Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats
Abstract
1. Introduction
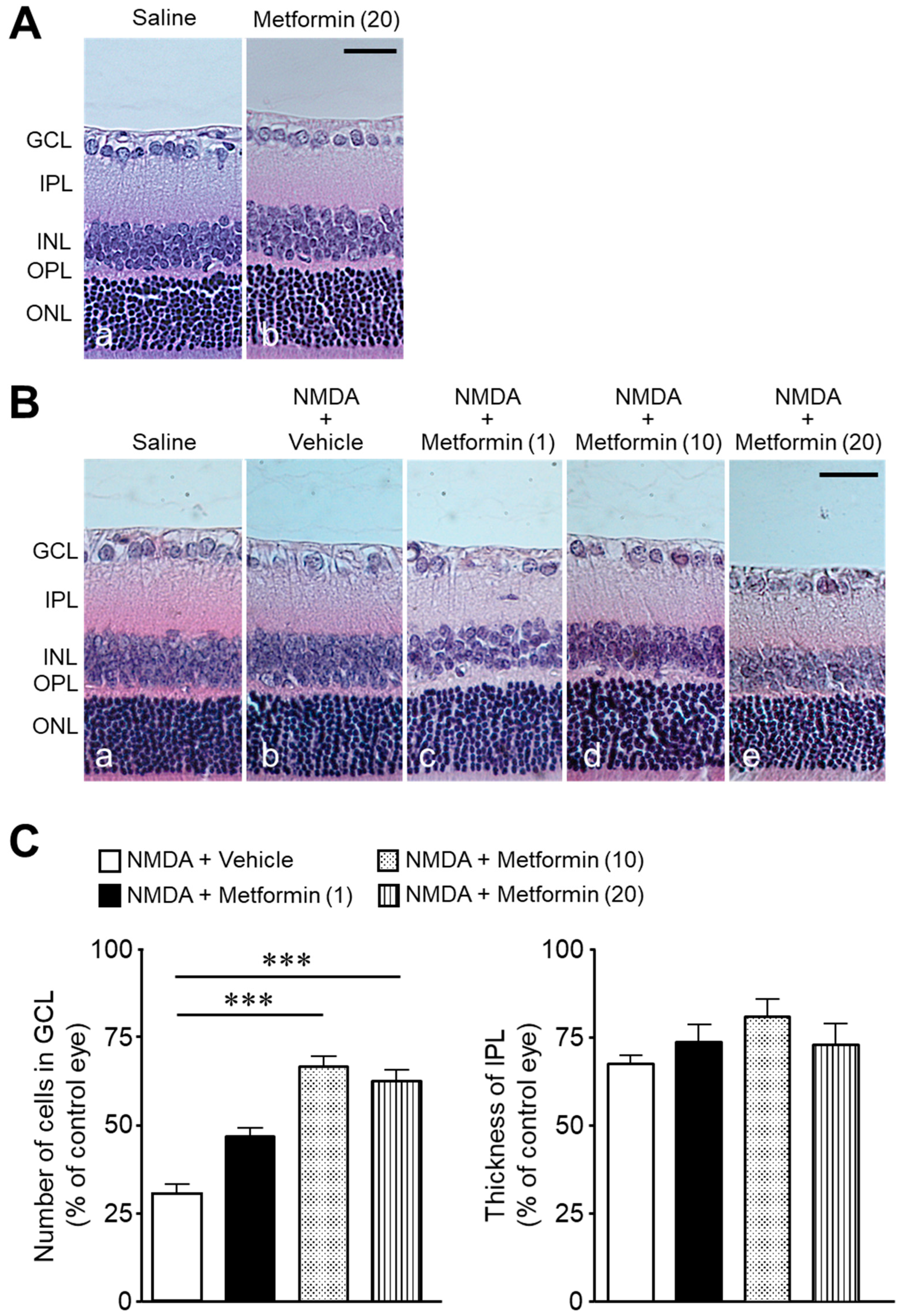
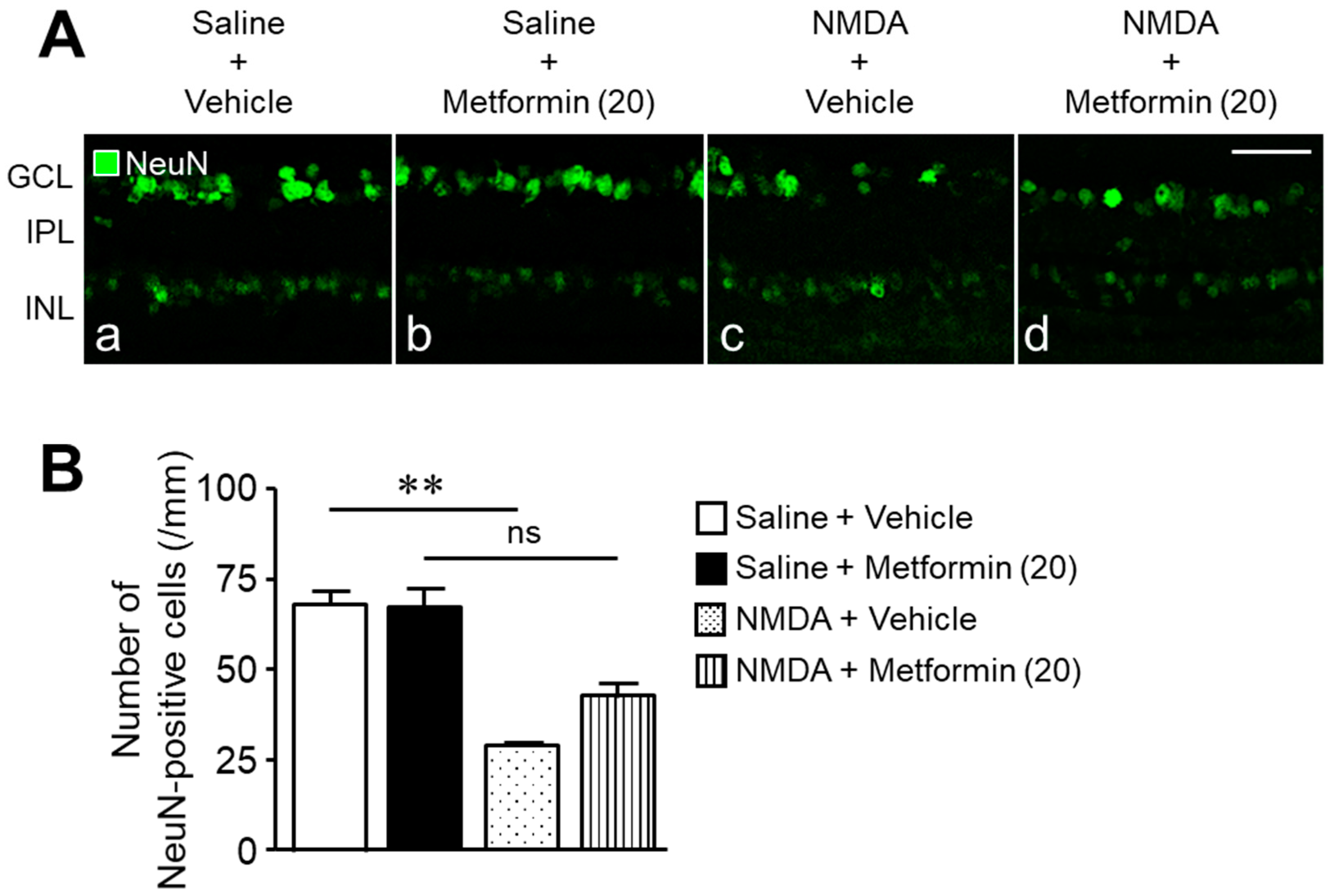
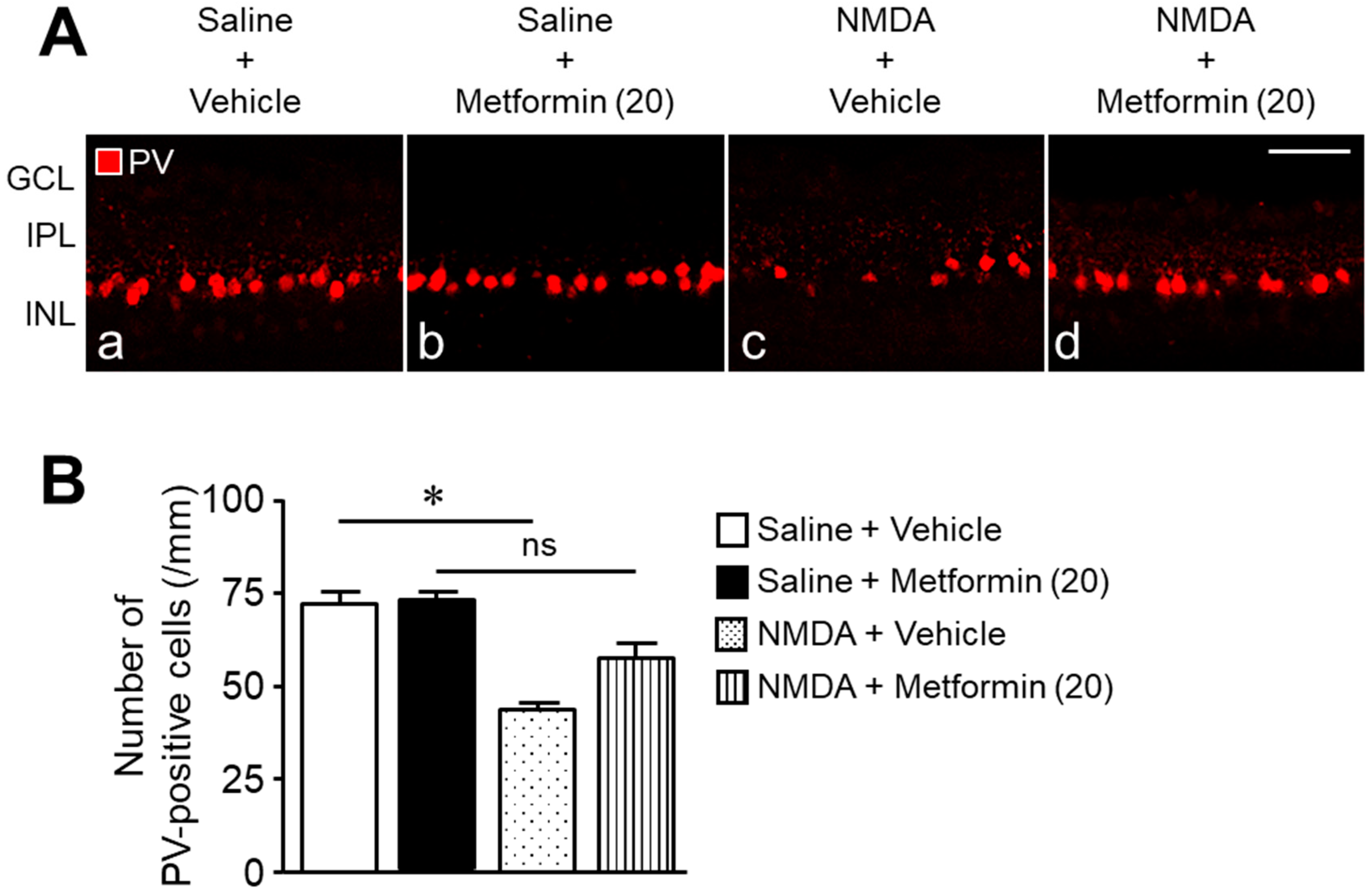
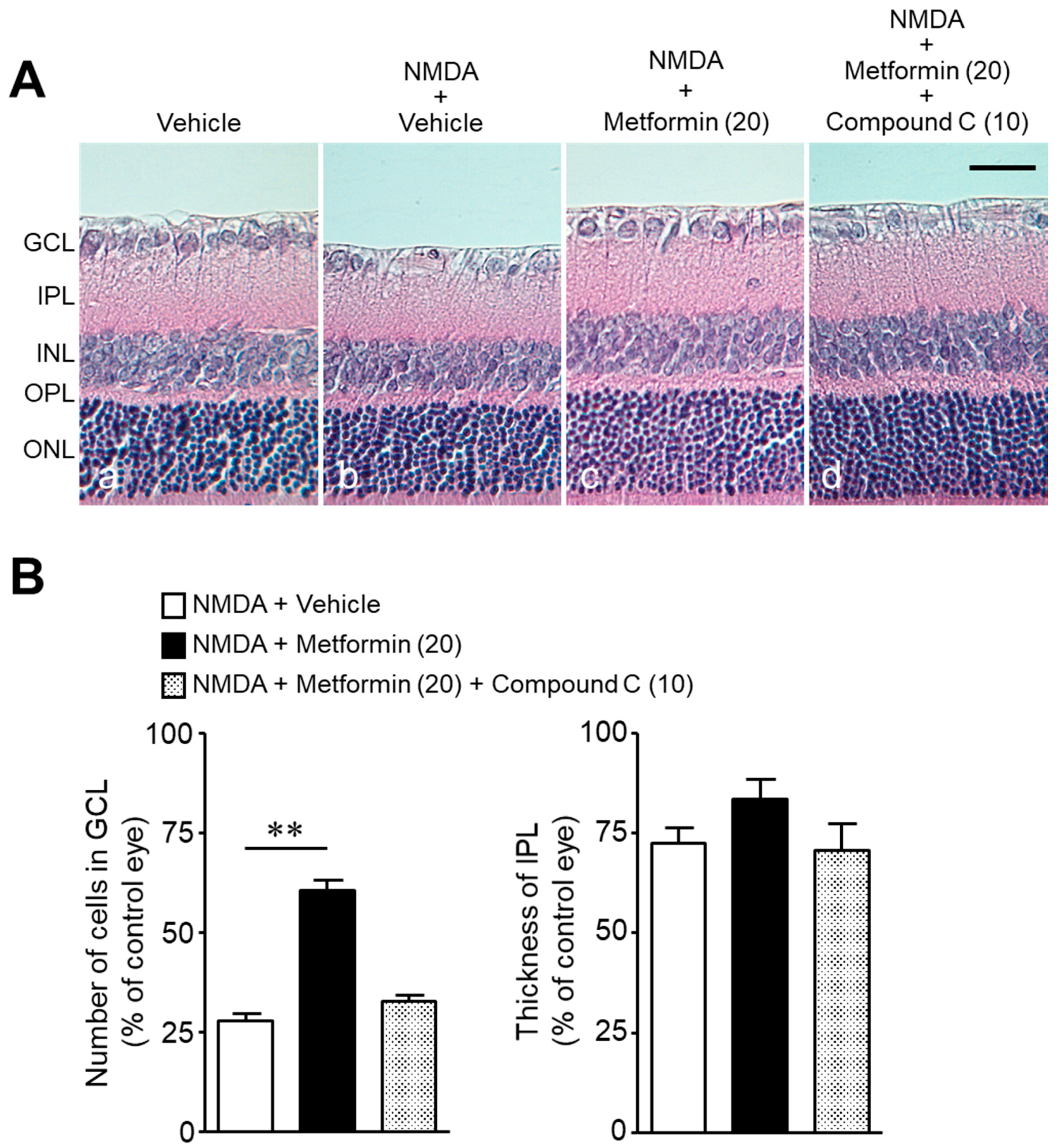
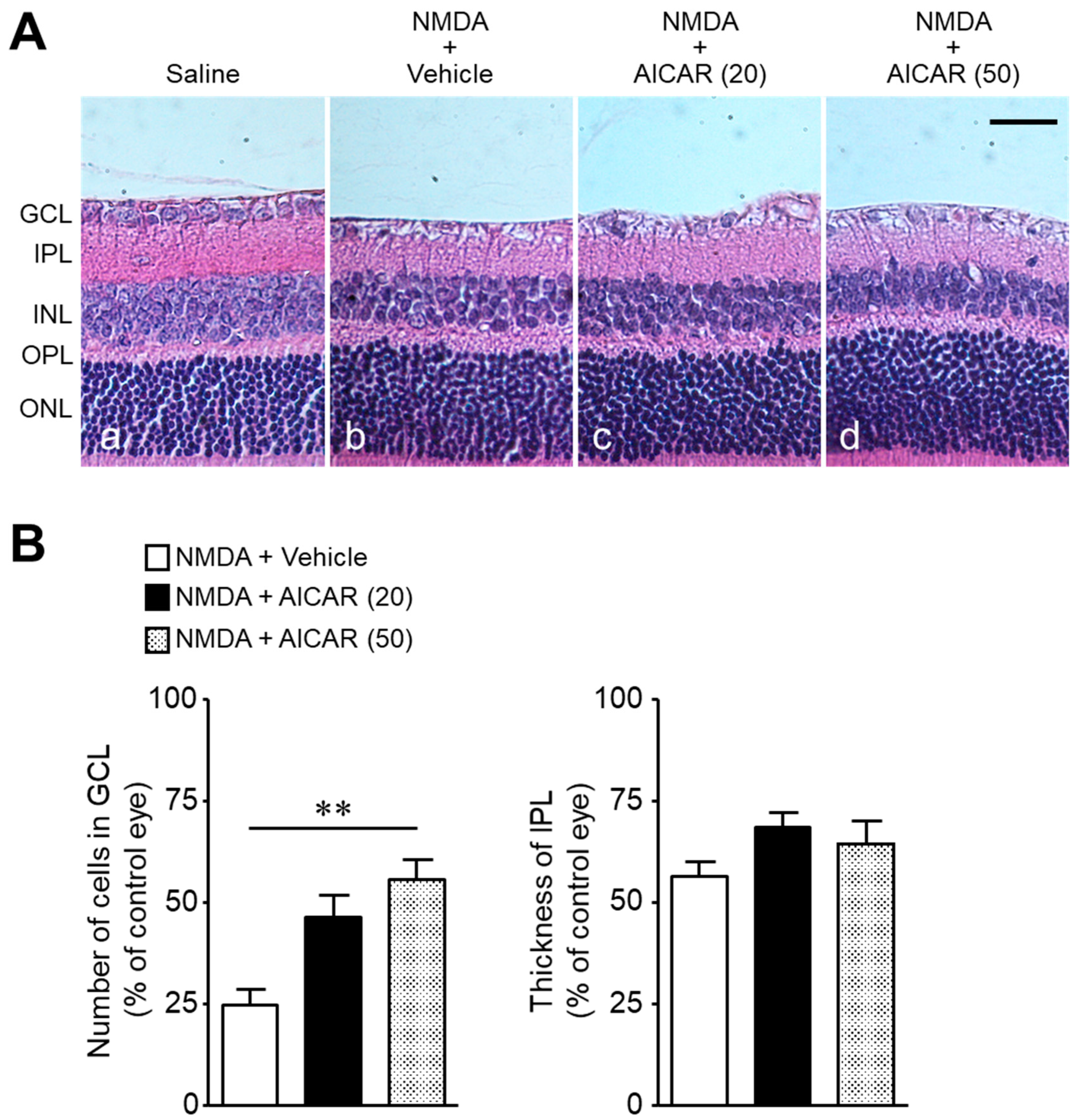
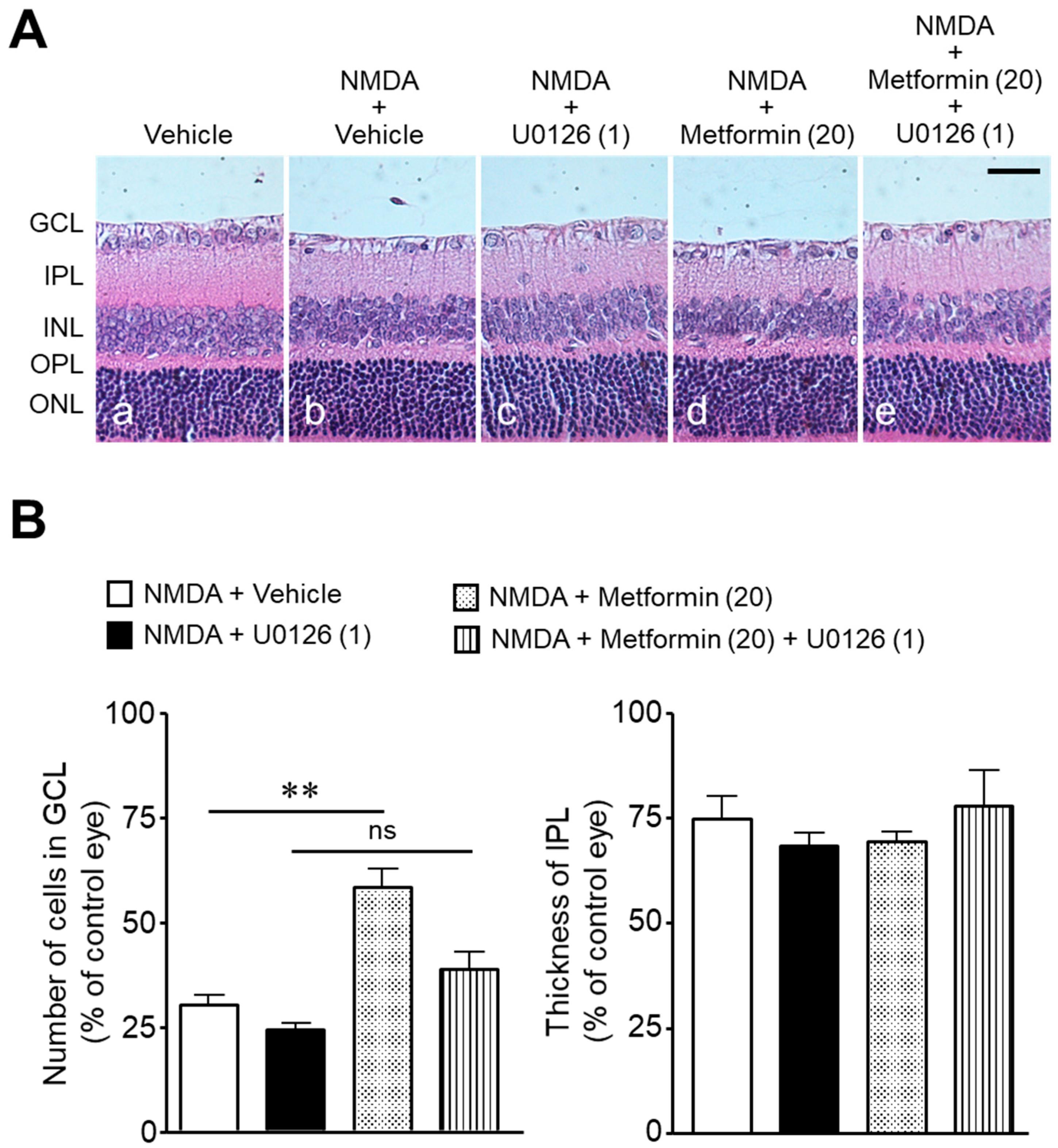
2. Results
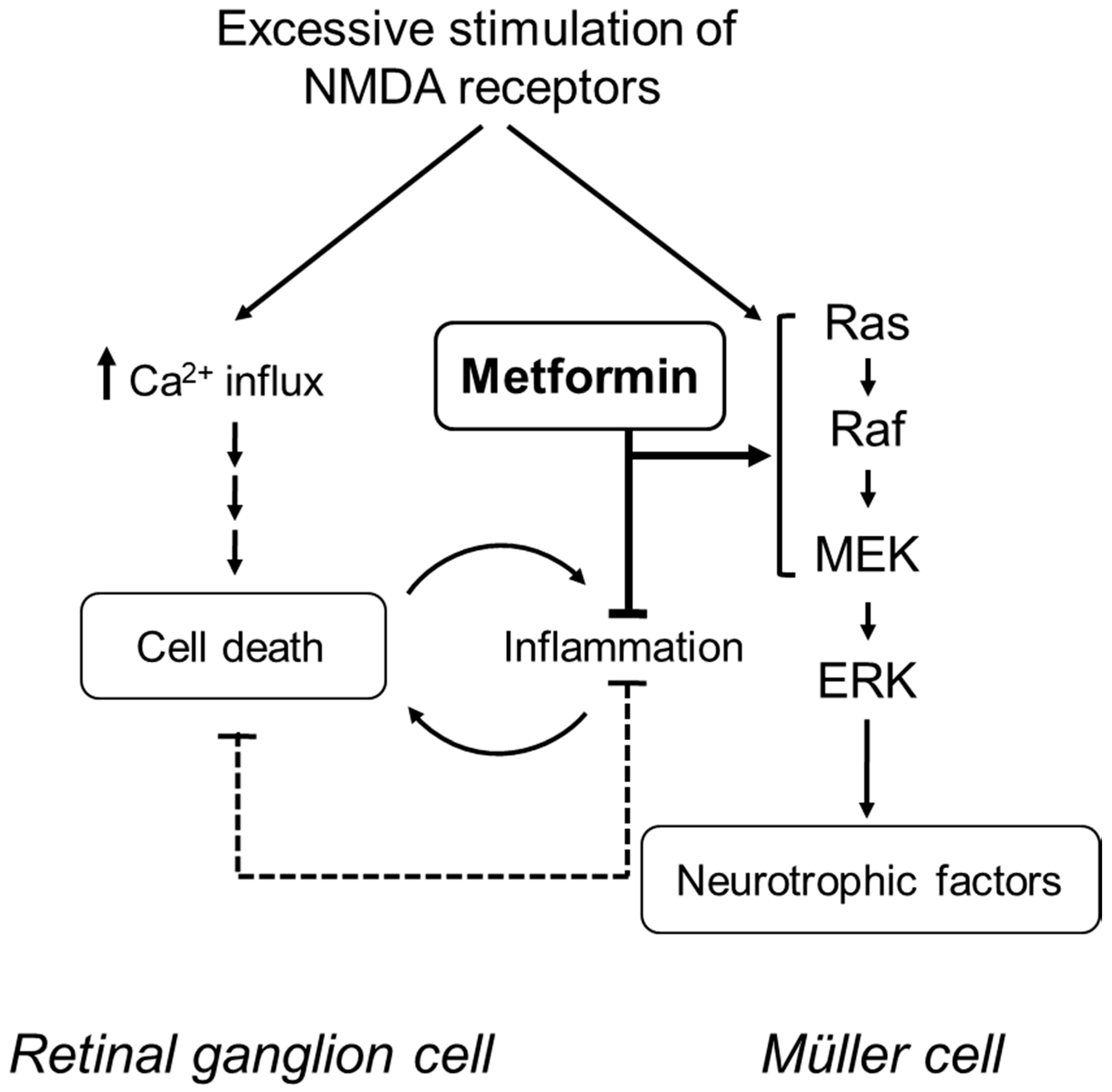
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Procedures
4.2.1. Experiment 1
4.2.2. Experiment 2
4.2.3. Experiment 3
4.2.4. Experiment 4
4.3. Histological Evaluation of the Retina
4.4. Immunohistochemistry
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An old drug with new applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Abdelgadir, E.; Ali, R.; Rashid, F.; Bashier, A. Effect of metformin on different non-diabetes related conditions, a special focus on malignant conditions: Review of literature. J. Clin. Med. Res. 2017, 9, 388–395. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Ashabi, G.; Khodagholi, F.; Khalaj, L.; Goudarzvand, M.; Nasiri, M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: Interference of AMPK/PGC-1α pathway. Metab. Brain Dis. 2014, 29, 47–58. [Google Scholar] [CrossRef]
- Porceddu, P.F.; Ishola, I.O.; Contu, L.; Morelli, M. Metformin prevented dopaminergic neurotoxicity induced by 3,4-Methylenedioxymethamphetamine Administration. Neurotox. Res. 2016, 30, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-H.; Zhu, G.-J.; Geng, D.-Q.; Zhang, H.-Z.; He, J.-M.; Guo, A.-Z.; Ma, L.-L.; Yu, D.-H. Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats. Physiol. Behav. 2017, 170, 115–123. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Liu, X.; Zhou, T.; Sun, H.; Edwards, P.; Gao, H.; Yu, F.-S.; Qiao, X. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS ONE 2018, 13, e0193031. [Google Scholar] [CrossRef]
- Joe, S.G.; Yoon, Y.H.; Choi, J.A.; Koh, J.-Y. Anti-angiogenic effect of metformin in mouse oxygen-induced retinopathy is mediated by reducing levels of the vascular endothelial growth factor receptor Flk-1. PLoS ONE 2015, 10, e0119708. [Google Scholar] [CrossRef]
- Mori, A.; Ishikawa, E.; Amano, T.; Sakamoto, K.; Nakahara, T. Anti-diabetic drug metformin dilates retinal blood vessels through activation of AMP-activated protein kinase in rats. Eur. J. Pharmacol. 2017, 798, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.; Choi, M.Y.; Lee, D.H.; Roh, G.S.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Kim, S.-J.; Yoo, J.-M.; et al. Metformin protects against retinal cell death in diabetic mice. Biochem. Biophys. Res. Commun. 2017, 492, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Mohamed, S.; Mustapha, N.M.; Lau, S.; Ishak, N.I.M.; Umran, N.S. Metformin attenuated histopathological ocular deteriorations in a streptozotocin-induced hyperglycemic rat model. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kong, L.; Wang, J.; Ash, J.D. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2018, 115, 10475–10480. [Google Scholar] [CrossRef]
- Luodan, A.; Zou, T.; He, J.; Chen, X.; Sun, D.; Fan, X.; Xu, H. Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front. Mol. Neurosci. 2019, 12, 102. [Google Scholar] [CrossRef]
- Kubota, S.; Ozawa, Y.; Kurihara, T.; Sasaki, M.; Yuki, K.; Miyake, S.; Noda, K.; Ishida, S.; Tsubota, K. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Investig. Opthalmol. Vis. Sci. 2011, 52, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2017, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Monte, M.D.; Casini, G. Relationships between neurodegeneration and vascular damage in diabetic retinopathy. Front. Neurosci. 2019, 13, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.; Siew, E.; Chu, R.; Tso, M.O. Ameliorative effect of MK-801 on retinal ischemia. J. Ocul. Pharmacol. Ther. 1997, 13, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Solberg, Y.; Rosner, M.; Turetz, J.; Belkin, M. MK-801 has neuroprotective and antiproliferative effects in retinal laser injury. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1380–1389. [Google Scholar]
- Nakazawa, T.; Takahashi, H.; Nishijima, K.; Shimura, M.; Fuse, N.; Tamai, M.; Hafezi-Moghadam, A.; Nishida, K. Pitavastatin prevents NMDA-induced retinal ganglion cell death by suppressing leukocyte recruitment. J. Neurochem. 2006, 100, 1018–1031. [Google Scholar] [CrossRef]
- Takeda, A.; Shinozaki, Y.; Kashiwagi, K.; Ohno, N.; Eto, K.; Wake, H.; Nabekura, J.; Koizumi, S. Microglia mediate non-cell-autonomous cell death of retinal ganglion cells. Glia 2018, 66, 2366–2384. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.H.; Abdelsaid, M.A.; Matragoon, S.; Pillai, B.A.; El-Remessy, A.B. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br. J. Pharmacol. 2011, 164, 170–180. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kitaoka, Y.; Munemasa, Y.; Ohtani-Kaneko, R.; Kikusui, T.; Uematsu, A.; Takeda, H.; Hirata, K.; Mori, Y.; Ueno, S. Neuroprotective effect of 17β-estradiol against N-methyl-D-aspartate-induced retinal neurotoxicity via p-ERK induction. J. Neurosci. Res. 2006, 85, 386–394. [Google Scholar] [CrossRef]
- Zhou, R.-H.; Yan, H.; Wang, B.-R.; Kuang, F.; Duan, X.-L.; Xu, Z. Role of Extracellular Signal-Regulated Kinase in Glutamate-Stimulated Apoptosis of Rat Retinal Ganglion Cells. Curr. Eye Res. 2007, 32, 233–239. [Google Scholar] [CrossRef]
- Nakazawa, T.; Shimura, M.; Ryu, M.; Nishida, K.; Pagès, G.; Pouysségur, J.; Endo, S. ERK1 plays a critical protective role againstN-methyl-D-aspartate-induced retinal injury. J. Neurosci. Res. 2007, 86, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Nakahara, T.; Kurauchi, Y.; Mori, A.; Sakamoto, K.; Ishii, K. Rapamycin prevents N-methyl-D-aspartate-induced retinal damage through an ERK-dependent mechanism in rats. J. Neurosci. Res. 2014, 92, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, I.; Aoki, Y.; Asano, D.; Ushikubo, H.; Mori, A.; Sakamoto, K.; Nakahara, T.; Ishii, K. Protective effects of everolimus against N-methyl-D-aspartic acid-induced retinal damage in rats. Biol. Pharm. Bull. 2015, 38, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Wäussle, H.; Grüunert, U.; Röhrenbeck, J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J. Comp. Neurol. 1993, 332, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.; Lam, T.T.; Li, W.W.Y.; Caprioli, J.; Kwong, J.M.K. Calpain and N-methyl-d-aspartate (NMDA)-induced excitotoxicity in rat retinas. Brain Res. 2005, 1046, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Shimazawa, M.; Suemori, S.; Inokuchi, Y.; Matsunaga, N.; Nakajima, Y.; Oka, T.; Yamamoto, T.; Hara, H. A Novel Calpain Inhibitor, ((1S)-1-((((1S)-1-Benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-methylbutyl)carbamic Acid 5-Methoxy-3-oxapentyl Ester (SNJ-1945), Reduces Murine Retinal Cell Death In Vitro and In Vivo. J. Pharmacol. Exp. Ther. 2009, 332, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.F.; Apers, S.; De Meyer, G.R.; Martinet, W. Metformin attenuates expression of endothelial cell adhesion molecules and formation of atherosclerotic plaques via autophagy induction. Ann. Clin. Exp. Metab. 2016, 5, 1–9. [Google Scholar]
- Devoldere, J.; Peynshaert, K.; De Smedt, S.C.; Remaut, K. Müller cells as a target for retinal therapy. Drug Discov. Today 2019, 24, 1483–1498. [Google Scholar] [CrossRef]
- Awai, M.; Koga, T.; Inomata, Y.; Oyadomari, S.; Gotoh, T.; Mori, M.; Tanihara, H. NMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153. J. Neurochem. 2005, 96, 43–52. [Google Scholar] [CrossRef]
- Inokuchi, Y.; Imai, S.; Nakajima, Y.; Shimazawa, M.; Aihara, M.; Araie, M.; Hara, H. Edaravone, a Free Radical Scavenger, Protects against Retinal Damage in Vitro and in Vivo. J. Pharmacol. Exp. Ther. 2009, 329, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Wong, K.Y.; Rupasinghe, C.N.; Tiwari, R.; Zhao, X.; Berberoglu, E.D.; Sinkler, C.; Liu, J.; Lee, I.; Parang, K.; et al. Inhibition of N-Methyl-D-aspartate-induced retinal neuronal death by polyarginine peptides is linked to the attenuation of stress-induced hyperpolarization of the inner mitochondrial membrane potential. J. Biol. Chem. 2015, 290, 22030–22048. [Google Scholar] [CrossRef] [PubMed]
- Gründer, T.; Kohler, K.; Kaletta, A.; Guenther, E. The distribution and developmental regulation of NMDA receptor subunit proteins in the outer and inner retina of the rat. J. Neurobiol. 2000, 44, 333–342. [Google Scholar] [CrossRef]
- Zhou, Y.; Tencerová, B.; Hartveit, E.; Veruki, M.L. Functional NMDA receptors are expressed by both AII and A17 amacrine cells in the rod pathway of the mammalian retina. J. Neurophysiol. 2016, 115, 389–403. [Google Scholar] [CrossRef]
- Oikawa, F.; Nakahara, T.; Akanuma, K.; Ueda, K.; Mori, A.; Sakamoto, K.; Ishii, K. Protective effects of the β3-adrenoceptor agonist CL316243 against N-methyl-D-aspartate-induced retinal neurotoxicity. Naunyn-Schmiedebergs Arch. Pharmacol. 2012, 385, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Naruoka, T.; Nakahara, T.; Tsuda, Y.; Kurauchi, Y.; Mori, A.; Sakamoto, K.; Nishihira, J.; Ishii, K. ISO-1, a macrophage migration inhibitory factor antagonist, prevents N-methyl-D-aspartate-induced retinal damage. Eur. J. Pharmacol. 2013, 718, 138–144. [Google Scholar] [CrossRef]
- Aoki, Y.; Nakahara, T.; Asano, D.; Ushikubo, H.; Mori, A.; Sakamoto, K.; Ishii, K. Preventive effects of rapamycin on inflammation and capillary degeneration in a rat model of NMDA-induced retinal injury. Biol. Pharm. Bull. 2015, 38, 321–324. [Google Scholar] [CrossRef]
- Hayashi, I.; Aoki, Y.; Ushikubo, H.; Asano, D.; Mori, A.; Sakamoto, K.; Nakahara, T.; Ishii, K. Protective effects of PF-4708671 againstN-methyl-d-aspartic acid-induced retinal damage in rats. Fundam. Clin. Pharmacol. 2016, 30, 529–536. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Asano, D.; Ushikubo, H.; Morita, A.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats. Int. J. Mol. Sci. 2021, 22, 4439. https://doi.org/10.3390/ijms22094439
Watanabe K, Asano D, Ushikubo H, Morita A, Mori A, Sakamoto K, Ishii K, Nakahara T. Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats. International Journal of Molecular Sciences. 2021; 22(9):4439. https://doi.org/10.3390/ijms22094439
Chicago/Turabian StyleWatanabe, Koki, Daiki Asano, Hiroko Ushikubo, Akane Morita, Asami Mori, Kenji Sakamoto, Kunio Ishii, and Tsutomu Nakahara. 2021. "Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats" International Journal of Molecular Sciences 22, no. 9: 4439. https://doi.org/10.3390/ijms22094439
APA StyleWatanabe, K., Asano, D., Ushikubo, H., Morita, A., Mori, A., Sakamoto, K., Ishii, K., & Nakahara, T. (2021). Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats. International Journal of Molecular Sciences, 22(9), 4439. https://doi.org/10.3390/ijms22094439





