaCO3 materials can potentially be used to treat inflammatory cells or diseases.
1. Introduction
Inorganic minerals have been widely used in drug delivery systems for biomedical applications [1]. Calcium carbonate (CaCO3), an inorganic biomineral, has been used as an antacid agent. It can be orally administered as a tablet, chewable tablet, capsule, or liquid. Furthermore, it has been used for the controlled and sustained delivery of chemical drugs [2,3,4], photosensitizers [5], and proteins [6,7] because of its biocompatibility and slow biodegradation [8]. CaCO3 is stable at physiological pH, but can be dissociated under acidic conditions [9,10]. Owing to their pH sensitivity, CaCO3-based delivery systems concentrate drugs into targeted cancer tissues within the acidic tumor microenvironment (TME) [4,5,10]. In addition, these systems react with protons (H+) to neutralize the acid [11,12]. CaCO3 can generate carbon dioxide (CO2) in the acidic TME, and this gas-generating property has extended its application as an ultrasound contrast agent for cancer imaging [5,10]. These previous studies have revealed the pH sensitivity, CO2 gas generation, and antacid properties of CaCO3 materials.
Polyphenol tannic acid (TA) is composed of five digalloyl ester groups that have been linked to a central glucose molecule, and exhibits various biological properties, such as antibacterial, anti-inflammatory, antioxidant, and anticancer activities [13,14]. Previous studies have revealed the TA-mediated scavenging of free radicals, leading to the inhibition of lipid oxidation and radical-mediated DNA damage [13,15,16]. Given their ability to undergo multiple interactions with various biomacromolecules (i.e., nucleic acids, peptides, proteins, and polysaccharides) through electrostatic and hydrogen bonding and/or hydrophobic interactions [17,18,19,20,21], TA–macromolecular complexes can be easily produced and used as surface modifiers on organic and inorganic substrates [22,23]. Thus, the TA-modified polymeric hydrogels and scaffolds greatly enhance anti-inflammatory effects in vitro or protect cells under reactive oxygen species (ROS) environments [23,24]. Moreover, as TA can coordinate with metal ions, it can be used to synthesize inorganic Ag and Au nanomaterials [25]. Our group recently prepared oxygen-generating calcium peroxide using TA through coordination between the catechol moieties of TA and calcium ions [26].
Based on these previous reports, we propose that the introduction of TA into CaCO3 materials can endow them with anti-inflammatory and antioxidant functions. As CaCO3 materials exert no anti-inflammatory or antioxidant functions, herein we fabricated tannylated CaCO3 (termed as TA-CaCO3) materials via the coordination of TA to calcium (Ca2+) ions and further nucleation of CaCO3 using carbonate ions (CO32−). We performed their physicochemical characterization and evaluated their antacid and antioxidant effects using colorimetric methods. In addition, we validated their in vitro antioxidant and anti-inflammatory properties in chondrocytes under inflammatory and ROS conditions.
2. Results and Discussion
2.1. Preparation of TA-CaCO3 Materials
TA-CaCO3 materials were synthesized by combining equimolar amounts of Ca2+ and CO32− at different concentrations with a fixed molar concentration of TA under constant stirring, as depicted in Figure 1. Given the tendency of TA to coordinate with metal ions [25,27,28], TA interacts with Ca2+ ions and facilitates the nucleation of CaCO3 to form TA-CaCO3 nanoparticles, which then agglomerate into microparticles due to the interactions between TA-CaCO3 nanoparticles.
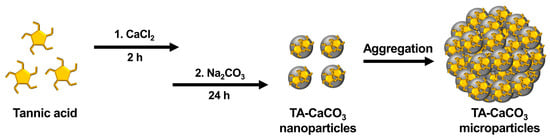
Figure 1.
Schematic illustration of the synthesis of TA-CaCO3 materials. TA was sequentially reacted with CaCl2 for 2 h and Na2CO3 for an additional 24 h in pure water (pH = 7.0).
2.2. Characterization of TA-CaCO3 Materials
The morphologies of the synthesized TA-CaCO3 materials were examined using scanning electron microscopy (SEM). As shown in Figure S1, at 25 molar ratios of calcium chloride (CaCl2)/sodium carbonate (Na2CO3), aggregates comprising small TA-CaCO3 particles were predominantly observed, and very small amounts of micron-sized spherical TA-CaCO3 particles were formed. More spherical TA-CaCO3 microparticles were gradually observed as the molar ratio of CaCl2/Na2CO3 was increased to 75. Interestingly, although spherical TA-CaCO3 microparticles were still observed when the molar ratios of TA and CaCl2/Na2CO3 were 100 or 150, more irregular and broken TA-CaCO3 particles were detected. These results suggested that small TA-CaCO3 particles were formed at lower molar ratios of CaCl2/Na2CO3 and that spherical and broken TA-CaCO3 particles were more common at higher molar ratios of TA and CaCl2/Na2CO3. Based on SEM images, 1:75 TA-CaCO3 particles were selected for further experiments because they produced more spherical TA-CaCO3 microparticles than other TA-CaCO3 particles.
Next, we investigated the particle size of the 1:75 TA-CaCO3 materials and found it to range from approximately 3 to 6 μm (Figure 2a and Figure S1). Interestingly, the magnified SEM images revealed the presence of small particles on the surface of the 1:75 TA-CaCO3 materials. These individual small particles ranged from 17 to 41 nm in size, and were approximately 26.18 ± 4.6 nm in diameter and spherical in shape (Figure 2b). SEM images revealed that the micron-sized TA-CaCO3 materials consisted of small TA-CaCO3 nanoparticles, probably owing to agglomeration following interactions between individual small nanoparticles.
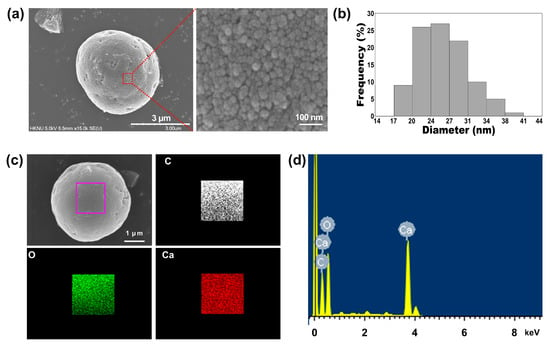
Figure 2.
(a) The representative SEM images and magnified surface of 1:75 TA-CaCO3 materials. The magnified SEM image revealed that the micron-sized TA-CaCO3 materials comprised small TA-CaCO3 nanoparticles. (b) Particle size distribution of the small TA-CaCO3 nanoparticles of 1:75 TA-CaCO3. (c) EDS mapping of elemental carbon (C; white), oxygen (O; green), and calcium (Ca; red). Scale bar: 1 μm. (d) EDS spectrum of 1:75 TA-CaCO3.
The preparation of TA-CaCO3 materials was confirmed using an SEM coupled with energy-dispersive (SEM-EDS) X-ray spectroscopy, inductively coupled plasma optical emission spectrometry (ICP-OES), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) patterns, and X-ray photoelectron spectroscopy (XPS). The EDS mapping and spectrum data revealed the presence of carbon, oxygen, and calcium on the surface of TA-CaCO3 materials (Figure 2c,d). Consistent with a previous report [29], the EDS spectrum showed the peaks of Kα (0.277 eV) corresponding to carbon, Kα (0.523 eV) corresponding to oxygen, and Kα (3.691 eV) and Lα (0.341 eV) corresponding to calcium. ICP-OES analysis showed that 0.1 mg of 1:75 TA-CaCO3 contained 18.6 μg of TA and 81.4 μg of CaCO3. The FT-IR spectrum of commercial CaCO3 showed the characteristic vibrations of carbonate ions (at 1805, 1410, 1090, and 874 cm−1) (Figure 3), as previously reported [30,31]. The preparation of TA-CaCO3 materials was confirmed by the presence of the main asymmetric vibrations at 1460 and 1410 cm−1. However, the symmetric vibration at 725 cm−1 disappeared, implying that TA-CaCO3 has an amorphous structure. Meanwhile, TA-CaCO3 showed characteristic peaks at 3300–3600 cm−1 (O-H stretching), 1445 cm−1 (C-C stretching of benzene ring and methylene; C-O stretching of phenolic), and 755 cm−1 (C-H torsion of benzene ring) [32], indicative of the presence of TA on the material.
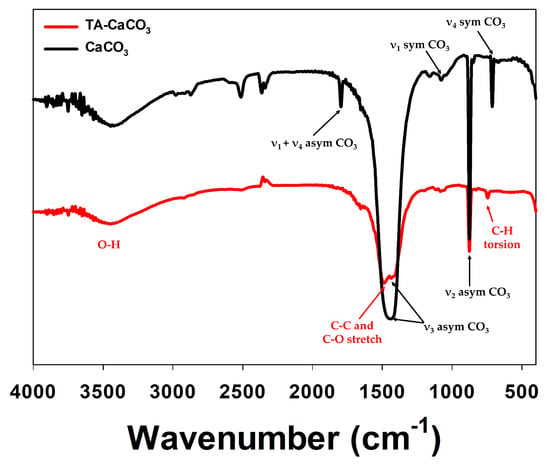
Figure 3.
FT-IR spectra of commercial CaCO3 and TA-CaCO3.
Next, commercial CaCO3 and synthesized TA-CaCO3 crystal phases were identified via XRD analysis (Figure 4a). CaCO3 exhibited the characteristic peaks, such as the plane of the calcite at 29.3° (104), and the calcite crystal faces at 23.02° (012), 35.9° (110), 39.4° (113), and 43.1° (202), respectively. Meanwhile, the diffraction peaks of TA-CaCO3 were observed at 24.8° (110), 27.08° (112), 32.7° (114), 43.8° (300), 49.1° (304), and 50.08° (118), respectively, and these diffraction peaks were consistent with the vaterite crystal faces [33,34]. Based on XRD data, we think that the prepared TA-CaCO3 materials are the vaterite form of calcium carbonate.
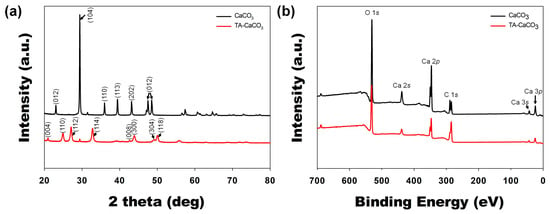
Figure 4.
XRD and XPS analyses of commercial CaCO3 and TA-CaCO3 materials. (a) XRD spectra of CaCO3 and TA-CaCO3. (b) Wide scan XPS spectra recorded from CaCO3 and TA-CaCO3.
XPS data revealed that CaCO3 and TA-CaCO3 showed Ca, O, and C signals (Figure 4b). The binding energy peaks of these two materials appeared O1s at 531 eV, Ca2s at 441 eV, two Ca2p at 351 and 347.2 eV, and two C1s peaks at 289.3 eV (CO3 in the CaCO3 surface) and 284.6 eV (adventitious carbon peak), respectively. These data demonstrated the successful synthesis of TA-CaCO3, and the synthesized TA-CaCO3 materials are vaterite calcium carbonate.
2.3. Antacid Effects of TA-CaCO3
CaCO3 exists as a stable crystalline solid at physiological pH, but can be dissociated into ionic species at or below weakly acidic pH [5,9]. Under acidic pH, CaCO3 neutralizes acids by reacting with the proton (H+) [11,12], and it has been used as an acid neutralizer [35].
To verify the antacid effects of TA-CaCO3 materials, commercial CaCO3 and TA-CaCO3 were dispersed in phosphate-buffered saline (PBS; physiological pH = 7.4) and simulated gastric fluid (SGF, pH 1.5) containing bromothymol blue (BTB). The color and absorption changes of BTB were monitored before and after the reaction, because BTB is a useful acid/base indicator to distinguish the acidity, neutrality, and alkalinity of an aqueous solution [36,37]. As shown in Figure 5a and Figure S2, the aqueous BTB solution without commercial CaCO3 and TA-CaCO3 exhibited a blue color at pH = 7.4 and turned to a yellowish color in the presence of SGF (pH = 1.5). CaCO3 and TA-CaCO3 showed a deep blue color of BTB at pH = 7.4, indicating the slight pH increases of the solution following the degradation of CaCO3 and TA-CaCO3, even at pH = 7.4. In contrast, the yellowish BTB solution at pH = 1.5 turned to a bluish green color after the reaction with CaCO3 and TA-CaCO3, indicating that the pH of the solution increased to a nearly neutral pH (approximately 7). Consistent with these color changes, the λmax shift of BTB occurred from 615 nm at pH = 7.4 to 433 nm under SGF (pH = 1.5) (Figure 5b). However, the absorptions of both CaCO3 and TA-CaCO3 groups increased at 615 nm, while the λmax of BTB at pH = 1.5 was blue-shifted from 433 nm to 403 nm, implying the pH increases of the solutions after reacting with two kinds of CaCO3 materials. Meanwhile, TA-CaCO3 showed significantly higher absorbance below 400 nm at both pH = 1.5 and pH = 7.4, indicating the presence of TA in the solutions. Furthermore, TA-CaCO3 had a lower absorbance than commercial CaCO3 at 615 nm. This is attributed to the fact that TA-CaCO3 contained a lower amount of CaCO3 than commercial CaCO3.
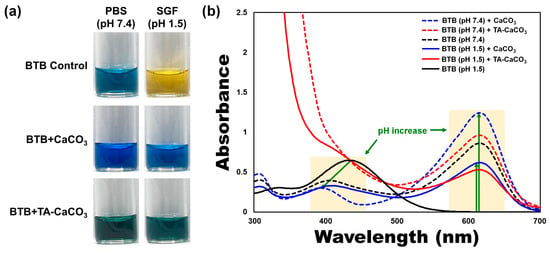
Figure 5.
Antacid effects of TA-CaCO3 using the colorimetric bromothymol blue (BTB) method. (a) Color changes and (b) UV/vis spectra of BTB under PBS (pH = 7.4) and simulated gastric fluid (SGF, pH = 1.5) after treatment with commercial CaCO3 and 1:75 TA-CaCO3. Green-colored arrows indicate a pH increase.
2.4. Antioxidant Effects of TA-CaCO3
The antioxidant effects of TA-CaCO3 were determined using the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [38]. As shown in Figure 6, the DPPH solution showed a deep violet color, with an absorbance at approximately 520 nm, owing to the delocalization of the spare electron over the molecule [38]. CaCO3-treated DPPH solution also had a deep violet color and an absorption band at around 520 nm, indicating that CaCO3 had no antioxidant effect. In contrast, TA-CaCO3-treated DPPH solution turned yellowish in color and lost its absorbance at 520 nm. This loss of violet color might be attributed to the presence of TA molecules within TA-CaCO3 because TA molecules have an effective radical-scavenging activity, as its hydroxyl groups easily reduce the free radicals of DPPH [39,40]. These data imply that TA-CaCO3 materials have antioxidant properties and effective ROS-scavenging activity.
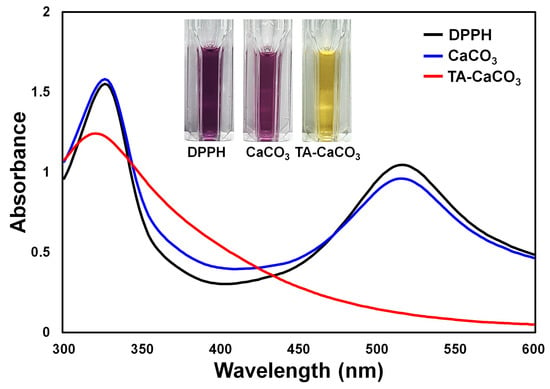
Figure 6.
Antioxidant effects of TA-CaCO3 determined using the colorimetric DPPH method. Changes in the color and UV/vis spectra of DPPH after treatment with commercial CaCO3 and 1:75 TA-CaCO3. Inset: Photos of the DPPH solution treated with commercial CaCO3 and 1:75 TA-CaCO3.
2.5. In Vitro Anti-Inflammatory Effects of TA-CaCO3 in Inflamed Chondrocytes
The cytotoxicity study of TA-CaCO3 was examined against normal chondrocytes. Figure S3 revealed that chondrocytes maintained their cell viabilities above 90% up to a 100 μg/mL concentration, indicating that TA-CaCO3 is non-toxic.
In addition to antioxidant effects, TA molecules also exhibit anti-inflammatory properties [41]. We examined the in vitro anti-inflammatory effects of TA-CaCO3 by analyzing the mRNA expression of pro-inflammatory factors, such as cyclooxygenase-2 (COX-2), interleukin (IL)-1β, IL-6, matrix metalloproteinase (MMP)-3, MMP-13, and tumor necrosis factor (TNF)-α in lipopolysaccharide (LPS)-stimulated chondrocytes, because LPS treatment leads to the overexpression of these pro-inflammatory factors [41,42,43].
As shown in Figure 7, LPS significantly upregulated the mRNA expression of all pro-inflammatory cytokines on day three compared to the control treatment. However, the mRNA levels of these pro-inflammatory cytokines were efficiently suppressed following treatment with TA-CaCO3 in a dose-dependent manner. These results indicate that TA-CaCO3 materials are highly effective in suppressing pro-inflammatory factors in LPS-stimulated chondrocytes. Consistent with previous studies, the present study also supports the anti-inflammatory effects of TA-based materials [24,44].
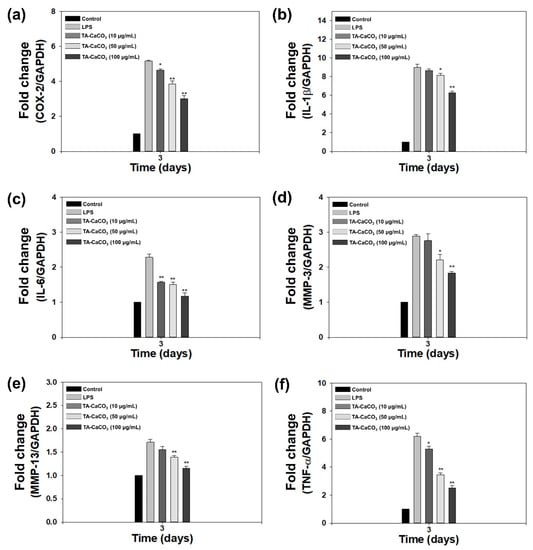
Figure 7.
In vitro anti-inflammatory effects of 1:75 TA-CaCO3 in inflamed chondrocytes. The mRNA levels of pro-inflammatory factors, including (a) cyclooxygenase-2 (COX-2), (b) interleukin-1β (IL-1β), (c) IL-6, (d) matrix metalloproteinase-3 (MMP-3), (e) MMP-13, and (f) tumor necrosis factor-α (TNF-α) in LPS-stimulated chondrocytes on day three. Data are represented as the mean ± SD (n = 5). ** p < 0.01 and * p < 0.05.
2.6. In Vitro Antioxidant and Protective Effects of TA-CaCO3 in H2O2-Treated Chondrocytes
The exogenous treatment of cells with H2O2 results in the induction of oxidative stress and produces intracellular ROS [45,46]. To demonstrate the antioxidant activities of TA-CaCO3 at the cellular level, H2O2 (300 μM)-pretreated chondrocytes were incubated with different concentrations of TA-CaCO3 extracts, and the intracellular ROS level was detected from the fluorescence signal of 2′,7-dichlorodihydrofluorescein diacetate (DCFDA) using a confocal laser scanning microscope. As shown in Figure 8a, no fluorescence was observed in normal cells, whereas a strong signal was detected in cells treated with 300 μM of H2O2 alone. The treatment of the extracts containing different concentrations of TA-CaCO3 led to a remarkable decrease in fluorescence intensities, indicative of the excellent scavenging of intracellular ROS by TA-CaCO3.
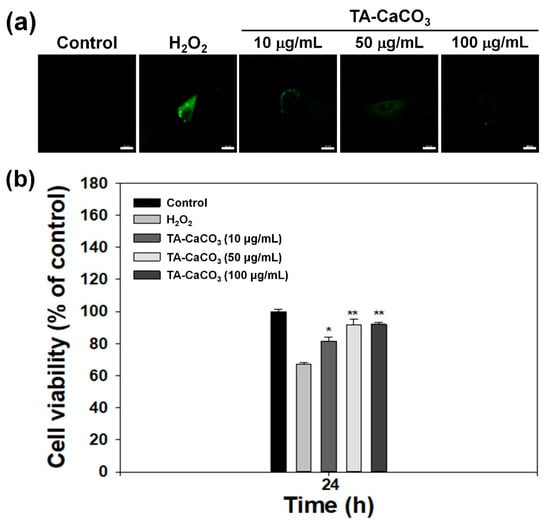
Figure 8.
In vitro antioxidant effects of TA-CaCO3. (a) Representative fluorescence images of intracellular ROS in chondrocytes treated with extracts containing different concentrations of 1:75 TA-CaCO3 for 24 h after pretreatment with 300 μM of H2O2 for 30 min. Scale bar: 20 μm. (b) Viability of chondrocytes treated with different concentrations of 1:75 TA-CaCO3 for 24 h after pretreatment with 300 μM of H2O2 for 30 min. Results are expressed as the mean ± SD (n = 5). ** p < 0.01 and * p < 0.05.
Previous studies have reported that H2O2 can be toxic to cells because it produces hydroxyl radicals [45,46]. Substances with antioxidant properties prevent cell damage and protect the human body from free radicals or ROS by supplying electrons from antioxidants to the damaged cells [47,48]. Based on these facts, we investigated whether TA-CaCO3 with antioxidant properties is effective in mediating cellular protection by measuring the viability of chondrocytes treated with 300 μM of H2O2 (Figure 8b). In comparison with the control group, the chondrocytes cultured in 300 μM of H2O2 showed a significant decrease in viability owing to the oxidative damage to cellular components [49]. However, the viability of the cells treated with different concentrations of TA-CaCO3 significantly increased in a concentration-dependent manner, and was much higher than that of the chondrocytes treated with 300 μM of H2O2 alone. Consistent with our previous studies showing that substances with antioxidant properties could protect the cells against ROS environments [23,50], TA-CaCO3 effectively protected the cells and prevented cellular damage under ROS conditions. This protective effect might be associated with the effective radical-scavenging activity of TA molecules within TA-CaCO3 materials.
3. Materials and Methods
3.1. Materials
Calcium chloride dihydrate (CaCl2·2H2O, abbreviated as CaCl2), sodium carbonate monohydrate (Na2CO3·H2O, abbreviated as Na2CO3), TA, potassium bromide (KBr), BTB, DPPH, sodium hydroxide (NaOH), 26% sodium chloride (NaCl) solution, and hydrochloric acid (HCl) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol (MeOH, 99.5%) and absolute ethanol (EtOH, 99.9%) were provided by Samchun (Pyeongtaek, Korea) and DUKSAN (Ansan, Korea), respectively. Commercial CaCO3 nanopowder (purity: 98%) was supplied by US Research Nanomaterials (Houston, TX, USA), and PBS by Lonza (Walkersville, MD, USA). SGF (pH = 1.5) was prepared using ultra-pure water (99.46% v/v), concentrated HCl (0.23% v/v), and 26% NaCl solution (0.21% v/v). To prepare the BTB solution, 10 mg of BTB was dissolved in 1 mL of 4% NaOH solution, and treated with 2 mL of 70% EtOH (2 mL) and 100 mL of ultra-pure water. Finally, a 10% NaOH solution was added dropwise to the BTB solution when the color of the BTB solution changed to blue.
3.2. Preparation of TA-CaCO3
For the preparation of TA-CaCO3 materials, TA (0.0294 mmol) was added to ultra-pure water (5 mL, pH = 7.0) and stirred to dissolve at 800 rpm for 1 h. Different moles of CaCl2 (0.735, 1.47, 2.20, 2.94, and 4.41 mmol) were dissolved in ultra-pure water (3 mL, pH = 7.0). The prepared CaCl2 solutions were added to the TA solution and stirred at 800 rpm for 2 h. In addition, different moles of Na2CO3 (0.735, 1.47, 2.20, 2.94, and 4.41 mmol) were dissolved in ultra-pure water (pH = 7.0) and added dropwise to the mixture solution of TA and CaCl2. The mixture was stirred at 800 rpm for 24 h, and the resulting solution was centrifuged at 1200 rpm for 5 min and washed with ultrapure water. The collected TA-CaCO3 materials were freeze-dried for two days. TA-CaCO3 materials synthesized at different molar ratios of TA (molar ratio: 1), CaCl2 (molar ratios: 25, 50, 75, 100, and 150), and Na2CO3 (molar ratios of 25, 50, 75, 100, and 150) were named as follows: 1:25, 1:50, 1:75, 1:100, and 1:150 TA-CaCO3.
3.3. Characterization of TA-CaCO3
For SEM analysis, the prepared TA-CaCO3 materials were coated with platinum. Then, the morphologies and sizes of the as-prepared TA-CaCO3 materials were examined using field-emission scanning electron microscopy (FE-SEM, S-4700, Hitachi, Japan). For size analysis of the prepared TA-CaCO3, randomly selected individual particles of each TA-CaCO3 type were analyzed using ImageJ software (Version 1.47; US National Institutes of Health, Bethesda, MD, USA). The surface elemental composition of TA-CaCO3 was examined using SEM-EDS.
To determine the amount of Ca2+ in TA-CaCO3, 1:75 TA-CaCO3 materials (0.1 mg) were co-treated with nitric acid and H2O2, and the prepared samples were analyzed using ICP-OES (Optima 7300 DV, PerkinElmer, Waltham, MA, USA). Through ICP-OES analysis, the amount of TA and CaCO3 within TA-CaCO3 was determined using a standard curve (Y = 10,807x + 1340.9; R2 = 0.999).
The prepared TA-CaCO3 materials were characterized using FT-IR (Shimadzu 8400S, Kyoto, Japan). The FT-IR spectrum was acquired using a KBr pellet at a resolution of 4 cm−1 between 4,000 and 400 cm−1.
XRD and XPS analyses of 1:75 TA-CaCO3 were done by using a K-alpha+ (Thermo Scientific, Waltham, MA, USA) with Cu Kα radiation and a D8 ADVANCE diffractometer (Bruker, Germany) with Cu Kα radiation, respectively.
3.4. Antacid Effects of TA-CaCO3 Materials
To investigate the in vitro antacid effects, CaCO3 (10 mg) or TA-CaCO3 (1:75, 10 mg) was dispersed in 2 mL of two different solutions, PBS (pH = 7.4) and SGF (pH = 1.5). Each solution (1.4 mL) was mixed well with the as-prepared BTB solution (0.6 mL) and stirred. After 1 h, each solution was centrifuged at 13,500 rpm for 30 min. The color of the collected supernatant was observed by taking photos, and the absorbance was analyzed using a UV/vis spectrophotometer (NEO-S490, NEOGEN, Daejeon, Korea).
3.5. Antioxidant Effects of TA-CaCO3 Materials
To examine and compare the in vitro antioxidant effects, CaCO3 (1 mg) or TA-CaCO3 (1 mg) was added to 1 mL of DPPH (0.1 mM) dissolved in MeOH and reacted at 600 rpm for 30 min. Then, each solution was centrifuged at 5,500 rpm for 10 min. The color of the supernatant was observed by taking photos, and the absorbance was recorded using a UV/vis spectrophotometer (NEO-S490).
3.6. In Vitro Cytotoxicity and Anti-Inflammatory Effects
Human knee articular chondrocytes were obtained from Lonza (Basel, Switzerland) and cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM, Welgene, Seoul, Korea), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37 °C.
The cytotoxicity of TA-CaCO3 materials was determined with a cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan). Chondrocytes (1 × 104 cells/well) were seeded and incubated in a 96-well plate. After 24 h of incubation, TA-CaCO3 materials at 0, 10, 50, and 100 μg/mL concentrations were treated into the cells. After 24 or 48 h of exposure, the CCK-8 reagent was additionally treated to the cells for 1 h, and the optical density was then recorded at 450 nm using a Multimode Reader. The cell viability of each group was represented as the percentage of viable cells versus the control group.
The in vitro anti-inflammatory effects of TA-CaCO3 against LPS-stimulated chondrocytes were evaluated by determining the mRNA levels of pro-inflammatory factors using a real-time polymerase chain reaction (PCR). Cells (1 × 105 cells/well) were seeded into 24-well plates and cultured overnight. Cells were simultaneously treated with both LPS (100 ng/mL) and different concentrations of TA-CaCO3 (0, 10, 50, and 100 μg/mL). After three-day treatment, the cells from each group were collected, and the total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s guidelines. Total RNA (1 μg) was reverse-transcribed into cDNA using AccuPower RT PreMix (Bioneer, Daejeon, Korea). The sequences of the target gene primers are provided in Supplementary Materials (Table S1). Real-time PCR analysis was conducted using an ABI7300 Real-Time Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The mRNA levels of the target genes were normalized to those of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and presented as relative levels.
3.7. Antioxidant Effects of TA-CaCO3 at the Cellular Level
To investigate the antioxidant effects of TA-CaCO3 at the cell level, we conducted DCFDA staining and a DCFDA assay. Cells (1 × 104 cells/well) were seeded and incubated in 24-well plates with microscope cover glasses for 24 h. Meanwhile, the extract solutions from the different concentrations of TA-CaCO3 (0, 10, 50, and 100 μg/mL) were prepared by incubating them in DMEM medium at 37 °C for 24 h, followed by collecting the supernatants from each group. Next, cells were treated with 300 μM of H2O2 at 37 °C for 30 min, followed by additional treatment of the collected extract solutions from each group for 24 h. After washing the cells with PBS, the cells were stained with DCFDA (25 μM) for 30 min under a dark condition, washed with PBS, and then fixed with 3.7% paraformaldehyde for 30 min. The fluorescence intensity within the cells was observed using a confocal laser scanning microscope (CLSM, LSM700, Zeiss, Germany).
3.8. Protection of Cell Viability in the ROS Environment
To investigate whether TA-CaCO3 can protect cell viability in an ROS environment, chondrocytes (1 × 105 cells/well) were seeded in a 24-well culture plate. Then, the cells were treated with 300 μM of H2O2 at 37 °C for 30 min, followed by additional treatment of TA-CaCO3 at 10, 50, and 100 μg/mL concentrations. After 24 h of treatment, the CCK-8 (Dojindo) reagent was added to the cells for 1 h. The supernatant from each group was transferred into a 96-well plate, and its optical density was measured at 450 nm using a multimode reader.
4. Conclusions
In the present study, we prepared TA-CaCO3 materials by reacting TA with CaCl2 and Na2CO3, which led to the interaction between TA and Ca2+ ions, followed by nucleation of CaCO3. Micron-sized 1:75 TA-CaCO3 materials (ranging from 3 to 6 μm) comprised small nanoparticles in a size range of 17–41 nm. TA-CaCO3 materials could effectively neutralize the SGF solution and scavenge free radicals. In addition, these particles significantly suppressed the mRNA expression of pro-inflammatory cytokines and mediators and scavenged intracellular ROS in cells. Their anti-inflammatory and antioxidant activities protected chondrocytes from ROS. These results suggest that TA-CaCO3 materials have excellent antacid, antioxidant, and anti-inflammatory properties. Importantly, TA molecules can undergo multiple interactions with nucleic acids, peptides, proteins, and polysaccharides. Furthermore, due to the molecular adsorption of CaCO3 materials, CaCO3-based materials can improve the incorporation efficacy of drugs. Thus, using TA-CaCO3 materials, we will develop dual drug delivery systems that can ferry both a chemical drug and protein drug, and then apply them to treat inflammatory cells or diseases.
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/article/10.3390/ijms22094614/s1.
Author Contributions
Conceptualization, K.P. and S.-E.K.; methodology, S.-Y.J., H.H., H.-S.J. and S.C.; formal analysis, S.-Y.J., H.H., H.-S.J. and S.C.; writing—original draft preparation, S.-Y.J. and K.P.; writing—review and editing, H.-J.K., K.P. and S.-E.K.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Research Foundation of Korea (NRF-2019M3A9E2066883) and by the Chung-Ang University Research Grants in 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Wei, W.; Ma, G.H.; Hu, G.; Yu, D.; McLeish, T.; Su, Z.G.; Shen, Z.Y. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J. Am. Chem. Soc. 2008, 130, 15808–15810. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, Q.; Gao, C. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Dong, Z.; Gong, Y.; Hao, Y.; Tian, L.; Yang, X.; Liu, Z.; Feng, L. CaCO3-Assisted Preparation of pH-Responsive Immune-Modulating Nanoparticles for Augmented Chemo-Immunotherapy. Nano-Micro Lett. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Park, D.J.; Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. Photosensitizer-loaded bubble-generating mineralized nanoparticles for ultrasound imaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.N.; Min, K.H.; Lee, H.J.; Jegal, J.H.; Lee, J.W.; Lee, S.C. Calcium Carbonate Mineralized Nanoparticles as an Intracellular Transporter of Cytochrome c for Cancer Therapy. Chem. Asian J. 2015, 10, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 2018, 122, 96–103. [Google Scholar] [CrossRef]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef]
- Ogomi, D.; Serizawa, T.; Akashi, M. Controlled release based on the dissolution of a calcium carbonate layer deposited on hydrogels. J. Control. Release 2005, 103, 315–323. [Google Scholar] [CrossRef]
- Min, K.H.; Min, H.S.; Lee, H.J.; Park, D.J.; Yhee, J.Y.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Silvestre, O.F.; Chen, X.; et al. pH-controlled gas-generating mineralized nanoparticles: A theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano 2015, 9, 134–145. [Google Scholar] [CrossRef]
- Rodriguez-Stanley, S.; Ahmed, T.; Zubaidi, S.; Riley, S.; Akbarali, H.I.; Mellow, M.H.; Miner, P.B. Calcium carbonate antacids alter esophageal motility in heartburn sufferers. Dig. Dis. Sci. 2004, 49, 1862–1867. [Google Scholar] [CrossRef]
- Raliya, R.; Som, A.; Shetty, N.; Reed, N.; Achilefu, S.; Biswas, P. Nano-antacids enhance pH neutralization beyond their bulk counterparts: Synthesis and characterization. RSC Adv. 2016, 6, 54331–54335. [Google Scholar] [CrossRef]
- Labieniec, M.; Gabryelak, T. Oxidatively modified proteins and DNA in digestive gland cells of the fresh-water mussel Unio tumidus in the presence of tannic acid and its derivatives. Mutat. Res. Toxicol. Environ. Mutagen. 2006, 603, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Turgut Cosan, D.; Saydam, F.; Ozbayer, C.; Doganer, F.; Soyocak, A.; Gunes, H.V.; Degirmenci, I.; Kurt, H.; Ustuner, M.C.; Bal, C. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology 2015, 67, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.K.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Andrade, R.G., Jr.; Dalvi, L.T.; Silva, J.M., Jr.; Lopes, G.K.; Alonso, A.; Hermes-Lima, M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch. Biochem. Biophys. 2005, 437, 1–9. [Google Scholar] [CrossRef]
- Shukla, A.; Fang, J.C.; Puranam, S.; Jensen, F.R.; Hammond, P.T. Hemostatic multilayer coatings. Adv. Mater. 2012, 24, 492–496. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Meng, F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N.; Silan, C. Inherently antioxidant and antimicrobial tannic acid release from poly(tannic acid) nanoparticles with controllable degradability. Colloids Surf. B Biointerfaces 2016, 142, 334–343. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H.A.; Lee, M.; Shin, Y.; Song, J.J.; Kang, S.W.; Nam, D.H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef]
- Hong, S.; Yeom, J.; Song, I.T.; Kang, S.M.; Lee, H.; Lee, H. Pyrogallol 2-Aminoethane: A Plant Flavonoid-Inspired Molecule for Material-Independent Surface Chemistry. Adv. Mater. Interfaces 2014, 1, 1400113. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. Int. J. Mol. Sci. 2018, 19, 3602. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Aromal, S.A.; Philip, D. Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys. E Low Dimens. Syst. Nanostructures 2012, 44, 1692–1696. [Google Scholar] [CrossRef]
- Park, J.S.; Song, Y.J.; Lim, Y.G.; Park, K. Facile Fabrication of Oxygen-Releasing Tannylated Calcium Peroxide Nanoparticles. Materials 2020, 13, 3864. [Google Scholar] [CrossRef]
- Lopes, L.C.S.; Brito, L.M.; Bezerra, T.T.; Gomes, K.N.; Carvalho, F.A.A.; Chaves, M.H.; Cantanhede, W. Silver and gold nanoparticles from tannic acid: Synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. An. Acad. Bras. Cienc. 2018, 90, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Abulateefeh, S.R.; Taha, M.O. Enhanced drug encapsulation and extended release profiles of calcium-alginate nanoparticles by using tannic acid as a bridging cross-linking agent. J. Microencapsul. 2015, 32, 96–105. [Google Scholar] [CrossRef]
- Gokhe, U.B.; Koparkar, K.A.; Omanwar, S.K. Synthesis and fluorescence properties of Ca2SiO4:Dy3+ phosphor for solid state lighting application. J. Mater. Sci. Mater. Electron. 2016, 27, 9286–9290. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brečević, L. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Cakar, S.; Ozacar, M. Fe-tannic acid complex dye as photo sensitizer for different morphological ZnO based DSSCs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 163, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, M.; Wołcyrz, M.; Ożyhar, A.; Dobryszycki, P. Phosphorylation of Intrinsically Disordered Starmaker Protein Increases Its Ability To Control the Formation of Calcium Carbonate Crystals. Cryst. Growth Des. 2012, 12, 158–168. [Google Scholar] [CrossRef]
- Dong, W.; Tu, C.; Tao, W.; Zhou, Y.; Tong, G.; Zheng, Y.; Li, Y.; Yan, D. Influence of the Mole Ratio of the Interacting to the Stabilizing Portion (RI/S) in Hyperbranched Polymers on CaCO3 Crystallization: Synthesis of Highly Monodisperse Microspheres. Cryst. Growth Des. 2012, 12, 4053–4059. [Google Scholar] [CrossRef]
- Torne, S.; Sheela, A.; Sarada, N.C. Investigation of the Role of the Alkalizing Agent in Sodium Alginate Liquid Anti-reflux Suspension. Curr. Drug Ther. 2020, 15, 53–60. [Google Scholar] [CrossRef]
- Puschett, J.B.; Rao, B.S.; Karandikar, B.M.; Matyjaszewski, K. Indicator characteristics of bromothymol blue derivatives. Talanta 1991, 38, 335–338. [Google Scholar] [CrossRef]
- De Meyer, T.; Hemelsoet, K.; Van der Schueren, L.; Pauwels, E.; De Clerck, K.; Van Speybroeck, V. Investigating the halochromic properties of azo dyes in an aqueous environment by using a combined experimental and theoretical approach. Chem. Eur. J. 2012, 18, 8120–8129. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Ozcelik, B.; Lee, J.H.; Min, D.B. Effects of Light, Oxygen, and pH on the Absorbance of 2,2-Diphenyl-1-picrylhydrazyl. J. Food Sci. 2003, 68, 487–490. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-kappaB activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, S.E.; Shim, K.S.; Kim, H.J.; Song, M.H.; Park, K.; Song, H.R. Exploring the In Vivo Anti-Inflammatory Actions of Simvastatin-Loaded Porous Microspheres on Inflamed Tenocytes in a Collagenase-Induced Animal Model of Achilles Tendinitis. Int. J. Mol. Sci. 2018, 19, 820. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yoon, J.S.; Lee, J.Y.; Kim, H.J.; Park, K.; Kim, S.E. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohydr. Polym. 2019, 209, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef]
- Mashimo, M.; Kato, J.; Moss, J. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 18964–18969. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, S.; Hong, J.Y.; Shim, K.S.; Kim, T.H.; Park, K.; Lee, S.H. Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials 2019, 10, 50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).