Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava
Abstract
1. Introduction
2. Results
2.1. Identification of TFs in the S. clava Genome
2.2. Comparison Analysis of S. clava TFs
2.3. Expression of S. clava TFs at Different Developmental Stages
2.4. Hox Genes
2.5. Zinc Finger Family
2.6. Forkhead Box Family
3. Discussion
4. Materials and Methods
4.1. Animals and Embryos
4.2. Transcriptome Sequencing and WGCNA Analysis
4.3. Identification and Classification of TFs
4.4. Heat Map, Phylogenetic Analysis, Domain Analysis, and Expression Correlation Analysis
4.5. Quantitative Real-Time PCR
4.6. Gene Ontology (GO) Enrichment Analysis
4.7. Data Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Tjian, R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 1989, 245, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bejerano, G.; Pheasant, M.; Makunin, I.; Stephen, S.; Kent, W.J.; Mattick, J.S.; Haussler, D. Ultraconserved elements in the human genome. Science 2004, 304, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell 2008, 134, 25–36. [Google Scholar] [CrossRef]
- Lee, T.I.; Young, R.A. Transcriptional regulation and its misregulation in disease. Cell 2013, 152, 1237–1251. [Google Scholar] [CrossRef]
- Arnold, H.-H.; Braun, T. The role of Myf-5 in somitogenesis and the development of skeletal muscles in vertebrates. J. Cell Sci. 1993, 104, 957–960. [Google Scholar]
- Peter, J. Nuclear Factor-kB—A pivotal transcription factor in chronic inflammatory diseases. Mech. Dis. 1997, 336, 1066–1071. [Google Scholar]
- Mallo, M. Reassessing the Role of Hox Genes during Vertebrate Development and Evolution. Trends Genet. 2018, 34, 209–217. [Google Scholar] [CrossRef]
- Wang, K.; Nishida, H. REGULATOR: A database of metazoan transcription factors and maternal factors for developmental studies. BMC Bioinform. 2015, 16, 114. [Google Scholar] [CrossRef]
- Hu, H.; Miao, Y.R.; Jia, L.H.; Yu, Q.Y.; Zhang, Q.; Guo, A.Y. AnimalTFDB 3.0: A comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019, 47, D33–D38. [Google Scholar] [CrossRef]
- Chiba, S.; Awazu, S.; Itoh, M.; Chin-Bow, S.T.; Satoh, N.; Satou, Y.; Hastings, K.E. A genomewide survey of developmentally relevant genes in Ciona intestinalis. IX. Genes for muscle structural proteins. Dev. Genes Evol. 2003, 213, 291–302. [Google Scholar] [CrossRef]
- Wada, S.; Tokuoka, M.; Shoguchi, E.; Kobayashi, K.; Di Gregorio, A.; Spagnuolo, A.; Branno, M.; Kohara, Y.; Rokhsar, D.; Levine, M.; et al. A genomewide survey of developmentally relevant genes in Ciona intestinalis. Dev. Genes Evol. 2003, 213, 222–234. [Google Scholar] [CrossRef]
- Yagi, K.; Satou, Y.; Mazet, F.; Shimeld, S.M.; Degnan, B.; Rokhsar, D.; Levine, M.; Kohara, Y.; Satoh, N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. III. Genes for Fox, ETS, nuclear receptors and NFkappaB. Dev. Genes Evol. 2003, 213, 235–244. [Google Scholar] [CrossRef]
- Yamada, L.; Kobayashi, K.; Degnan, B.; Satoh, N.; Satou, Y. A genomewide survey of developmentally relevant genes in Ciona intestinalis. IV. Genes for HMG transcriptional regulators, bZip and GATA/Gli/Zic/Snail. Dev. Genes Evol. 2003, 213, 245–253. [Google Scholar] [CrossRef]
- Imai, K.S.; Hino, K.; Yagi, K.; Satoh, N.; Satou, Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: Towards a comprehensive understanding of gene networks. Development 2004, 131, 4047–4058. [Google Scholar] [CrossRef]
- Miwata, K.; Chiba, T.; Horii, R.; Yamada, L.; Kubo, A.; Miyamura, D.; Satoh, N.; Satou, Y. Systematic analysis of embryonic expression profiles of zinc finger genes in Ciona intestinalis. Dev. Biol. 2006, 292, 546–554. [Google Scholar] [CrossRef]
- Katikala, L.; Aihara, H.; Passamaneck, Y.J.; Gazdoiu, S.; Jose-Edwards, D.S.; Kugler, J.E.; Oda-Ishii, I.; Imai, J.H.; Nibu, Y.; Di Gregorio, A. Functional Brachyury binding sites establish a temporal read-out of gene expression in the Ciona notochord. PLoS Biol. 2013, 11, e1001697. [Google Scholar] [CrossRef]
- Di Gregorio, A. The notochord gene regulatory network in chordate evolution: Conservation and divergence from Ciona to vertebrates. Curr. Top. Dev. Biol. 2020, 139, 325–374. [Google Scholar] [CrossRef]
- Yasuo, H.; Satoh, N. An ascidian homolog of the mouse Brachyury (T) gene is expressed exclusively in notochord cells at the fate restricted stage. Dev. Growth Differ. 1994, 36, 9–18. [Google Scholar] [CrossRef]
- Corbo, J.C.; Levine, M.; Zeller, R.W. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 1997, 124, 589–602. [Google Scholar]
- Jose-Edwards, D.S.; Kerner, P.; Kugler, J.E.; Deng, W.; Jiang, D.; Di Gregorio, A. The identification of transcription factors expressed in the notochord of Ciona intestinalis adds new potential players to the brachyury gene regulatory network. Dev. Dyn. 2011, 240, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Jose-Edwards, D.S.; Oda-Ishii, I.; Nibu, Y.; Di Gregorio, A. Tbx2/3 is an essential mediator within the Brachyury gene network during Ciona notochord development. Development 2013, 140, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.S.; Satoh, N.; Satou, Y. An essential role of a FoxD gene in notochord induction in Ciona embryos. Development 2002, 129, 3441–3453. [Google Scholar] [PubMed]
- Yagi, K.; Satou, Y.; Satoh, N. A zinc finger transcription factor, ZicL, is a direct activator of Brachyury in the notochord specification of Ciona intestinalis. Development 2004, 131, 1279–1288. [Google Scholar] [CrossRef]
- Imai, K.S.; Satou, Y.; Satoh, N. Multiple functions of a Zic-like gene in the differentiation of notochord, central nervous system and muscle in Ciona savignyi embryos. Development 2002, 129, 2723–2732. [Google Scholar]
- Lemons, D.; McGinnis, W. Genomic Evolution of Hox Gene Clusters. Science 2006, 313, 1918–1922. [Google Scholar] [CrossRef]
- DeBiasse, M.B.; Colgan, W.N.; Harris, L.; Davidson, B.; Ryan, J.F. Inferring Tunicate Relationships and the Evolution of the Tunicate Hox Cluster with the Genome of Corella inflata. Genome Biol. Evol. 2020, 12, 948–964. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Lu, Q.; Ren, P.; Guo, X.; Wang, J.; Li, X.; Chang, Y.; Duan, S.; Wang, S.; et al. Genomic basis of environmental adaptation in the leathery sea squirt (Styela clava). Mol. Ecol. Resour. 2020. [Google Scholar] [CrossRef]
- Goldstien, S.J.; Schiel, D.R.; Gemmell, N.J. Regional connectivity and coastal expansion: Differentiating pre-border and post-border vectors for the invasive tunicate Styela clava. Mol. Ecol. 2010, 19, 874–885. [Google Scholar] [CrossRef]
- Goldstien, S.J.; Dupont, L.; Viard, F.; Hallas, P.J.; Nishikawa, T.; Schiel, D.R.; Gemmell, N.J.; Bishop, J.D. Global phylogeography of the widely introduced North West Pacific ascidian Styela clava. PLoS ONE 2011, 6, e16755. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Ikuta, T.; Yoshida, N.; Satoh, N.; Saiga, H. Ciona intestinalis Hox gene cluster: Its dispersed structure and residual colinear expression in development. Proc. Natl. Acad. Sci. USA 2004, 101, 15118–15123. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-C.; Edvardsen, R.B.; Maeland, A.D. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature 2004, 431, 67–71. [Google Scholar] [CrossRef]
- Sekigami, Y.; Kobayashi, T.; Omi, A.; Nishitsuji, K.; Ikuta, T.; Fujiyama, A.; Satoh, N.; Saiga, H. Hox gene cluster of the ascidian, Halocynthia roretzi, reveals multiple ancient steps of cluster disintegration during ascidian evolution. Zoological. Lett. 2017, 3, 17. [Google Scholar] [CrossRef]
- Blanchoud, S.; Rutherford, K.; Zondag, L.; Gemmell, N.J.; Wilson, M.J. De novo draft assembly of the Botrylloides leachii genome provides further insight into tunicate evolution. Sci. Rep. 2018, 8, 5518. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Li, Y.; Wei, H.; Guo, Z.; Wang, S.; Zhang, L.; Bao, Z. Identification and expression profiles of Fox transcription factors in the Yesso scallop (Patinopecten yessoensis). Gene 2020, 733, 144387. [Google Scholar] [CrossRef]
- Tu, Q.; Brown, C.T.; Davidson, E.H.; Oliveri, P. Sea urchin Forkhead gene family: Phylogeny and embryonic expression. Dev. Biol. 2006, 300, 49–62. [Google Scholar] [CrossRef]
- Berna, L.; Alvarez-Valin, F. Evolutionary Genomics of Fast Evolving Tunicates. Genome Biol. Evol. 2014, 6, 1724–1738. [Google Scholar] [CrossRef]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar] [CrossRef]
- Ikushima, H.; Negishi, H.; Taniguchi, T. The IRF family transcription factors at the interface of innate and adaptive immune responses. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, J.; Ren, R.; Zhang, X. Anti-virus effects of interferon regulatory factors (IRFs) identified in ascidian Ciona savignyi. Fish Shellfish. Immunol. 2020, 106, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Glass, C.K. Signaling by Nuclear Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef] [PubMed]
- Plateroti, M.; Kress, E.; Mori, J.I.; Samarut, J. Thyroid Hormone Receptor α1 Directly Controls Transcription of the β-Catenin Gene in Intestinal Epithelial Cells. Mol. Cell. Biol. 2006, 26, 3204–3214. [Google Scholar] [CrossRef]
- Vincent Bertrand, C.H.; Caillol, D.; Popovici, C.; Lemaire, P. Neural Tissue in Ascidian Embryos Is Induced by FGF9/16/20, Acting via a Combination of Maternal GATA and Ets Transcription Factors. Cell 2003, 115, 615–627. [Google Scholar] [CrossRef]
- Miya, T.; Nishida, H. An Ets transcription factor, HrEts, is target of FGF signaling and involved in induction of notochord, mesenchyme, and brain in ascidian embryos. Dev. Biol. 2003, 261, 25–38. [Google Scholar] [CrossRef]
- Hotta, K. Brachyury-downstream gene sets in a chordate, Ciona intestinalis: Integrating notochord specification, morphogenesis and chordate evolution. Evol. Dev. 2008, 10, 37–51. [Google Scholar] [CrossRef]
- Gainous, T.B.; Wagner, E.; Levine, M. Diverse ETS transcription factors mediate FGF signaling in the Ciona anterior neural plate. Dev. Biol. 2015, 399, 218–225. [Google Scholar] [CrossRef]
- Schep, A.N.; Adryan, B. A comparative analysis of transcription factor expression during metazoan embryonic development. PLoS ONE 2013, 8, e66826. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef]
- Wang, H.; Mo, P.; Ren, S.; Yan, C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J. Biol. Chem. 2010, 285, 13201–13210. [Google Scholar] [CrossRef]
- Dou, L.; Liang, H.F.; Geller, D.A.; Chen, Y.F.; Chen, X.P. The regulation role of interferon regulatory factor-1 gene and clinical relevance. Hum. Immunol. 2014, 75, 1110–1114. [Google Scholar] [CrossRef]
- Nam, S.; Lim, J.S. Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch. Pharm. Res. 2016, 39, 1548–1555. [Google Scholar] [CrossRef]
- Davidson, B.; Swalla, B.J. A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Development 2002, 129, 4739–4751. [Google Scholar]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef]
- Nikolenko, J.V.; Krasnov, A.N. Nuclear receptors: Structure and mechanisms of action. Russ. J. Genet. 2007, 43, 234–240. [Google Scholar] [CrossRef]
- Shao, C.; Bao, B.; Xie, Z.; Chen, X.; Li, B.; Jia, X.; Yao, Q.; Ortí, G.; Li, W.; Li, X.; et al. The genome and transcriptome of Japanese flounder provide insights into flatfish asymmetry. Nat. Genet. 2016, 49, 119–124. [Google Scholar] [CrossRef]
- Izpisu’a-Belmonte, J.-C.; Falkenstein, H.; Dollé, P.; Duboule, A.R.D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991, 10, 2279–2289. [Google Scholar] [CrossRef]
- Pascual-Anaya, J.; Sato, I.; Sugahara, F.; Higuchi, S.; Paps, J.; Ren, Y.; Takagi, W.; Ruiz-Villalba, A.; Ota, K.G.; Wang, W.; et al. Hagfish and lamprey Hox genes reveal conservation of temporal colinearity in vertebrates. Nat. Ecol. Evol. 2018, 2, 859–866. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Jiao, W.; Li, J.; Xun, X.; Sun, Y.; Guo, X.; Huan, P.; Dong, B.; Zhang, L.; et al. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat. Ecol. Evol. 2017, 1, 120. [Google Scholar] [CrossRef]
- Ikuta, T.; Satoh, N.; Saiga, H. Limited functions of Hox genes in the larval development of the ascidian Ciona intestinalis. Development 2010, 137, 1505–1513. [Google Scholar] [CrossRef]
- Weigel, D.; Jürgens, G.; Küttner, F.; Seifert, E.; Jäckle, H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 1989, 57, 645–658. [Google Scholar] [CrossRef]
- Carlsson, P.; Mahlapuu, M. Forkhead transcription factors: Key players in development and metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.S.; Kudoh, T.; Dawid, I.B.; Fritz, A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development 2003, 130, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.S.; Logsdon, J.M., Jr.; Fritz, A. Expression and phylogenetic analyses of three zebrafish FoxI class genes. Dev. Dyn. 2003, 228, 301–307. [Google Scholar] [CrossRef]
- Zilinski, C.; Brownell, I.; Hashimoto, R.; Medina-Martinez, O.; Swindell, E.C.; Jamrich, M. Expression of FoxE4 and Rx visualizes the timing and dynamics of critical processes taking place during initial stages of vertebrate eye development. Dev. Neurosci. 2004, 26, 294–307. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids. Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids. Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef]
- Dardaillon, J.; Dauga, D.; Simion, P.; Faure, E.; Onuma, T.A.; DeBiasse, M.B.; Louis, A.; Nitta, K.R.; Naville, M.; Besnardeau, L.; et al. ANISEED 2019: 4D exploration of genetic data for an extended range of tunicates. Nucleic Acids Res. 2020, 48, D668–D675. [Google Scholar] [CrossRef]
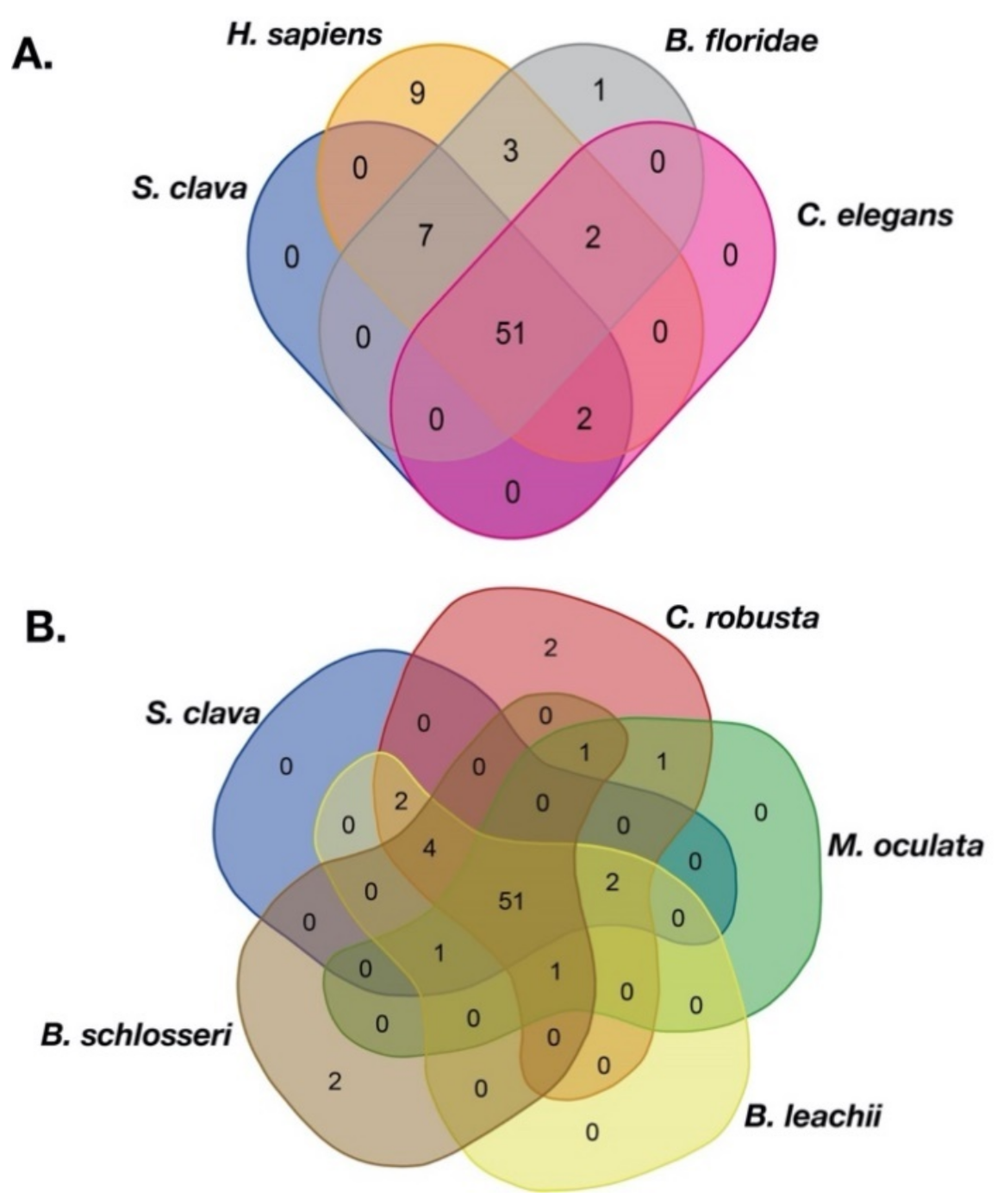

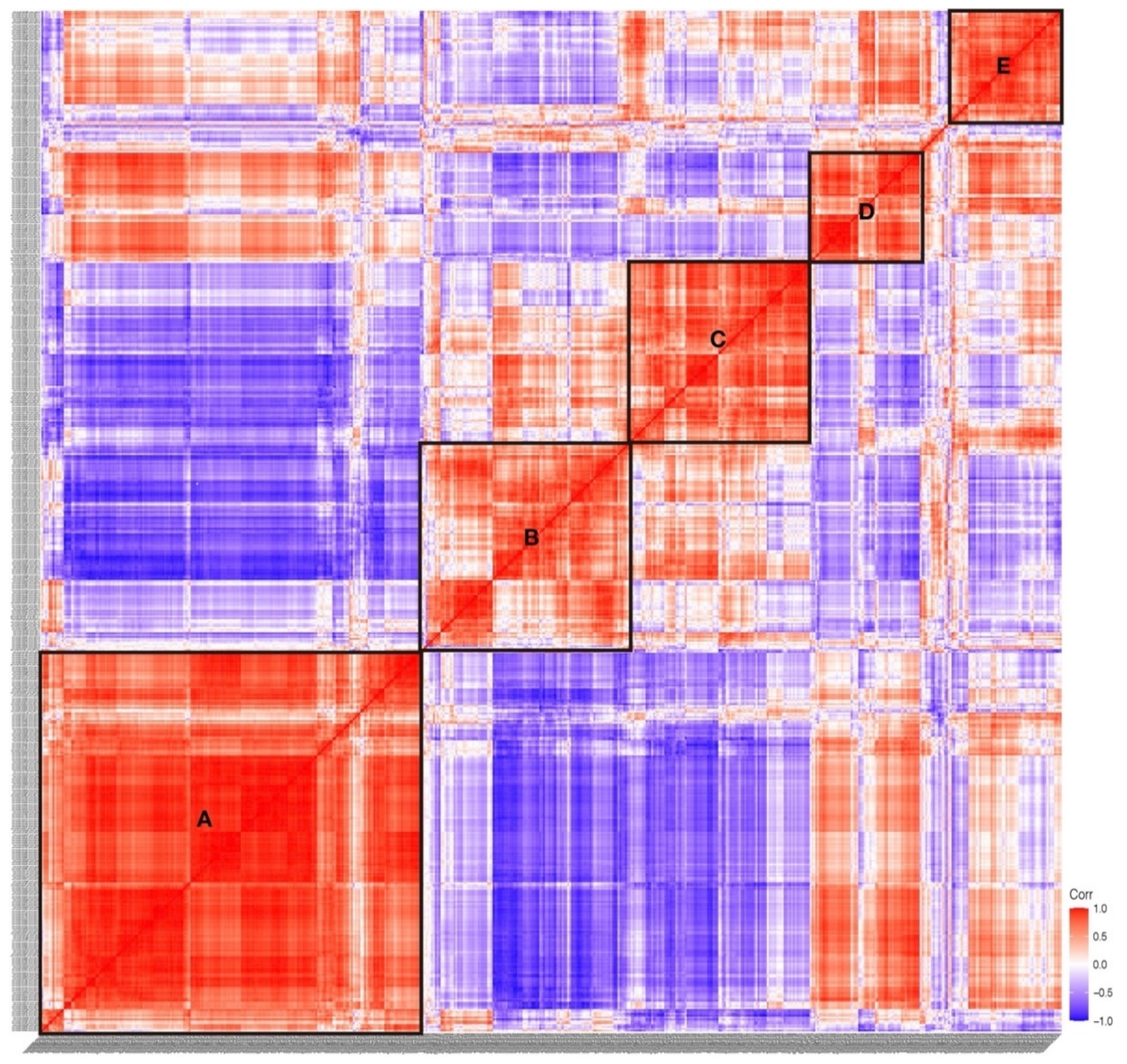
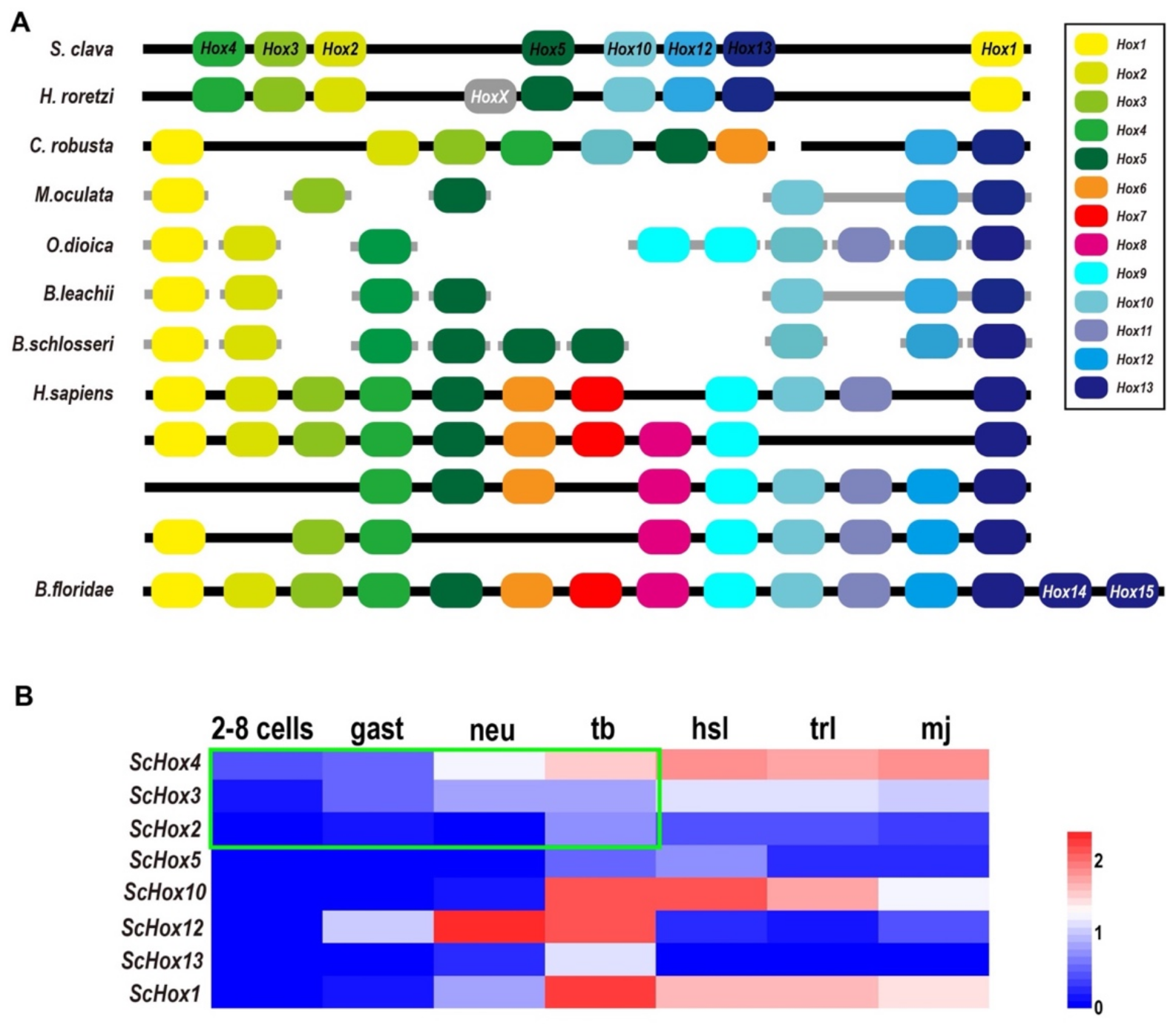



| TF Superfamily | TF Family | S. clava | C. robusta | M. oculata | O. dioica | B. leachii | B. schlosseri | H. sapiens | B. floridae | C. elegans | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AF-4 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | 1 | 0 | ||
| ARID | 4 | 5 | 7 | 4 | 4 | 2 | 15 | 4 | 5 | ||
| BTD | 2 | 2 | 2 | 2 | 2 | 3 | 6 | 2 | 1 | ||
| bZIP | bZIP_1 | 13 | 16 | 12 | 21 | 15 | 7 | 37 | 14 | 11 | |
| bZIP_2 | 6 | 7 | 7 | 8 | 6 | 2 | 12 | 8 | 17 | ||
| CBF_beta | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| NF-Y | CBFB_NFYA | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | |
| CBFD_NFYB_HMF | 8 | 9 | 8 | 13 | 8 | 4 | 45 | 16 | 9 | ||
| CG-1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | ||
| CP2 | 2 | 3 | 2 | 2 | 2 | 3 | 8 | 2 | 1 | ||
| CSD | 9 | 5 | 4 | 7 | 4 | 4 | 17 | 2 | 5 | ||
| CSRNP_N | 1 | 1 | 0 | 2 | 1 | 2 | 3 | 1 | 1 | ||
| MH1 | CTF_NFI | 2 | 1 | 0 | 0 | 1 | 0 | 4 | 1 | 0 | |
| MH1 | 4 | 5 | 7 | 11 | 4 | 5 | 11 | 4 | 7 | ||
| E2F_TDP | 2 | 4 | 4 | 3 | 3 | 2 | 20 | 4 | 5 | ||
| ETS | Ets | 12 | 15 | 12 | 11 | 14 | 12 | 29 | 12 | 10 | |
| ETS_PEA3_N | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | ||
| Forkhead box | 23 | 25 | 24 | 26 | 27 | 27 | 55 | 28 | 16 | ||
| GCFC | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 1 | 2 | ||
| GCM | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | ||
| GTF2I | 0 | 1 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | ||
| bHLH | bHLH | 41 | 39 | 36 | 22 | 37 | 20 | 102 | 73 | 38 | |
| Myc_N | 0 | 1 | 1 | 0 | 0 | 1 | 4 | 1 | 0 | ||
| SIM_C | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | ||
| HMG_box | 20 | 20 | 14 | 19 | 18 | 21 | 102 | 29 | 16 | ||
| Homeobox | Homeodomain | 73 | 76 | 74 | 70 | 74 | 45 | 264 | 108 | 85 | |
| CUT | 2 | 1 | 3 | 3 | 3 | 2 | 8 | 3 | 7 | ||
| PBC | 1 | 1 | 1 | 1 | 1 | 5 | 11 | 1 | 2 | ||
| Pou | 2 | 3 | 3 | 5 | 3 | 2 | 17 | 6 | 3 | ||
| HPD | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | ||
| HSF_DNA-binding | 1 | 1 | 0 | 4 | 1 | 2 | 9 | 5 | 1 | ||
| HTH_psq | 6 | 2 | 3 | 0 | 9 | 0 | 2 | 5 | 1 | ||
| IRF | IRF | 5 | 5 | 6 | 2 | 8 | 5 | 3 | 4 | 0 | |
| IRF-3 | 1 | 3 | 2 | 0 | 4 | 9 | 9 | 4 | 0 | ||
| LAG1-DNAbinding | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| LRRFIP | 0 | 1 | 3 | 1 | 1 | 1 | 5 | 1 | 1 | ||
| MBD | 3 | 2 | 4 | 0 | 3 | 2 | 7 | 3 | 2 | ||
| Myb_DNA-binding | 12 | 13 | 11 | 7 | 13 | 11 | 25 | 16 | 7 | ||
| NCU-G1 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | ||
| NDT80_PhoG | 1 | 1 | 3 | 0 | 1 | 2 | 2 | 1 | 2 | ||
| Nrf1_DNA-binding | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 0 | ||
| P53 | 3 | 2 | 1 | 1 | 3 | 5 | 3 | 2 | 1 | ||
| PAX | 7 | 6 | 5 | 8 | 5 | 2 | 9 | 5 | 9 | ||
| PC4 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 2 | 1 | ||
| RFX_DNA_binding | 2 | 3 | 3 | 1 | 3 | 2 | 10 | 5 | 1 | ||
| RHD_DNA_binding | 3 | 2 | 3 | 2 | 4 | 5 | 13 | 2 | 0 | ||
| Runt | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | ||
| SAND | 3 | 2 | 1 | 2 | 1 | 2 | 7 | 4 | 4 | ||
| SRF-TF | 3 | 2 | 2 | 3 | 2 | 1 | 5 | 3 | 2 | ||
| STAT | STAT_alpha | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | |
| STAT_binding | 2 | 2 | 2 | 2 | 2 | 2 | 5 | 1 | 2 | ||
| STAT_int | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | ||
| TAFH | 2 | 1 | 1 | 1 | 1 | 1 | 6 | 3 | 1 | ||
| T-box | 10 | 8 | 8 | 8 | 8 | 13 | 19 | 10 | 19 | ||
| TEA | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 1 | 1 | ||
| TF_AP-2 | 1 | 2 | 1 | 1 | 1 | 2 | 5 | 1 | 4 | ||
| TF_Otx | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| TIG | 11 | 7 | 6 | 12 | 16 | 29 | 18 | 15 | 4 | ||
| TSC22 | 2 | 1 | 1 | 0 | 2 | 1 | 4 | 1 | 5 | ||
| Tub | 1 | 1 | 1 | 2 | 1 | 1 | 5 | 2 | 2 | ||
| Vert_HS_TF | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | ||
| zinc finger | THAP | 21 | 8 | 27 | 0 | 40 | 49 | 9 | 7 | 3 | |
| GATA | 5 | 4 | 4 | 5 | 5 | 2 | 17 | 7 | 12 | ||
| DM | 5 | 2 | 0 | 2 | 7 | 3 | 7 | 8 | 11 | ||
| Nuclear Receptor | Hormone_receptor | 6 | 6 | 6 | 7 | 5 | 9 | 28 | 10 | 151 | |
| Androgen_receptor | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Oest_receptor | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Prog_receptor | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| GCR | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| zf-C4 | 15 | 12 | 14 | 33 | 11 | 21 | 32 | 21 | 124 | ||
| zf-BED | 17 | 2 | 6 | 0 | 8 | 9 | 3 | 0 | 9 | ||
| zf-C2H2 | 154 | 95 | 193 | 72 | 110 | 310 | 748 | 742 | 58 | ||
| zf-C2HC | 5 | 3 | 2 | 2 | 4 | 5 | 8 | 2 | 1 | ||
| zf-LITAF-like | 4 | 1 | 2 | 5 | 3 | 2 | 4 | 11 | 14 | ||
| zf-MIZ | 2 | 2 | 3 | 2 | 1 | 0 | 6 | 2 | 2 | ||
| zf-NF-X1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Total TFs | 553 | 456 | 557 | 425 | 522 | 693 | 1867 | 1240 | 703 | ||
| Total TF families | 60 | 64 | 57 | 51 | 61 | 60 | 74 | 64 | 55 | ||
| TF Family | Turquoise | Blue | Magenta | Pink | Brown | Yellow | Red | Green | Black | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| AF-4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| ARID | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
| BTD | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| bZIP_1 | 3 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 5 | 13 |
| bZIP_2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| CBF_beta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| CBFB_NFYA | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| CBFD_NFYB_HMF | 2 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 8 |
| CP2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| CSD | 2 | 1 | 1 | 0 | 1 | 1 | 3 | 0 | 0 | 9 |
| CSRNP_N | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| CTF_NFI | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| CUT | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| DM | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| E2F_TDP | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Ets | 3 | 0 | 0 | 3 | 2 | 3 | 0 | 1 | 0 | 12 |
| Forkhead box | 4 | 1 | 0 | 3 | 5 | 4 | 3 | 1 | 2 | 23 |
| GATA | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 |
| GCFC | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| HLH | 10 | 7 | 0 | 3 | 13 | 2 | 3 | 0 | 3 | 41 |
| HMG_box | 9 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 20 |
| Homeodomain | 5 | 4 | 2 | 6 | 37 | 8 | 3 | 4 | 3 | 72 |
| Hormone_recepter | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 6 |
| HPD | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
| HSF_DNA-binding | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HTH_psq | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 6 |
| IRF | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 |
| IRF-3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| MBD | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 |
| MH1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Myb_DNA-binding | 6 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 11 |
| NCU-G1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| NDT80_PhoG | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Nrf1_DNA-binding | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| P53 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| PAX | 2 | 0 | 0 | 1 | 3 | 0 | 1 | 0 | 0 | 7 |
| PBC | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PC4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pou | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| RFX_DNA_binding | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| RHD_DNA_binding | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 3 |
| Runt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| SAND | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| SRF-TF | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| STAT_alpha | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| STAT_binding | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| TAFH | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| T-box | 1 | 1 | 3 | 1 | 3 | 1 | 0 | 0 | 0 | 10 |
| TEA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| TF_AP-2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| THAP | 6 | 1 | 0 | 4 | 6 | 0 | 0 | 2 | 2 | 21 |
| TIG | 6 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 11 |
| TSC22 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Tub | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| zf-BED | 3 | 2 | 0 | 7 | 2 | 2 | 0 | 1 | 0 | 17 |
| zf-C2H2 | 67 | 24 | 20 | 12 | 7 | 5 | 7 | 3 | 5 | 150 |
| zf-C2HC | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| zf-C4 | 0 | 1 | 1 | 3 | 2 | 3 | 2 | 1 | 2 | 15 |
| zf-LITAF-like | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| zf-MIZ | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| zf-NF-X1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 174 | 61 | 36 | 52 | 100 | 44 | 23 | 20 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wei, J.; Yu, H.; Dong, B. Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava. Int. J. Mol. Sci. 2021, 22, 4317. https://doi.org/10.3390/ijms22094317
Zhang J, Wei J, Yu H, Dong B. Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava. International Journal of Molecular Sciences. 2021; 22(9):4317. https://doi.org/10.3390/ijms22094317
Chicago/Turabian StyleZhang, Jin, Jiankai Wei, Haiyan Yu, and Bo Dong. 2021. "Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava" International Journal of Molecular Sciences 22, no. 9: 4317. https://doi.org/10.3390/ijms22094317
APA StyleZhang, J., Wei, J., Yu, H., & Dong, B. (2021). Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava. International Journal of Molecular Sciences, 22(9), 4317. https://doi.org/10.3390/ijms22094317







