Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
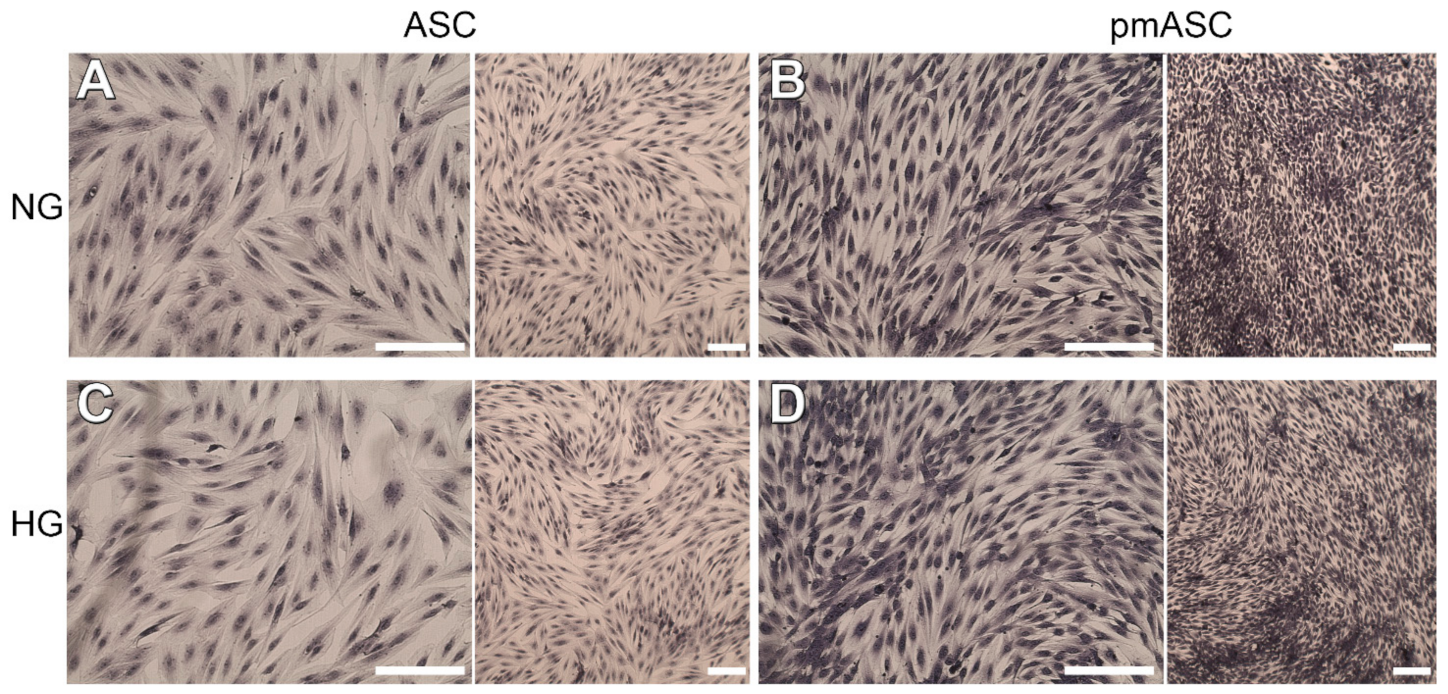
2.1. Cell Proliferation and Viability
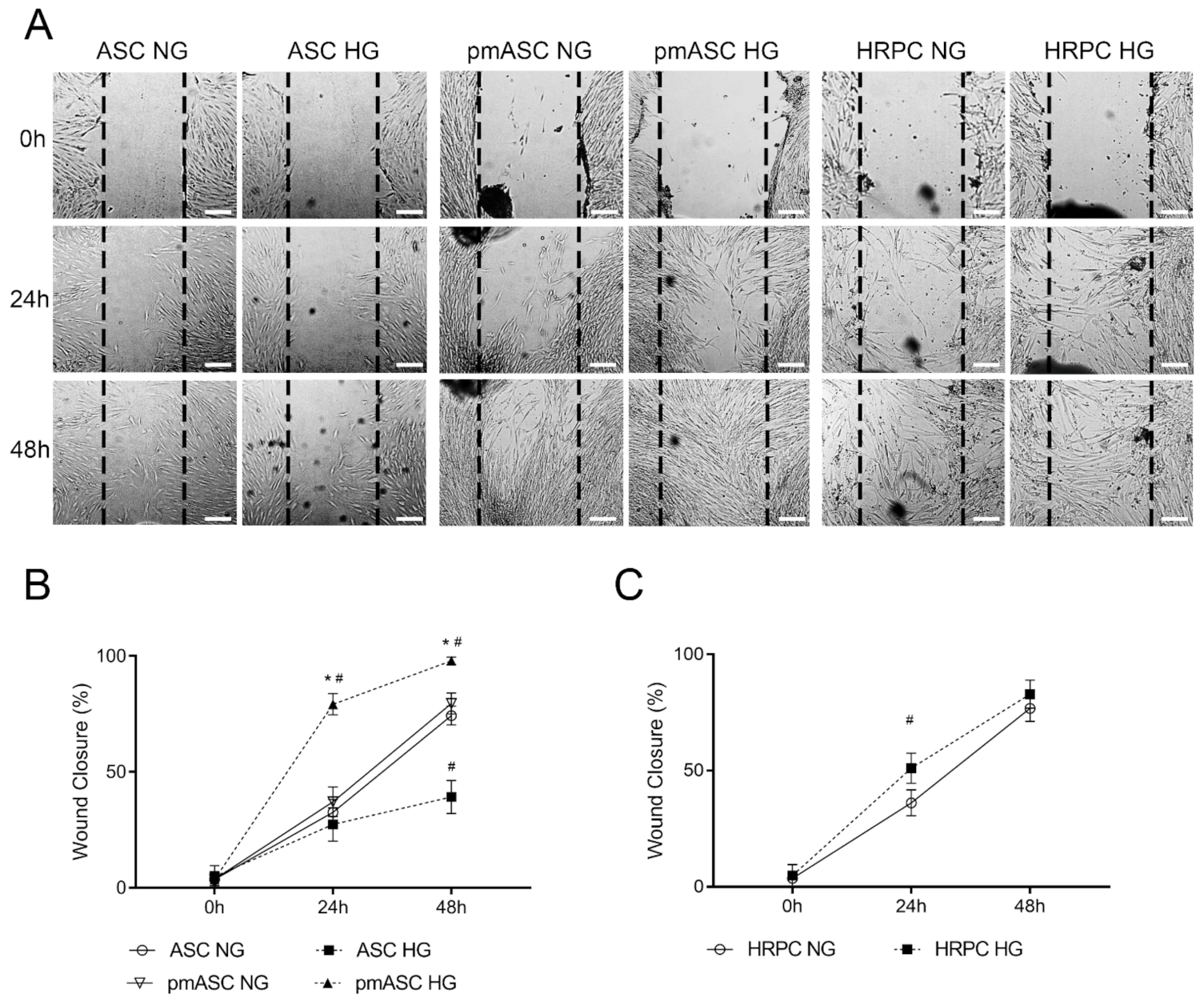
2.2. Cell Migration
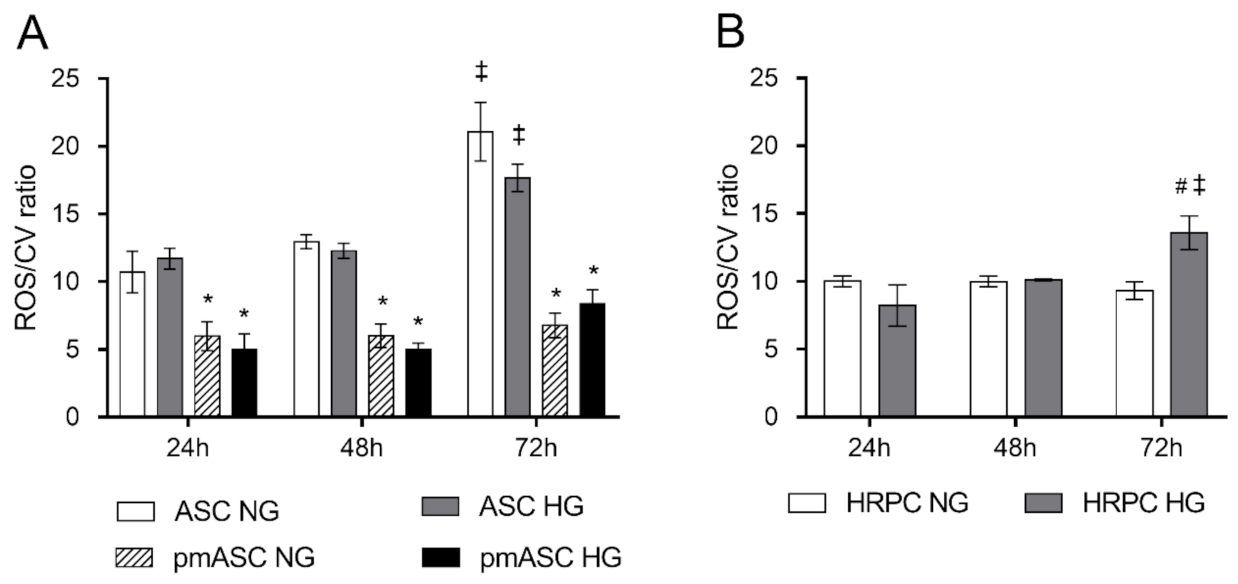
2.3. ROS Level Measurements
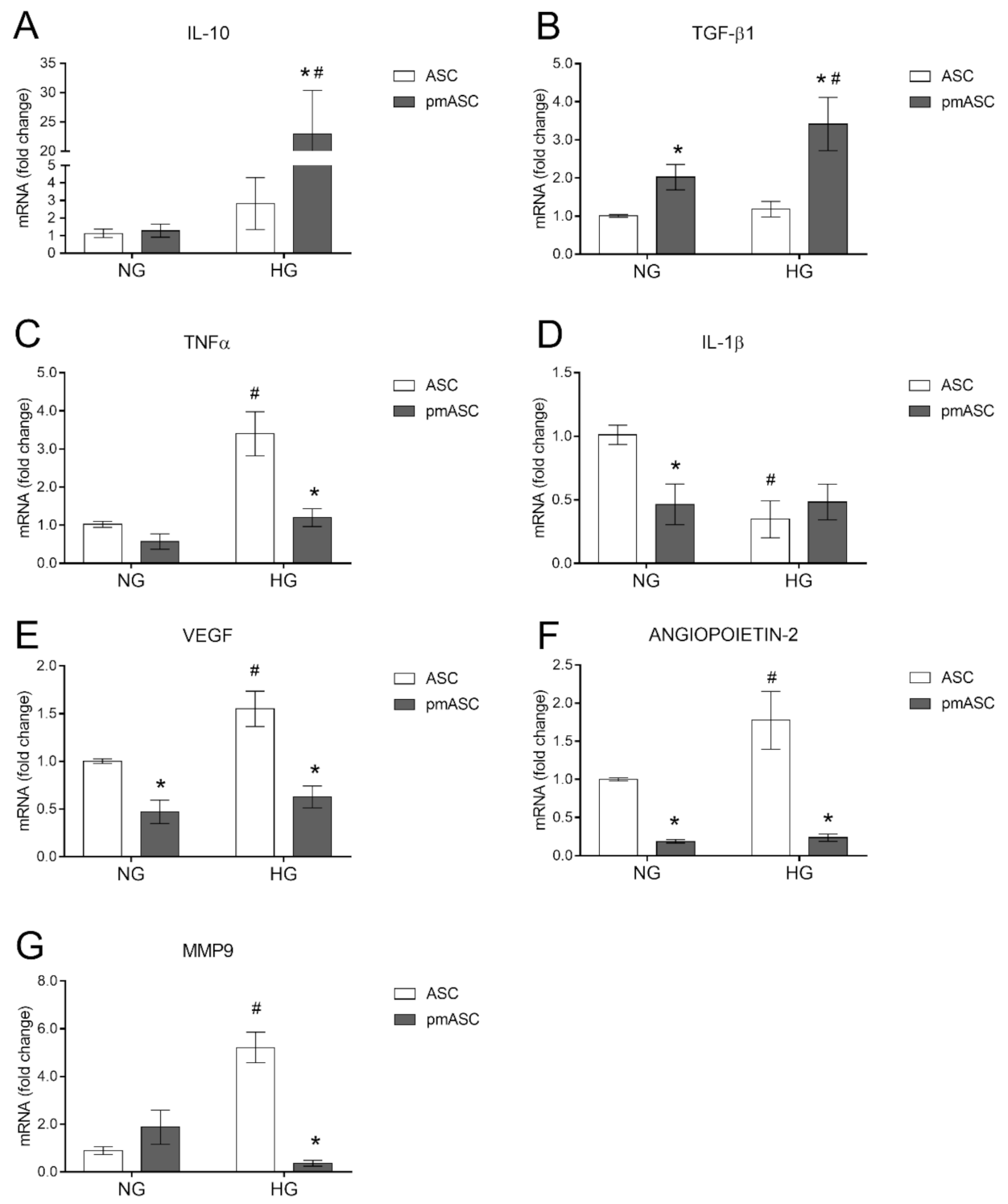
2.4. High Glucose Effects on ASC mRNA Levels of Anti-Inflammatory Cytokines, Pro-Inflammatory Cytokines, and Angiogenic Factors
2.5. Three-Dimensional Cultures in Matrigel
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.2.1. HRPC Cultures
4.2.2. HREC Cultures
4.2.3. ASC Cultures
4.2.4. ASCs Undergoing Pericyte-Like Differentiation
4.3. Cell Proliferation Assay
4.4. Cell Viability Assay
4.5. Wound-Healing Assay
4.6. ROS Measurements
4.7. Extraction of Total RNA and Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.8. Three-Dimensional Cultures in Matrigel
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCs | human adipose-derived mesenchymal stem cells |
| DR | diabetic retinopathy |
| ROS | reactive oxygen species |
| PmASCs | ASCs pre-cultured in the pericyte medium |
| TGF-β1 | transforming growth factor-β1 |
| IL-10 | interleukin-10 |
| IL-1β | interleukin-1β |
| TNF-α | tumor necrosis factor-α |
| BRB | blood retinal barrier |
| VEGF | vascular endothelial growth factor |
| PM | pericyte medium |
| α-SMA | smooth muscle actin α |
| NG2 | neural/glial antigen 2 |
| HRECs | human retinal endothelial cells |
| MMP-9 | matrix metallopeptidase-9 |
| HRPCs | pericytes |
| NG | normal glucose |
| HG | high glucose |
| ECM | endothelial cell medium |
| DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| MTT | 3-[4,5-dimethylthiazol-2-y l]-2,5-diphenyl tetrasodium bromide |
References
- Lo Furno, D.; Graziano, A.C.; Avola, R.; Giuffrida, R.; Perciavalle, V.; Bonina, F.; Mannino, G.; Cardile, V. A citrus bergamia extract decreases adipogenesis and increases lipolysis by modulating PPAR levels in mesenchymal stem cells from human adipose tissue. PPAR Res. 2016, 2016, 4563815. [Google Scholar] [CrossRef] [PubMed]
- Furno, D.L.; Graziano, A.C.E.; Caggia, S.; Perrotta, R.E.; Tarico, M.S.; Giuffrida, R.; Cardile, V. Decrease of apoptosis markers during adipogenic differentiation of mesenchymal stem cells from human adipose tissue. Apoptosis 2013, 18, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Parrinello, N.L.; Forte, S.; Castrogiovanni, P.; Pricoco, E.; Imbesi, R.; Castorina, S.; Leonardi, R.; et al. Cycloastragenol as an Exogenous Enhancer of Chondrogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. A Morphological Study. Cells 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ge, R.; Wu, H.; Ding, X.; Song, H.; Ji, H.; Li, M.; Ma, Y.; Li, S.; Wang, C.; et al. The osteogenic differentiation of human adipose-derived stem cells is regulated through the let-7i-3p/LEF1/β-catenin axis under cyclic strain. Stem Cell Res. Ther. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Furno, D.L.; Mannino, G.; Giuffrida, R.; Gili, E.; Vancheri, C.; Tarico, M.S.; Perrotta, R.E.; Pellitteri, R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J. Cell. Physiol. 2018, 233, 7091–7100. [Google Scholar] [CrossRef]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef]
- Santos, A.D.L.; Da Silva, C.G.; Barretto, L.S.D.S.; Franciozi, C.E.D.S.; Tamaoki, M.J.S.; De Almeida, F.G.; Faloppa, F. Biomechanical evaluation of tendon regeneration with adipose-derived stem cell. J. Orthop. Res. 2018, 37, 1281–1286. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Evans, W.; Pentecost, M.; Lenin, R.; Periasamy, R.; Jha, K.A.; Alli, S.; Gentry, J.; Thomas, S.M.; Sohl, N.; et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Res. Ther. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Fiori, A.; Terlizzi, V.; Kremer, H.; Gebauer, J.; Hammes, H.-P.; Harmsen, M.C.; Bieback, K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiol. 2018, 223, 729–743. [Google Scholar] [CrossRef]
- Kremer, H.; Gebauer, J.; Elvers-Hornung, S.; Uhlig, S.; Hammes, H.-P.; Beltramo, E.; Steeb, L.; Harmsen, M.C.; Sticht, C.; Klueter, H.; et al. Pro-angiogenic Activity Discriminates Human Adipose-Derived Stromal Cells From Retinal Pericytes: Considerations for Cell-Based Therapy of Diabetic Retinopathy. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Giurdanella, G.; Anfuso, C.D.; Olivieri, M.; Lupo, G.; Caporarello, N.; Eandi, C.M.; Drago, F.; Bucolo, C.; Salomone, S. Aflibercept, bevacizumab and ranibizumab prevent glucose-induced damage in human retinal pericytes in vitro, through a PLA2/COX-2/VEGF-A pathway. Biochem. Pharmacol. 2015, 96, 278–287. [Google Scholar] [CrossRef]
- Giurdanella, G.; Lupo, G.; Gennuso, F.; Conti, F.; Furno, D.L.; Mannino, G.; Anfuso, C.D.; Drago, F.; Salomone, S.; Bucolo, C. Activation of the VEGF-A/ERK/PLA2 Axis Mediates Early Retinal Endothelial Cell Damage Induced by High Glucose: New Insight from an In Vitro Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 7528. [Google Scholar] [CrossRef]
- Alikhani, M.; Roy, S.; Graves, D.T. FOXO1 plays an essential role in apoptosis of retinal pericytes. Mol. Vis. 2010, 16, 408–415. [Google Scholar]
- Beltramo, E. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Peters, S.; Cree, I.A.; Alexander, R.; Turowski, P.; Ockrim, Z.; Patel, J.; Boyd, S.R.; Joussen, A.M.; Ziemssen, F.; Hykin, P.G.; et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine 2007, 40, 144–150. [Google Scholar] [CrossRef]
- Benest, A.V.; Kruse, K.; Savant, S.; Thomas, M.; Laib, A.M.; Loos, E.K.; Fiedler, U.; Augustin, H.G. Angiopoietin-2 Is Critical for Cytokine-Induced Vascular Leakage. PLoS ONE 2013, 8, e70459. [Google Scholar] [CrossRef]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. 2014, 163, 296–306. [Google Scholar] [CrossRef]
- Spencer, B.G.; Estevez, J.J.; Liu, E.; Craig, J.E.; Finnie, J.W. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology 2020, 28, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Gennuso, F.; Giurdanella, G.; Conti, F.; Drago, F.; Salomone, S.; Furno, D.L.; Bucolo, C.; Giuffrida, R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J. Stem Cells 2020, 12, 1152–1170. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- Vancheri, C.; Mastruzzo, C.; Trovato-Salinaro, E.; Gili, E.; Furno, D.L.; Pistorio, M.P.; Caruso, M.; La Rosa, C.; Crimi, C.; Failla, M.; et al. Interaction between human lung fibroblasts and T-lymphocytes prevents activation of CD4+ cells. Respir. Res. 2005, 6, 103. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and im-munomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, L.; Huang, R.; Wu, P.; He, L. Comparison of inflammatory cytokines levels in the aqueous humor with diabetic retinopathy. Int. Ophthalmol. 2020, 40, 2763–2769. [Google Scholar] [CrossRef]
- Mtairag, E.M.; Chollet-Martin, S.; Oudghiri, M.; Laquay, N.; Jacob, M.-P.; Michel, J.-B.; Feldman, L.J. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc. Res. 2001, 49, 882–890. [Google Scholar] [CrossRef]
- Gimeno, M.J.; Pascual, G.; García-Honduvilla, N.; Prieto, A.; De Mon, M.A.; Bellón, J.M.; Buján, J. Modulatory role of IL10 in endothelial cell damage and platelet adhesion. Histol. Histopathol. 2003, 18, 14670. [Google Scholar]
- Kinzenbaw, D.A.; Chu, Y.; Silva, R.A.P.; Didion, S.P.; Faraci, F.M. Interleukin-10 protects against aging-induced endothelial dysfunction. Physiol. Rep. 2013, 1, e00149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Zhang, Z.; Jiang, F.; Meng, X.; Yan, H. Interleukin-10 overexpression improves the function of endothelial progenitor cells stimulated with TNF-? Through the activation of the STAT3 signaling pathway. Int. J. Mol. Med. 2014, 35, 471–477. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.C.; Zhang, N.; Van Crombruggen, K.; Hu, G.H.; Hong, S.L.; Bachert, C. Transforming growth factor-beta1 in inflammatory airway disease: A key for understanding inflammation and remodeling. Allergy 2012, 67, 1193–1202. [Google Scholar] [CrossRef]
- Abdoli, A.; Maspi, N.; Ghaffarifar, F. Wound healing in cutaneous leishmaniasis: A double edged sword of IL-10 and TGF-β. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 15–26. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [CrossRef]
- Scholz, A.; Plate, K.H.; Reiss, Y. Angiopoietin-2: A multifaceted cytokine that functions in both angiogenesis and inflammation. Ann. New York Acad. Sci. 2015, 1347, 45–51. [Google Scholar] [CrossRef]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood–retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic Degradation of VE-Cadherin Alters the Blood-Retinal Barrier in Diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef]
- Devi, T.S.; Hosoya, K.-I.; Terasaki, T.; Singh, L.P. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: Implications for diabetic retinopathy. Exp. Cell Res. 2013, 319, 1001–1012. [Google Scholar] [CrossRef]
- May, J.M.; Jayagopal, A.; Qu, Z.-C.; Parker, W.H. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 2014, 452, 112–117. [Google Scholar] [CrossRef]
- Haspula, D.; Vallejos, A.K.; Moore, T.M.; Tomar, N.; Dash, R.K.; Hoffmann, B.R. Influence of a Hyperglycemic Microenvironment on a Diabetic Versus Healthy Rat Vascular Endothelium Reveals Distinguishable Mechanistic and Phenotypic Responses. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence (5′-3′) | Amplicon (bp) | Accession Number |
|---|---|---|---|
| IL-10 | Fw: GACTTTAAGGGTTACCTGGGTTG | 112 | NM_000572.3 |
| Rv: TCACATGCGCCTTGATGTCTG | |||
| TGF-β1 | Fw: CGTCTGCTGAGGCTCAAGT | 74 | NM_000660.7 |
| Rv: CGCCAGGAATTGTTGCTGTA | |||
| TNF-α | Fw: AGCCCATGTTGTAGCAAACC | 134 | NM_000594.4 |
| Rv: TGAGGTACAGGCCCTCTGAT | |||
| IL-1β | Fw: AGCTACGAATCTCCGACCAC | 186 | NM_000576.3 |
| Rv: CGTTATCCCATGTGTCGAAGAA | |||
| VEGF | Fw: ATCTTCAAGCCATCCTGTGTGC | 121 | NM_001025366.3 |
| Rv: GAGGTTTGATCCGCATAATCTG | |||
| Angiopoietin-2 | Fw: CTCGAATACGATGACTCGGTG | 76 | NM_001386337.1 |
| Rv: TCATTAGCCACTGAGTGTTGTTT | |||
| MMP9 | Fw: CACTGTCCACCCCTCAGAGC | 264 | NM_004994.3 |
| Rv: GCCAACTTGTCGGCGATAAGG | |||
| 18S rRNA | Fw: TAAGTCCCTGCCCTTTGTACACA | 69 | NR_146119 |
| Rv: GATCCGAGGGCCTCACTAAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, G.; Longo, A.; Gennuso, F.; Anfuso, C.D.; Lupo, G.; Giurdanella, G.; Giuffrida, R.; Lo Furno, D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4604. https://doi.org/10.3390/ijms22094604
Mannino G, Longo A, Gennuso F, Anfuso CD, Lupo G, Giurdanella G, Giuffrida R, Lo Furno D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2021; 22(9):4604. https://doi.org/10.3390/ijms22094604
Chicago/Turabian StyleMannino, Giuliana, Anna Longo, Florinda Gennuso, Carmelina Daniela Anfuso, Gabriella Lupo, Giovanni Giurdanella, Rosario Giuffrida, and Debora Lo Furno. 2021. "Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 22, no. 9: 4604. https://doi.org/10.3390/ijms22094604
APA StyleMannino, G., Longo, A., Gennuso, F., Anfuso, C. D., Lupo, G., Giurdanella, G., Giuffrida, R., & Lo Furno, D. (2021). Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 22(9), 4604. https://doi.org/10.3390/ijms22094604






