Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation?
Abstract
:1. Introduction
2. Role of Mast Cells in Barrier Integrity and Host Defense
2.1. Mast Cells in Skin Homeostasis
2.2. Mast Cells as a Link between Innate and Adaptive Immunity
2.3. The Role of Mast Cells in Venom Detoxification
2.4. The Role of Mast Cells in Bacterial Infections
2.5. The Role of Mast Cells in Virus Infections
2.6. The Role of Mast Cells in Parasite Infections
2.7. The Role of Mast Cells in Fungi Infections
3. Mast Cell Contribution to Inflammatory Skin Disorders
3.1. IgE-Mediated Acute Allergic Cutaneous Responses
3.2. The Role of Mast Cells in Atopic Dermatitis
3.3. Mast Cell Functions in Contact Hypersensitivity
3.4. Mast Cells in Psoriasis
4. Mast Cell-Driven Skin Diseases
4.1. The Role of Mast Cells in Urticaria
4.2. Mast Cell-Driven Mechanisms in Mastocytosis
4.3. Mast Cell Activation Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsepkolenko, A.; Tsepkolenko, V.; Dash, S.; Mishra, A.; Bader, A.; Melerzanov, A.; Giri, S. The regenerative potential of skin and the immune system. Clin. Cosmet. Investig. Dermatol. 2019, 12, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.K.; Armstrong, C.A.; Ansel, J.C. The skin as an immune organ. West. J. Med. 1994, 160, 146–152. [Google Scholar] [PubMed]
- Sumpter, T.L.; Balmert, S.C.; Kaplan, D.H. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight 2019. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F.; Kim, E.Y.; Brenner, M.B.; Shaw, L.; Yu, B.; Goldrath, A.; Mostafavi, S.; Regev, A.; et al. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef]
- Janssens, A.S. Mast cell distribution in normal adult skin. J. Clin. Pathol. 2005, 58, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, Z.; Li, Z.; Wu, Y. Molecular regulation of mast cell development and maturation. Mol. Biol. Rep. 2010, 37, 1993–2001. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 2018, 49, 640–653.e5. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, A.; Naumann, R.; Dudeck, A.; Zerjatke, T.; Gerbaulet, A.; Roers, A. Mast Cells Occupy Stable Clonal Territories in Adult Steady-State Skin. J. Investig. Dermatol. 2020, 140, 2433–2441.e5. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Dudeck, A.; Suender, C.A.; Kostka, S.L.; von Stebut, E.; Maurer, M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur. J. Immunol. 2011, 41, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, J.; Kotrba, J.; Immler, R.; Hoffmann, A.; Voss, M.; Alexaki, V.I.; Morton, L.; Jahn, S.R.; Katsoulis-Dimitriou, K.; Winzer, S.; et al. Directional mast cell degranulation of tumor necrosis factor into blood vessels primes neutrophil extravasation. Immunity 2021, 54. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellman, L.T.; Akula, S.; Thorpe, M.; Fu, Z. Tracing the origins of IgE, mast cells, and allergies by studies of wild animals. Front. Immunol. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, E.; Lam, R.S.; Wölbing, F.; Matzner, N.; Zemtsova, I.M.; Sobiesiak, M.; Mahmud, H.; Sausbier, U.; Biedermann, T.; Ruth, P.; et al. Blunted IgE-Mediated Activation of Mast Cells in Mice Lacking the Ca2+—Activated K+ Channel KCa3.1. J. Immunol. 2008, 180, 8040–8047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunder, C.A.; St. John, A.L.; Li, G.; Leong, K.W.; Berwin, B.; Staats, H.F.; Abraham, S.N. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009, 206, 2455–2467. [Google Scholar] [CrossRef] [Green Version]
- Bax, H.J.; Keeble, A.H.; Gould, H.J. Cytokinergic IgE action in mast cell activation. Front. Immunol. 2012, 3, 229. [Google Scholar] [CrossRef] [Green Version]
- Weller, C.L.; Collington, S.J.; Williams, T.; Lamb, J.R. Mast cells in health and disease. Clin. Sci. 2011, 120, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang-lin, H.; Wei, G.; Hong-ying, L.; Jian, T. The Role of the Mast Cell in Skin Aging. J. Dermatol. Res. Ther. 2016, 2. [Google Scholar] [CrossRef]
- Abdel Hafez, S.M.N. Age related changes in the dermal mast cells and the associated changes in the dermal collagen and cells: A histological and electron microscopy study. Acta Histochem. 2019, 121, 619–627. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Barron, M.J.; Watson, R.E.B.; Griffiths, C.E.M.; Bulfone-Paus, S. Aged human skin accumulates mast cells with altered functionality that localize to macrophages and vasoactive intestinal peptide-positive nerve fibres. Br. J. Dermatol. 2019, 180, 849–858. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T.; Crivellato, E. The role of mast cells in human skin cancers. Clin. Exp. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 247–261. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Kurashima, Y.; Amiya, T.; Fujisawa, K.; Shibata, N.; Suzuki, Y.; Kogure, Y.; Hashimoto, E.; Otsuka, A.; Kabashima, K.; Sato, S.; et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity 2014, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Roediger, B.; Weninger, W. The role of chemokines in cutaneous immunosurveillance. Immunol. Cell Biol. 2015, 93, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Richter, A.; Puddicombe, S.M.; Lordan, J.L.; Bucchieri, F.; Wilson, S.J.; Djukanovic, R.; Dent, G.; Holgate, S.T.; Davies, D.E. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am. J. Respir. Cell Mol. Biol. 2001, 25, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, A.; Toksoy, A.; Engelhardt, E.; Bröcker, E.B.; Gillitzer, R. Mast cell involvement in normal human skin wound healing: Expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J. Pathol. 2000, 190, 100–106. [Google Scholar] [CrossRef]
- Brown Lobbins, M.L.; Shivakumar, B.R.; Postlethwaite, A.E.; Hasty, K.A. Chronic exposure of interleukin-13 suppress the induction of matrix metalloproteinase-1 by tumour necrosis factor α in normal and scleroderma dermal fibroblasts through protein kinase B/Akt. Clin. Exp. Immunol. 2018, 191, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Huang, X.; Ahmadi, P.; Stenberg, P.; Liebler, J.M.; Le, A.C.; Planck, S.R.; Rosenbaum, J.T. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int. Arch. Allergy Immunol. 1998, 115, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef] [Green Version]
- Leist, M.; Sünder, C.A.; Drube, S.; Zimmermann, C.; Geldmacher, A.; Metz, M.; Dudeck, A.; Maurer, M. Membrane-bound stem cell factor is the major but not only driver of fibroblast-induced murine skin mast cell differentiation. Exp. Dermatol. 2017, 26, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Smrž, D.; Jung, M.-Y.; Bandara, G.; Desai, A.; Smržová, Š.; Kuehn, H.S.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Stem Cell Factor Programs the Mast Cell Activation Phenotype. J. Immunol. 2012, 188, 5428–5437. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Binstadt, B.A.; Monach, P.A.; Johnsen, A.; Gurish, M.; Iwakura, Y.; Benoist, C.; Mathis, D.; Lee, D.M. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.L.; Marichal, T.; Sokolove, J.; Starkl, P.; Gaudenzio, N.; Iwakura, Y.; Karasuyama, H.; Schwartz, L.B.; Robinson, W.H.; Tsai, M.; et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014, 66, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Hu-Li, J.; Shevach, E.M.; Mizuguchi, J.; Ohara, J.; Mosmann, T.; Paul, W.E. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J. Exp. Med. 1987, 165, 157–172. [Google Scholar] [CrossRef]
- Bradding, P.; Feather, I.H.; Howarth, P.H.; Mueller, R.; Roberts, J.A.; Britten, K.; Bews, J.P.; Hunt, T.C.; Okayama, Y.; Heusser, C.H. Interleukin 4 is localized to and released by human mast cells. J. Exp. Med. 1992, 176, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradding, P.; Feather, I.H.; Wilson, S.; Bardin, P.G.; Heusser, C.H.; Holgate, S.T.; Howarth, P.H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J. Immunol. 1993, 151, 3853–3865. [Google Scholar] [PubMed]
- Horsmanheimo, L.; Harvima, I.T.; Järvikallio, A.; Harvima, R.J.; Naukkarinen, A.; Horsmanheimo, M. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br. J. Dermatol. 1994, 131, 348–353. [Google Scholar] [CrossRef]
- Bradding, P.; Feather, I.H.; Wilson, S.; Holgate, S.T.; Howarth, P.H. Cytokine immunoreactivity in seasonal rhinitis: Regulation by a topical corticosteroid. Am. J. Respir. Crit. Care Med. 1995, 151, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Artuc, M.; Steckelings, U.M.; Henz, B.M. Mast cell-fibroblast interactions: Human mast cells as source and inducers of fibroblast and epithelial growth factors. J. Investig. Dermatol. 2002, 118, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.A.; Albino, A.P.; McNutt, N.S. Human cutaneous mast cells express basic fibroblast growth factor. Lab. Investig. 1995, 72, 215–222. [Google Scholar]
- Qu, Z.; Liebler, J.M.; Powers, M.R.; Galey, T.; Ahmadi, P.; Huang, X.N.; Ansel, J.C.; Butterfield, J.H.; Planck, S.R.; Rosenbaum, J.T. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am. J. Pathol. 1995, 147, 564–573. [Google Scholar]
- Pennington, D.W.; Lopez, A.R.; Thomas, P.S.; Peck, C.; Gold, W.M. Dog mastocytoma cells produce transforming growth factor beta1. J. Clin. Investig. 1992, 90, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Grützkau, A.; Krüger-Krasagakes, S.; Baumeister, H.; Schwarz, C.; Kögel, H.; Welker, P.; Lippert, U.; Henz, B.M.; Möller, A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol. Biol. Cell 1998, 9, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Heissig, B.; Rafii, S.; Akiyama, H.; Ohki, Y.; Sato, Y.; Rafael, T.; Zhu, Z.; Hicklin, D.J.; Okumura, K.; Ogawa, H.; et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J. Exp. Med. 2005, 202, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Fang, L.; Tang, X.; Lu, S.; Tamm, M.; Stolz, D.; Roth, M. TGF-β Upregulated Mitochondria Mass through the SMAD2/3→C/EBPβ→PRMT1 Signal Pathway in Primary Human Lung Fibroblasts. J. Immunol. 2019, 202, 37–47. [Google Scholar] [CrossRef]
- Lacy, S.H.; Woeller, C.F.; Thatcher, T.H.; Pollock, S.J.; Small, E.M.; Sime, P.J.; Phipps, R.P. Activated Human Lung Fibroblasts Produce Extracellular Vesicles with Antifibrotic Prostaglandins. Am. J. Respir. Cell Mol. Biol. 2019, 60, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-A.; Park, M.; Kim, Y.-H.; Woo, S.-Y. Th17 cell-mediated immune responses promote mast cell proliferation by triggering stem cell factor in keratinocytes. Biochem. Biophys. Res. Commun. 2017, 487, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mascarenhas, N.; Eckmann, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216.e6. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-A.; Kim, H.J.; Kim, Y.-H.; Park, M.; Woo, S.-Y. Dexamethasone Promotes Keratinocyte Proliferation by Triggering Keratinocyte Growth Factor in Mast Cells. Int. Arch. Allergy Immunol. 2019, 179, 53–61. [Google Scholar] [CrossRef]
- Feuerherm, A.J.; Jørgensen, K.M.; Sommerfelt, R.M.; Eidem, L.E.; Lægreid, A.; Johansen, B. Platelet-activating factor induces proliferation in differentiated keratinocytes. Mol. Cell. Biochem. 2013, 384, 83–94. [Google Scholar] [CrossRef]
- Huttunen, M.; Hyttinen, M.; Nilsson, G.; Butterfield, J.H.; Horsmanheimo, M.; Harvima, I.T. Inhibition of keratinocyte growth in cell culture and whole skin culture by mast cell mediators. Exp. Dermatol. 2001, 10, 184–192. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Mildner, M.; Mlitz, V.; Gruber, F.; Eckhart, L.; Werfel, T.; Gutzmer, R.; Elias, P.M.; Tschachler, E. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy 2013, 68, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Artuc, M.; Steckelings, U.M.; Grützkau, A.; Smorodchenko, A.; Henz, B.M. A long-term coculture model for the study of mast cell-keratinocyte interactions. J. Investig. Dermatol. 2002, 119, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehra, S.; Serezani, A.P.M.; Ocaña, J.A.; Travers, J.B.; Kaplan, M.H. Mast Cells Regulate Epidermal Barrier Function and the Development of Allergic Skin Inflammation. J. Investig. Dermatol. 2016, 136, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Swerlick, R.A. Angiogenesis. J. Dermatol. 1995, 22, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016, 146–151. [Google Scholar] [CrossRef]
- McHale, C.; Mohammed, Z.; Gomez, G. Human Skin-Derived Mast Cells Spontaneously Secrete Several Angiogenesis-Related Factors. Front. Immunol. 2019, 1445. [Google Scholar] [CrossRef] [PubMed]
- Kunder, C.A.; St John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Toda, S. Mast cells and angiogenesis. Microsc. Res. Tech. 2003, 60, 64–69. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hültner, L.; Röcken, M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000, 192, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Kneilling, M.; Mailhammer, R.; Hültner, L.; Schönberger, T.; Fuchs, K.; Schaller, M.; Bukala, D.; Massberg, S.; Sander, C.A.; Braumüller, H.; et al. Direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia mediates local tissue inflammation. Blood 2009, 114, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc. Natl. Acad. Sci. USA 1986, 83, 2204–2208. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.M.; Huang, Z.L.; Xu, X.H.; Aritake, K.; Eguchi, N.; Nambu, F.; Narumiya, S.; Urade, Y.; Hayaishi, O. Lipocalin-type prostaglandin D syntase produces prostaglandin D2 involved in regulation of physiological sleep. Proc. Natl. Acad. Sci. USA 2006, 103, 17949–17954. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, E.; Barlow, C.L.; Ramirez, S.H.; Abood, M.E.; Brailoiu, G.C. Effects of Platelet-Activating Factor on Brain Microvascular Endothelial Cells. Neuroscience 2018, 377, 105–113. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e5. [Google Scholar] [CrossRef]
- Takagi, K.; Yamakuchi, M.; Matsuyama, T.; Kondo, K.; Uchida, A.; Misono, S.; Hashiguchi, T.; Inoue, H. IL-13 enhances mesenchymal transition of pulmonary artery endothelial cells via down-regulation of miR-424/503 in vitro. Cell. Signal. 2018, 42, 270–280. [Google Scholar] [CrossRef]
- Mohr, T.; Haudek-Prinz, V.; Slany, A.; Grillari, J.; Micksche, M.; Gerner, C. Proteome profiling in IL-1 β and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS ONE 2017, 12, e0179065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Koh, M.; Noguchi, S.; Araki, M.; Otsuka, H.; Yokosuka, M.; Soeta, S. Expressions of vascular endothelial growth factor receptors, Flk1 and Flt1, in rat skin mast cells during development. J. Vet. Med. Sci. 2020, 82, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; De Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123. [Google Scholar] [CrossRef]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e5. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Riquer, Z.P.; Segura-Villalobos, D.; Ramírez-Moreno, I.G.; Pérez Rodríguez, M.J.; Lamas, M.; Gonzalez-Espinosa, C. Signal Transduction Pathways Activated by Innate Immunity in Mast Cells: Translating Sensing of Changes into Specific Responses. Cells 2020, 9, 2411. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Katsoulis-Dimitriou, K.; Edler, H.J.; Dudeck, J.; Drube, S.; Dudeck, A. Mast cells initiate the vascular response to contact allergens by sensing cell stress. J. Allergy Clin. Immunol. 2020, 145, 1476–1479.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frandji, P.; Tkaczyk, C.; Oskeritzian, C.; David, B.; Desaymard, C.; Mécheri, S.; Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; et al. Mast cells signal their importance in health and disease. Immunol. Rev. 2018, 282, 17949–17954. [Google Scholar] [CrossRef]
- Espinosa, E.; Valitutti, S. New roles and controls of mast cells. Curr. Opin. Immunol. 2018, 50, 39–47. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros-Martinez, C.; Mendez-Barbero, N.; Montalvo-Yuste, A.; Jensen, B.M.; Gomez-Cardenosa, A.; Klitfod, L.; Garrido-Arandia, M.; Alvarez-Llamas, G.; Pastor-Vargas, C.; Vivanco, F.; et al. Endothelial Regulator of Calcineurin 1 Promotes Barrier Integrity and Modulates Histamine-Induced Barrier Dysfunction in Anaphylaxis. Front. Immunol. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez-Barbero, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Jensen, B.M.; Gutiérrez-Muñoz, C.; Tome-Amat, J.; Garrido-Arandia, M.; Díaz-Perales, A.; Ballesteros-Martinez, C.; Laguna, J.J.; et al. The TNF-like weak inducer of the apoptosis/fibroblast growth factor-inducible molecule 14 axis mediates histamine and platelet-activating factor-induced subcutaneous vascular leakage and anaphylactic shock. J. Allergy Clin. Immunol. 2020, 145, 583–596.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef] [Green Version]
- Rönnberg, E.; Melo, F.R.; Pejler, G. Mast cell proteoglycans. J. Histochem. Cytochem. 2012, 60, 950–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AhYoung, A.P.; Eckard, S.C.; Gogineni, A.; Xi, H.; Lin, S.J.; Gerhardy, S.; Cox, C.; Phung, Q.T.; Hackney, J.A.; Katakam, A.K.; et al. Neutrophil serine protease 4 is required for mast cell-dependent vascular leakage. Commun. Biol. 2020, 3, 687. [Google Scholar] [CrossRef]
- Syenina, A.; Jagaraj, C.J.; Aman, S.A.B.; Sridharan, A.; St John, A.L. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcγ receptors. eLife 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef] [Green Version]
- Nyekel, F.N.; Pacreau, E.; Benadda, S.; Msallam, R.; Abrink, M.; Pejler, G.; Davoust, J.; Benhamou, M.; Charles, N.; Launay, P.; et al. Mast cell degranulation exacerbates skin rejection by enhancing neutrophil recruitment. Front. Immunol. 2018, 9, 2690. [Google Scholar] [CrossRef]
- Schramm, R.; Schaefer, T.; Menger, M.D.; Thorlacius, H. Acute mast cell-dependent neutrophil recruitment in the skin is mediated by KC and LFA-1: Inhibitory mechanisms of dexamethasone. J. Leukoc. Biol. 2002, 72, 1122–1132. [Google Scholar] [PubMed]
- Sutherland, R.E.; Olsen, J.S.; McKinstry, A.; Villalta, S.A.; Wolters, P.J. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J. Immunol. 2008, 181, 5598–5605. [Google Scholar] [CrossRef] [Green Version]
- Doener, F.; Michel, A.; Reuter, S.; Friedrich, P.; Böhm, L.; Relle, M.; Codarri, L.; Tenzer, S.; Klein, M.; Bopp, T.; et al. Mast cell-derived mediators promote murine neutrophil effector functions. Int. Immunol. 2013, 25, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast cell: An emerging partner in immune interaction. Front. Immunol. 2012, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Carroll-Portillo, A.; Surviladze, Z.; Cambi, A.; Lidke, D.S.; Wilson, B.S. Mast cell synapses and exosomes: Membrane contacts for information exchange. Front. Immunol. 2012, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of mast cell secretory granules on skin inflammation boosts dendritic cell migration and priming efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864.e4. [Google Scholar] [CrossRef]
- Caron, G.; Delneste, Y.; Roelandts, E.; Duez, C.; Herbault, N.; Magistrelli, G.; Bonnefoy, J.Y.; Pestel, J.; Jeannin, P. Histamine induces CD86 expression and chemokine production by human immature dendritic cells. J. Immunol. 2001, 166, 6000–6006. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Dudeck, J.; Medyukhina, A.; Fröbel, J.; Svensson, C.-M.M.; Kotrba, J.; Gerlach, M.; Gradtke, A.-C.C.; Schröder, B.; Speier, S.; Figge, M.T.; et al. Mast cells acquire MHC II from dendritic cells during skin inflammation. J. Exp. Med. 2017, 214, 3791–3811. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC Class II Expression by Mast Cells Supports Effector and Regulatory T Cell Activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzio, N.; Espagnolle, N.; Mars, L.T.; Liblau, R.; Valitutti, S.; Espinosa, E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood 2009, 114, 4979–4988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantri, C.K.; St. John, A.L. Immune synapses between mast cells and γδ T cells limit viral infection. J. Clin. Investig. 2019, 129, 1094–1108. [Google Scholar] [CrossRef] [Green Version]
- Stelekati, E.; Bahri, R.; D’Orlando, O.; Orinska, Z.; Mittrücker, H.-W.W.; Langenhaun, R.; Glatzel, M.; Bollinger, A.; Paus, R.; Bulfone-Paus, S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 2009, 31, 665–676. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Riedel, C.; Caulfield, J.P.; Wasserman, S.I.; Austen, K.F. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J. Immunol. 1981, 126, 2071–2078. [Google Scholar]
- Admyre, C.; Telemo, E.; Almqvist, N.; Lötvall, J.; Lahesmaa, R.; Scheynius, A.; Gabrielsson, S. Exosomes—Nanovesicles with possible roles in allergic inflammation. Allergy 2008, 63, 404–408. [Google Scholar] [CrossRef]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.R.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Suurmond, J.; Habets, K.L.L.; Dorjée, A.L.; Huizinga, T.W.; Toes, R.E.M. Expansion of Th17 Cells by Human Mast Cells Is Driven by Inflammasome-Independent IL-1 β. J. Immunol. 2016, 197, 4473–4481. [Google Scholar] [CrossRef] [Green Version]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.-C.C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamon, P.; Shefler, I.; Moshkovits, I.; Munitz, A.; Horwitz Klotzman, D.; Mekori, Y.A.; Hershko, A.Y. IL-33 and IgE stimulate mast cell production of IL-2 and regulatory T cell expansion in allergic dermatitis. Clin. Exp. Allergy 2017, 47, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.-L.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; Pan, Y.; Pierini, A.; Galli, S.J.; Negrin, R.S. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood 2013, 122, 3659–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Profet, M. The function of allergy: Immunological defense against toxins. Q. Rev. Biol. 1991, 66, 23–62. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Starkl, P.; Reber, L.L.; Kalesnikoff, J.; Oettgen, H.C.; Tsai, M.; Metz, M.; Galli, S.J. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity 2013, 39, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. Testing the “toxin hypothesis of allergy”: Mast cells, IgE, and innate and acquired immune responses to venoms. Curr. Opin. Immunol. 2015, 36, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Starkl, P.; Marichal, T.; Tsai, M. Mast Cells and IgE can Enhance Survival During Innate and Acquired Host Responses to Venoms. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 193–221. [Google Scholar]
- Bruni, F.M.; Coutinho, E.M.M.; Andrade-Barros, A.I.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. Anaphylaxis induced by Thalassophryne nattereri venom in mice is an IgE/IgG1-mediated, IL-4-dependent phenomenon. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahoshi, M.; Song, C.H.; Piliponsky, A.M.; Metz, M.; Guzzetta, A.; Åbrink, M.; Schlenner, S.M.; Feyerabend, T.B.; Rodewald, H.R.; Pejler, G.; et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Investig. 2011, 121, 4180–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, M.; Piliponsky, A.M.; Chan, C.C.; Lammel, V.; Åbrink, M.; Pejler, G.; Tsai, M.; Galli, S.J. Mast cells can enhance resistance to snake and honeybee venoms. Science 2006, 313, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.; Stavenhagen, K.; Kolarich, D.; Sommerhoff, C.P.; Maurer, M.; Metz, M. Human mast cell tryptase is a potential treatment for snakebite envenoming across multiple snake species. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Bonadonna, P.; Bonifacio, M.; Lombardo, C.; Zanotti, R. Hymenoptera Allergy and Mast Cell Activation Syndromes. Curr. Allergy Asthma Rep. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Johnzon, C.F.; Rönnberg, E.; Pejler, G. The Role of Mast Cells in Bacterial Infection. Am. J. Pathol. 2016, 186, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, D.; Lyubynska, N.; Wolters, P.J.; Killeen, N.P.; Baluk, P.; McDonald, D.M.; Hawgood, S.; Caughey, G.H. Mast cells protect mice from mycoplasma pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Kulka, M.; Fukuishi, N.; Rottem, M.; Mekori, Y.A.; Metcalfe, D.D. Mast cells, which interact with Escherichia coli, up-regulate genes associated with innate immunity and become less responsive to FcεRI-mediated activation. J. Leukoc. Biol. 2006, 79, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Wei, O.L.; Hilliard, A.; Kalman, D.; Sherman, M. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect. Immun. 2005, 73, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Ketavarapu, J.M.; Rodriguez, A.R.; Yu, J.J.; Cong, Y.; Murthy, A.K.; Forsthuber, T.G.; Guentzel, M.N.; Klose, K.E.; Berton, M.T.; Arulanandam, B.P. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc. Natl. Acad. Sci. USA 2008, 105, 9313–9318. [Google Scholar] [CrossRef] [Green Version]
- Velin, D.; Bachmann, D.; Bouzourene, H.; Michetti, P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology 2005, 129, 142–155. [Google Scholar] [CrossRef]
- Torres-Atencio, I.; Rosero, S.; Ordoñez, C.; Ruiz, M.; Goodridge, A. Mycobacterial lipids induce calcium mobilization and degranulation of mast cells. Am. J. Respir. Crit. Care Med. 2018, 198, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Syska, W.; Weller, K.; Magerl, M.; Zuberbier, T.; Metz, M.; Maurer, M. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am. J. Pathol. 2007, 170, 1910–1916. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, C.; Troeltzsch, D.; Giménez-Rivera, V.A.; Galli, S.J.; Metz, M.; Maurer, M.; Siebenhaar, F. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc. Natl. Acad. Sci. USA 2019, 116, 20500–20504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rönnberg, E.; Johnzon, C.-F.; Calounova, G.; Garcia Faroldi, G.; Grujic, M.; Hartmann, K.; Roers, A.; Guss, B.; Lundequist, A.; Pejler, G. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef] [Green Version]
- Von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Möllerherm, H.; von Köckritz-Blickwede, M.; Branitzki-Heinemann, K. Antimicrobial activity of mast cells: Role and relevance of extracellular DNA traps. Front. Immunol. 2016, 7, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.L.; Wang, Z.; Igawa, S.; Choi, J.E.; Werbel, T.; Di Nardo, A. Lipocalin 2: A New Antimicrobial in Mast Cells. Int. J. Mol. Sci. 2019, 20, 2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nardo, A.; Yamasaki, K.; Dorschner, R.A.; Lai, Y.; Gallo, R.L. Mast Cell Cathelicidin Antimicrobial Peptide Prevents Invasive Group A Streptococcus Infection of the Skin. J. Immunol. 2008, 180, 7565–7573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.; Zhang, D.; Lu, B.; Zhou, W.; Wang, D. Activation of mast cells in skin abscess induced by Staphylococcus aureus (S. aureus) infection in mice. Res. Vet. Sci. 2018, 118, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Muñoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.C.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkl, P.; Watzenboeck, M.L.; Popov, L.M.; Zahalka, S.; Hladik, A.; Lakovits, K.; Radhouani, M.; Haschemi, A.; Marichal, T.; Reber, L.L.; et al. IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus. Immunity 2020, 53, 793–804.e9. [Google Scholar] [CrossRef]
- Pundir, P.; Liu, R.; Vasavda, C.; Serhan, N.; Limjunyawong, N.; Yee, R.; Zhan, Y.; Dong, X.X.; Wu, X.; Zhang, Y.; et al. A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe 2019, 26, 114–122.e8. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Nishiyama, C.; Kanada, S.; Niwa, Y.; Shimokawa, N.; Ushio, H.; Nishiyama, M.; Okumura, K.; Ogawa, H. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood 2007, 109, 4846–4855. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast cell responses to viruses and pathogen products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef] [Green Version]
- Jolly, S.; Detilleux, J.; Desmecht, D. Extensive mast cell degranulation in bovine respiratory syncytial virus-associated paroxystic respiratory distress syndrome. Vet. Immunol. Immunopathol. 2004, 97, 125–136. [Google Scholar] [CrossRef]
- Shirato, K.; Taguchi, F. Mast cell degranulation is induced by A549 airway epithelial cell infected with respiratory syncytial virus. Virology 2009, 386, 88–93. [Google Scholar] [CrossRef]
- Graziano, F.M.; Tilton, R.; Hirth, T.; Segaloff, D.; Mullins, T.; Dick, E.; Buckner, C.K.; Busse, W.W. The effect of parainfluenza 3 infection on guinea pig basophil and lung mast cell histamine release. Am. Rev. Respir. Dis. 1989, 139, 715–720. [Google Scholar] [CrossRef]
- Xiong, L.; Zhen, S.; Yu, Q.; Gong, Z. HCV-E2 inhibits hepatocellular carcinoma metastasis by stimulating mast cells to secrete exosomal shuttle microRNAs. Oncol. Lett. 2017, 14, 2141–2146. [Google Scholar] [CrossRef] [Green Version]
- Sundstrom, J.B.; Ellis, J.E.; Hair, G.A.; Kirshenbaum, A.S.; Metcalfe, D.D.; Yi, H.; Cardona, A.C.; Lindsay, M.K.; Ansari, A.A. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood 2007, 109, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Demeure, C.E.; Brahimi, K.; Hacini, F.; Marchand, F.; Péronet, R.; Huerre, M.; St.-Mezard, P.; Nicolas, J.-F.; Brey, P.; Delespesse, G.; et al. Anopheles Mosquito Bites Activate Cutaneous Mast Cells Leading to a Local Inflammatory Response and Lymph Node Hyperplasia. J. Immunol. 2005, 174, 3932–3940. [Google Scholar] [CrossRef] [Green Version]
- Depinay, N.; Hacini, F.; Beghdadi, W.; Peronet, R.; Mécheri, S. Mast Cell-Dependent Down-Regulation of Antigen-Specific Immune Responses by Mosquito Bites. J. Immunol. 2006, 176, 4141–4146. [Google Scholar] [CrossRef] [Green Version]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.W.; Wu-Hsieh, B.A.; Lin, Y.S.; Chen, W.Y.; Huang, Y.; Anderson, R. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J. Biomed. Sci. 2018, 25, 77. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.T.; Wan, S.W.; Chang, Y.C.; Lee, C.K.; Wu-Hsieh, B.A.; Anderson, R.; Lin, Y.S. Antibodies against nonstructural protein 1 protect mice from dengue virus-induced mast cell activation. Lab. Investig. 2017, 97, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Troupin, A.; Shirley, D.; Londono-Renteria, B.; Watson, A.M.; McHale, C.; Hall, A.; Hartstone-Rose, A.; Klimstra, W.B.; Gomez, G.; Colpitts, T.M. A Role for Human Skin Mast Cells in Dengue Virus Infection and Systemic Spread. J. Immunol. 2016, 197, 4382–4391. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.T.; Wan, S.W.; Anderson, R.; Lin, Y.S. Mast cell-macrophage dynamics in modulation of dengue virus infection in skin. Immunology 2015, 146, 163–172. [Google Scholar] [CrossRef] [Green Version]
- John, A.L.S.; Rathore, A.P.S.; Yap, H.; Ng, M.L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef] [Green Version]
- Furuta, T.; Murao, L.A.; Lan, N.T.P.; Huy, N.T.; Huong, V.T.Q.; Thuy, T.T.; Tham, V.D.; Nga, C.T.P.; Ha, T.T.N.; Ohmoto, Y.; et al. Association of mast cell-derived VEGF and proteases in dengue shock syndrome. PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Tissera, H.; Rathore, A.P.S.; Leong, W.Y.; Pike, B.L.; Warkentien, T.E.; Farouk, F.S.; Syenina, A.; Ooi, E.E.; Gubler, D.J.; Wilder-Smith, A.; et al. Chymase level is a predictive biomarker of dengue hemorrhagic fever in pediatric and adult patients. J. Infect. Dis. 2017, 216, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.; Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Nishida, A.; John, A.L.S. Transcriptional Profiling Confirms the Therapeutic Effects of Mast Cell Stabilization in a Dengue Disease Model. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Sager, G.; Gabaglio, S.; Sztul, E.; Belov, G.A. Role of host cell secretory machinery in zika virus life cycle. Viruses 2018, 10, 559. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, K.; Gonçalves, A.J.d.S.; de Souza, L.J.; Sales, A.P.; de Lima, S.M.B.; Trindade, G.F.; Ciambarella, B.T.; Amorim Tasmo, N.R.; Diaz, B.L.; de Carvalho, J.J.; et al. Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells 2020, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Coish, J.M.; Crozier, R.W.E.; Schieffelin, J.S.; Coorssen, J.R.; Hunter, F.F.; MacNeil, A.J. Mast Cell Infection by Zika Virus and Augmentation by Pre-existing Dengue Virus Immunity. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Lu, F.; Huang, S. The roles of mast cells in parasitic protozoan infections. Front. Immunol. 2017, 8, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choumet, V.; Attout, T.; Chartier, L.; Khun, H.; Sautereau, J.; Robbe-Vincent, A.; Brey, P.; Huerre, M.; Bain, O. Visualizing Non Infectious and Infectious Anopheles gambiae Blood Feedings in Naive and Saliva-Immunized Mice. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Wilainam, P.; Nintasen, R.; Viriyavejakul, P. Mast cell activation in the skin of Plasmodium falciparum malaria patients. Malar. J. 2015, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Huang, S.; Chen, X.; Liu, X.B.; Wu, Q.; Wang, Y.; Li, X.; Li, K.; Gao, H.; Cen, S.; et al. Activation of Mast Cells Promote Plasmodium berghei ANKA Infection in Murine Model. Front. Cell. Infect. Microbiol. 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Tonkal, A.M.D.J.; Croft, S.; Roy, S. Mast cells at the host-pathogen interface: Host-protection versus immune evasion in leishmaniasis. Clin. Exp. Immunol. 2004, 137, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Bidri, M.; Vouldoukis, I.; Djavad Mossalayi, M.; Debré, P.; Guillosson, J.J.; Mazier, D.; Arock, M. Evidence for direct interaction between mast cells and Leishmania parasites. Parasite Immunol. 1997, 19, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Amato, V.S.; Bacha, H.A.; AlMusawi, T.; Duarte, M.I.; Neto, V.A. Toll-like receptors and leishmaniasis. Infect. Immun. 2008, 76, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, K.S.; Cortada, V.M.C.L.; Dorval, M.E.C.; Souza Lima, M.A.A.; Oshiro, E.T.; Souza, C.S.F.; Silva-Almeida, M.; Carvalho, L.O.P.; Gonçalves da Costa, S.C.; Abreu-Silva, A.L. Leishmania (Leishmania) infantum/chagasi: Histopathological aspects of the skin in naturally infected dogs in two endemic areas. Exp. Parasitol. 2010, 124, 253–257. [Google Scholar] [CrossRef]
- Maurer, M.; Lopez Kostka, S.; Siebenhaar, F.; Moelle, K.; Metz, M.; Knop, J.; Stebut, E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006, 20, 2460–2467. [Google Scholar] [CrossRef]
- Naqvi, N.; Ahuja, K.; Selvapandiyan, A.; Dey, R.; Nakhasi, H.; Puri, N. Role of Mast Cells in clearance of Leishmania through extracellular trap formation. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, C.; Wolff, S.; Zapf, T.; Raifer, H.; Feyerabend, T.B.; Bollig, N.; Camara, B.; Trier, C.; Schleicher, U.; Rodewald, H.R.; et al. Mast cells have no impact on cutaneous leishmaniasis severity and related Th2 differentiation in resistant and susceptible mice. Eur. J. Immunol. 2016, 46, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Q.; Luo, Y.; Scheffel, J.; Zhao, Z.; Maurer, M. The complex role of mast cells in fungal infections. Exp. Dermatol. 2019, 28, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Croft, C.A.; Culibrk, L.; Moore, M.M.; Tebbutt, S.J. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: A critical review. Front. Microbiol. 2016, 7, 472. [Google Scholar] [CrossRef] [Green Version]
- Radsak, M.; Platzbecker, U.; Schmidt, C.S.; Hofmann, W.K.; Nolte, F. Infectious complications in patients with myelodysplastic syndromes: A review of the literature with emphasis on patients treated with 5-azacitidine. Eur. J. Haematol. 2017, 99, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Margalit, A.; Kavanagh, K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Patlán, A.; Campillo-Navarro, M.; Rodríguez-Cortés, O.; Muñoz-Cruz, S.; Wong-Baeza, I.; Estrada-Parra, S.; Estrada-García, I.; Serafín-López, J.; Chacón-Salinas, R. Recognition of Candida albicans by Dectin-1 induces mast cell activation. Immunobiology 2015, 220, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, E.; Vita, F.; Medic, N.; Soranzo, M.R.; Zabucchi, G.; Borelli, V. Mast Cells Kill Candida albicans in the Extracellular Environment but Spare Ingested Fungi from Death. Inflammation 2014, 37, 2174–2189. [Google Scholar] [CrossRef]
- Carsin, A.; Romain, T.; Ranque, S.; Reynaud-Gaubert, M.; Dubus, J.C.; Mège, J.L.; Vitte, J. Aspergillus fumigatus in cystic fibrosis: An update on immune interactions and molecular diagnostics in allergic bronchopulmonary aspergillosis. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 1632–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.X.; Fan, L.C.; Li, M.H.; Cao, W.J.; Xu, J.F. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: A synthesis review of published literature. Respir. Med. 2017, 122, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Urb, M.; Pouliot, P.; Gravelat, F.N.; Olivier, M.; Sheppard, D.C. Aspergillus fumigatus induces immunoglobulin E—Independent mast cell degranulation. J. Infect. Dis. 2009, 200, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, D.; Nawrot, U. Contribution of Malassezia spp. to the development of atopic dermatitis. Mycoses 2019, 62, 588–596. [Google Scholar] [CrossRef]
- Barbosa-Lorenzi, V.C.; Peyda, S.; Scheynius, A.; Nilsson, G.; Lunderius-Andersson, C. Curdlan induces selective mast cell degranulation without concomitant release of LTC4, IL-6 or CCL2. Immunobiology 2017, 222, 647–650. [Google Scholar] [CrossRef]
- Ribbing, C.; Engblom, C.; Lappalainen, J.; Lindstedt, K.; Kovanen, P.T.; Karlsson, M.A.; Lundeberg, L.; Johansson, C.; Nilsson, G.; Lunderius-Andersson, C.; et al. Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy 2011, 66, 110–119. [Google Scholar] [CrossRef]
- Cheng, L.E.; Hartmann, K.; Roers, A.; Krummel, M.F.; Locksley, R.M. Perivascular Mast Cells Dynamically Probe Cutaneous Blood Vessels to Capture Immunoglobulin E. Immunity 2013, 38, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Sala-Cunill, A.; Björkqvist, J.; Senter, R.; Guilarte, M.; Cardona, V.; Labrador, M.; Nickel, K.F.; Butler, L.; Luengo, O.; Kumar, P.; et al. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J. Allergy Clin. Immunol. 2015, 135, 1031–1043.e6. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.F.; Brown, S.G.A. Mediators released during human anaphylaxis. Curr. Allergy Asthma Rep. 2012, 12, 33–41. [Google Scholar] [CrossRef]
- Yang, L.; Fu, J.; Zhou, Y. Research Progress in Atopic March. Front. Immunol. 2020, 11, 1907. [Google Scholar] [CrossRef]
- Matsumoto, K.; Iikura, K.; Morita, H.; Saito, H. Barrier dysfunction in the atopic march—How does atopic dermatitis lead to asthma in children? J. Allergy Clin. Immunol. 2020, 145, 1551–1553. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Leung, D.Y.M. Role of IgE in atopic dermatitis. Curr. Opin. Immunol. 1993, 5, 956–962. [Google Scholar] [CrossRef]
- Grabbe, J.; Haas, N.; Hamann, K.; Kolde, G.; Hakimi, J.; Czarnetzki, B.M. Demonstration of the high-affinity IgE receptor on human Langerhans cells in normal and diseased skin. Br. J. Dermatol. 1993, 129, 120–123. [Google Scholar] [CrossRef]
- Barata, L.T.; Ying, S.; Meng, Q.; Barkans, J.; Rajakulasingam, K.; Durham, S.R.; Kay, A.B. IL-4- and IL-5-positive T lymphocytes, eosinophils, and mast cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J. Allergy Clin. Immunol. 1998, 101, 222–230. [Google Scholar] [CrossRef]
- Imayama, S.; Shibata, Y.; Hori, Y. Epidermal mast cells in atopic dermatitis. Lancet 1995, 346, 1559. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Bester, C.; Grützkau, A.; Serowka, F.; Fischer, A.; Henz, B.M.; Welker, P. Mast cells and vasculature in atopic dermatitis—Potential stimulus of neoangiogenesis. Allergy Eur. J. Allergy Clin. Immunol. 2005, 60, 90–97. [Google Scholar] [CrossRef]
- Obara, W.; Kawa, Y.; Ra, C.; Nishioka, K.; Soma, Y.; Mizoguchi, M. T cells and mast cells as a major source of interleukin-13 in atopic dermatitis. Dermatology 2002, 205, 11–17. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. IL-4 and human skin mast cells revisited: Reinforcement of a pro-allergic phenotype upon prolonged exposure. Arch. Dermatol. Res. 2016, 308, 665–670. [Google Scholar] [CrossRef]
- Suurmond, J.; Habets, K.L.L.; Tatum, Z.; Schonkeren, J.J.; Hoen, P.A.C.T.; Huizinga, T.W.J.; Laros, J.F.J.; Toes, R.E.M.; Kurreeman, F. Repeated FcεRI triggering reveals modified mast cell function related to chronic allergic responses in tissue. J. Allergy Clin. Immunol. 2016, 138, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taube, C.; Stassen, M. Mast cells and mast cell-derived factors in the regulation of allergic sensitization. Chem. Immunol. Allergy 2008, 94, 58–66. [Google Scholar] [PubMed]
- Leyva-Castillo, J.M.; Das, M.; Artru, E.; Yoon, J.; Galand, C.; Geha, R.S. Mast cell–derived IL-13 downregulates IL-12 production by skin dendritic cells to inhibit the TH1 cell response to cutaneous antigen exposure. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Alenius, H.; Laouini, D.; Woodward, A.; Mizoguchi, E.; Bhan, A.K.; Castigli, E.; Oettgen, H.C.; Geha, R.S. Mast cells regulate IFN-γ expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J. Allergy Clin. Immunol. 2002, 109, 106–113. [Google Scholar] [CrossRef]
- Gordon, J.R. Monocyte chemoattractant peptide-1 expression during cutaneous allergic reactions in mice is mast cell dependent and largely mediates the monocyte recruitment response. J. Allergy Clin. Immunol. 2000, 106, 110–116. [Google Scholar] [CrossRef]
- Steinhoff, M.; Steinhoff, A.; Homey, B.; Luger, T.A.; Schneider, S.W. Role of vasculature in atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgate, S.T. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007, 28, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.P.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef]
- Jariwala, S.P.; Abrams, E.; Benson, A.; Fodeman, J.; Zheng, T. The role of thymic stromal lymphopoietin in the immunopathogenesis of atopic dermatitis. Clin. Exp. Allergy 2011, 41, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, N.; Sae-Wong, C.; Kangsanant, S.; Nabe, T.; Yoshino, S. Thymic stromal lymphopoietin-induced interleukin-17A is involved in the development of IgE-mediated atopic dermatitis-like skin lesions in mice. Immunology 2015, 146, 568–581. [Google Scholar] [CrossRef] [Green Version]
- Babina, M.; Wang, Z.; Franke, K.; Zuberbier, T. Thymic Stromal Lymphopoietin Promotes MRGPRX2-Triggered Degranulation of Skin Mast Cells in a STAT5-Dependent Manner with Further Support from JNK. Cells 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cells. Cytokine 2011, 54, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Zoltowska, A.; Ketelaar, M.E.; Nilsson, G. IL-33 and Thymic Stromal Lymphopoietin in mast cell functions. Eur. J. Pharmacol. 2016, 778, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Drube, S.; Kraft, F.; Dudeck, J.; Müller, A.-L.; Weber, F.; Göpfert, C.; Meininger, I.; Beyer, M.; Irmler, I.; Häfner, N.; et al. MK2/3 Are Pivotal for IL-33–Induced and Mast Cell–Dependent Leukocyte Recruitment and the Resulting Skin Inflammation. J. Immunol. 2016, 197, 3662–3668. [Google Scholar] [CrossRef]
- Imai, Y.; Yasuda, K.; Sakaguchi, Y.; Haneda, T.; Mizutani, H.; Yoshimoto, T.; Nakanishi, K.; Yamanishi, K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 13921–13926. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Egusa, C.; Maeda, T.; Numata, T.; Nakano, N.; Nishiyama, C.; Tsuboi, R. Il-33 promotes mhc class ii expression in murine mast cells. Immun. Inflamm. Dis. 2015, 3, 196–208. [Google Scholar] [CrossRef]
- Hao, Y.; Peng, B.; Che, D.; Zheng, Y.; Kong, S.; Liu, R.; Shi, J.; Han, H.; Wang, J.; Cao, J.; et al. Imiquimod-related dermatitis is mainly mediated by mast cell degranulation via Mas-related G-protein coupled receptor B2. Int. Immunopharmacol. 2020, 81. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, H.; Kolkhir, P.; Babina, M.; Düll, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-related G protein–coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 2021, 147, 456–469. [Google Scholar] [CrossRef]
- Thapaliya, M.; Chompunud Na Ayudhya, C.; Amponnawarat, A.; Roy, S.; Ali, H. Mast Cell-Specific MRGPRX2: A Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr. Allergy Asthma Rep. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Kwang, H.L.; Ji, Y.K.; Kang, D.S.; Yoo, J.C.; Lee, W.J.; Jai, Y.R. Increased expression of endothelial cell adhesion molecules due to mediator release from human foreskin mast cells stimulated by autoantibodies in chronic urticaria sera. J. Investig. Dermatol. 2002, 118, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Babina, M. MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis? Exp. Dermatol. 2020, 29, 1104–1111. [Google Scholar] [CrossRef]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Corbière, A.; Loste, A.; Gaudenzio, N. MRGPRX2 sensing of cationic compounds—A bridge between nociception and skin diseases? Exp. Dermatol. 2020, 30. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Hirasawa, N. The role of histamine H1 and H4 receptors in atopic dermatitis: From basic research to clinical study. Allergol. Int. 2014, 63, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Cowden, J.M.; Zhang, M.; Dunford, P.J.; Thurmond, R.L. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Investig. Dermatol. 2010, 130, 1023–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Pan, W.H.; Wang, X.R.; Liu, Y.; Chen, M.; Xu, X.G.; Liao, W.Q.; Hu, J.H. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharmacol. 2015, 28, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Buddenkotte, J.; Lerner, E.A. Role of mast cells and basophils in pruritus. Immunol. Rev. 2018, 282, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.L.B.; Kim, B.S. Pruritus in allergy and immunology. J. Allergy Clin. Immunol. 2019, 144, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, T.; Matsumoto, K.; Namiranian, S.; Yamashita, H.; Glatthorn, H.; Kimura, M.; Dolan, B.R.; Lee, J.J.; Galli, S.J.; Kawakami, Y.; et al. Mast cells are required for full expression of allergen/SEB-Induced skin inflammation. J. Investig. Dermatol. 2013, 133, 2695–2705. [Google Scholar] [CrossRef] [Green Version]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Castillo, J.M.; Galand, C.; Kam, C.; Burton, O.; Gurish, M.; Musser, M.A.; Goldsmith, J.D.; Hait, E.; Nurko, S.; Brombacher, F.; et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019, 50, 1262–1275.e4. [Google Scholar] [CrossRef] [PubMed]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef] [Green Version]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.T.T.; Sugita, K.; Akdis, C.A. Novel Biologicals for the Treatment of Allergic Diseases and Asthma. Curr. Allergy Asthma Rep. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Kaegi, C.; Akdis, M.; Bavbek, S.; Bossios, A.; Chatzipetrou, A.; Eiwegger, T.; Firinu, D.; Harr, T.; Knol, E.; et al. EAACI IG Biologicals task force paper on the use of biologic agents in allergic disorders. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 727–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in allergic inflammation and therapeutic perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.E.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Ring, J. Occupational skin disease—A major health problem in Europe. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 919–920. [Google Scholar] [CrossRef] [Green Version]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F.; Rustemeyer, T.; Thyssen, J.P. Recent advances in understanding and managing contact dermatitis. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.F. Immunological mechanisms in allergic contact dermatitis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fujisawa, H.; Zhuang, L.; Freed, I.; Howell, B.G.; Shahid, S.; Shivji, G.M.; Mak, T.W.; Sauder, D.N. CD4 + Th1 and CD8 + Type 1 Cytotoxic T Cells Both Play a Crucial Role in the Full Development of Contact Hypersensitivity. J. Immunol. 2000, 165, 6783–6790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspari, A.A.; Katz, S.I.; Martin, S.F. Contact Hypersensitivity. Curr. Protoc. Immunol. 2016, 113, 4.2.1–4.2.7. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef]
- Silvestre, M.C.; dos Reis, V.M.S.; Sato, M.N. Innate immunity and effector and regulatory mechanisms involved in allergic contact dermatitis. An. Bras. Dermatol. 2018, 93, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Vocanson, M.; Hennino, A.; Rozières, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1699–1714. [Google Scholar] [CrossRef]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.F.; Hennino, A. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J. Investig. Dermatol. 2007, 127, 1430–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askenase, P.W.; Van Loveren, H.; Kraeuter-Kops, S.; Ron, Y.; Meade, R.; Theoharides, T.C.; Nordlund, J.J.; Scovern, H.; Gerhson, M.D.; Ptak, W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J. Immunol. 1983, 131, 2687–2694. [Google Scholar]
- Thomas, W.R.; Schrader, J.W. Delayed hypersensitivity in mast-cell-deficient mice. J. Immunol. 1983, 130, 2565–2567. [Google Scholar] [PubMed]
- Mekori, Y.A.; Galli, S.J. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J. Immunol. 1985, 135, 879–885. [Google Scholar]
- Kerdel, F.A.; Belsito, D.V.; Scotto-Chinnici, R.; Soter, N.A. Mast cell participation during the elicitation of murine allergic contact hypersensitivity. J. Investig. Dermatol. 1987, 88, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Nunomura, S.; Gon, Y.; Endo, D.; Kishiro, S.; Fukunaga, M.; Kitahata, Y.; Terui, T.; Ra, C. Abrogation of high-affinity IgE receptor-mediated mast cell activation at the effector phase prevents contact hypersensitivity to oxazolone. J. Investig. Dermatol. 2010, 130, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryce, P.J.; Miller, M.L.; Miyajima, I.; Tsai, M.; Galli, S.J.; Oettgen, H.C. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity 2004, 20, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Kambe, N.; Nakamura, Y.; Saito, M.; Nishikomori, R. The inflammasome, an innate immunity guardian, participates in skin urticarial reactions and contact hypersensitivity. Allergol. Int. 2010, 59, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Mraz, V.; Geisler, C.; Bonefeld, C.M. Dendritic Epidermal T Cells in Allergic Contact Dermatitis. Front. Immunol. 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Andersson, S.I.; Stenfeldt, A.L.; Simonsson, C.; Bergström, J.; Ericson, M.B.; Jonsson, C.A.; Broo, K.S. Modification and expulsion of keratins by human epidermal keratinocytes upon hapten exposure in vitro. Chem. Res. Toxicol. 2011, 24, 737–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, A.; Nicholas, B.; Darley, R.; Parkinson, E.; Teo, Y.; Aleksic, M.; Maxwell, G.; Elliott, T.; Ardern-Jones, M.; Skipp, P. Characterization of the Class I MHC Peptidome Resulting From DNCB Exposure of HaCaT Cells. Toxicol. Sci. 2021, 180, 136–147. [Google Scholar] [CrossRef]
- Simonsson, C.; Andersson, S.I.; Stenfeldt, A.L.; Bergström, J.; Bauer, B.; Jonsson, C.A.; Ericson, M.B.; Broo, K.S. Caged fluorescent haptens reveal the generation of cryptic epitopes in allergic contact dermatitis. J. Investig. Dermatol. 2011, 131, 1486–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Gaide, O.; Pétrilli, V.; Martinon, F.; Contassot, E.; Roques, S.; Kummer, J.A.; Tschopp, J.; French, L.E. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Investig. Dermatol. 2007, 127, 1956–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastião, A.I.; Ferreira, I.; Brites, G.; Silva, A.; Neves, B.M.; Teresa Cruz, M. NLRP3 Inflammasome and Allergic Contact Dermatitis: A Connection to Demystify. Pharmaceutics 2020, 12, 867. [Google Scholar] [CrossRef]
- Esser, P.R.; Wölfle, U.; Dürr, C.; von Loewenich, F.D.; Schempp, C.M.; Freudenberg, M.A.; Jakob, T.; Martin, S.F. Contact Sensitizers Induce Skin Inflammation via ROS Production and Hyaluronic Acid Degradation. PLoS ONE 2012, 7, e41340. [Google Scholar] [CrossRef] [Green Version]
- Weber, F.C.; Esser, P.R.; Müller, T.; Ganesan, J.; Pellegatti, P.; Simon, M.M.; Zeiser, R.; Idzko, M.; Jakob, T.; Martin, S.F. Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J. Exp. Med. 2010, 207, 2609–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onami, K.; Kimura, Y.; Ito, Y.; Yamauchi, T.; Yamasaki, K.; Aiba, S. Nonmetal haptens induce ATP release from keratinocytes through opening of pannexin hemichannels by reactive oxygen species. J. Investig. Dermatol. 2014, 134, 1951–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia Minciullo, P.; Guarneri, F.; MinciuLLo, P.; Calapai, F.; Cannavò, P. IL-31 and IL-33 circulating levels in allergic contact dermatitis. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 156–158. [Google Scholar]
- Taniguchi, K.; Yamamoto, S.; Hitomi, E.; Inada, Y.; Suyama, Y.; Sugioka, T.; Hamasaki, Y. Interleukin 33 is induced by Tumor necrosis factor alpha and interferon gamma in keratinocytes and contributes to allergic contact dermatitis. J. Investig. Allergol. Clin. Immunol. 2013, 23, 428–434. [Google Scholar] [PubMed]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of Poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, Y.; Yasuda, K.; Sakaguchi, Y.; Futatsugi-Yumikura, S.; Yoshimoto, T.; Nakanishi, K.; Yamanishi, K. Immediate-type contact hypersensitivity is reduced in interleukin-33 knockout mice. J. Dermatol. Sci. 2014, 74, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Seike, M.; Furuya, K.; Omura, M.; Hamada-Watanabe, K.; Matsushita, A.; Ohtsu, H. Histamine H4 receptor antagonist ameliorates chronic allergic contact dermatitis induced by repeated challenge. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.C.; Németh, T.; Csepregi, J.Z.; Dudeck, A.; Roers, A.; Ozsvári, B.; Oswald, E.; Puskás, L.G.; Jakob, T.; Mócsai, A.; et al. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J. Exp. Med. 2015, 212, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.W.; Tedla, N.; Lloyd, A.R.; Wakefield, D.; McNeil, H.P. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Investig. 1998, 102, 1617–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, A.; Kubo, M.; Honda, T.; Egawa, G.; Nakajima, S.; Tanizaki, H.; Kim, B.; Matsuoka, S.; Watanabe, T.; Nakae, S.; et al. Requirement of Interaction between Mast Cells and Skin Dendritic Cells to Establish Contact Hypersensitivity. PLoS ONE 2011, 6, e25538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suto, H.; Nakae, S.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast Cell-Associated TNF Promotes Dendritic Cell Migration. J. Immunol. 2006, 176, 4102–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, J.; Ghouse, S.M.; Lehmann, C.H.K.; Hoppe, A.; Schubert, N.; Nedospasov, S.A.; Dudziak, D.; Dudeck, A. Mast-Cell-Derived TNF Amplifies CD8+ Dendritic Cell Functionality and CD8+ T Cell Priming. Cell Rep. 2015, 13, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakurai, M.; Monteforte, R.; Suto, H.; Tsai, M.; Nakae, S.; Galli, S.J. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am. J. Pathol. 2006, 169, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef]
- Shimada, Y.; Hasegawa, M.; Kaburagi, Y.; Hamaguchi, Y.; Komura, K.; Saito, E.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. L-Selectin or ICAM-1 Deficiency Reduces an Immediate-Type Hypersensitivity Response by Preventing Mast Cell Recruitment in Repeated Elicitation of Contact Hypersensitivity. J. Immunol. 2003, 170, 4325–4334. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Rivera, V.-A.; Siebenhaar, F.; Zimmermann, C.; Siiskonen, H.; Metz, M.; Maurer, M. Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J. Immunol. 2016, 197, 4240–4246. [Google Scholar] [CrossRef] [Green Version]
- Babina, M.; Wang, Z.; Franke, K.; Guhl, S.; Artuc, M.; Zuberbier, T. Yin-Yang of IL-33 in Human Skin Mast Cells: Reduced Degranulation, but Augmented Histamine Synthesis through p38 Activation. J. Investig. Dermatol. 2019, 139, 1516–1525.e3. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Fan, H.; Chen, M.; Wang, J.; Brand, D.; He, X.; Quesniaux, V.; Ryffel, B.; Zhu, L.; Liang, D.; et al. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-β1. J. Allergy Clin. Immunol. 2012, 130. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzio, N.; Marichal, T.; Galli, S.J.; Reber, L.L. Genetic and imaging approaches reveal pro-inflammatory and immunoregulatory roles of mast cells in contact hypersensitivity. Front. Immunol. 2018, 9, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.U.; Hwang, J.; Hulliger, S.; Bonder, C.S.; Yamanouchi, J.; Santamaria, P.; Kubes, P. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am. J. Pathol. 2008, 172, 1638–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reber, L.L.; Sibilano, R.; Starkl, P.; Roers, A.; Grimbaldeston, M.A.; Tsai, M.; Gaudenzio, N.; Galli, S.J. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsai, M.; Galli, S.J. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.H.S.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The regulatory B cell–mediated peripheral tolerance maintained by mast cell IL-5 suppresses oxazolone-induced contact hypersensitivity. Sci. Adv. 2019, 5, 8152–8169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Honda, T.; Kanameishi, S.; Honda, Y.; Egawa, G.; Kitoh, A.; Nakajima, S.; Otsuka, A.; Nomura, T.; Dainichi, T.; et al. PD-L1 on mast cells suppresses effector CD8+ T-cell activation in the skin in murine contact hypersensitivity. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Ronconi, G.; Caraffa, A.; Kritas, S.K. Activation of mast cells mediates inflammatory response in psoriasis: Potential new therapeutic approach with IL-37. Dermatol. Ther. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Prim. 2016, 2, 1–17. [Google Scholar] [CrossRef]
- Brody, I. Mast cell degranulation in the evolution of acute eruptive guttate psoriasis vulgaris. J. Investig. Dermatol. 1984, 82, 460–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.J.; Hansen, U.; Kristensen, J.K.; Nielsen, H.; Skov, P.S.; Nielsen, H.J. Studies on mast cells and histamine release in psoriasis: The effect of ranitidine. Acta Derm. Venereol. 1998, 78, 190–193. [Google Scholar] [CrossRef]
- Oishi, N.; Iwata, H.; Kambe, N.; Kobayashi, N.; Fujimoto, K.; Sato, H.; Hisaka, A.; Ueno, K.; Yamaura, K. Expression of precipitating factors of pruritus found in humans in an imiquimod-induced psoriasis mouse model. Heliyon 2019, 5, e01981. [Google Scholar] [CrossRef] [Green Version]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvima, I.T.; Nilsson, G.; Suttle, M.M.; Naukkarinen, A. Is there a role for mast cells in psoriasis? Arch. Dermatol. Res. 2008, 300, 461–478. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balato, A.; Lembo, S.; Mattii, M.; Schiattarella, M.; Marino, R.; De Paulis, A.; Balato, N.; Ayala, F. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp. Dermatol. 2012, 21, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359.e1. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuberbier, T.; Asero, R.; Bindslev-Jensen, C.; Walter Canonica, G.; Church, M.K.; Giménez-Arnau, A.; Grattan, C.E.H.; Kapp, A.; Merk, H.F.; Rogala, B.; et al. EAACI/GA2LEN/EDF/WAO guideline: Definition, classification and diagnosis of urticaria. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1417–1426. [Google Scholar] [CrossRef]
- Jacques, P.; Lavoie, A.; Bédard, P.M.; Brunet, C.; Hebert, J. Chronic idiopathic urticaria: Profiles of skin mast cell histamine release during active disease and remission. J. Allergy Clin. Immunol. 1992, 89, 1139–1143. [Google Scholar] [CrossRef]
- Guida, B.; De Martino, C.; De Martino, S.; Tritto, G.; Patella, V.; Trio, R.; D’Agostino, C.; Pecoraro, P.; D’Agostino, L. Histamine plasma levels and elimination diet in chronic idiopathic urticaria. Eur. J. Clin. Nutr. 2000, 54, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.F.; Austen, K.F.; Fonferko, E.; Sheffer, A.L. Morphologically distinctive forms of cutaneous mast cell degranulation induced by cold and mechanical stimuli: An ultrastructural study. J. Allergy Clin. Immunol. 1987, 80, 603–611. [Google Scholar] [CrossRef]
- Garafalo, J.; Kaplan, A.P. Histamine release and therapy of severe dermatographism. J. Allergy Clin. Immunol. 1981, 68, 103–105. [Google Scholar] [CrossRef]
- Kring Tannert, L.; Stahl Skov, P.; Bjerremann Jensen, L.; Maurer, M.; Bindslev-Jensen, C. Cold urticaria patients exhibit normal skin levels of functional mast cells and histamine after tolerance induction. Dermatology 2012, 224, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P.; Gray, L.; Shaff, R.E.; Horakova, Z.; Beaven, M.A. In vivo studies of mediator release in cold urticaria and cholinergic urticaria. J. Allergy Clin. Immunol. 1975, 55, 394–402. [Google Scholar] [CrossRef]
- Koh, Y.I.; Choi, I.S.; Lee, S.H.; Lee, J.B.; Park, C.H.; Hong, S.N. Localized heat urticaria associated with mast cell and eosinophil degranulation. J. Allergy Clin. Immunol. 2002, 109, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.B.; Lieberman, P.; Friedman, M.M.; Kaliner, M.; Kaplan, R.; Bale, G.; Treadwell, G.; Yoo, T.J. Mediator release in local heat urticaria: Protection with combined H1 and H2 antagonists. J. Allergy Clin. Immunol. 1985, 76, 35–39. [Google Scholar] [CrossRef]
- Hawk, J.L.; Eady, R.A.; Challoner, A.V.; Kobza-Black, A.; Keahey, T.M.; Greaves, M.W. Elevated blood histamine levels and mast cell degranulation in solar urticaria. Br. J. Clin. Pharmacol. 1980, 9, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Mayou, S.C.; Black, A.K.; Eady, R.A.J.; Greaves, M.W. Cholinergic dermographism. Br. J. Dermatol. 1986, 115, 371–377. [Google Scholar] [CrossRef]
- Mekori, Y.A.; Dobozin, B.S.; Schocket, A.L.; Kohler, P.F.; Clark, R.A.F. Delayed Pressure Urticaria Histologically Resembles Cutaneous Late-Phase Reactions. Arch. Dermatol. 1988, 124, 230–235. [Google Scholar] [CrossRef]
- Schaefer, P. Urticaria: Evaluation and Treatment. Am. Fam. Physician 2011, 83, 1078–1084. [Google Scholar] [PubMed]
- Kolkhir, P.; Church, M.K.; Weller, K.; Metz, M.; Schmetzer, O.; Maurer, M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J. Allergy Clin. Immunol. 2017, 139, 1772–1781.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, M.; Rosén, K.; Hsieh, H.-J.; Saini, S.; Grattan, C.; Gimenéz-Arnau, A.; Agarwal, S.; Doyle, R.; Canvin, J.; Kaplan, A.; et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N. Engl. J. Med. 2013, 368, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, M.; Metz, M.; Brehler, R.; Hillen, U.; Jakob, T.; Mahler, V.; Pföhler, C.; Staubach, P.; Treudler, R.; Wedi, B.; et al. Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J. Allergy Clin. Immunol. 2018, 141, 638–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyden, S.E.; Desai, A.; Cruse, G.; Young, M.L.; Bolan, H.C.; Scott, L.M.; Eisch, A.R.; Long, R.D.; Lee, C.-C.R.; Satorius, C.L.; et al. Vibratory Urticaria Associated with a Missense Variant in ADGRE2. N. Engl. J. Med. 2016, 374, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wallengren, J.; Möller, H.; Ekman, R. Occurrence of substance P, vasoactive intestinal peptide, and calcitonin gene-related peptide in dermographism and cold urticaria. Arch. Dermatol. Res. 1987, 279, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Borici-Mazi, R.; Kouridakis, S.; Kontou-Fili, K. Cutaneous responses to substance P and calcitonin gene-related peptide in chronic urticaria: The effect of cetirizine and dimethindene. Allergy Eur. J. Allergy Clin. Immunol. 1999, 54, 46–56. [Google Scholar] [CrossRef]
- Fujisawa, D.; Kashiwakura, J.-I.I.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633.e9. [Google Scholar] [CrossRef]
- Toledo, M.A.S.; Gatz, M.; Sontag, S.; Gleixner, K.V.; Eisenwort, G.; Feldberg, K.; Hamouda, A.E.I.; Kluge, F.; Guareschi, R.; Rossetti, G.; et al. Nintedanib targets KIT D816V neoplastic cells derived from induced pluripotent stem cells of systemic mastocytosis. Blood 2021, 137, 2070–2084. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ghosh, J.; Kapur, R. Mastocytosis—A mutated KIT receptor induced myeloproliferative disorder. Oncotarget 2015, 6, 18250–18264. [Google Scholar] [CrossRef]
- Piao, X.; Bernstein, A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood 1996, 87, 3117–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, K.; Artuc, M.; Baldus, S.E.; Zirbes, T.K.; Hermes, B.; Thiele, J.; Mekori, Y.A.; Henz, B.M. Expression of Bcl-2 and Bcl-xL in Cutaneous and Bone Marrow Lesions of Mastocytosis. Am. J. Pathol. 2003, 163, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Aichberger, K.J.; Gleixner, K.V.; Mirkina, I.; Cerny-Reiterer, S.; Peter, B.; Ferenc, V.; Kneidinger, M.; Baumgartner, C.; Mayerhofer, M.; Gruze, A.; et al. Identification of proapoptotic Bim as a tumor suppressor in neoplastic mast cells: Role of KIT D816V and effects of various targeted drugs. Blood 2009, 114, 5342–5351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.; Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.; Lange, M.; et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; The American Academy of Allergy, Asthma & Immunology; And the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 2016, 137, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Akin, C.; Bindslev-Jensen, C.; Maurer, M.; Broesby-Olsen, S. Treatment Strategies in Mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.; Schwaab, J.; Naumann, N.; Horny, H.-P.; Sotlar, K.; Haferlach, T.; Metzgeroth, G.; Fabarius, A.; Valent, P.; Hofmann, W.-K.; et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood 2017, 130, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P.; Akin, C.; Nedoszytko, B.; Bonadonna, P.; Hartmann, K.; Niedoszytko, M.; Brockow, K.; Siebenhaar, F.; Triggiani, M.; Arock, M.; et al. Diagnosis, classification and management of mast cell activation syndromes (Mcas) in the era of personalized medicine. Int. J. Mol. Sci. 2020, 21, 9030. [Google Scholar] [CrossRef] [PubMed]
- Molderings, G.J.; Haenisch, B.; Bogdanow, M.; Fimmers, R.; Nöthen, M.M. Familial Occurrence of Systemic Mast Cell Activation Disease. PLoS ONE 2013, 8, e76241. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Ackerley, M.B.; Bluestein, L.S.; Brewer, J.H.; Brook, J.B.; Buchanan, A.D.; Cuni, J.R.; Davey, W.P.; Dempsey, T.T.; Dorff, S.R.; et al. Diagnosis of mast cell activation syndrome: A global “consensus-2”. Diagnosis 2020. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H. Survey of Mast Cell Mediator Levels from Patients Presenting with Symptoms of Mast Cell Activation. Int. Arch. Allergy Immunol. 2020, 181, 43–50. [Google Scholar] [CrossRef]
- Valent, P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin. Exp. Allergy 2014, 44, 914–920. [Google Scholar] [CrossRef] [PubMed]
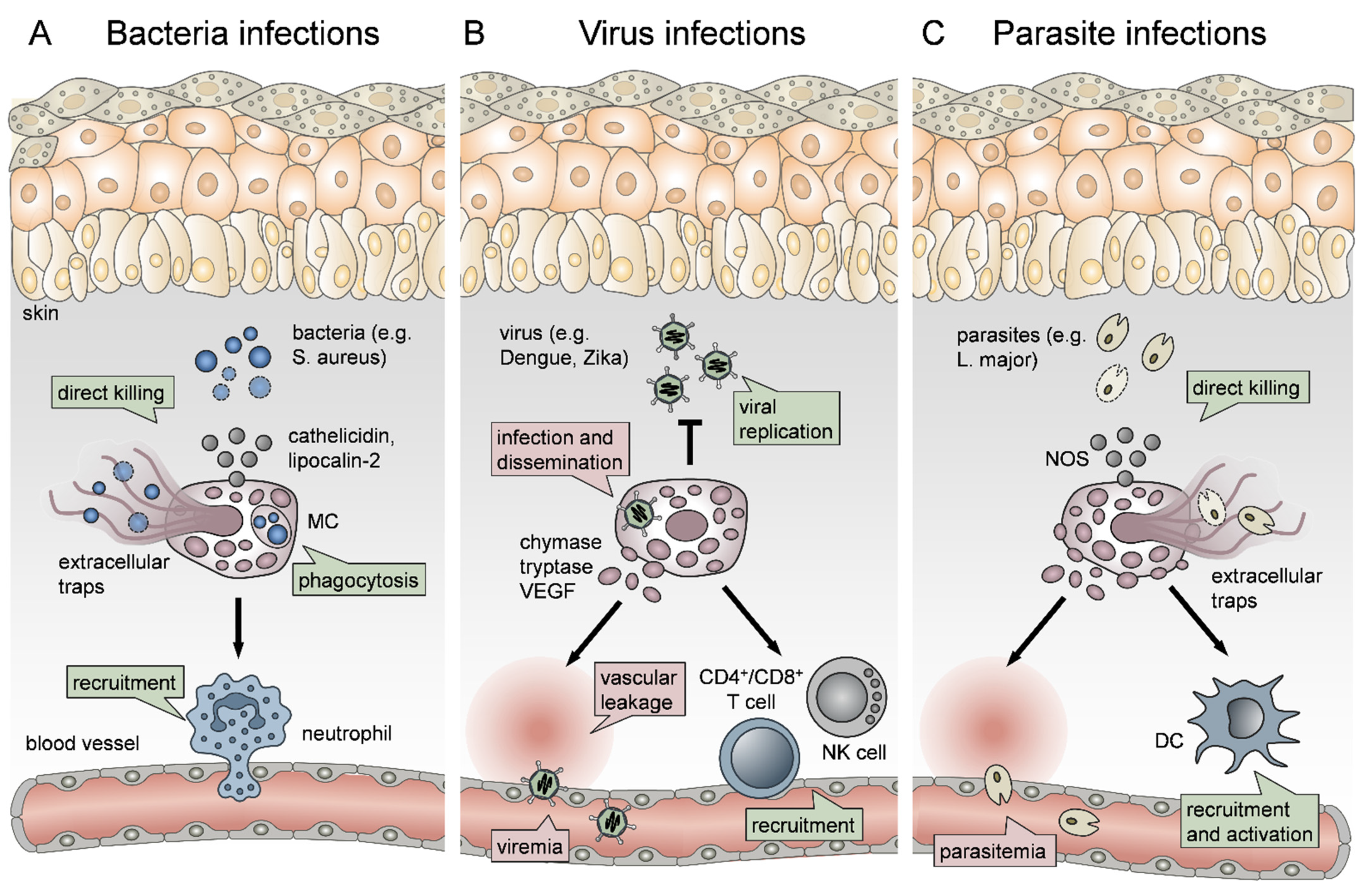
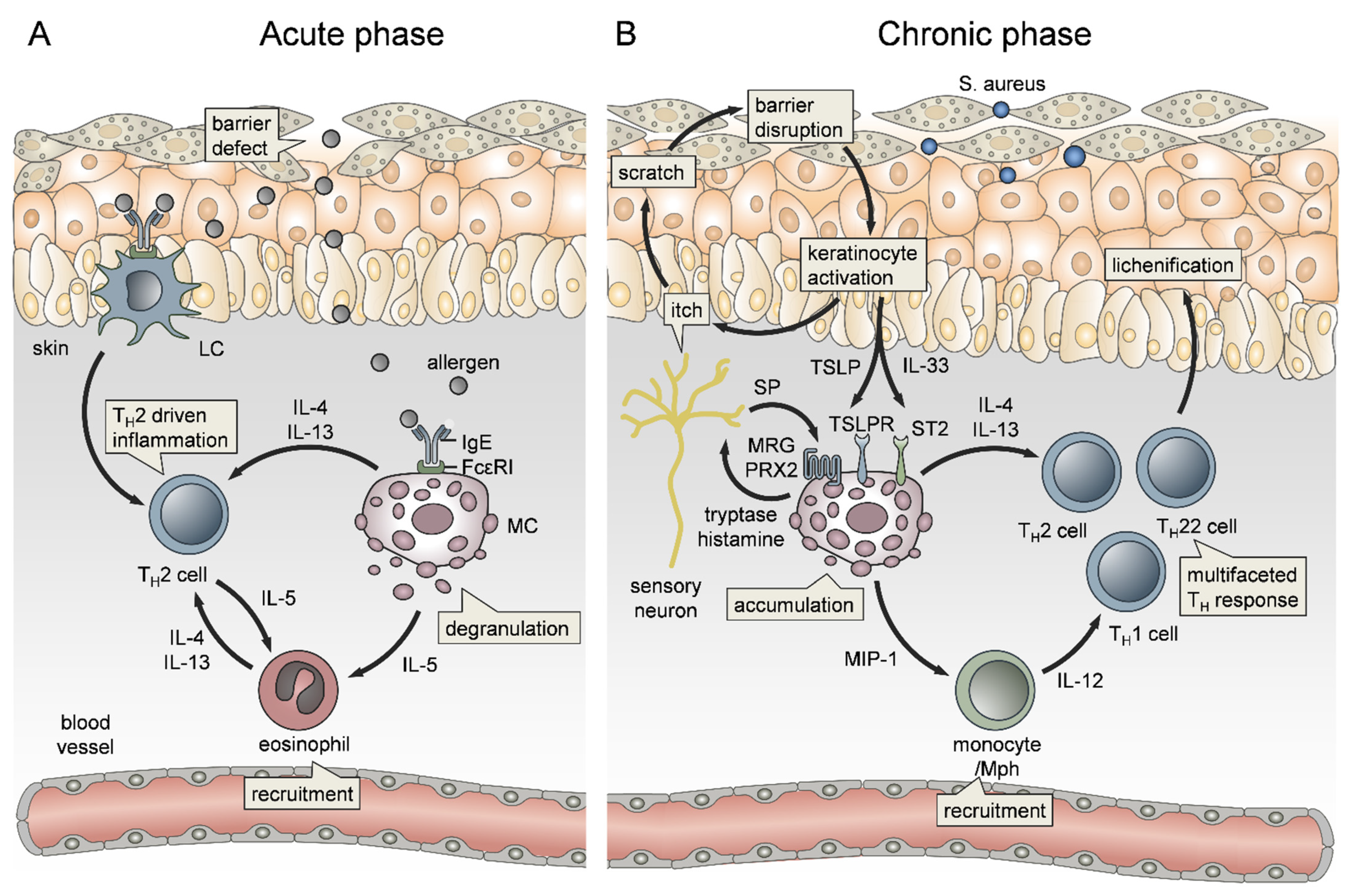
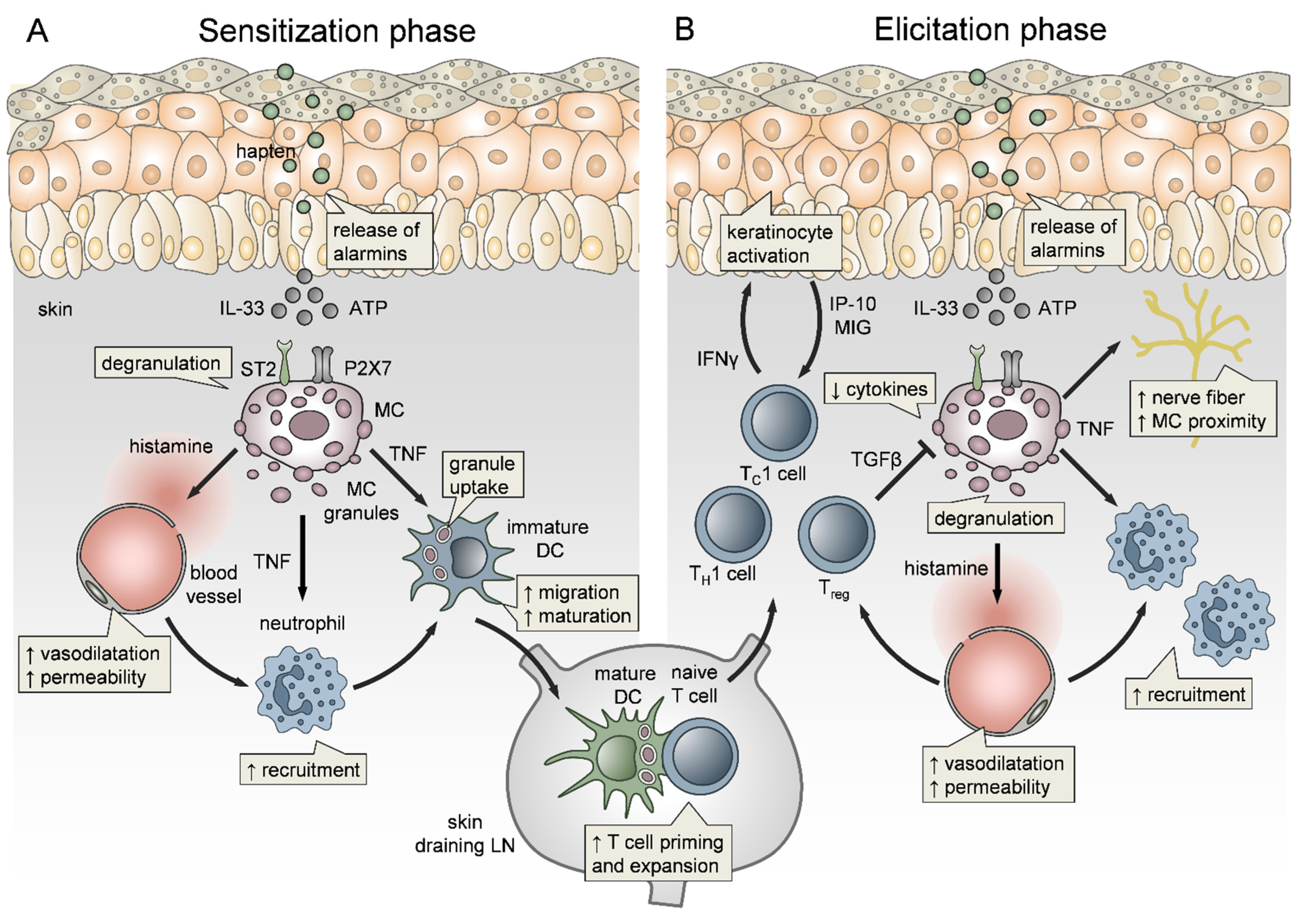
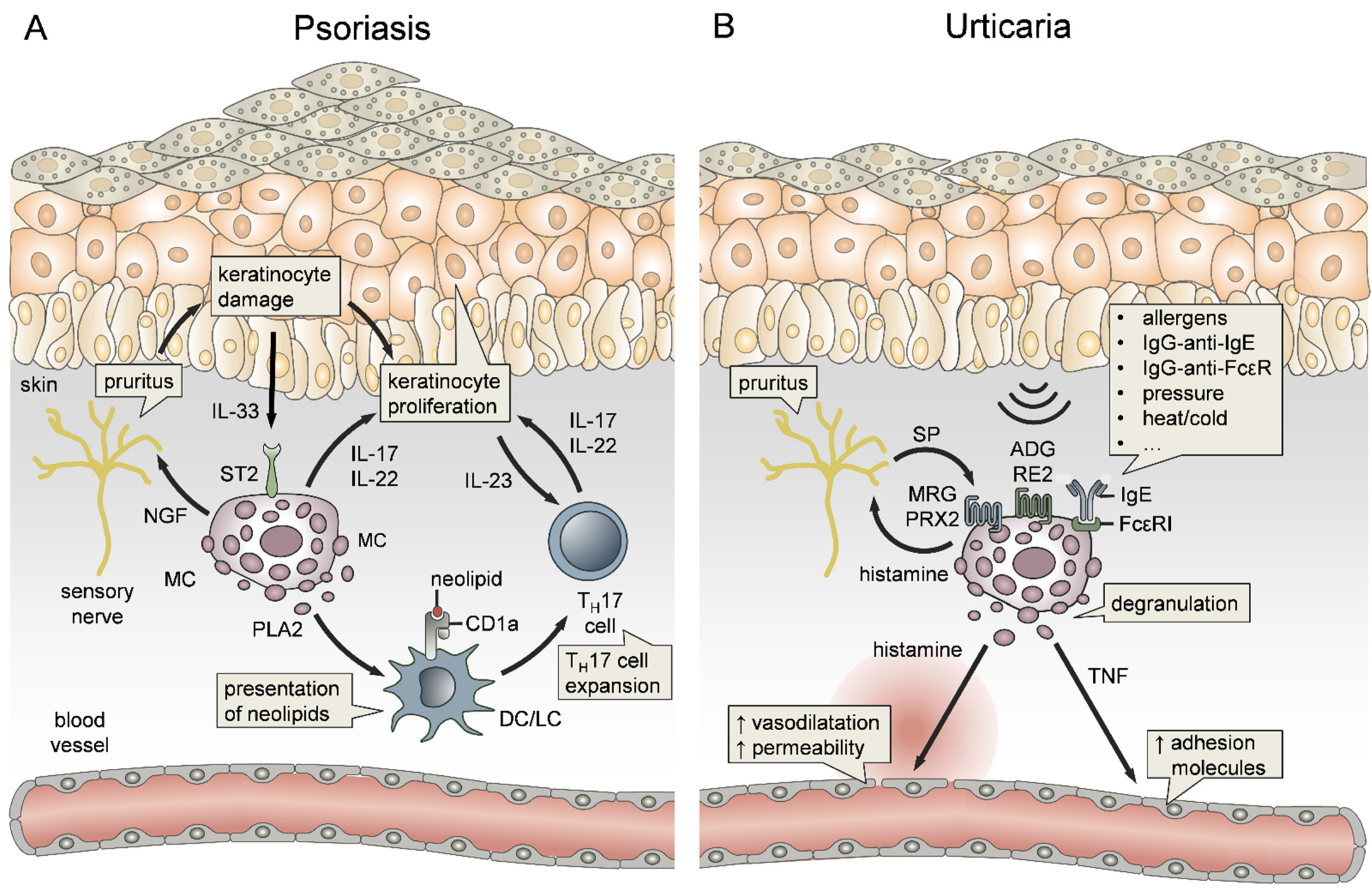
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. https://doi.org/10.3390/ijms22094589
Voss M, Kotrba J, Gaffal E, Katsoulis-Dimitriou K, Dudeck A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? International Journal of Molecular Sciences. 2021; 22(9):4589. https://doi.org/10.3390/ijms22094589
Chicago/Turabian StyleVoss, Martin, Johanna Kotrba, Evelyn Gaffal, Konstantinos Katsoulis-Dimitriou, and Anne Dudeck. 2021. "Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation?" International Journal of Molecular Sciences 22, no. 9: 4589. https://doi.org/10.3390/ijms22094589






