The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development
Abstract
1. Introduction
2. Oxidative Stress and Bladder Cancer
3. Inflammation and Bladder Cancer
4. Modulation of Inflammatory System in Course of BCG Therapy
- (1)
- Attachment of BCG to the urothelium cells through the interaction between molecules in the bacterial wall and fibronectin in the urothelium;
- (2)
- Internalisation of BCG into resident immune cells, regular cells and urothelial tumour cells through increased macropinocytosis;
- (3)
- BCG-mediated induction of innate immunity, which is characterised by urothelial cells and antigen-presenting cells (APCs) activation and then induction of cytokine and chemokine production (including IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNF-α that attract granulocytes and mononuclear cells to the bladder. Interestingly, the levels of IL-1β, IL-8, IL-15, IL-18, CXC-chemokine ligand 10 (CXCL10), GM-CSF, CC-chemokine ligand 2 (CCL2) and CCL3 in urine are detectable within the first 24 h after BCG infusion. In addition, in the urinary tract and the bladder, the presence of neutrophils, monocytes, macrophages, T cells, B cells and NK cells was observed after BCG therapy.
- (4)
- BCG-mediated initiation of tumour-specific immunity. APC and urothelial cell activity may lead to BCG antigens presentation of these cells surface via MHC class II. These MHC affect CD4+ T cell receptors, resulting in primarily T helper 1 (TH1) cell immune activation and differentiation. TH1 activation induces the generation of IL-2, IL-12, IFN-γ, TNF-α and TNF-β and leads to the activation of cytotoxic CD8+ T lymphocytes, which destroy cancer cells. On the other hand, in the BCG therapy response, the production of IL-4, IL-5, IL-6 and IL-10 by primary T helper 2 (TH2) was associated with BCG non-responsiveness and cancer progression [241].
5. Angiogenesis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]; International Agency for Research on Cancer: Lyon, France, 2010; Available online: http://globocan.iarc.fr (accessed on 6 May 2012).
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Stern, M.C.; Lin, J.; Figueroa, J.D.; Kelsey, K.T.; Kiltie, A.E.; Yuan, J.M.; Matullo, G.; Fletcher, T.; Benhamou, S.; Taylor, J.A.; et al. International Consortium of Bladder Cancer. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: Findings from the interna-tional consortium of bladder cancer. Cancer Res. 2009, 69, 6857–6864. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Boffetta, P.; Adami, H.-O. Tobacco use and cancer causation: Association by tumour type. J. Intern. Med. 2002, 252, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; Bogillot, O.; Cordier, S.; Greiser, E.; Schill, W.; Vineis, P.; Lopez-Abente, G.; Tzonou, A.; Chang-Claude, J.; Bolm-Audorff, U.; et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case-control studies. Int. J. Cancer 2000, 86, 289–294. [Google Scholar] [CrossRef]
- Snyderwine, E.G.; Sinha, R.; Felton, J.S.; Ferguson, L.R. Highlights of the eighth international conference on carcinogen-ic/mutagenic N-substituted aryl compounds. Mutat. Res. 2002, 506–507, 1–8. [Google Scholar] [CrossRef]
- García-Pérez, J.; Pollán, M.; Boldo, E.; Pérez-Gómez, B.; Aragonés, N.; Lope, V.; Ramis, R.; Vidal, E.; López-Abente, G. Mortality due to lung, laryngeal and bladder cancer in towns lying in the vicinity of combustion installations. Sci. Total Environ. 2009, 407, 2593–2602. [Google Scholar] [CrossRef]
- Case, R.A.M.; Hosker, M.E. Tumour of the Urinary Bladder as an Occupational Disease in the Rubber Industry in England and Wales. J. Epidemiol. Commun. Heal. 1954, 8, 39–50. [Google Scholar] [CrossRef][Green Version]
- Golka, K.; Wiese, A.; Assennato, G.; Bolt, H.M. Occupational exposure and urological cancer. World J. Urol. 2004, 21, 382–391. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, G.; Qin, Y.; Lu, D.; Golka, K.; Geller, F.; Chen, J.; Shen, J. GSTP1 A1578G (Ile105Val) polymorphism in benzi-dine-exposed workers: An association with cytological grading of exfoliated urothelial cells. Pharmacogenetics 2003, 13, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Meliker, J.R.; Nriagu, J.O. Arsenic in drinking water and bladder cancer: Review of epidemiological evidence. Trace Metals Other Contam. Environ. 2007, 9, 551–584. [Google Scholar]
- Navarro Silvera, S.A.; Rohan, T.E. Trace elements and cancer risk: A review of the epidemiologic evidence. Cancer Causes Control 2007, 18, 7–27. [Google Scholar] [CrossRef]
- Bladder Cancer Risk Factors. Available online: https://www.cancer.org/cancer/bladder-cancer/causes-risks-prevention/risk-factors.html (accessed on 1 February 2021).
- Michaud, D.S.; Kogevinas, M.; Cantor, K.P.; Villanueva, C.M.; Garcia-Closas, M.; Rothman, N.; Malats, N.; Real, F.X.; Serra, C.; García-Closas, R.; et al. Total Fluid and Water Consumption and the Joint Effect of Exposure to Disinfection By-Products on Risk of Bladder Cancer. Environ. Health Perspect. 2007, 115, 1569–1572. [Google Scholar] [CrossRef]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Curhan, G.C.; Willett, W.C.; Giovannucci, E.L. Fluid intake and the risk of bladder cancer in men. N. Engl. J. Med. 1999, 340, 1390–1397. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, B.; Tian, Y.; Chen, Y.; Luo, D.; Lin, Y.; Li, H.; Wang, K.-J. Total fluid consumption and risk of bladder cancer: A meta-analysis with updated data. Oncotarget 2017, 8, 55467–55477. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.F.; Cohen, M.B. Urinary system. Cancer 1995, 75, 316–329. [Google Scholar] [CrossRef]
- American Cancer Society Cancer Facts & Figures 2008. Available online: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf (accessed on 1 January 2021).
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef]
- European Cancer Patient Coalition White Paper on Bladder Cancer 2016. Available online: https://ecpc.org/wp-content/uploads/2019/08/ECPC–White–Paper–Bladd (accessed on 1 February 2021).
- Epidemiology of Bladder Cancer in Europe. Available online: https://ec.europa.eu/jrc/en/publication/epidemiology-bladder-cancer-europe (accessed on 1 February 2021).
- Howlader, N.N.A.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Cho, H.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, USA, 2010. Available online: http://seer.cancer.gov/csr/1975_2010/ (accessed on 1 January 2021).
- Yee, D.S.; Ishill, N.M.; Lowrance, W.T.; Herr, H.W.; Elkin, E.B. Ethnic Differences in Bladder Cancer Survival. Urology 2011, 78, 544–549. [Google Scholar] [CrossRef]
- Madeb, R.; Messing, E.M. Gender, racial and age differences in bladder cancer incidence and mortality. Urol. Oncol. Semin. Orig. Investig. 2004, 22, 86–92. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Smith, A.B.; Meyer, A.-M.; Kuo, T.-M.; Tyree, S.; Kim, W.Y.; Milowsky, M.I.; Pruthi, R.S.; Millikan, R.C. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 2013, 120, 86–95. [Google Scholar] [CrossRef]
- Ries, L.A.G.; Kosary, C.L.; Hankey, B.F.; Miller, B.A.; Clegg, L.; Mariotto, A.; Feuer, E.J.; Edwards, B.K. (Eds.) SEER Cancer Statistics Review, 1975–2002; National Cancer Institute: Bethesda, MD, USA, 2002. Available online: http://seer.cancer.gov/csr/1975_2002/ (accessed on 1 January 2021).
- Scosyrev, E.; Noyes, K.; Feng, C.; Messing, E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2008, 115, 68–74. [Google Scholar] [CrossRef]
- Taub, D.A.; Hollenbeck, B.K.; Cooper, K.L.; Dunn, R.L.; Miller, D.C.; Taylor, J.M.; Wei, J.T. Racial disparities in resource utiliza-tion for cystectomy. Urology 2006, 67, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Aben, K.K.; Witjes, J.A.; Schoenberg, M.P.; De Kaa, C.H.-V.; Kiemeney, L.A. Familial aggregation of urothelial cell carcinoma. Int. J. Cancer 2001, 98, 274–278. [Google Scholar] [CrossRef]
- Kramer, A.A.; Graham, S.; Burnett, W.S.; Nasca, P. Familial Aggregation of Bladder Cancer Stratified by Smoking Status. Epidemiology 1991, 2, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, X. Genetic susceptibility to bladder cancer risk and outcome. Pers. Med. 2011, 8, 365–374. [Google Scholar] [CrossRef] [PubMed]
- García-Closas, M.; Malats, N.; Silverman, D.; Dosemeci, M.; Kogevinas, M.; Hein, D.W.; Tardón, A.; Serra, C.; Carrato, A.; García-Closas, R.; et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: Results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 2005, 366, 649–659. [Google Scholar] [CrossRef]
- Kiemeney, L.A.; Sulem, P.; Besenbacher, S.; Vermeulen, S.H.; Sigurdsson, A.; Thorleifsson, G.; Gudbjartsson, D.F.; Stacey, S.N.; Gudmundsson, J.; Zanon, C.; et al. A sequence variant at 4p16.3 confers susceptibility to urinary bladder cancer. Nat. Genet. 2010, 42, 415–419. [Google Scholar] [CrossRef]
- Vatsis, K.P.; Weber, W.W.; Bell, D.A.; Dupret, J.-M.; Evans, D.A.P.; Grant, D.M.; Hein, D.W.; Lin, H.J.; Meyer, U.A.; Relling, M.V.; et al. Nomenclature for N-acetyltransferases. Pharmacogenetics 1995, 5, 1–17. [Google Scholar] [CrossRef]
- Hein, D.W. Molecular genetics and function of NAT1 and NAT2: Role in aromatic amine metabolism and carcino-genesis. Mutat. Res. 2002, 506–507, 65–77. [Google Scholar] [CrossRef]
- Rothman, N.; Garcia-Closas, M.; Chatterjee, N.; Malats, N.; Wu, X.; Figueroa, J.D.; Real, F.X.; Van Den Berg, D.; Matullo, G.; Ba-ris, D. A multi-stage ge-nome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010, 42, 978–984. [Google Scholar] [CrossRef]
- Sidransky, D.; Von Eschenbach, A.; Tsai, Y.C.; Jones, P.; Summerhayes, I.; Marshall, F.; Paul, M.; Green, P.; Hamilton, S.R.; Frost, P.; et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 1991, 252, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y. Bladder Cancer and Genetic Mutations. Cell Biophys. 2015, 73, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Real, F.X. 70% of Bladder Cancers Involve a Specific Mutation in a Particular Gene; Pompeu Fabra University: Barcelona, Spain, 2013. [Google Scholar]
- Yokomizo, A.; Mai, M.; Tindall, D.J.; Cheng, L.; Bostwick, D.G.; Naito, S.; I Smith, D.; Liu, W. Overexpression of the wild type p73 gene in human bladder cancer. Oncogene 1999, 18, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Beukers, W.; Hercegovac, A.; Zwarthoff, E.C. HRAS mutations in bladder cancer at an early age and the possible asso-ciation with the Costello Syndrome. Eur. J. Hum. Genet. 2014, 22, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Kim, J.-S.; Bondaruk, J.; Shariat, S.F.; Wang, Z.-F.; Elkahloun, A.G.; Ozawa, T.; Gerard, J.; Zhuang, D.; Zhang, S.; et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat. Genet. 2013, 45, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Rafnar, T.; Sulem, P.; Thorleifsson, G.; Vermeulen, S.H.; Helgason, H.; Saemundsdottir, J.; Gudjonsson, S.A.; Sigurdsson, A.; Stacey, S.N.; Gudmundsson, J.; et al. Genome-wide association study yields variants at 20p12.2 that associate with urinary bladder cancer. Hum. Mol. Genet. 2014, 23, 5545–5557. [Google Scholar] [CrossRef]
- Lipunova, N.; Wesselius, A.; Cheng, K.K.; Van Schooten, F.-J.; Bryan, R.T.; Cazier, J.-B.; Galesloot, T.E.; Kiemeney, L.A.; Zeegers, M.P. Genome-wide Association Study for Tumour Stage, Grade, Size, and Age at Diagnosis of Non–muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2019, 2, 381–389. [Google Scholar] [CrossRef]
- Kiemeney, L.A.; Thorlacius, S.; Sulem, P.; Geller, F.; Aben, K.K.H.; Stacey, S.N.; Gudmundsson, J.; Jakobsdottir, M.; Bergthorsson, J.T.; Sigurdsson, A.; et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat. Genet. 2008, 40, 1307–1312. [Google Scholar] [CrossRef]
- Dudek, A.M.; Vermeulen, S.H.; Kolev, D.; Grotenhuis, A.J.; Kiemeney, L.A.L.M.; Verhaegh, G.W. Identification of an enhancer region within the TP63/LEPREL1 locus containing genetic variants associated with bladder cancer risk. Cell. Oncol. 2018, 41, 555–568. [Google Scholar] [CrossRef]
- Garcia-Closas, M.; Ye, Y.; Rothman, N.; Figueroa, J.D.; Malats, N.; Dinney, C.P.; Chatterjee, N.; Prokunina-Olsson, L.; Wang, Z.; Lin, J. A genome-wide association study of bladder cancer identifies a new susceptibility locus within SLC14A1, a urea transporter gene on chromosome 18q12.3. Hum. Mol. Genet. 2011, 20, 4282–4289. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Y.; Shi, M.-J.; Chen, J.-F.; Liao, Y.; Hu, B.-W.; Hireche, A. Association between the TACC3 rs798766 Polymorphism and Risk of Urinary Bladder Cancer: A Synthesis Based on Current Evidence. Dis. Markers 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chu, H.; Lv, Q.; Wang, L.; Yuan, L.; Fu, G.; Tong, N.; Qin, C.; Yin, C.; Zhang, Z.; et al. Cumulative effect of genome-wide as-sociation study-identified genetic variants for bladder cancer. Int. J. Cancer 2014, 135, 2653–2660. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Ye, Y.; Siddiq, A.; Garcia-Closas, M.; Chatterjee, N.; Prokunina-Olsson, L.; Cortessis, V.K.; Kooperberg, C.; Cussenot, O.; Benhamou, S. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum. Mol. Genet. 2014, 23, 1387–1398. [Google Scholar] [CrossRef]
- Selinski, S.; Lehmann, M.-L.; Blaszkewicz, M.; Ovsiannikov, D.; Moormann, O.; Guballa, C.; Kress, A.; Truß, M.C.; Gerullis, H.; Otto, T.; et al. Rs11892031[A] on chromosome 2q37 in an intronic region of the UGT1A locus is associated with urinary bladder cancer risk. Arch. Toxicol. 2012, 86, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Wang, H.; Xie, Y.-T.; Li, Y.; Zheng, L.-Y.; Ruan, Y.; Song, A.-P.; Tian, X.-X.; Fang, W.-G. Association of Germline Variation in CCNE1 and CDK2 with Breast Cancer Risk, Progression and Survival among Chinese Han Women. PLoS ONE 2012, 7, e49296. [Google Scholar] [CrossRef]
- Rafnar, T.; Vermeulen, S.H.; Sulem, P.; Thorleifsson, G.; Aben, K.K.; Witjes, J.A.; Grotenhuis, A.J.; Verhaegh, G.W.; De Kaa, C.A.H.-V.; Besenbacher, S.; et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Hum. Mol. Genet. 2011, 20, 4268–4281. [Google Scholar] [CrossRef]
- Ebbinghaus, D.; Bánfi, G.; Selinski, S.; Blaszkewicz, M.; Bürger, H.; Hengstler, J.G.; Nyirády, P.; Golka, K. Polymorphisms of xenobi-otic metabolizing enzymes in bladder cancer patients of the Semmelweis University Budapest, Hungary. J. Toxicol. Environ. Health A 2017, 80, 423–429. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Ashfaq, R.; Thompson, M.; Sagalowsky, A.I.; Hsieh, J.-T.; Lotan, Y. Expression of Cyclooxygenase-2 in Normal Urothelium, and Superficial and Advanced Transitional Cell Carcinoma of Bladder. J. Urol. 2007, 177, 1163–1168. [Google Scholar] [CrossRef]
- Wadhwa, P.; Goswami, A.K.; Joshi, K.; Sharma, S.K. Cyclooxygenase-2 expression increases with the stage and grade in transi-tional cell carcinoma of the urinary bladder. Int. Urol. Nephrol. 2005, 37, 47–53. [Google Scholar] [CrossRef]
- Tadin, T.; Krpina, K.; Stifter, S.; Babarovic, E.; Fuckar, Z.; Jonjic, N. Lower cyclooxygenase-2 expression is associated with recur-rence of solitary non-muscle invasive bladder carcinoma. Diagn. Pathol. 2012, 7, 152. [Google Scholar] [CrossRef][Green Version]
- Shimada, K.; Fujii, T.; Anai, S.; Fujimoto, K.; Konishi, N. ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Sandes, E.O.; Lodillinsky, C.; Langle, Y.; Belgorosky, D.; Marino, L.; Gimenez, L.; Casabé, A.R.; Eiján, A.M. Inducible nitric oxide syn-thase and PPARγ are involved in bladder cancer progression. J. Urol. 2012, 188, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Eiján, A.M.; E Davel, L.; A Rueda, H.; Rozenberg, G.; De Lustig, E.S.; A Jasnis, M. Differential nitric oxide release and sensitivity to injury in different murine mammary tumor cell lines. Int. J. Mol. Med. 1998, 2, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, S.; Bayraktar, N.; Beytur, A.; Ergin, H.; Bayraktar, M.; Eǧri, M.; Egri, M. Can the levels of nitric oxide in the urine, serum and tumor tissue be putative markers for bladder cancer? Int. J. Urol. 2006, 13, 1079–1085. [Google Scholar] [CrossRef]
- Gecit, I.; Aslan, M.; Gunes, M.; Pirincci, N.; Esen, R.; Demir, H.; Ceylan, K. Serum prolidase activity, oxidative stress, and nitric ox-ide levels in patients with bladder cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.R. Association of superoxide dismutase, glutathione peroxidase, catalse, and xanthine oxidase with incidence of bladder cancer. Cancer Res. J. 2015, 3, 17–27. [Google Scholar] [CrossRef]
- Pirinççi, N.; Geçit, I.; Güneş, M.; Yüksel, M.B.; Kaba, M.; Tanık, S.; Demir, H.; Aslan, M. Serum adenosine deaminase, catalase and carbonic anhydrase activities in patients with bladder cancer. Clinics 2012, 67, 1443–1446. [Google Scholar] [CrossRef]
- Hempel, N.; Ye, H.; Abessi, B.; Mian, B.; Melendez, J.A. Altered redox status accompanies progression to metastatic human blad-der cancer. Free Radic Biol. Med. 2009, 46, 42–50. [Google Scholar] [CrossRef]
- Wieczorek, E.; Jablonowski, Z.; Tomasik, B.; Gromadzinska, J.; Jablonska, E.; Konecki, T.; Fendler, W.; Sosnowski, M.; Wasowicz, W.; Reszka, E. Different Gene Expression and Activity Pattern of Antioxidant Enzymes in Bladder Cancer. Anticancer Res. 2017, 37, 841–848. [Google Scholar] [CrossRef]
- Hung, R.J.; Boffetta, P.; Brennan, P.; Malaveille, C.; Gelatti, U.; Placidi, D.; Carta, A.; Hautefeuille, A.; Porru, S. Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogen 2004, 25, 973–978. [Google Scholar] [CrossRef]
- Utangac, M.M.; Yeni, E.; Savas, M.; Altunkol, A.; Ciftci, H.; Gumus, K.; Demir, M. Paraoxonase and arylesterase activity in bladder cancer. Türk Üroloji Dergisi Turk. J. Urol. 2017, 43, 147–151. [Google Scholar] [CrossRef]
- Iftimie, S.; García-Heredia, A.; Pujol-Bosch, F.; Pont-Salvadó, A.; López-Azcona, A.F.; Hernández-Aguilera, A.; Cabré, N.; Luci-ano-Mateo, F.; Fort-Gallifa, I.; Castro, A.; et al. Serum Paraoxonase-1 Concentration as a Potential Predictor of Uri-nary Bladder Cancer Recurrence. A Five Year Follow-Up Study. Arch. Med. Res. 2018, 49, 119–122. [Google Scholar] [CrossRef]
- Oztürk, O.; Kağnici, O.F.; Oztürk, T.; Durak, H.; Tüzüner, B.M.; I Kisakesen, H.; Cakalir, C.; Isbir, T. 192R allele of paraoxanase 1 (PON1) gene as a new marker for susceptibility to bladder cancer. Anticancer Res. 2009, 29, 4041–4046. [Google Scholar] [PubMed]
- Bacchetti, T.; Sartini, D.; Pozzi, V.; Cacciamani, T.; Ferretti, G.; Emanuelli, M. Exploring the role of paraoxonase-2 in bladder can-cer: Analyses performed on tissue samples, urines and cell cultures. Oncotarget 2017, 8, 28785–28795. [Google Scholar] [CrossRef] [PubMed]
- Chung-man Ho, J.; Zheng, S.; Comhair, S.A.; Farver, C.; Erzurum, S.C. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001, 61, 8578–8585. [Google Scholar]
- Punnonen, K.; Ahotupa, M.; Asaishi, K.; Kudo, R. Antioxidant enzyme activities and oxidative stress in human breast cancer. J. Cancer Res. Clin. Oncol. 1994, 120, 374–377. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Kozuszko, B.; Sulkowska, M.; Bogdan, Z.; Kozlowski, M.; Snarska, J.; Puchalski, Z.; Sulkowski, S.; Skrzydlewski, Z. Antioxidant potential in esophageal, stomach and colorectal cancers. Hepatogastroenterology 2003, 50, 126–131. [Google Scholar] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Does Oxidative Stress Change During Orthotopic Liver Transplantation? J. Controv. Biomed. Res. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Lepara, Z.; Lepara, O.; Fajkić, A.; Rebić, D.; Alić, J.; Spahović, H. Serum malondialdehyde (MDA) level as a potential biomarker of cancer progression for patients with bladder cancer. Rom. J. Intern. Med. 2020, 58, 146–152. [Google Scholar]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, B.; Sawicka, E.; Matuszewski, M.; Dembowski, J.; Piwowar, A. The Dependence between Urinary Levels of Angiogenesis Factors, 8-Iso-prostaglandin F2α, ɣ-Synuclein, and Interleukin-13 in Patients with Bladder Cancer: A Pilot Study. J. Oncol. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, N.; Kilic, S.; Bayraktar, M.R.; Aksoy, N. Lipid peroxidation and antioxidant enzyme activities in cancerous bladder tissue and their relation with bacterial infection: A controlled clinical study. J. Clin. Lab. Anal. 2010, 24, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gecit, İ.; Eryılmaz, R.; Kavak, S.; Meral, İ.; Demir, H.; Pirinççi, N.; Güneş, M.; Taken, K. The Prolidase Activity, Oxidative Stress, and Nitric Oxide Levels of Bladder Tissues with or Without Tumor in Patients with Bladder Cancer. J. Membr. Biol. 2017, 250, 455–459. [Google Scholar] [CrossRef]
- Durak, I.; Perk, H.; Kavutçu, M.; Canbolat, O.; Akyol, Ö.; Bedük, Y. Adenosine deaminase, 5′nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human bladder tissues. Free. Radic. Biol. Med. 1994, 16, 825–831. [Google Scholar] [CrossRef]
- Jeon, S.H.; Park, J.H.; Chang, S.G. Expression of antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxi-dase) in human bladder cancer. Korean J. Urol. 2007, 48, 921–926. [Google Scholar] [CrossRef]
- Badjatia, N.; Satyam, A.; Singh, P.; Seth, A.; Sharma, A. Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 360–367. [Google Scholar] [CrossRef]
- Schäfer, G.; Cramer, T.; Suske, G.; Kemmner, W.; Wiedenmann, B.; Höcker, M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J. Biol. Chem. 2003, 278, 8190–8198. [Google Scholar] [CrossRef]
- Hagen, G.; Müller, S.; Beato, M.; Suske, G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994, 13, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef]
- Höcker, M.; Rosenberg, I.; Xavier, R.; Henihan, R.J.; Wiedenmann, B.; Rosewicz, S.; Podolsky, D.K.; Wang, T.C.J. Oxidative stress activates the human histidine decarboxylase promoter in AGS gastric cancer cells. Biol. Chem. 1998, 273, 23046–23054. [Google Scholar]
- Wang, H.H.; Hsieh, H.L.; Wu, C.Y.; Sun, C.C.; Yang, C.M. Oxidized low-density lipoprotein induces matrix metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1 pathway in brain astrocytes. Glia 2009, 57, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Koike, L.; Miyata, Y.; Hirata, M.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3- kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue in-hibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002, 62, 1025–1029. [Google Scholar]
- Mook, O.R.F.; Frederiks, W.M.; Van Noorden, C.J.F. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta (BBA) Bioenerg. 2004, 1705, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Cho, H.J.; Kang, J.H.; Kwak, J.Y.; Lee, T.S.; Lee, I.S.; Park, N.G.; Nakajima, H.; Magae, J.; Chang, Y.C. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mecha-nisms. Carcinogenesis 2007, 28, 1104–1110. [Google Scholar] [CrossRef]
- Lee, G.H.; Jin, S.W.; Kim, S.J.; Pham, T.H.; Choi, J.H.; Jeong, H.G. Tetrabromobisphenol A Induces MMP-9 Expression via NADPH Oxidase and the activation of ROS, MAPK, and Akt Pathways in Human Breast Cancer MCF-7 Cells. Toxicol. Res. 2019, 35, 93–101. [Google Scholar] [CrossRef]
- Yang, C.-R.; Ou, Y.-C.; Kuo, J.-H.; Kao, Y.-L.; Chen, C.-L.; Yean, S.-Y.; Horng, Y.-Y.; Yang, C.-S. Intracellular glutathione content of urothelial cancer in correlation to chemotherapy response. Cancer Lett. 1997, 119, 157–162. [Google Scholar] [CrossRef]
- Yalcin, O.; Karataş, F.; Erulaş, F.; Özdemir, E. The levels of glutathione peroxidase, vitamin A, E, C and lipid peroxidation in patients with transitional cell carcinoma of the bladder. BJU Int. 2004, 93, 863–866. [Google Scholar] [CrossRef]
- Ichimura, Y.; Habuchi, T.; Tsuchiya, N.; Wang, L.; Oyama, C.; Sato, K.; Nishiyama, H.; Ogawa, O.; Kato, T. INCREASED RISK OF BLADDER CANCER ASSOCIATED WITH A GLUTATHIONE PEROXIDASE 1 CODON 198 VARIANT. J. Urol. 2004, 172, 728–732. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, D.; Grossman, H.B.; Wu, X. Glutathione peroxidase 1 gene polymorphism and risk of recurrence in patients with superficial bladder cancer. Urology 2005, 66, 769–774. [Google Scholar] [CrossRef]
- Devarajan, A.; Shih, D.; Reddy, S.T. Inflammation, infection, cancer and all that…the role of paraoxonases. Adv. Exp. Med. Biol. 2014, 824, 33–41. [Google Scholar]
- Witte, I.; Altenhöfer, S.; Wilgenbus, P.; Amort, J.; Clement, A.M.; Pautz, A.; Li, H.; Förstermann, U.; Horke, S. Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells. Cell Death Dis. 2011, 2, e112. [Google Scholar] [CrossRef] [PubMed]
- Witte, I.; Foerstermann, U.; Devarajan, A.; Reddy, S.T.; Horke, S. Protectors or Traitors: The Roles of PON2 and PON3 in Athero-sclerosis and Cancer. J. Lipids 2012, 2012, 342806. [Google Scholar] [CrossRef] [PubMed]
- Horke, S.; Witte, I.; Wilgenbus, P.; Krüger, M.; Strand, D.; Förstermann, U. Paraoxonase-2 Reduces Oxidative Stress in Vascular Cells and Decreases Endoplasmic Reticulum Stress–Induced Caspase Activation. Circulation 2007, 115, 2055–2064. [Google Scholar] [CrossRef]
- Altenhofer, S.; Witte, I.; Teiber, J.F.; Wilgenbus, P.; Pautz, A.; Li, H.; Daiber, A.; Witan, H.; Clement, A.M.; Forstermann, U. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010, 285, 24398–24403. [Google Scholar] [CrossRef]
- Devarajan, A.; Bourquard, N.; Hama, S.; Navab, M.; Grijalva, V.R.; Morvardi, S.; Clarke, C.F.; Vergnes, L.; Reue, K.; Teiber, J.F.; et al. Paraoxonase 2 Deficiency Alters Mitochondrial Function and Exacerbates the Development of Atherosclerosis. Antioxid. Redox. Sig. 2011, 14, 341–351. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Tang, R.; Xu, H.; Qiu, M.; Chen, Q.; Chen, J.; Fu, Z.; Ying, K.; Xie, Y.; et al. Discovery and analysis of hepatocellular car-cinoma genes using cDNA microarrays. J. Cancer Res. Clin. Oncol. 2002, 128, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ribarska, T.; Ingenwerth, M.; Goering, W.; Engers, R.; Schulz, A.W. Epigenetic inactivation of the placentally imprinted tumor suppressor gene TFPI2 in prostate carcinoma. Cancer Genom. Proteom. 2010, 7, 51–60. [Google Scholar]
- Ross, M.E.; Zhou, X.; Song, G.; Shurtleff, S.A.; Girtman, K.; Williams, W.K.; Liu, H.-C.; Mahfouz, R.; Raimondi, S.C.; Lenny, N.; et al. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood 2003, 102, 2951–2959. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Milkovic, L.; Siems, W.; Siems, R.; Zarkovic, N. Oxidative Stress and Antioxidants in Carcinogenesis and Integrative Therapy of Cancer. Curr. Pharm. Des. 2014, 20, 6529–6542. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Ciccarese, C.; Massari, F.; Blanca, A.; Tortora, G.; Montironi, R.; Cheng, L.; Scarpelli, M.; Raspollini, M.R.; Vau, N.; Fonseca, J.; et al. Tp53 and its potential therapeutic role as a target in bladder cancer. Expert Opin. Ther. Targets 2017, 21, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Akbani, R.; Creighton, C.J.; Lerner, S.P.; Weinstein, J.N.; Getz, G.; Kwiatkowski, D.J. Invasive bladder cancer: Genomic in-sights and therapeutic promise. Clin. Cancer Res. 2015, 21, 4514–4524. [Google Scholar] [CrossRef] [PubMed]
- Furlan, D.; Trapani, D.; Berrino, E.; Debernardi, C.; Panero, M.; Libera, L.; Sahnane, N.; Riva, C.; Tibiletti, M.G.; Sessa, F.; et al. Oxidative DNA damage induces hypomethylation in a compromised base excision repair colorectal tumourigenesis. Br. J. Cancer 2017, 116, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, S.; Ye, S.; Zhang, Y.; Xu, W.; Zhang, B.; Liu, X.; Mo, F.; Hua, W. Aberrant CpG Islands’ Hypermethylation of ABCB1 in Mesenchymal Stem Cells of Patients with Steroid-associated Osteonecrosis. J. Rheumatol. 2013, 40, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.J.; Lee, W.J.; Chang, J.H.; Chow, J.M.; Chung, C.L.; Hung, W.Y.; Chien, M.H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung can-cer. Environ. Toxicol. 2017, 32, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-G.; Kim, S.-H.; Kim, K.-Y.; Yu, S.-N.; Choi, H.-D.; Kim, Y.-W.; Nam, H.-W.; Seo, Y.-K.; Ahn, S.-C. Toyocamycin induces apoptosis via the crosstalk between reactive oxygen species and p38/ERK MAPKs signaling pathway in human prostate cancer PC-3 cells. Pharmacol. Rep. 2017, 69, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.; Bai, C.; Yao, C.; Zhong, H.; Zou, C.; Chen, X. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Cao, R.; Wang, G.; Qian, K.; Chen, L.; Qian, G.; Xie, C.; Dan, H.C.; Jiang, W.; Wu, M.; Wu, C.L.; et al. Silencing of HJURP induces dysreg-ulation of cell cycle and ROS metabolism in bladder cancer cells via PPARgamma-SIRT1 feedback loop. J. Cancer. 2017, 8, 2282–2295. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Fulton, A.M.; E Loveless, S.; Heppner, G.H. Mutagenic activity of tumor-associated macrophages in Salmonella typhimurium strains TA98 and TA 100. Cancer Res. 1984, 44, 4308–4311. [Google Scholar]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.D.; Shoaibi, M.A.; Maestro, R.; Carnero, A.; Hannon, G.J.; Beach, D.H. A Proinflammatory Cytokine Inhibits P53 Tumor Suppressor Activity. J. Exp. Med. 1999, 190, 1375–1382. [Google Scholar] [CrossRef]
- Petrenko, O.; Moll, U.M. Macrophage Migration Inhibitory Factor MIF Interferes with the Rb-E2F Pathway. Mol. Cell 2005, 17, 225–236. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Pirianov, G.; Colston, K.W. Interactions of vitamin D analogue CB1093, TNFalpha and ceramide on breast cancer cell apopto-sis. Mol. Cell Endocrinol. 2001, 172, 69–78. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Kawai, K.; A Reznikoff, C.; Oyasu, R. Transformation in vitro of a nontumorigenic rat urothelial cell line by hydrogen peroxide. Cancer Res. 1996, 56, 139–144. [Google Scholar]
- Mercogliano, M.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Leek, R.D.; Landers, R.; Fox, S.B.; Ng, F.; Harris, A.L.; Lewis, C.E. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br. J. Cancer 1998, 77, 2246–2251. [Google Scholar] [CrossRef]
- O’Brien, T.S.; Fox, S.B.; Dickinson, A.J.; Turley, H.; Westwood, M.; Moghaddam, A.; Gatter, K.C.; Bicknell, R.; Harris, A.L. Expression of the angiogenic factor thymidine phosphorylase/platelet-derived endothelial cell growth factor in primary bladder cancers. Cancer Res. 1996, 56, 4799–4804. [Google Scholar] [PubMed]
- Taniguchi, K.; Koga, S.; Nishikido, M.; Yamashita, S.; Sakuragi, T.; Kanetake, H.; Saito, Y. Systemic immune response after in-travesical instillation of bacille Calmette-Guerin (BCG) for superficial bladder cancer. Clin. Exp. Immunol. 1999, 115, 131–135. [Google Scholar] [CrossRef]
- Raziuddin, S.; Masihuzzaman, M.; Shetty, S.; Ibrahim, A. Tumor necrosis factor alpha production in schistosomiasis with carci-noma of urinary bladder. J. Clin. Immunol. 1993, 13, 23–29. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, Y.; Lv, Y.; Wang, Y. Meta-analysis shows strong positive association of the TNF-alpha gene with tumor stage in bladder cancer. Urol. Int. 2012, 89, 337–341. [Google Scholar] [CrossRef]
- Ahirwar, D.; Kesarwani, P.; Manchanda, P.K.; Mandhani, A.; Mittal, R.D. Anti- and proinflammatory cytokine gene polymorphism and genetic predisposition: Association with smoking, tumor stage and grade, and bacillus Calmette-Guérin immunotherapy in bladder cancer. Cancer Genet. Cytogenet. 2008, 184, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.P.; Haldar, N.A.; Bunce, M.; Marshall, S.E.; le Monier, K.; Winsey, S.L.; Christodoulos, K.; Cranston, D.; Welsh, K.I.; Harris, A.L. Polymorphisms in tumour necrosis factor (TNF) are associated with risk of bladder cancer and grade of tumour at presenta-tion. Br. J. Cancer. 2003, 89, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Jeong, P.; Kim, E.-J.; Kim, E.-G.; Byun, S.-S.; Kim, C.S.; Kim, W.-J. Association of bladder tumors and GA genotype of −308 nucleotide in tumor necrosis factor-alpha promoter with greater tumor necrosis factor-alpha expression. Urology 2004, 64, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.J.; Lu, H.F.; Yeh, L.S.; Hsu, C.D.; Chen, W.C. Lack of evidence for the association of tumor necrosis factor-alpha gene pro-moter polymorphism with calcium oxalate stone and bladder cancer patients. Urol. Res. 2001, 29, 412–416. [Google Scholar] [CrossRef]
- Yang, J.J.; Ko, K.P.; Cho, L.Y.; Shin, A.; Gwack, J.; Chang, S.H.; Shin, H.R.; Yoo, K.Y.; Kang, D.; Park, S.K. The role of TNF genetic variants and the interaction with cigarette smoking for gastric cancer risk: A nested case-control study. BMC Cancer. 2009, 9, 238. [Google Scholar] [CrossRef]
- Li, Y.-T.; He, B.; Wang, Y.-Z. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal. Toxicol. 2009, 21, 641–647. [Google Scholar] [CrossRef]
- Lima, L.; Oliveira, D.; Ferreira, J.A.; Tavares, A.; Cruz, R.; Medeiros, R.; Santos, L.L. The role of functional polymorphisms in immune response genes as biomarkers of bacille Calmette-Guérin (BCG) immunotherapy outcome in bladder cancer: Establishment of a predictive profile in a Southern Europe population. BJU Int. 2015, 116, 753–763. [Google Scholar] [CrossRef]
- Davies, B.; Waxman, J.; Wasan, H.; Abel, P.; Williams, G.; Krausz, T.; Neal, D.; Thomas, D.; Hanby, A.; Balkwill, F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993, 53, 5365–5369. [Google Scholar]
- Nutt, J.; Durkan, G.; Mellon, J.; Lunec, J. Matrix metalloproteinases (MMPs) in bladder cancer: The induction of MMP9 by epidermal growth factor and its detection in urine. BJU Int. 2003, 91, 99–104. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Kim, C.H. ERK1/2 mediates TNF-alphainduced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J. Cell Physiol. 2004, 198, 417–427. [Google Scholar] [CrossRef]
- Shin, K.Y.; Moon, H.S.; Park, H.Y.; Lee, T.Y.; Woo, Y.N.; Kim, H.J.; Lee, S.J.; Kong, G. Effects of tumor necrosis factor-alpha and interfer-on-gamma on expressions of matrix metalloproteinase-2 and -9 in human bladder cancer cells. Cancer Lett. 2000, 159, 127–134. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, S.-S.; Cho, Y.-H.; Park, K.; Kim, E.-J.; Jung, K.-H.; Kim, S.-K.; Kim, W.-J.; Moon, S.-K. Activation of matrix metalloproteinase-9 by TNF-α in human urinary bladder cancer HT1376 cells: The role of MAP kinase signaling pathways. Oncol. Rep. 2008, 19, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Rinco´n, M.; Anguita, J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4þ T cells. J. Exp. Med. 1997, 185, 461–469. [Google Scholar] [CrossRef]
- Luo, Y.; Ye, Z.; Li, K.; Chen, R.; Li, S.; Pang, J. Associations between polymorphisms in the IL-4 and IL-4 receptor genes and uri-nary carcinomas: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 1227–1233. [Google Scholar] [PubMed]
- Lee, S.-J.; Lee, E.-J.; Kim, S.-K.; Jeong, P.; Cho, Y.-H.; Yun, S.J.; Kim, S.; Kim, G.-Y.; Choi, Y.H.; Cha, E.-J.; et al. Identification of Pro-Inflammatory Cytokines Associated with Muscle Invasive Bladder Cancer; The Roles of IL-5, IL-20, and IL-28A. PLoS ONE 2012, 7, e40267. [Google Scholar] [CrossRef]
- Chen, M.-F.; Lin, P.-Y.; Wu, C.-F.; Chen, W.-C.; Wu, C.-T. IL-6 Expression Regulates Tumorigenicity and Correlates with Prognosis in Bladder Cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.; Shariat, S.F.; Kim, J.H.; Wheeler, T.M.; Slawin, K.M.; Lerner, S.P. Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J. Urol. 2002, 167, 1475–1481. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Jorcyk, C.; Tawara, K. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: Potential of anti-IL-6 therapies. Cancer Manag. Res. 2011, 3, 177. [Google Scholar] [CrossRef]
- Kishimoto, T. INTERLEUKIN-6: From Basic Science to Medicine—40 Years in Immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Schafer, Z.T.; Brugge, J.S. IL-6 involvement in epithelial cancers. J. Clin. Investig. 2007, 117, 3660–3663. [Google Scholar] [CrossRef] [PubMed]
- Rieger-Christ, K.M.; Ng, L.; Hanley, R.S.; Durrani, O.; Ma, H.; Yee, A.S.; A Libertino, J.; Summerhayes, I.C. Restoration of plakoglobin expression in bladder carcinoma cell lines suppresses cell migration and tumorigenic potential. Br. J. Cancer 2005, 92, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune mark-ers in cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Gautam, K.A.; Muktanand, T.; Sankhwar, S.N.; Goel, A.; Sankhwar, P.L.; Rajender, S. Functional polymorphisms in the IL6 gene promoter and the risk of urinary bladder cancer in India. Cytokine 2016, 77, 152–156. [Google Scholar] [CrossRef]
- Naugler, W.E.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef]
- Mikucki, M.E.; Fisher, D.T.; Ku, A.W.; Appenheimer, M.M.; Muhitch, J.B.; Evans, S.S. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int. J. Hyperth. 2013, 29, 464–473. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Karashima, T.; Sweeney, P.; Kamat, A.; Huang, S.; Kim, S.J.; Bar-Eli, M.; McConkey, D.J.; Dinney, C.P. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin. Cancer Res. 2003, 9, 2786–2797. [Google Scholar] [PubMed]
- Kanarek, N.; London, N.; Schueler-Furman, O.; Ben-Neriah, Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2010, 2, a000166. [Google Scholar] [CrossRef]
- Inoue, K.; Slaton, J.W.; Kim, S.J.; Perrotte, P.; Eve, B.Y.; Bar-Eli, M.; Radinsky, R.; Dinney, C.P. Interleukin 8 expression regulates tu-morigenicity and metastasis in human bladder cancer. Cancer Res. 2000, 60, 2290–2299. [Google Scholar] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Martin, G.R.; Jain, R.K. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluores-cence ratio imaging microscopy. Cancer Res. 1994, 54, 5670–5674. [Google Scholar]
- Folkman, J. Tumor angiogenesis and tissue factor. Nat. Med. 1996, 2, 167–168. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Turner, K.J.; Crew, J.P.; Wykoff, C.C.; Watson, P.H.; Poulsom, R.; Pastorek, J.; Ratcliffe, P.J.; Cranston, D.; Harris, A.L. The hypox-ia-inducible genes VEGF and CA9 are differentially regulated in superficial versus invasive bladder cancer. Br. J. Cancer 2002, 86, 1276–1282. [Google Scholar] [CrossRef]
- Xia, G.; Kageyama, Y.; Hayashi, T.; Hyochi, N.; Kawakami, S.; Kihara, K. Positive expression of HIF-2α/EPAS1 in invasive bladder cancer. Urology 2002, 59, 774–778. [Google Scholar] [CrossRef]
- Onita, T.; Ji, P.G.; Xuan, J.W.; Sakai, H.; Kanetake, H.; Maxwell, P.H.; Fong, G.H.; Gabril, M.Y.; Moussa, M.; Chin, J.L. Hypoxia-induced, perinecrotic expression of endothelial Per-ARNT-Sim domain protein-1/hypoxia-inducible factor-2α correlates with tumor progression, vascularization, and focal macrophage infiltration in bladder cancer. Clin. Cancer Res. 2002, 8, 471–480. [Google Scholar]
- Sun, S.; Elwood, J.; Greene, W.C. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol. Cell Biol. 1996, 16, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Franzoso, G.; Baldi, L.; Carlson, L.; Mills, L.; Lin, Y.C.; Gerstberger, S.; Siebenlist, U. The signal response of IκBα is regu-lated by transferable N- and C-terminal domains. Mol. Cell Biol. 1997, 17, 3021–3027. [Google Scholar] [CrossRef]
- Haskill, S.; Beg, A.A.; Tompkins, S.M.; Morris, J.S.; Yurochko, A.D.; Sampson-Johannes, A.; Mondal, K.; Ralph, P.; Baldwin, A.S., Jr. Char-acterization of an immediate-early gene induced in adherent monocytes that encodes IκB-like activity. Cell 1991, 65, 1281–1289. [Google Scholar] [CrossRef]
- Huang, S.; Robinson, J.B.; Deguzman, A.; Bucana, C.D.; Fidler, I.J. Blockade of nuclear factor-κB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and inter-leukin 8. Cancer Res. 2000, 60, 5334–5339. [Google Scholar]
- Huang, S.; Pettaway, C.A.; Uehara, H.; Bucana, C.D.; Fidler, I.J. Blockade of NF-κB activity in human prostate cancer cells is associ-ated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001, 20, 4188–4197. [Google Scholar] [CrossRef]
- Ahirwar, D.K.; Mandhani, A.; Mittal, R.D. IL-8 −251 T > A Polymorphism Is Associated with Bladder Cancer Susceptibility and Outcome after BCG Immunotherapy in a Northern Indian Cohort. Arch. Med Res. 2010, 41, 97–103. [Google Scholar] [CrossRef]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 Family Members and Inflammation. Immunology 2004, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Baharlou, R.; Vasmehjani, A.A.; Dehghani, A.; Ghobadifar, M.A.; Khoubyari, M. Reduced Interleukin-17 and Transforming Growth Factor Beta Levels in Peripheral Blood as Indicators for Following the Course of Bladder Cancer. Immune Netw. 2014, 14, 156–163. [Google Scholar] [CrossRef]
- Straus, D.S. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Mol. Cancer 2013, 12, 78. [Google Scholar] [CrossRef]
- Li, L.; Boussiotis, V.A. The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clin. Immunol. 2013, 148, 246–253. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, T.; Wang, J. Expression of IL-23R and IL-17 and the pathology and prognosis of urinary bladder carci-noma. Oncol. Lett. 2018, 16, 4325–4330. [Google Scholar] [PubMed]
- Levidou, G.; Saetta, A.A.; Korkolopoulou, P.; Papanastasiou, P.; Gioti, K.; Pavlopoulos, P.; Diamantopoulou, K.; Thomas-Tsagli, E.; Xiromeritis, K.; Patsouris, E. Clinical significance of nuclear factor (NF)-kappaB levels in urothelial carcinoma of the urinary bladder. Virchows Arch. 2008, 452, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Suzuki, E.; Mizuno, R.; Shinojima, T.; Miyajima, A.; Umezawa, K.; Oya, M. Down-regulation of NF kappa B activation is an effective therapeutic modality in acquired platinum-resistant bladder cancer. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.; Chen, H.; Xu, J.; Zhang, G.; Gu, C.; Ding, Q.; Wei, Q.; Zhu, Y.; Ye, D. The Rare Variant rs35356162 in UHRF1BP1 In-creases Bladder Cancer Risk in Han Chinese Population. Front. Oncol. 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer. 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.N.; Hilyard, A.C.; Lagna, G.; Hata, A. SMAD proteinscontrol DROSHA-mediated microRNA maturation. Nature 2008, 454, 56–61. [Google Scholar] [CrossRef]
- Ahirwar, D.K.; Agrahari, A.; Mandhani, A.; Mittal, R.D. Cytokine gene polymorphisms are associated with risk of urinary blad-der cancer and recurrence after BCG immunotherapy. Biomarkers 2009, 14, 213–218. [Google Scholar] [CrossRef]
- D’Inzeo, S.; Nicolussi, A.; Donini, C.F.; Zani, M.; Mancini, P.; Nardi, F. A novel human Smad4 mutation is involved in papil-lary thyroid carcinoma progression. Endocr. Relat. Cancer 2012, 19, 39–55. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Landström, M.; Moustakas, A. Mechanism of TGF-β signaling to growth arrest, apoptosis, and epitheli-al-mesenchymal transition. Curr. Opin. Cell Biol. 2009, 21, 166–176. [Google Scholar] [CrossRef]
- Viel, S.; Marçais, A.; Guimaraes, F.S.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-β inhibits the activa-tion and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Genrich, G.; Kruppa, M.; Lenk, L.; Helm, O.; Broich, A.; Freitag-Wolf, S.; Röcken, C.; Sipos, B.; Schäfer, H.; Sebens, S. The anti-oxidative transcription factor Nuclear factor E2 related factor-2 (Nrf2) counteracts TGF-β1 mediated growth inhibition of pancreatic ductal epithelial cells -Nrf2 as determinant of pro-tumorigenic functions of TGF-β1. BMC Cancer 2016, 16, 155. [Google Scholar] [CrossRef]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferation in human bladder cancer cells. Toxicol. Sci. 2008, 104, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Cen, L.; Kohout, J.; Hutzen, B.; Chan, C.; Hsieh, F.-C.; Loy, A.; Huang, V.; Cheng, G.; Lin, J. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol. Cancer 2008, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ross, J.L.; Cowell, J.K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAK STAT 2014, 3, e28086. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Z.; Hou, Y.; Hu, J.; Wang, C. The effects of STAT3 and Survivin silencing on the growth of human bladder carci-noma cells. Tumour. Biol. 2014, 35, 5401–5407. [Google Scholar] [CrossRef]
- Ho, P.L.; Lay, E.J.; Jian, W.; Parra, D.; Chan, K.S. Stat3 Activation in Urothelial Stem Cells Leads to Direct Progression to Invasive Bladder Cancer. Cancer Res. 2012, 72, 3135–3142. [Google Scholar] [CrossRef]
- Shen, H.-B.; Gu, Z.-Q.; Jian, K.; Qi, J. CXCR4-mediated Stat3 activation is essential for CXCL12-induced cell invasion in bladder cancer. Tumor Biol. 2013, 34, 1839–1845. [Google Scholar] [CrossRef]
- Zhao, D.; Besser, A.H.; Wander, S.A.; Sun, J.; Zhou, W.; Wang, B.; Ince, T.; Durante, M.A.; Guo, W.; Mills, G.; et al. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregula-tion. Oncogene 2015, 34, 5447–5459. [Google Scholar] [CrossRef]
- Wu, C.-C.; Huang, Y.-K.; Huang, C.-Y.; Shiue, H.-S.; Pu, Y.-S.; Su, C.-T.; Lin, Y.-C.; Hsueh, Y.-M. Polymorphisms of TNF-α -308 G/A and IL-8 -251 T/A Genes Associated with Urothelial Carcinoma: A Case-Control Study. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Leibovici, D.; Grossman, H.B.; Dinney, C.P.; Millikan, R.E.; Lerner, S.; Wang, Y.; Gu, J.; Dong, Q.; Wu, X. Polymorphisms in Inflammation Genes and Bladder Cancer: From Initiation to Recurrence, Progression, and Survival. J. Clin. Oncol. 2005, 23, 5746–5756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lerner, S.; Leibovici, D.; Dinney, C.P.; Grossman, H.B.; Wu, X. Polymorphisms in the Inflammatory Genes IL-6, IL-8, TNF-α, NFKB1, and PPARG and Bladder Cancer Risk. Epidemiology 10: Smoking and Cancer: Lung, Head and Neck, and Bladder Cancer. 2004. Available online: https://cancerres.aacrjournals.org/content/64/7_Supplement/918.3 (accessed on 23 April 2021).
- Chiang, Y.-T.; Ho, C.-H.; Hu, S.-W.; Yang, T.-Y.; Sung, C.-W.; Wang, Y.-H.; Wu, C.-C. Association between the rs1800795G>C polymorphism in the promoter of interleukin-6 gene and bladder cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 3598–3604. [Google Scholar]
- Ebadi, N.; Jahed, M.; Mivehchi, M.; Majidizadeh, T.; Asgary, M.; Hosseini, S.A. Interleukin-12 and interleukin-6 gene polymor-phisms and risk of bladder cancer in the Iranian population. Asian Pac. J. Cancer Prev. 2014, 15, 7869–7873. [Google Scholar] [CrossRef]
- Rein, T.; Förster, R.; Krause, A.; Winnacker, E.L.; Zorbas, H. Organization of the a-globin promoter and possible role of nuclear factor I in an aglobin-inducible and a noninducible cell line. J. Biol. Chem. 1995, 270, 19643–19650. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Hope, J.C.; Cumberbatch, M.; Fielding, I.; Dearman, R.J.; Kimber, I.; Hopkins, S.J. Identification of dendritic cells as a major source of interleukin-6 in draining lymph nodes following skin sensitization of mice. Immunology 1995, 86, 441–447. [Google Scholar]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, P.; Wang, Y.; Shi, S.; Zhang, K.; Liao, H.; Zhang, L. Interleukin-17 gene polymorphisms are associated with blad-der cancer in a Chinese Han population. Mol. Carcinog. 2013, 52, 871–878. [Google Scholar] [CrossRef]
- Qian, X.; Chen, H.; Wu, X.; Hu, L.; Huang, Q.; Jin, Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tu-morigenesis. Cytokine 2017, 89, 34–44. [Google Scholar] [CrossRef]
- Lv, L.; Pan, K.; Li, X.-D.; She, K.-L.; Zhao, J.-J.; Wang, W.; Chen, J.-G.; Chen, Y.-B.; Yun, J.-P.; Xia, J.-C. The Accumulation and Prognosis Value of Tumor Infiltrating IL-17 Producing Cells in Esophageal Squamous Cell Carcinoma. PLoS ONE 2011, 6, e18219. [Google Scholar] [CrossRef]
- Garcia-Hernandez Mde, L.; Hamada, H.; Reome, J.B.; Misra, S.K.; Tighe, M.P.; Dutton, R.W. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J. Immunol. 2010, 184, 4215–4227. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Su, H.; Zhong, W.; Yuan, Y.; Yu, Z.; Fang, Y.; Zhou, H.; Li, C.; Huang, K. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin. Transl. Oncol. 2014, 17, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Katano, M.; Torisu, M. Neutrophil-Mediated Tumor Cell Destruction in Cancer Ascites. Cancer 1982, 50, 62–68. [Google Scholar] [CrossRef]
- Hirahara, N.; Nio, Y.; Sasaki, S.; Minari, Y.; Takamura, M.; Iguchi, C.; Dong, M.; Yamasawa, K.; Tamura, K. Inoculation of human in-terleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. On-cology 2001, 61, 79–89. [Google Scholar]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell re-sponses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- E Heuvers, M.; Aerts, J.G.; Cornelissen, R.; Groen, H.; Hoogsteden, H.C.; Hegmans, J.P. Patient-tailored modulation of the immune system may revolutionize future lung cancer treatment. BMC Cancer 2012, 12, 580. [Google Scholar] [CrossRef]
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef]
- Zwirner, N.W.; Ziblat, A. Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol. 2017, 8, 25. [Google Scholar] [CrossRef]
- Oka, N.; Markova, T.; Tsuzuki, K.; Li, W.; El-Darawish, Y.; Pencheva-Demireva, M.; Yamanishi, K.; Yamanishi, H.; Sakagami, M.; Tanaka, Y.; et al. IL-12 regulates the expansion, phenotype, and function of murine NK cells activated by IL-15 and IL-18. Cancer Immunol. Immunother. 2020, 69, 1699–1712. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Chen, F.; Cao, A.; Yao, S.; Evans-Marin, H.L.; Liu, H.; Wu, W.; Carlsen, E.D.; Dann, S.M.; Soong, L.; Sun, J.; et al. mTOR Mediates IL-23 Induction of Neutrophil IL-17 and IL-22 Production. J. Immunol. 2016, 196, 4390–4399. [Google Scholar] [CrossRef]
- Lee, P.W.; Smith, A.J.; Yang, Y.; Selhorst, A.J.; Liu, Y.; Racke, M.K.; Lovett-Racke, A.E. Il-23r–activated stat3/stat4 is essential for th1/th17-mediated cns autoimmunity. JCI Insight 2017, 2, e91663. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hedl, M.; Abraham, C. Il23 group> il23r recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the ibd-protective il23r r381q variant modulates these outcomes. Gut 2020, 69, 264–273. [Google Scholar] [CrossRef]
- Guéry, L.; Hugues, S. Th17 Cell Plasticity and Functions in Cancer Immunity. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nat. Cell Biol. 2006, 442, 461–465. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Wu, X.-R. Urothelial tumorigenesis: A tale of divergent pathways. Nat. Rev. Cancer 2005, 5, 713–725. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Compérat, E.; Larré, S.; Rouprêt, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houede, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015, 466, 589–594. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.; Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Han, R.F.; Pan, J.G. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 2006, 67, 1216–1223. [Google Scholar] [CrossRef]
- Malmström, P.-U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.L. Efficacy and safety of bacille Calmette–Guérin immunotherapy in superficial bladder cancer. Clin. Infect. Dis. 2000, 31, 86–90. [Google Scholar] [CrossRef]
- Lamm, D.L.; Blumenstein, B.A.; Crissman, J.D.; Montie, J.E.; Gottesman, J.E.; Lowe, B.A.; Sarosdy, M.F.; Bohl, R.D.; Grossman, H.B.; Beck, T.M.; et al. Maintenance bacillus calmette-guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study. J. Urol. 2000, 163, 1124–1129. [Google Scholar] [CrossRef]
- Oddens, J.; Brausi, M.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Hoeltl, W.; Turkeri, L.; Marreaud, S.; et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guérin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. Eur. Urol. 2013, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.A.; Birkhäuser, F.D.; Biot, C.; Gsponer, J.R.; Bisiaux, A.; Wetterauer, C.; Lagranderie, M.; Marchal, G.; Orgeur, M.; Bouchier, C.; et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur. Urol. 2014, 66, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Vegt, P.D.; Witjes, J.A.; Witjes, W.P.; Doesburg, W.H.; Debruyne, F.M.; van der Meijden, A.P. A randomized study of intravesical mi-tomycin C, bacillus Calmette–Guerin Tice and bacillus Calmette-Guerin RIVM treatment in pTa-pT1 papillary carcinoma and carcinoma in situ of the bladder. J. Urol. 1995, 153, 929–933. [Google Scholar] [CrossRef]
- Del Giudice, F.; Busetto, G.M.; Gross, M.S.; Maggi, M.; Sciarra, A.; Salciccia, S.; Ferro, M.; Sperduti, I.; Flammia, S.; Canale, V.; et al. Efficacy of three BCG strains (Connaught, TICE and RIVM) with or without secondary resection (re-TUR) for intermediate/high-risk non-muscle-invasive bladder cancers: Results from a retrospective single-institution cohort analysis. J. Cancer Res. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Dalbagni, G.; Karnes, R.J.; Shariat, S.; Joniau, S.; Palou, J.; Serretta, V.; Larré, S.; di Stasi, S.; Colombo, R.; et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2099 patients with T1G3 non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 484.e19–484.e25. [Google Scholar] [CrossRef]
- Miyazaki, J.; Hinotsu, S.; Ishizuka, N.; Naito, S.; Ozono, S.; Akaza, H.; Nishiyama, H. Adverse reactions related to treatment com-pliance during BCG maintenance therapy for non-muscle-invasive bladder cancer. Jpn. J. Clin. Oncol. 2013, 43, 827–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC nomograms and risk groups for predicting re-currence, progression, and disease-specifc and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder can-cer patients treated with 1–3 years of maintenance bacillus Calmette–Guérin. Eur. Urol. 2016, 6, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; Ferro, M.; Cantiello, F.; Lucarelli, G.; Di Stasi, S.; Hurle, R.; Guazzoni, G.; Busetto, G.M.; De Berardinis, E.; Damiano, R.; et al. Validation of Neutrophil-to-lymphocyte Ratio in a Multi-institutional Cohort of Patients with T1G3 Non–muscle-invasive Bladder Cancer. Clin. Genitourin. Cancer 2018, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; Porav-Hodade, D.; Ferro, M.; Mathieu, R.; Abufaraj, M.; Foerster, B.; Kimura, S.; Shariat, S.F. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A sys-tematic review and meta-analysis. Urol. Oncol. 2018, 36, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Xylinas, E.; Crivelli, J.J.; Passoni, N.; Comploj, E.; Pycha, A.; Chrystal, J.; Sun, M.; Karakiewicz, P.I.; Gontero, P.; et al. Obesity is associated with worse outcomes in patients with T1 high grade urothe-lial carcinoma of the bladder. J. Urol. 2013, 190, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Bolenz, C.; Herrmann, E.; Bastian, P.J.; Michel, M.S.; Wülfing, C.; Tiemann, A.; Buchner, A.; Stief, C.G.; Fritsche, H.M.; Burger, M.; et al. Lymphovascular invasion is an independent predictor of oncological outcomes in patients with lymph nodenegative urothelial bladder cancer treated by radical cystec-tomy: A multicentre validation trial. BJU Int. 2010, 106, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Di Lorenzo, G.; Vartolomei, M.D.; Bruzzese, D.; Cantiello, F.; Lucarelli, G.; Musi, G.; Di Stasi, S.; Hurle, R.; Guazzoni, G.; et al. Absolute basophil count is associated with time to recurrence in patients with high-grade T1 bladder cancer receiving bacillus Calmette–Guérin after transurethral re-section of the bladder tumor. World J. Urol. 2020, 38, 143–150. [Google Scholar] [CrossRef]
- Voehringer, D. Recent advances in understanding basophil functions in vivo. F1000Research 2017, 6, 1464. [Google Scholar] [CrossRef]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T helper type 2 cell infltrate correlates with cancer-associated fbroblast thymic stromal lymphopoietin production and re-duced survival in pancreatic cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef]
- Akan, S.; Ediz, C.; Sahin, A.; Tavukcu, H.H.; Urkmez, A.; Horasan, A.; Yilmaz, O.; Verit, A. Can the systemic immune inflammation index be a predictor of BCG response in patients with high-risk non-muscle invasive bladder cancer? Int. J. Clin. Pract. 2021, 75. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Ma, W.; Wu, Y.; Maskey, N.; Guo, Y.; Liu, J.; Mao, S.; Zhang, J.; Yao, X.; et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann. Transl. Med. 2019, 7, 431. [Google Scholar] [CrossRef]
- Gorgel, S.N.; Akin, Y.; Koc, E.M.; Kose, O.; Ozcan, S.; Yilmaz, Y. Retrospective study of systemic immune-inflammation index in muscle invasive bladder cancer: Initial results of single centre. Int. Urol. Nephrol. 2020, 52, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, K.; Mao, S.; Zhang, J.; Wang, L.; Zhang, Z.; Liu, M.; Zhang, W.; Wu, Y.; Yan, Y.; et al. Preoperative C-reactive pro-tein/albumin ratio is a significant predictor of survival in bladder cancer patients after radical cystectomy: A retrospective study. Cancer Manag. Res. 2018, 10, 4789–4804. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Gu, J.; Ye, Y.; Williams, S.B.; Dinney, C.P.; Wu, X.; Kamat, A. High baseline levels of interleukin-8 in leukocytes and urine predict tumor recurrence in non-muscle invasive bladder cancer patients receiving bacillus Calmette–Guerin therapy: A long-term survival analysis. OncoImmunology 2017, 6, e1265719. [Google Scholar] [CrossRef]
- Da Silva, F.M.C.; Videira, P.A.; Ligeiro, D.; Cabral, M.G.; Sylvester, R.; Trindade, H. Systemic humoral responses of non-muscle-invasive bladder cancer during BCG treatment: Less is more. J. Cancer Metast. Treat. 2017, 3, 116. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Moscow) 1998, 63, 854–865. [Google Scholar]
- Kaempfer, R.; Gerez, L.; Farbstein, H.; Madar, L.; Hirschman, O.; Nussinovich, R.; Shapiro, A. Prediction of response to treatment in superficial bladder carcinoma through pattern of interleukin-2 gene expression. J. Clin. Oncol. 1996, 14, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, D.K.; Mandhani, A.; Dharaskar, A.; Kesarwani, P.; Mittal, R.D. Association of tumour necrosis factor-α gene (T-1031C, C-863A, and C-857T) polymorphisms with bladder cancer susceptibility and outcome after bacille Calmette-Guérin immu-notherapy. BJU Int. 2009, 104, 867–873. [Google Scholar] [CrossRef]
- Fus, Ł.P.; Górnicka, B. Role of angiogenesis in urothelial bladder carcinoma. Cent. Eur. J. Urol. 2016, 69, 258–263. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage re-cruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chu, K.-C.; Yeh, W.-M. The expression of vascular endothelial growth factor in transitional cell carcinoma of urinary bladder is correlated with cancer progression. Urol. Oncol. Semin. Orig. Investig. 2004, 22, 1–6. [Google Scholar] [CrossRef]
- Donmez, G.; Sullu, Y.; Baris, S.; Yildiz, L.; Aydin, O.; Karagoz, F.; Kandemir, B. Vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), and thrombospondin-1 (TSP-1) expression in urothelial carcinomas. Pathol. Res. Pract. 2009, 205, 854–857. [Google Scholar] [CrossRef]
- Fauconnet, S.; Bernardini, S.; Lascombe, I.; Boiteux, G.; Clairotte, A.; Monnien, F.; Chabannes, E.; Bittard, H. Expression analysis of VEGF-A and VEGF-B: Relationship with clinicopathological parameters in bladder cancer. Oncol. Rep. 2009, 21, 1495–1504. [Google Scholar] [CrossRef]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and its re-ceptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar]
- Quentin, T.; Schlott, T.; Korabiowska, M.; Käthei, N.; Zöller, G.; Glaser, F.; Kunze, E. Alteration of the vascular endothelial growth factor and angiopoietins-1 and -2 pathways in transitional cell carcinomas of the urinary bladder associated with tumor progression. Anticancer Res. 2004, 24, 2745–2756. [Google Scholar]
- Berse, B.; Brown, L.F.; Van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth fac-tor) gene is expressed differentially in normal tissue. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar] [CrossRef]
- Crew, J.P. Vascular endothelial growth factor: An important angiogenic mediator in bladder cancer. Eur. Urol. 1999, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Crew, J.P.; Fuggle, S.; Bickwell, R.; Cranston, D.W.; de Benedetti, A.; Harris, A.L. Eukaryotic initiaton factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular growth factor expression and tumour progression. Br. J. Cancer 2000, 82, 161–166. [Google Scholar] [CrossRef]
- O’Brien, T.; Cranston, D.; Fuggle, S.; Bicknell, R.; Harris, A.L. Different angiogenic pathways characterize superficial and in-vasive bladder cancer. Cancer Res. 1995, 55, 510–513. [Google Scholar]
- Bernardini, S.; Fauconnet, S.; Chabannes, E.; Henry, P.C.; Adessi, G.; Bittard, H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J. Urol. 2001, 166, 1275–1279. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Lee, S.-J.; Chang, S.-G. Clinical significance of urinary vascular endothelial growth factor in patients with superficial bladder tumors. Oncol. Rep. 2001, 8, 1265–1267. [Google Scholar] [CrossRef]
- Garcia-Closas, M.; Malats, N.; Real, F.X.; Yeager, M.; Welch, R.; Silverman, D.; Kogevinas, M.; Dosemeci, M.; Figueroa, J.; Chatterjee, N.; et al. Large-Scale Evaluation of Candidate Genes Identifies Associations between VEGF Polymorphisms and Bladder Cancer Risk. PLoS Genet. 2007, 3, e29. [Google Scholar] [CrossRef]
- Zhang, L.F.; Ren, K.W.; Zuo, L.; Zou, J.G.; Song, N.H.; Mi, Y.Y.; Wang, Z.J.; Zhang, W. VEGF gene rs3025039C/T and rs833052C/A vari-ants are associated with bladder cancer risk in Asian descendants. J. Cell Biochem. 2019, 120, 10402–10412. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1) α: Its protein stability and biological func-tions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef]
- Theodoropoulos, V.E.; Lazaris, A.C.; Kastriotis, I.; Spiliadi, C.; Theodoropoulos, G.E.; Tsoukala, V.; Patsouris, E.; Sofras, F. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005, 95, 425–431. [Google Scholar] [CrossRef]
- Chai, C.Y.; Chen, W.T.; Hung, W.C.; Kang, W.Y.; Huang, Y.C.; Su, Y.C.; Yang, C.H. Hypoxia-inducible factor-1alpha expression corre-lates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J. Clin. Pathol. 2008, 61, 658–664. [Google Scholar] [CrossRef]
- Deniz, H.; Karakok, M.; Yagcı, F.; Güldür, M.E.; Yaĝci, F. Evaluation of relationship between HIF-1α immunoreactivity and stage, grade, angiogenic profile and proliferative index in bladder urothelial carcinomas. Int. Urol. Nephrol. 2010, 42, 103–107. [Google Scholar] [CrossRef]
- Nadaoka, J.; Kumazawa, T.; Yuasa, T.; Horikawa, Y.; Saito, M.; Inoue, T.; Narita, S.; Satoh, S.; Nishiyama, H.; Ogawa, O.; et al. Prognostic significance of HIF-1α polymorphisms in transitional cell carcinoma of the bladder. Int. J. Cancer 2007, 122, 1297–1302. [Google Scholar] [CrossRef]
- Fingleton, B.; Vargo-Gogola, T.; Crawford, H.C.; Matrisian, L.M. Matrilysin [MMP-7] Expression Selects for Cells with Reduced Sensitivity to Apoptosis. Neoplasia 2001, 3, 459–468. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorganic Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Gangwar, R.; Kapoor, R.; Mittal, R.D. Bladder Cancer Risk Associated with Genotypic Polymorphism of the Ma-trix Metalloproteinase-1 and 7 in North Indian Population. Dis. Mark. 2010, 29, 37–46. [Google Scholar] [CrossRef]
- Tasci, A.I.; Tugcu, V.; Ozbek, E.; Ozbay, B.; Simsek, A.; Koksal, V. A single-nucleotide polymorphism in the matrix metallopro-teinase-1 promoter enhances bladder cancer susceptibility. BJU Int. 2008, 101, 503–507. [Google Scholar]
- Elkin, M.; Ariel, I.; Miao, H.Q.; Nagler, A.; Pines, M.; de-Groot, N.; Hochberg, A.; Vlodavsky, I. Inhibition of bladder carcinoma an-giogenesis, stromal support, and tumor growth by halofuginone. Cancer Res. 1999, 59, 4111–4118. [Google Scholar]
- Kanayama, H.; Yokota, K.; Kurokawa, Y.; Murakami, Y.; Nishitani, M.; Kagawa, S. Prognostic values of matrix metalloprotein-ase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer 1998, 82, 1359–1366. [Google Scholar] [CrossRef]
- Hara, I.; Miyake, H.; Hara, S.; Arakawa, S.; Kamidono, S. Significance of matrix metalloproteinases and tissue inhibitors of met-alloproteinase expression in the recurrence of superficial transitional cell carcinoma of the bladder. J. Urol. 2001, 165, 1769–1772. [Google Scholar] [CrossRef]
- Offersen, B.V.; Knap, M.M.; Horsman, M.R.; Verheijen, J.; Hanemaaijer, R.; Overgaard, J. Matrix metalloproteinase-9 measured in urine from bladder cancer patients is an independent prognostic marker of poor survival. Acta Oncol. 2010, 49, 1283–1287. [Google Scholar] [CrossRef]
- Szarvas, T.; Singer, B.B.; Becker, M.; Dorp, F.V.; Jäger, T.; Szendrői, A.; Riesz, P.; Romics, I.; Rübben, H.; Ergün, S. Urinary matrix metalloproteinase-7 level is associated with the presence of metastasis in bladder cancer. BJU Int. 2010, 107, 1069–1073. [Google Scholar] [CrossRef]
- Durkan, G.C.; Nutt, J.E.; Rajjayabun, P.H.; Neal, D.E.; Lunec, J.; Mellon, J.K. Prognostic significance of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in voided urine samples from patients with transitional cell carcinoma of the blad-der. Clin. Cancer Res. 2001, 7, 3450–3456. [Google Scholar]
- Mohammad, M.A.; Ismael, N.R.; Shaarawy, S.M. El-Merzabani MM. Prognostic value of membrane type 1 and 2 matrix metallo-proteinase expression and gelatinase A activity in bladder cancer. Int. J. Biol. Markers. 2010, 25, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vasala, K.; Turpeenniemi-Hujanen, T. Serum tissue inhibitor of metalloproteinase-2 (TIMP-2) and matrix metalloproteinase-2 in complex with the inhibitor (MMP-2:TIMP-2) as prognostic markers in bladder cancer. Clin. Biochem. 2007, 40, 640–644. [Google Scholar] [CrossRef]
- Miao, C.; Liang, C.; Zhu, J.; Xu, A.; Zhao, K.; Hua, Y.; Zhang, J.; Chen, W.; Suo, C.; Zhang, C.; et al. Prognostic role of matrix metalloproteinases in bladder carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 32309–32321. [Google Scholar] [CrossRef]
- Kader, A.K.; Liu, J.; Shao, L.; Dinney, C.P.; Lin, J.; Wang, Y.; Gu, J.; Grossman, H.B.; Wu, X. Matrix Metalloproteinase Polymorphisms Are Associated with Bladder Cancer Invasiveness. Clin. Cancer Res. 2007, 13, 2614–2620. [Google Scholar] [CrossRef]
- Wieczorek, E.; Reszka, E.; Wasowicz, W.; Grzegorczyk, A.; Konecki, T.; Sosnowski, M.; Jablonowski, Z. MMP7 and MMP8 genetic polymorphisms in bladder cancer patients. Cent. Eur. J. Urol. 2013, 66, 405–410. [Google Scholar] [CrossRef]
- Wieczorek, E.; Reszka, E.; Jablonowski, Z.; Jablonska, E.; Krol, M.B.; Grzegorczyk, A.; Gromadzinska, J.; Sosnowski, M.; Wasowicz, W. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013, 112, 1207–1214. [Google Scholar] [CrossRef]
- Mao, F.; Niu, X.B.; Gu, S.; Ji, L.; Wei, B.J.; Wang, H.B. The association between matrix metalloproteinase-7 genetic variant and blad-der cancer risk in a Chinese Han population. Clin. Exp. Med. 2019, 19, 565–570. [Google Scholar] [CrossRef]
- Campbell, S.C.; Volpert, O.V.; Ivanovich, M.; Bouck, N.P. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998, 58, 1298–1304. [Google Scholar]
- Shariat, S.F.; Youssef, R.F.; Gupta, A.; Chade, D.C.; Karakiewicz, P.I.; Isbarn, H.; Jeldres, C.; Sagalowsky, A.I.; Ashfaq, R.; Lotan, Y. Associ-ation of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J. Urol. 2010, 183, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Grossfeld, G.D.; Ginsberg, D.A.; Stein, J.P.; Bochner, B.H.; Esrig, D.; Nichols, P.W.; Taylor, C.R.; Cote, R.J.; Groshen, S.; Dunn, M.; et al. Thrombospondin-1 Expression in Bladder Cancer: Association with p53 Alterations, Tumor Angiogenesis, and Tumor Progression. J. Natl. Cancer Inst. 1997, 89, 219–227. [Google Scholar] [CrossRef]
- Gu, J.; Tao, J.; Yang, X.; Li, P.; Yang, X.; Qin, C.; Cao, Q.; Cai, H.; Zhang, Z.; Wang, M.; et al. Effects of TSP-1-696 C/T pol-ymorphism on bladder cancer susceptibility and clinicopathologic features. Cancer Genet. 2014, 206, 247–252. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Yang, X.; Qin, C.; Cao, Q.; Zhang, Z.; Wang, M.; Cai, H.; Gu, J.; Tao, J. TSP-1-1223 A/G Polymor-phism as a Potential Predictor of the Recurrence Risk of Bladder Cancer in a Chinese Population. Int. J. Genom. 2013, 2013, 473242. [Google Scholar]
- Nakamura, Y.; Miyata, Y.; Takehara, K.; Asai, A.; Mitsunari, K.; Araki, K.; Matsuo, T.; Ohba, K.; Sakai, H. The Pathological Significance and Prognostic Roles of Thrombospondin-1, and -2, and 4N1K-peptide in Bladder Cancer. Anticancer. Res. 2019, 39, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Burgess, W.H.; Maciag, T. The Heparin-Binding (Fibroblast) Growth Factor Family of Proteins. Annu. Rev. Biochem. 1989, 58, 575–606. [Google Scholar] [CrossRef]
- Gan, Y.; Wientjes, M.G.; Au, J.L.-S. Expression of Basic Fibroblast Growth Factor Correlates with Resistance to Paclitaxel in Human Patient Tumors. Pharm. Res. 2006, 23, 1324–1331. [Google Scholar] [CrossRef]
- Szarvas, T.; Jäger, T.; Laszlo, V.; Kramer, G.; Klingler, H.C.; Dorp, F.V.; Romics, I.; Ergun, S.; Rübben, H. Circulating Angiostatin, bFGF, and Tie2/TEK Levels and Their Prognostic Impact in Bladder Cancer. Urology 2012, 80, 737.e13–737.e18. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Gandini, O.; Gradilone, A.; Silvestri, I.; Giuliani, L.; Magnanti, M.; Gallucci, M.; Saccani, G.; Frati, L.; Agliano, A.M. De-tection of basic fibroblast growth factor mRNA in urinary bladder cancer: Correlation with local relapses. Int. J. Oncol. 1999, 14, 1123–1127. [Google Scholar] [PubMed]
- Knowles, M.A. Role of FGFR3 in urothelial cell carcinoma: Biomarker and potential therapeutic target. World J. Urol. 2007, 25, 581–593. [Google Scholar] [CrossRef][Green Version]
- Billerey, C.; Chopin, D.; Aubriot-Lorton, M.H.; Ricol, D.; Diez, G.; de Medina, S.; Van Rhijn, B.; Bralet, M.P.; Lefrere-Belda, M.A.; Lahaye, J.B. Radvanyi F Frequent FGFR3 mutations in papillary noninvasive bladder (pTa) tumors. Am. J. Pathol. 2001, 158, 1955–1959. [Google Scholar] [CrossRef]
- van Oers, J.M.; Wild, P.J.; Burger, M.; Denzinger, S.; Stoehr, R.; Rosskopf, E.; Hofstaedter, F.; Steyerberg, E.W.; Klinkhammer-Schalke, M.; Zwarthoff, E.C. FGFR3 mutations and a normal CK20 staining pattern define low-grade noninvasive urothelial bladder tumors. Eur. Urol. 2007, 52, 760–768. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.W.; Lurkin, I.; Radvanyi, F.; Kirkels, W.J.; van der Kwast, T.H.; Zwarthoff, E.C. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001, 61, 1265–1268. [Google Scholar]

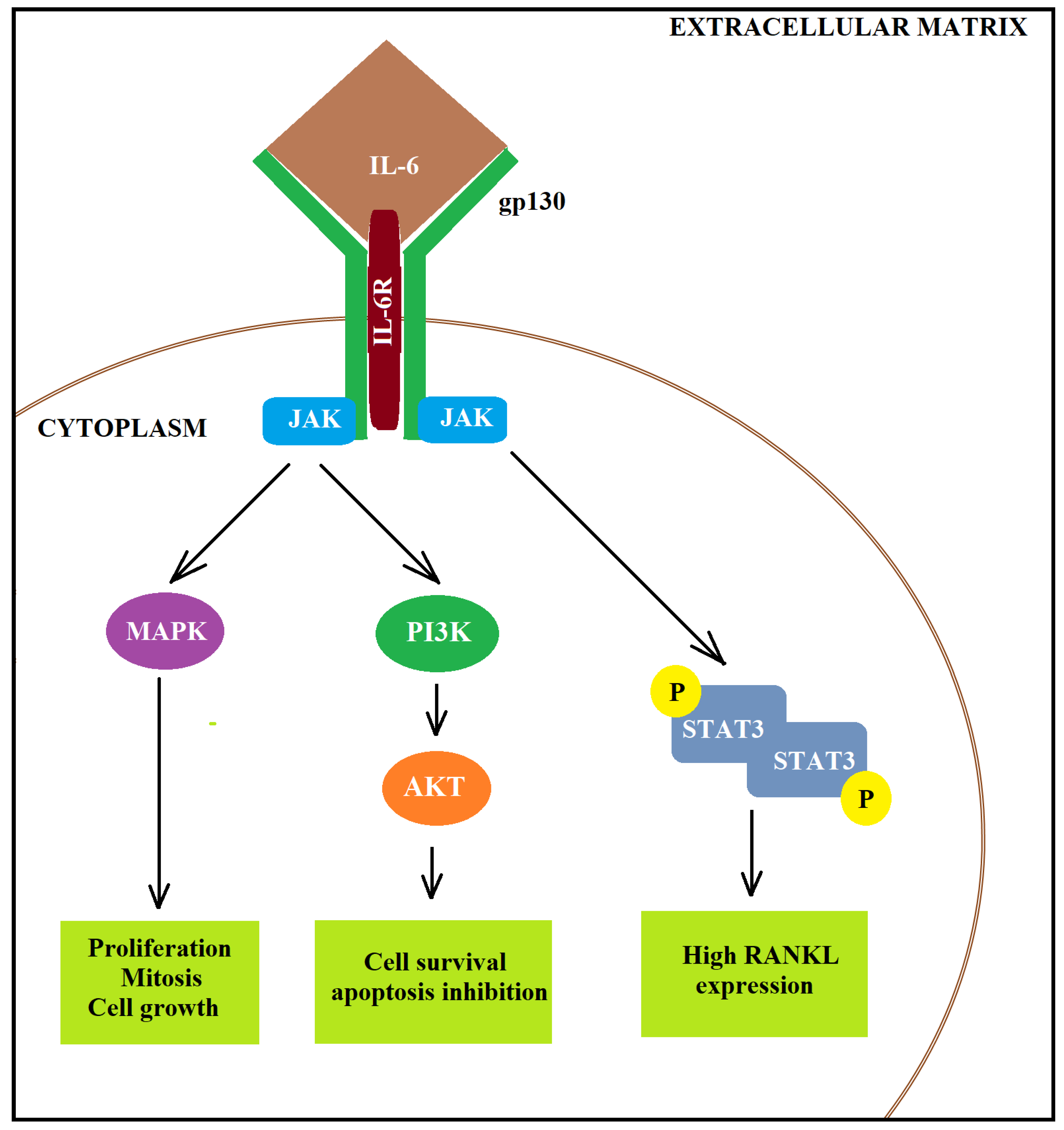
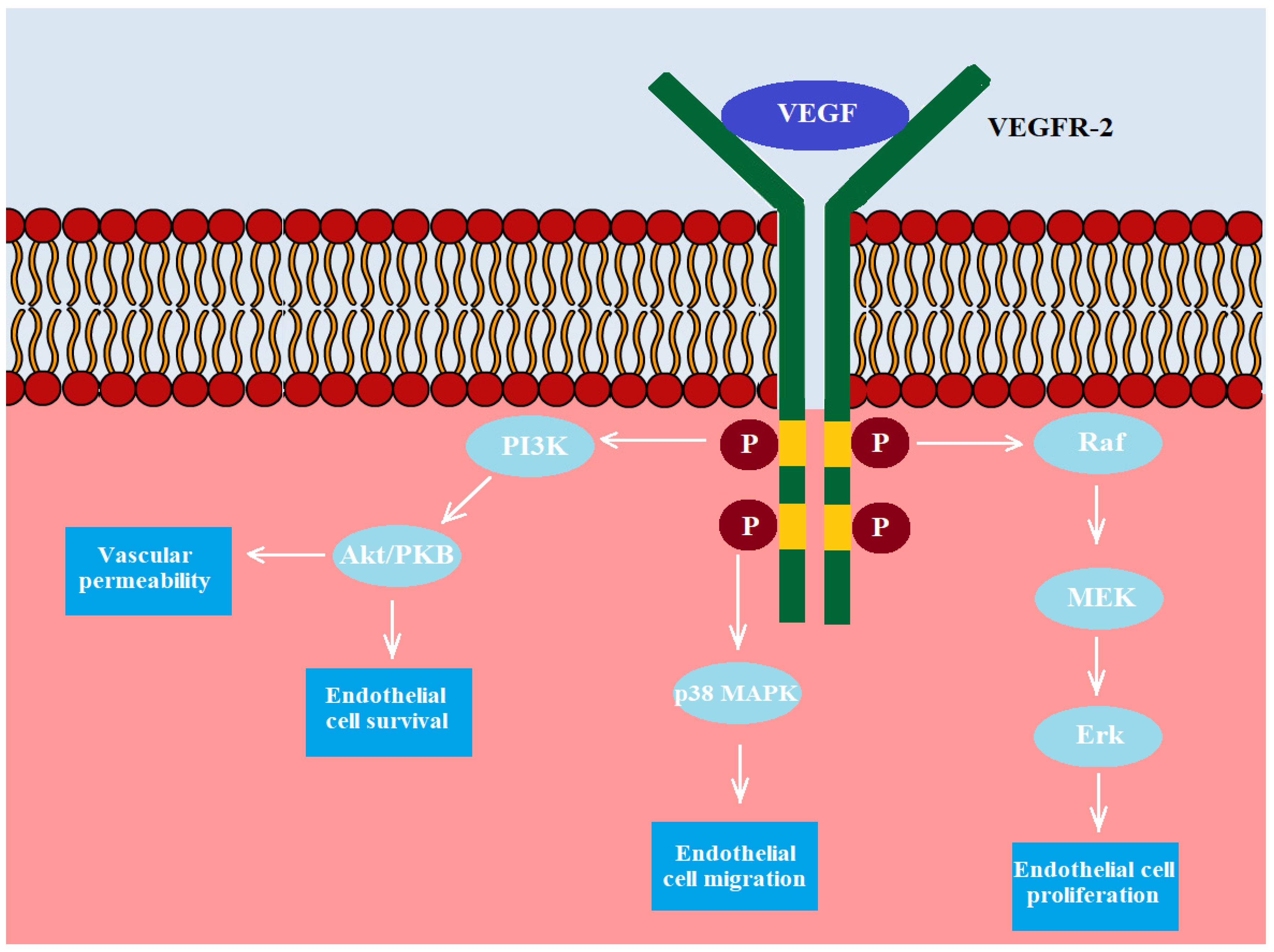
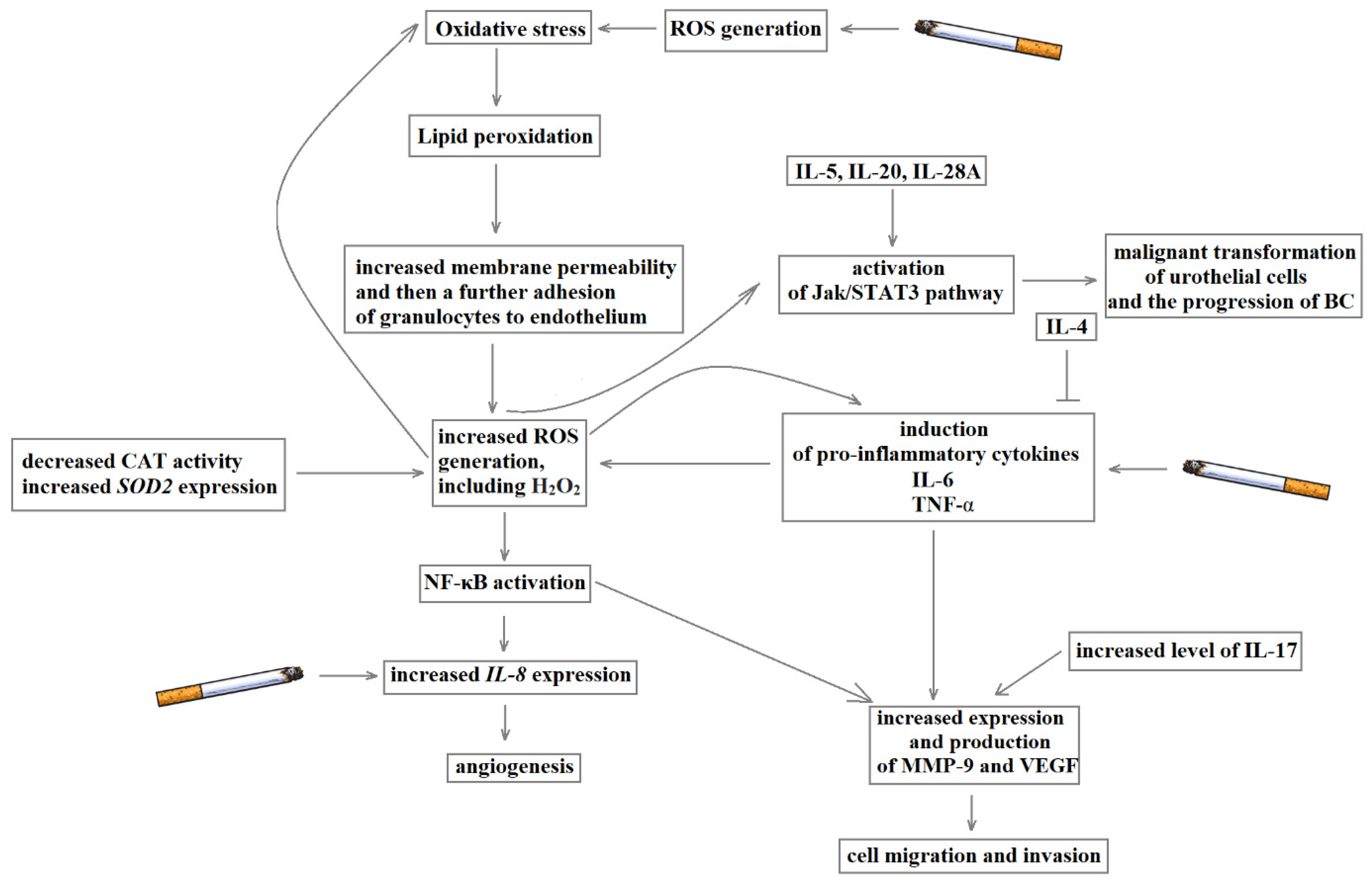
| Gene (Protein Product) | Polymorphism | Chromosome Location | Position | Nucleotide Change | SNP Function | Risk Allele (Frequency) | Allelic OR (95%CI) | p-Value | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AL050403.2 | rs62185668 | 20p12.2 | chr20:10981287 | C/A | intron variant | 0.22012 | 1.19 (1.13–1.26) | 2 × 10−11 | [46] |
| PSCA, JRK | rs2294008 | 8q24.3 | chr8:142680513 | C/T | 5′ UTR variant | 0.451005 | 1.15 (1.10–1.20) | 2 × 10−10 | [47,48] |
| TP63, P3H2 | rs710521 | 3q28 | chr3:189928144 | T/C | intergenic variant | 0.257688 | 1.19 (1.12–1.27) | 6 × 10−8 | [49] |
| CASC11, MYC | rs9642880 | 8q24.21 | chr8:127705823 | G/A G/T | intron variant | 0.000000 0.455939 | 1.21 (1.15–1.28) | 7 × 10−12 | [48] |
| AC023421.2, SLC14A1 | rs7238033 | 18q12.3 | chr18:45737001 | T/C | intron variant | 0.566372 | 1.2 (1.13–1.28) | 9 × 10−9 | [50] |
| TACC3 | rs798766 | 4p16.3 | chr4:1732512 | T/C | intron variant | 0.795826 | 1.24 (1.17–1.32) | 1 × 10−11 | [36,51] |
| PSD3, NAT2 | rs1495741 | 8p22 | chr8:18415371 | G/A | regulatory region variant | 0.756073 | 1.14 (1.09–1.18) | 2 × 10−10 | [52,53] |
| UGT1A8, UGT1A10 | rs11892031 | 2q37.1 | chr2:233656637 | A/C A/T | intron variant | 0.078850, 0.000000 | 1.19 (1.12–1.27) | 1 × 10−7 | [39,54] |
| CCNE1, AC008798.3 | rs8102137 | 19q12 | chr19:29805946 | T/C | regulatory region variant | 0.306569 | 1.13 (1.09–1.17) | 2 × 10−11 | [39,55] |
| APOBEC3A, AL022318.1 | rs1014971 | 22q13.1 | chr22:38936618 | C/T | regulatory region variant | 0.628195 | 1.18 (1.10–1.18) | 8 × 10−12 | [39,53] |
| CLPTM1L | rs401681 | 5p15.33 | chr5:1321972 | G/A | intron variant | 0.433217 | 1.12 (1.08–1.16) | 4 × 10−11 | [53,56] |
| AC023421.2, SLC14A1 | rs17674580 | 18q12.3 | chr18:45729946 | C/A C/T | 5′ UTRvariant | 0.000000, 0.332221 | 1.17 (1.11–1.22) | 8 × 10−11 | [56,57] |
| LINC02871 | rs6104690 | 20p12.2 | chr20:11007451 | G/A G/T | intron variant | 0.553042, 0.000000 | 1.12 (1.08–1.18) | 7 × 10−7 | [53] |
| LSP1, TNNI2 | rs907611 | 11p15.5 | Chr11:1852842 | G/A | regulatory region variant | 0.304845 | 1.15 (1.09–1.21) | 4 × 10−8 | [53] |
| MYNN | rs10936599 | 3q26.2 | chr3:169774313 | C/T | synonymous variant | 0.245632 | 1.18 (1.11–1.23) | 5 × 10−9 | [52,53] |
| AL513188.1, CDKAL1 | rs4510656 | 6p22.3 | chr6:20766466 | C/A | intron variant | 0.38803 | 1.12 (1.08–1.18) | 7 × 10−7 | [53] |
| PAG1 | rs5003154 | 8q21.13 | chr8:81074718 | T/C T/G | intron variant | 0.51809, 0.00000 | 1.11 (1.06–1.16) | 1 × 10−6 | [53] |
| MCF2L | rs4907479 | 13q34 | chr13:113004794 | G/A G/C | intron variant | 0.23256, 0.00000 | 1.13 (1.07–1.18) | 3 × 10−6 | [53] |
| Gene | Enzyme | Gene Location | Characteristics of the Enzyme | References |
|---|---|---|---|---|
| COX-2 | prostaglandin-endoperoxide synthase 2 (cyclooxygenase) | 1q31.1 | is the key enzyme in prostaglandin biosynthesis and acts both as a dioxygenase and as a peroxidase—converts arachidonic acid to prostaglandin endoperoxide H2; COX-2 is naturally inhibited by calcitriol (the active form of Vitamin D) | [58,59,60] |
| NOX-4 | NADPH oxidase 4 | 11q14.3 | protects the vasculature against inflammatory stress; NOX-dependent ROS modulation by amino endoperoxides may induce apoptosis in high Nox4-expressing cancer cells | [61] |
| iNOS | inducible nitric oxide synthase | 17q11.2. | iNOS produces large quantities of NO upon stimulation, such as by proinflammatory cytokines | [61,62,63,64,65] |
| CAT | catalase | 11p13 | is a heme enzyme and catalyses the decomposition of hydrogen peroxide to water and oxygen and thereby mitigates the toxic effects of hydrogen peroxide | [66,67,68] |
| GPx3 | glutathione peroxidase 3 | 5q33.1 | catalyze the reduction of organic hydroperoxides and hydrogen peroxide (H2O2) by glutathione, and thereby protect cells against oxidative damage; this isozyme is secreted, and is abundantly found in plasma | [69] |
| SOD1 | superoxide dismutase 1 [Cu-Zn] | 21q22.11 | is a soluble cytoplasmic protein, acting as a homodimer to convert naturally occurring but harmful superoxide radicals to molecular oxygen and hydrogen peroxide; this protein also contains an antimicrobial peptide that displays antibacterial, antifungal, and anti-MRSA activity against E. coli, E. faecalis, S. aureus, S. aureus MRSA LPV+, S. agalactiae, and yeast C. krusei | [69] |
| SOD2 | manganese-dependent superoxide dismutase (MnSOD) | 6q25 | transforms toxic superoxide, a by-product of the mitochondrial electron transport chain, into hydrogen peroxide and diatomic oxygen | [69,70] |
| PON1 | serum paraoxonase/arylesterase 1 | 7q21.3 | is secreted mainly by the liver; is responsible for hydrolysing organophosphate pesticides and nerve gasses, and mediates enzymatic protection of low-density lipoproteins against oxidative modification | [71,72,73] |
| PON2 | serum paraoxonase/arylesterase 2 | 7q21.3 | may act as a cellular antioxidant, protecting cells from oxidative stress—prevents LDL lipid peroxidation, reverses the oxidation of mildly oxidised LDL, and inhibits the ability of MM-LDL to induce monocyte chemotaxis. Hydrolytic activity against acylhomoserine lactones, important bacterial quorum-sensing mediators, suggests the encoded protein may also play a role in defence responses to pathogenic bacteria | [74] |
| Oxidative Stress | ||||
|---|---|---|---|---|
| Gene/Protein | Biological Specimens | Molecular Change in Bladder Cancer Course | Comments | References |
| MDA level | serum | increased | [80] | |
| 8-iso-PGF2 α | urine | increased | no correlation was observed between 8-iso-PGF2 α level and the degree of malignancy and invasiveness of BC | [53,82] |
| COX-2 expression | bladder cells | increased | COX-2 expression was inversely correlated with existing of recurrence of NMIBC; high level of COX-2 expression was associated with an advancing grade and T stage of superficial transitional cell carcinoma | [59,60] |
| NOX-4 expression | bladder tissue | overexpression | [61] | |
| NO | bladder tissue, urine and serum | increased | [64,65] | |
| iNOS expression | bladder tissue | increased | overexpression of iNOS was correlated with a transition to more advanced stages of bladder cancer | [62] |
| CAT level | serum, blood | reduced | [66] | |
| CAT expression and activity | bladder tissue | the low expression of CAT may contribute to the recurrence of BC | [67,69,79] | |
| GPx3 activity | plasma | reduced | [69] | |
| GPx1 activity and expression | erythrocytes, leucocytes | increased | [69,98,99] | |
| SOD activity | cell carcinoma, erythrocytes | reduced | [69] | |
| SOD expression | cell carcinoma, peripheral blood leucocytes | [83,84,85,86] | ||
| SOD level | serum | SOD level in serum and blood was negatively correlated with the stage of bladder cancer—the lowest level of SOD was observed in patients with the most advanced cancer | [66,87] | |
| PON1 concentration | serum | reduced | [71,72] | |
| PON2 expression | bladder tissue | increased | [74] | |
| Gene | Name | Location | Characteristic | References |
|---|---|---|---|---|
| TNF-α | tumour necrosis factor alpha | 6p21.33 | TNF-α is mainly secreted by macrophages and can bind to and thus functions through its receptors TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR. This cytokine is involved in regulating a broad spectrum of biological processes, including cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation. It has been implicated in various diseases, including autoimmune diseases, insulin resistance, psoriasis, rheumatoid arthritis, ankylosing spondylitis, tuberculosis, autosomal dominant polycystic kidney disease, and cancer. | [128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150] |
| IL-4 | interleukin 4 | 5q31.1 | This cytokine is a ligand for the interleukin 4 receptor. This receptor also binds to IL13. IL4 is considered an important cytokine for tissue repair, counterbalancing the effects of proinflammatory type 1 cytokines; however, it also promotes allergic airway inflammation. IL-4 has an essential role in the production of allergen-specific immunoglobin (Ig) E. | [151,152] |
| IL-5 | interleukin 5 | 5q31.1 | The cytokine acts as a growth and differentiation factor for both B cells and eosinophils and plays a significant role in regulating eosinophil formation, maturation, recruitment and survival. | [153] |
| IL-6 | interleukin 6 | 7p15.3 | The cytokine is involved in inflammation and the maturation of B cells. An elevated level of the encoded protein has been finding in virus infections, including COVID-19. | [154,155,156,157,158,159,160,161,162,163,164,165,166,167,168] |
| IL-8 | interleukin 8 | 4q13.3 | IL-8 is a major mediator of the inflammatory response and is secreted by mononuclear macrophages, neutrophils, eosinophils, T lymphocytes, epithelial cells, and fibroblasts. It functions as a chemotactic factor by guiding the neutrophils to the site of infection. This chemokine is also a potent angiogenic factor. The binding of IL-8 to one of its receptors (IL-8RB/CXCR2) increases the permeability of blood vessels, and an elevated level of IL-8 is positively correlated with the greater severity of multiple disease outcomes. | [169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184] |
| IL-17 | interleukin 17 | 6p12.2 | The cytokine is produced by activated T cells. IL-17-mediated downstream pathways induce the production of inflammatory molecules, chemokines, antimicrobial peptides, and remodelling proteins. The cytokine elicits crucial impacts on host defence, cell trafficking, immune modulation, and tissue repair, with a critical role in the induction of innate immune defences. A high level of this cytokine is associated with several chronic inflammatory diseases, including rheumatoid arthritis, psoriasis and multiple sclerosis. The lung damage induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is largely a result of the inflammatory response promoted by cytokines such as IL-17 (cytokine storm). | [185,186,187,188,189,190] |
| IL-20 | interleukin 20 | 1q32.1 | The cytokine is part of the larger IL-10 cytokine family. It is produced by keratinocytes and generally functions to enhance innate defence mechanisms. It promotes wound healing by increasing keratinocyte proliferation. IL-20 is considered critical in skin inflammation, and upregulation of its receptor has been shown in patients with psoriasis. | [152] |
| IL-28A | interleukin 28A/interferon lambda 2 | 19q13.2 | IL-28A expression can be induced by a viral infection. | [152] |
| NF-κB | nuclear factor kappa B | 4q24 | NF-κB is a transcription regulator activated by various intra- and extra-cellular stimuli such as cytokines, oxidant-free radicals, ultraviolet irradiation, and bacterial or viral products. | [169,170,191,192,193] |
| TGF-β | transforming growth factor beta 1 | 19q13.2 | The factor regulates cell proliferation, differentiation, and growth and modulates the expression and activation of different growth factors, including interferon-gamma and TNF-α. This gene is frequently upregulated in tumour cells, and mutations in this gene result in Camurati-Engelmann disease. | [194,195,196,197,198,199,200] |
| IFN-α | Interferon alpha | 9p21.3 | It is produced in response to viral infection as a key part of the innate immune response with potent antiviral, antiproliferative and immunomodulatory properties. | [201,202] |
| IFN-β | Interferon beta | 9p21.3 | It is released as part of the innate immune response to pathogens. | [201,202] |
| IFN-γ | interferon gamma | 12q15 | It is secreted by cells of both the innate and adaptive immune systems and binds to the interferon-gamma receptor, which triggers a cellular response to viral and microbial infections | [201,202] |
| Inflammation | ||||
|---|---|---|---|---|
| Gene/Protein | Biological Specimens | Molecular Change in Bladder Cancer Course | Comments | References |
| NF-κB expression | bladder tissue | increased | NF-κB expression is associated with histologic grade and T category in bladder urothelial cancer | [191] |
| IL-8 expression | bladder tissue | increased | IL-8 expression may be associated with the metastatic potential of human transitional cell carcinoma | [171] |
| TNF-α level | urine | increased | the enzyme level is associated with bladder cancer progression | [135,136] |
| IL-6 level | serum | increased | high serum level of IL-6 was associated with metastasis and poor prognosis | [155] |
| IL-17 expression | peripheral blood | increased | worse prognosis may be correlated with high expression of IL-17 | [187,188] |
| Gene | Gene Localised | Name (Aliases) | Location | Substrates | Activation Pathway | References |
|---|---|---|---|---|---|---|
| MMP-1 | 11q22.2 | interstitial collagenase (CLG, CLGN) | secreted | collagens: I, II, III, VII, VIII, X, gelatin | The plasmin has been described and other serine proteases, i.e., kallikrein, trypsin, neutrophil elastase, cathepsin G, tryptase and chymase may be involved in the activation of proMMP-1. | [293,294] |
| MMP-2 | 16q12.2 | gelatinase-A, 72 kDa gelatinase | secreted | gelatin, collagens: I, II, III, IV, Vii, X | A complex of membrane-type 1 MMP (MT1-MMP/MMP14) and tissue inhibitor of MMP-2 recruits pro-MMP 2 from the extracellular milieu to the cell surface. Activation then requires an active molecule of MT1-MMP and autocatalytic cleavage. Clustering of integrin chains promotes MMP-2 activation. Another factor that will support the activation of MMP-2 is cell-cell clustering. A wild-type activated leukocyte cell adhesion molecule (ALCAM) is also required to activate the MMP-2. | [146,147,293,295,296,297,298,299,300,301,302,303,304,305,306] |
| MMP-7 | 11q22.2 | matrilysin, PUMP 1 (MMP-7, MPSL-1, PUMP-1) | secreted | fibronectin, laminin, collagen IV, gelatin | Pro-MMP7 is converted from the latent form to the active form by endoproteinases and plasmin. Plasmin cleaves at the site recognisable to trypsin is considered as the possible physiological activator. | [299,307] |
| MMP-9 | 20q13.12 | gelatinase-B, 92 kDa gelatinase (CLG4B, GELB, MANDP2, MMP-9) | secreted | gelatin, collagen IV, V | The proMMP-9 includes a cysteine residue in the N-terminal pro-domain that binds to the zinc atom in the active site thus maintaining latency. Activation of MMP-9 requires a disruption of the cysteine interaction with the zinc atom. MMP-9 activators include MMP-2, MMP-3, MMP-7, MMP-10, MMP-13, cathepsin G and urokinase/plasmin. | [296,297,298,299,304] |
| MMP12 | 11q22.2 | macrophage metalloelastase (HME, ME, MME, MMP-12) | secreted | elastin, fibronectin, collagen IV | Neutrophil elastase may be required for the proteolytic activation of pro-MMP-12 | [304] |
| Angiogenesis | ||||
|---|---|---|---|---|
| Gene/Protein | Biological Specimens | Molecular Change in Bladder Cancer Course | Comments | References |
| VEGF expression | bladder tissue | increased | The VEGF-A level in tissue was correlated with BC grade; VEGF expression was higher in deeper tumours than superficial tumours and invasive tumours compared to non-invasive tumours. | [272,275] |
| VEGFR-1 expression | bladder tissue | increased | mRNA level of VEGFR was correlated to the pathologic stage of BC. | [275] |
| HIF-1 expression | bladder tissue | increased | The increased HIF-1 expression was positive correlated with disease progression and recurrence and was also associated with poor overall survival. | [288,289] |
| MMP-9 expression | bladder tissue | increased | MMP-9 expression was higher in deeper tumours compared to superficial tumours, in invasive tumours compared to non-invasive tumours and high-grade tumours than in low-grade tumours; overexpression of MMP-9 in tissue was associated with a higher risk of tumour recurrence and poor prognosis. | [295,297,298] |
| MMP-2 level | bladder tissue | increased | [301,302] | |
| TSP-1 level | bladder cells (in vitro study) | reduced | [308] | |
| TSP-1 expression | bladder tissue | The lower expression of TSP-1 was observed in high-grade tumours than in low-grade tumours. | [309,310] | |
| bFGF expression | bladder tissue | increased | The overexpression of bFGF was positively correlated with muscle invasion, high tumour grade, chemotherapy resistance, high recurrence rate and poor prognosis in BC patients. | [309,315] |
| bFGF level | urine, serum | [316,317] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigner, P.; Grębowski, R.; Bijak, M.; Saluk-Bijak, J.; Szemraj, J. The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. Int. J. Mol. Sci. 2021, 22, 4483. https://doi.org/10.3390/ijms22094483
Wigner P, Grębowski R, Bijak M, Saluk-Bijak J, Szemraj J. The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. International Journal of Molecular Sciences. 2021; 22(9):4483. https://doi.org/10.3390/ijms22094483
Chicago/Turabian StyleWigner, Paulina, Radosław Grębowski, Michał Bijak, Joanna Saluk-Bijak, and Janusz Szemraj. 2021. "The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development" International Journal of Molecular Sciences 22, no. 9: 4483. https://doi.org/10.3390/ijms22094483
APA StyleWigner, P., Grębowski, R., Bijak, M., Saluk-Bijak, J., & Szemraj, J. (2021). The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. International Journal of Molecular Sciences, 22(9), 4483. https://doi.org/10.3390/ijms22094483







