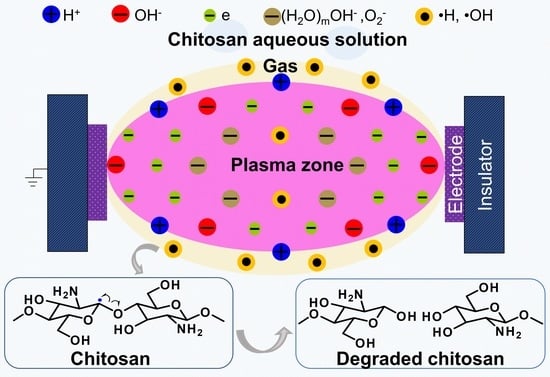
Insight on Solution Plasma in Aqueous Solution and Their Application in Modification of Chitin and Chitosan
Abstract
1. Introduction
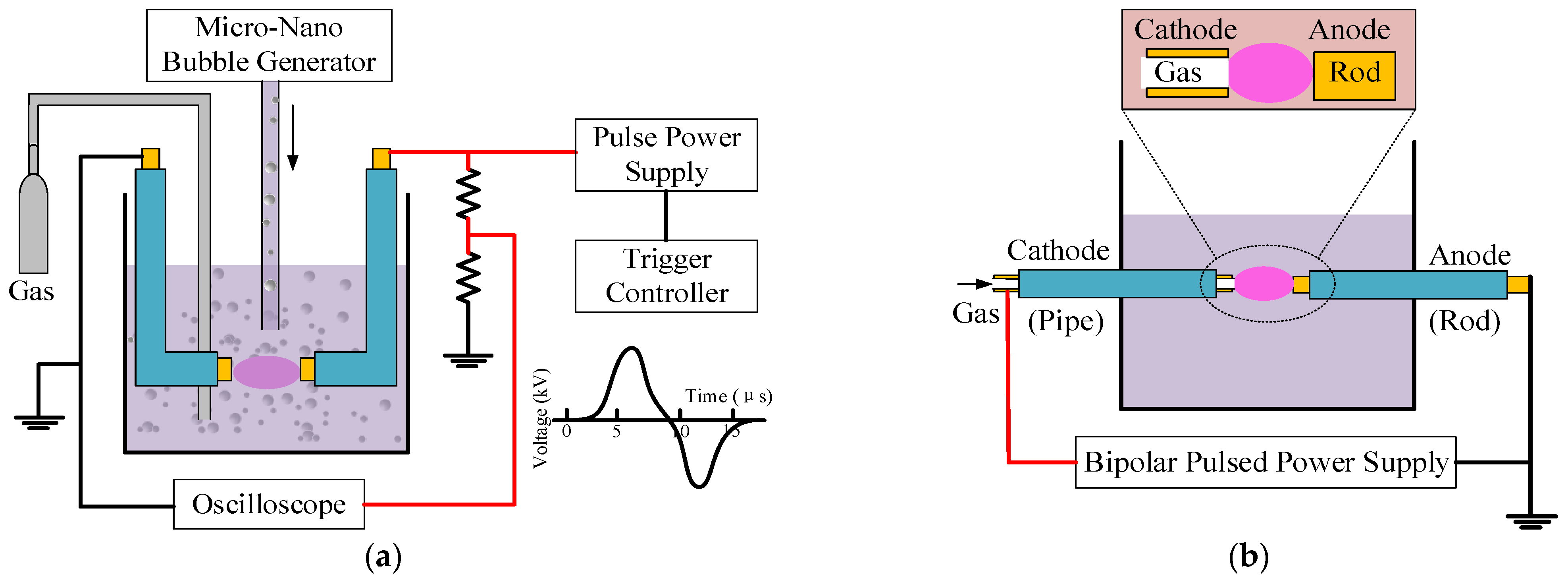
2. Solution Plasma Process (SPP): Chemistry and Influencing Parameters
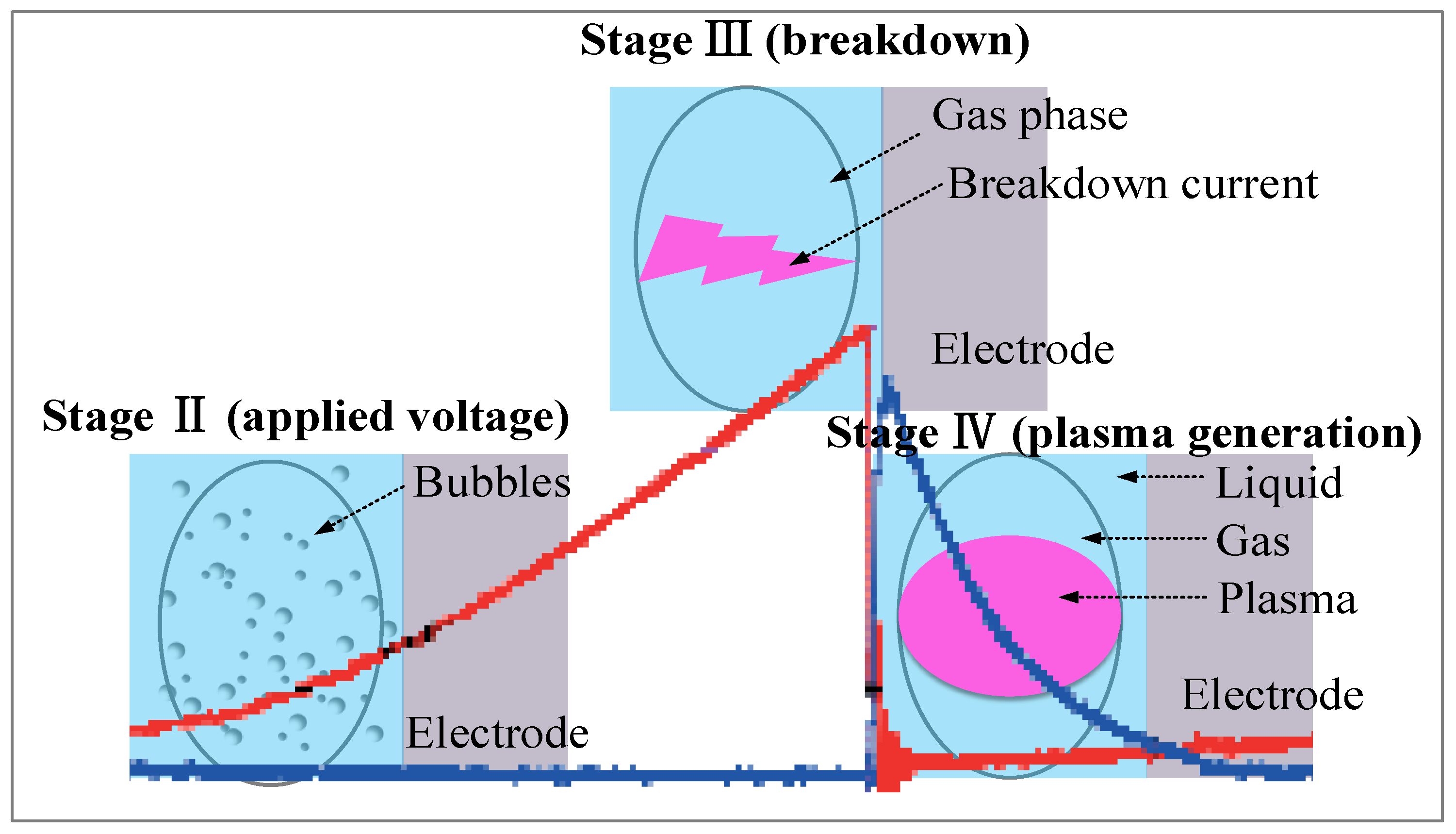
2.1. Electrical Breakdown
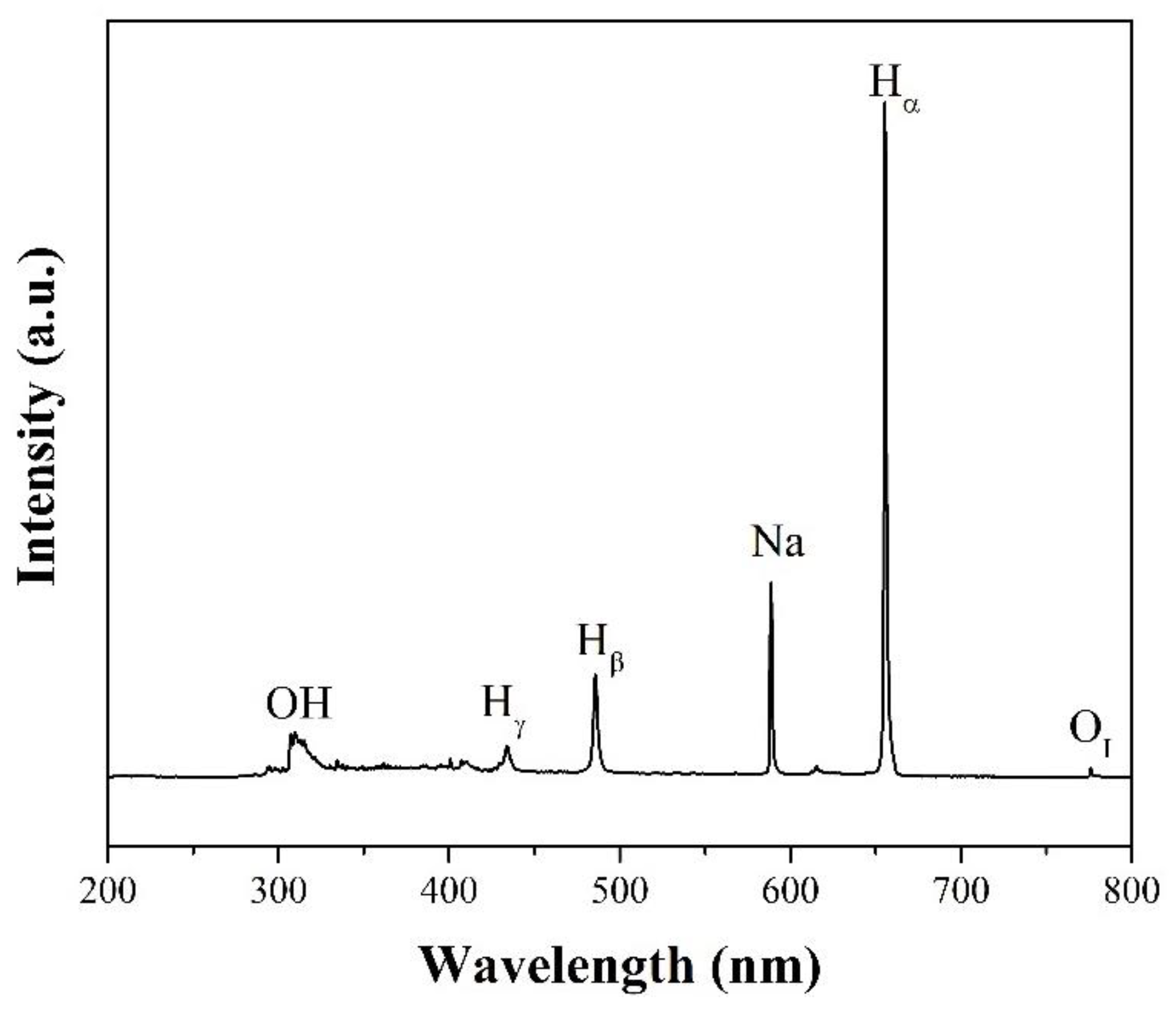
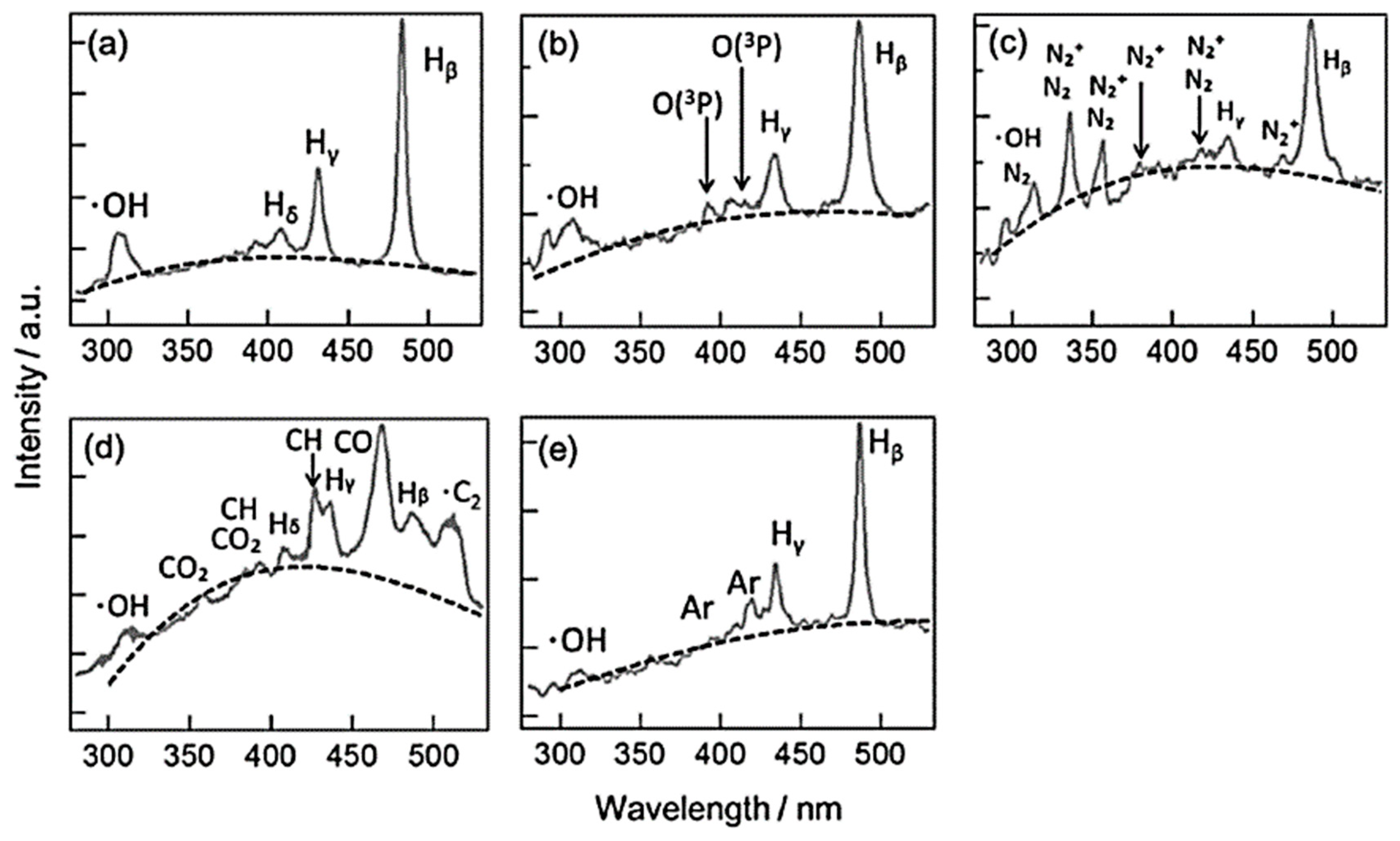
2.2. Formation of Reactive Species
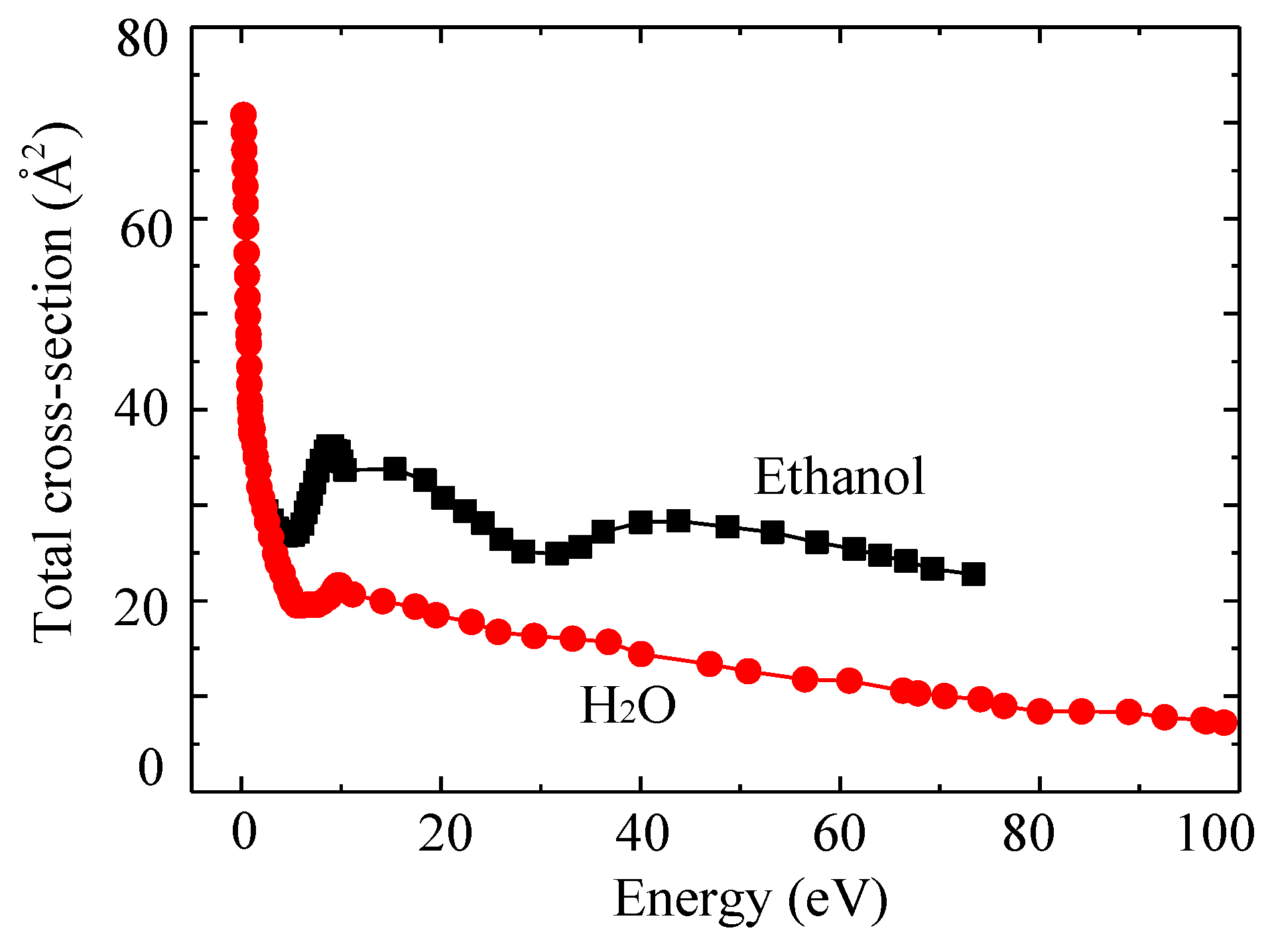
2.3. Influencing Parameters in SPP under Aqueous Solutions
3. Solution Plasma Process for Modification of Chitin and Chitosan
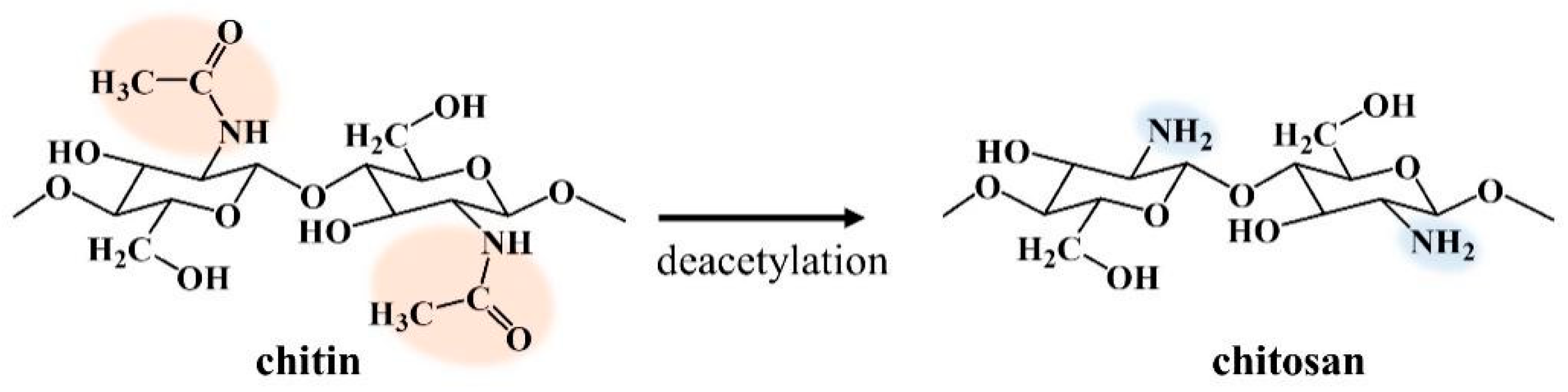
3.1. Chitin and Chitosan
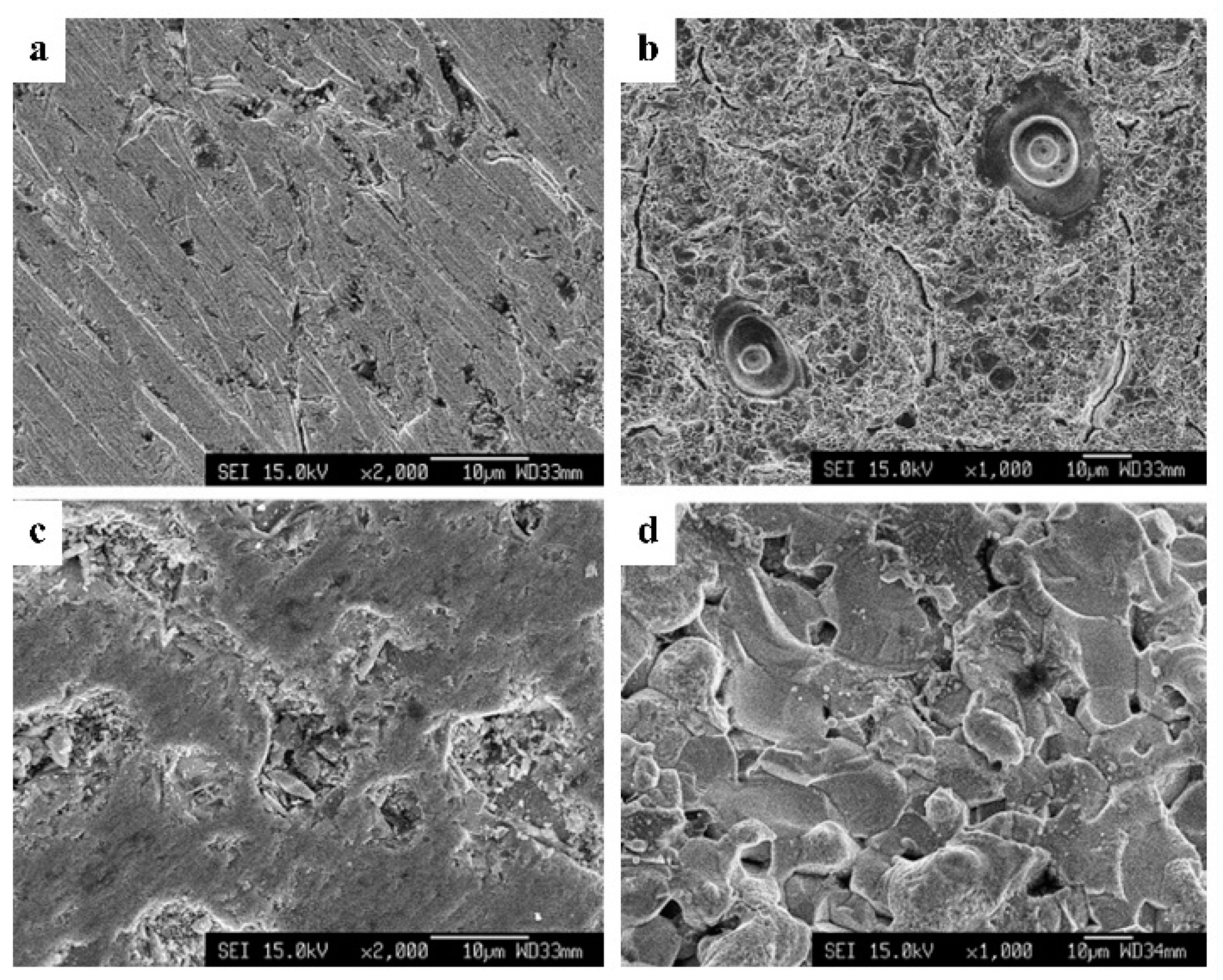
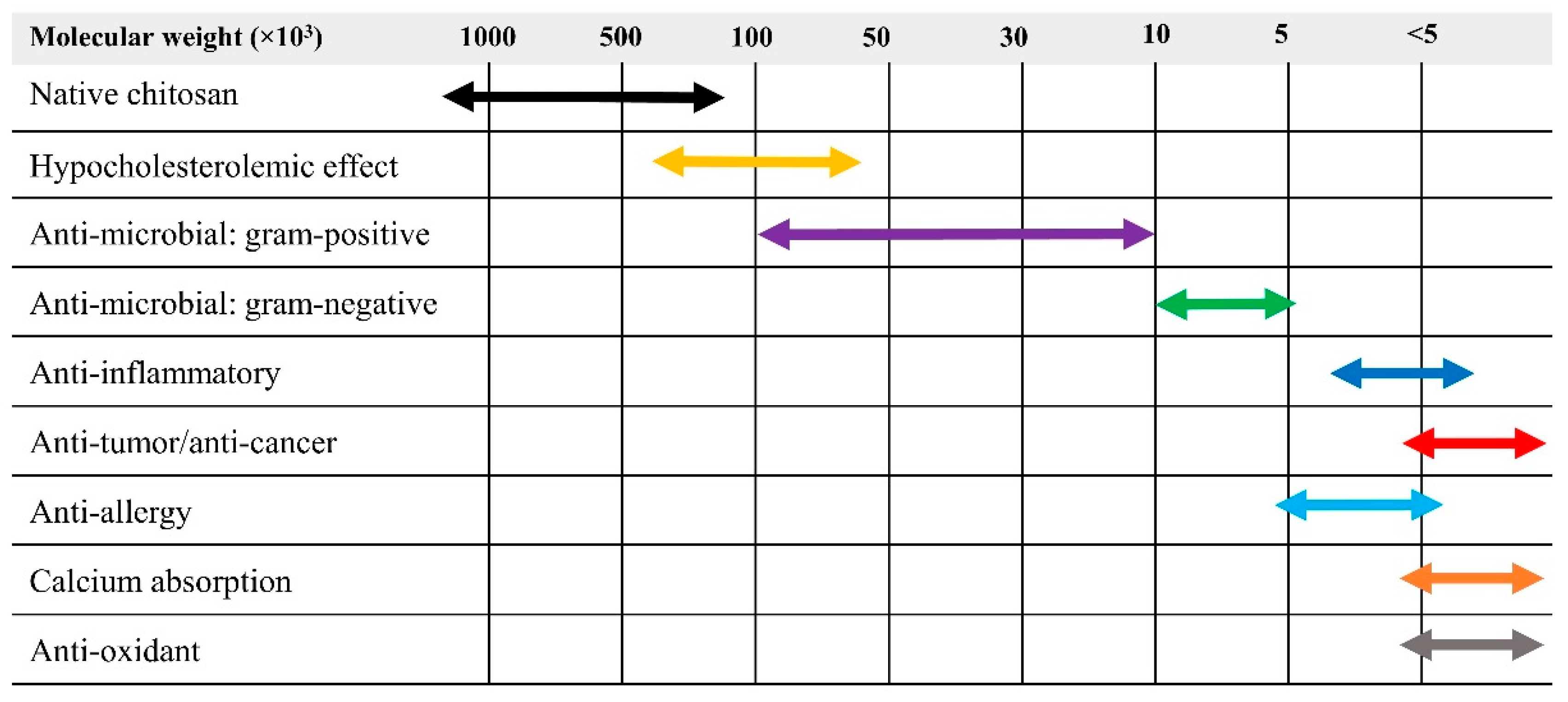
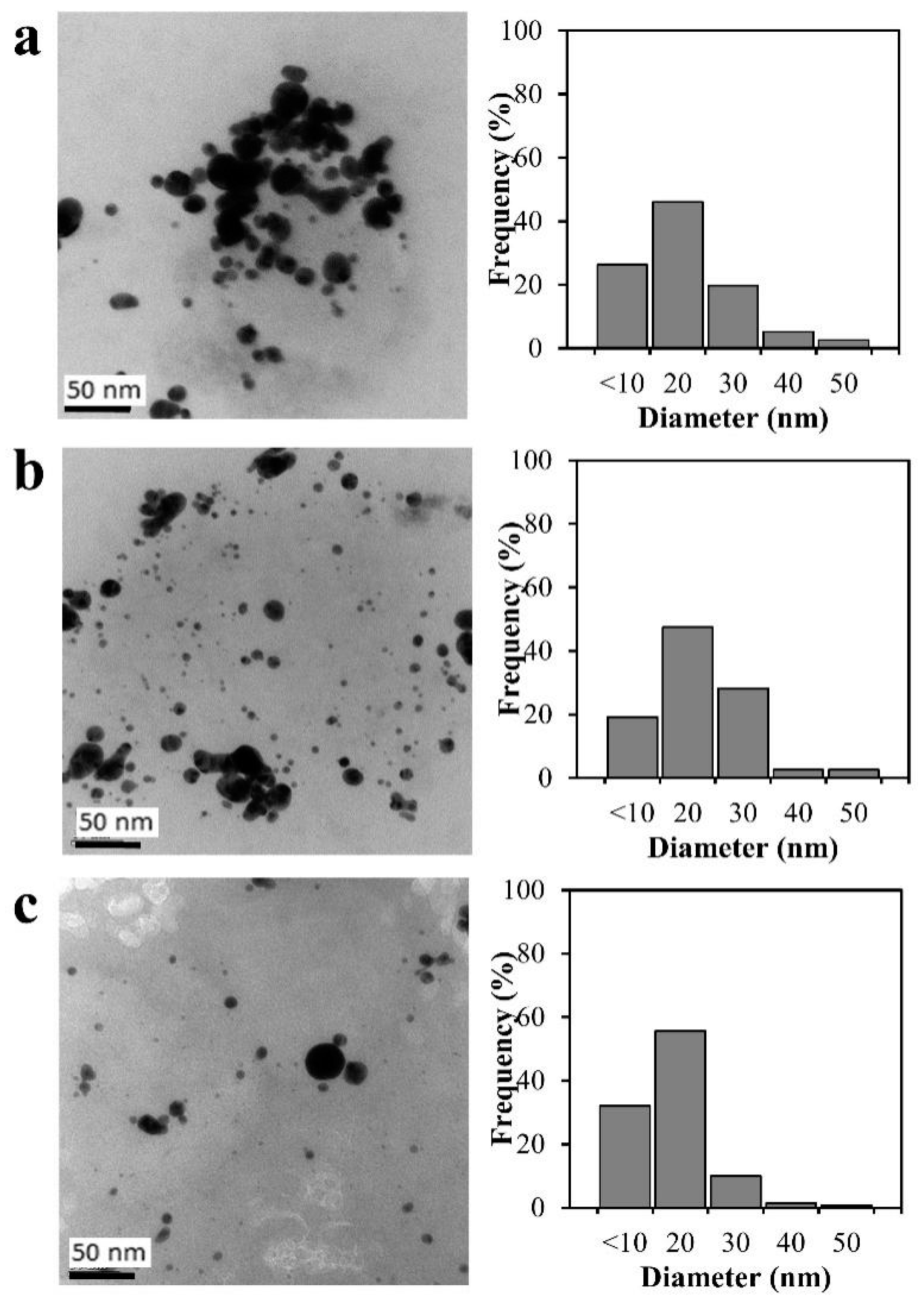
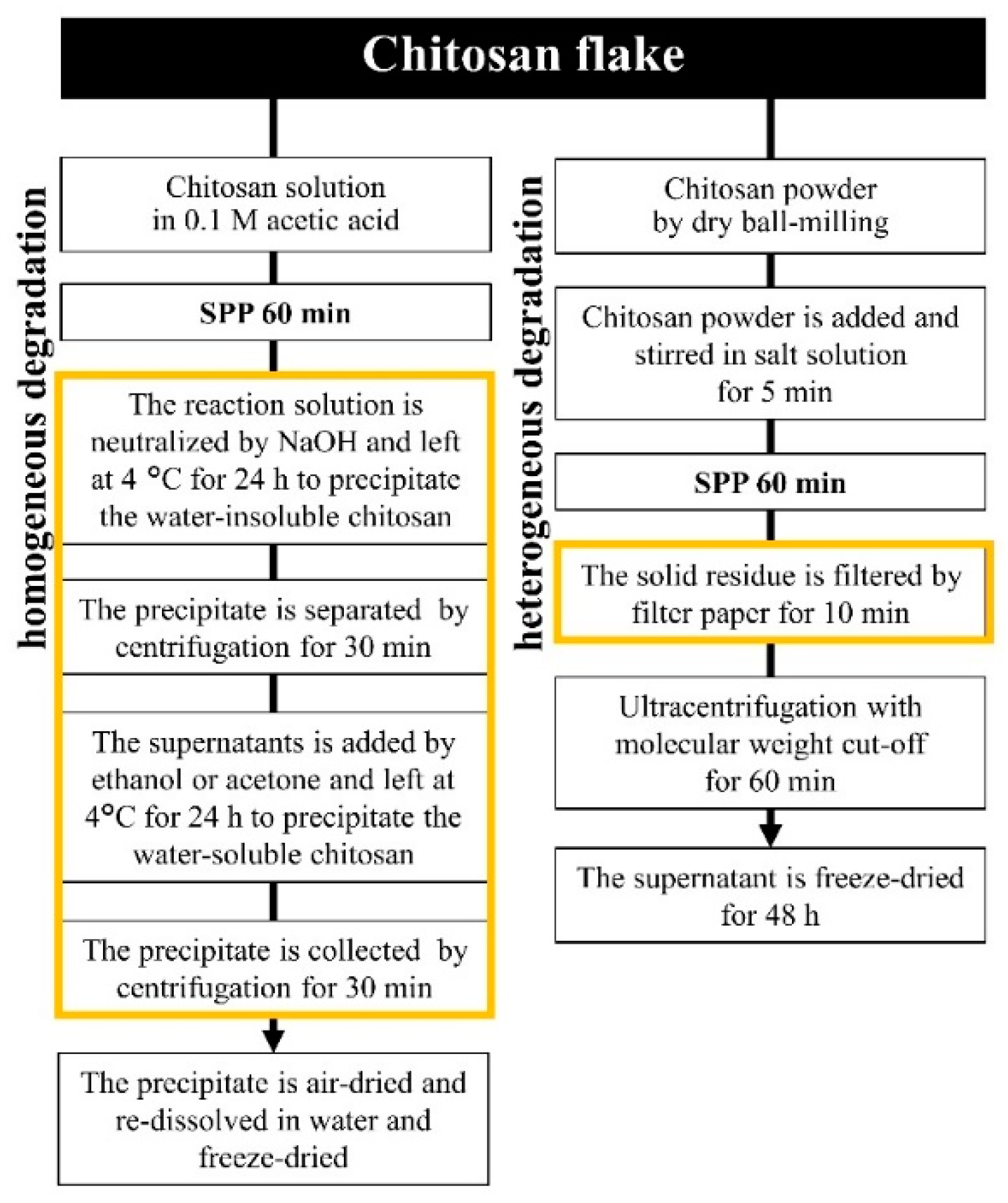
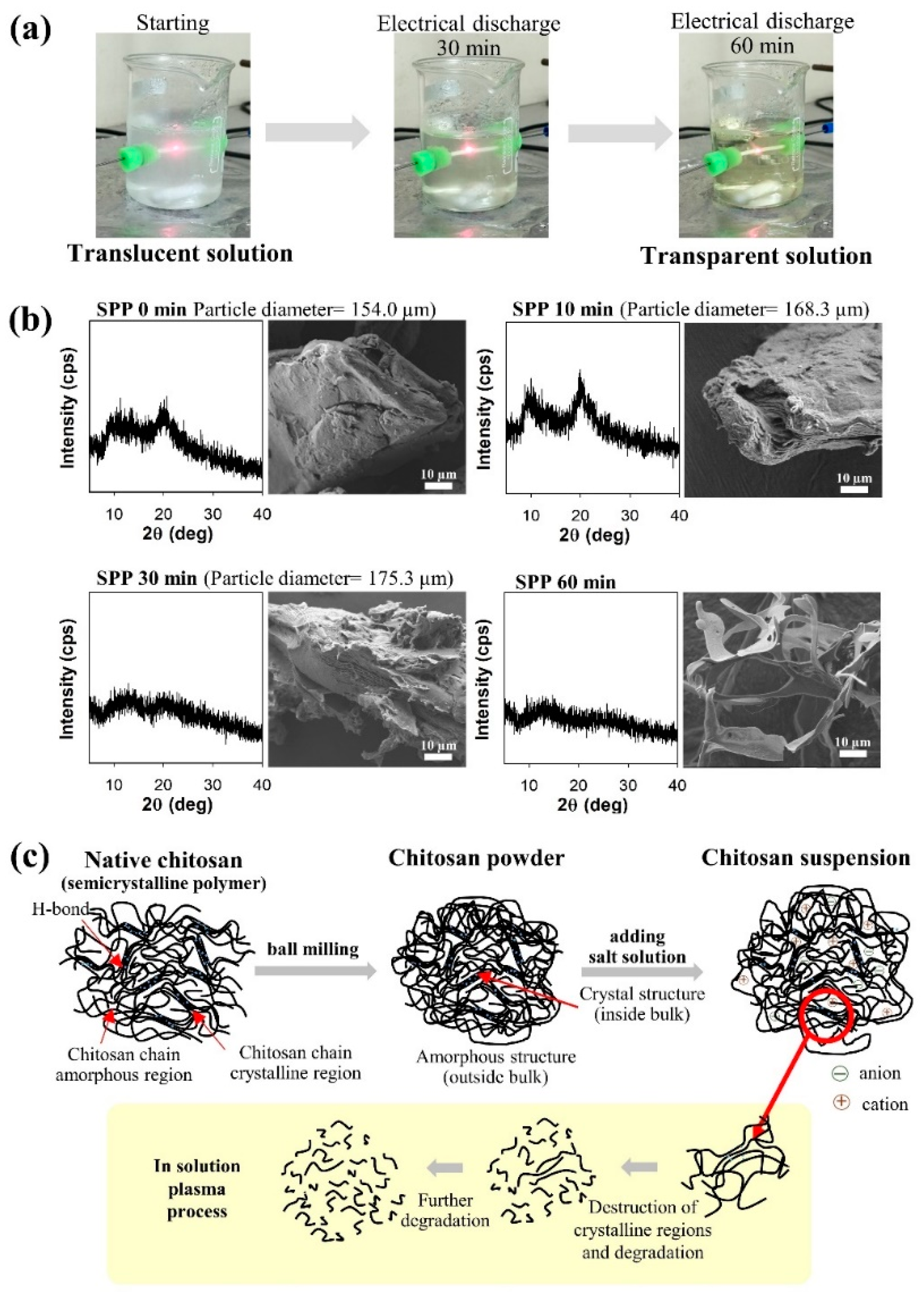
3.2. Reduction of Molecular Weight and Destruction of Crystallinity Via SPP
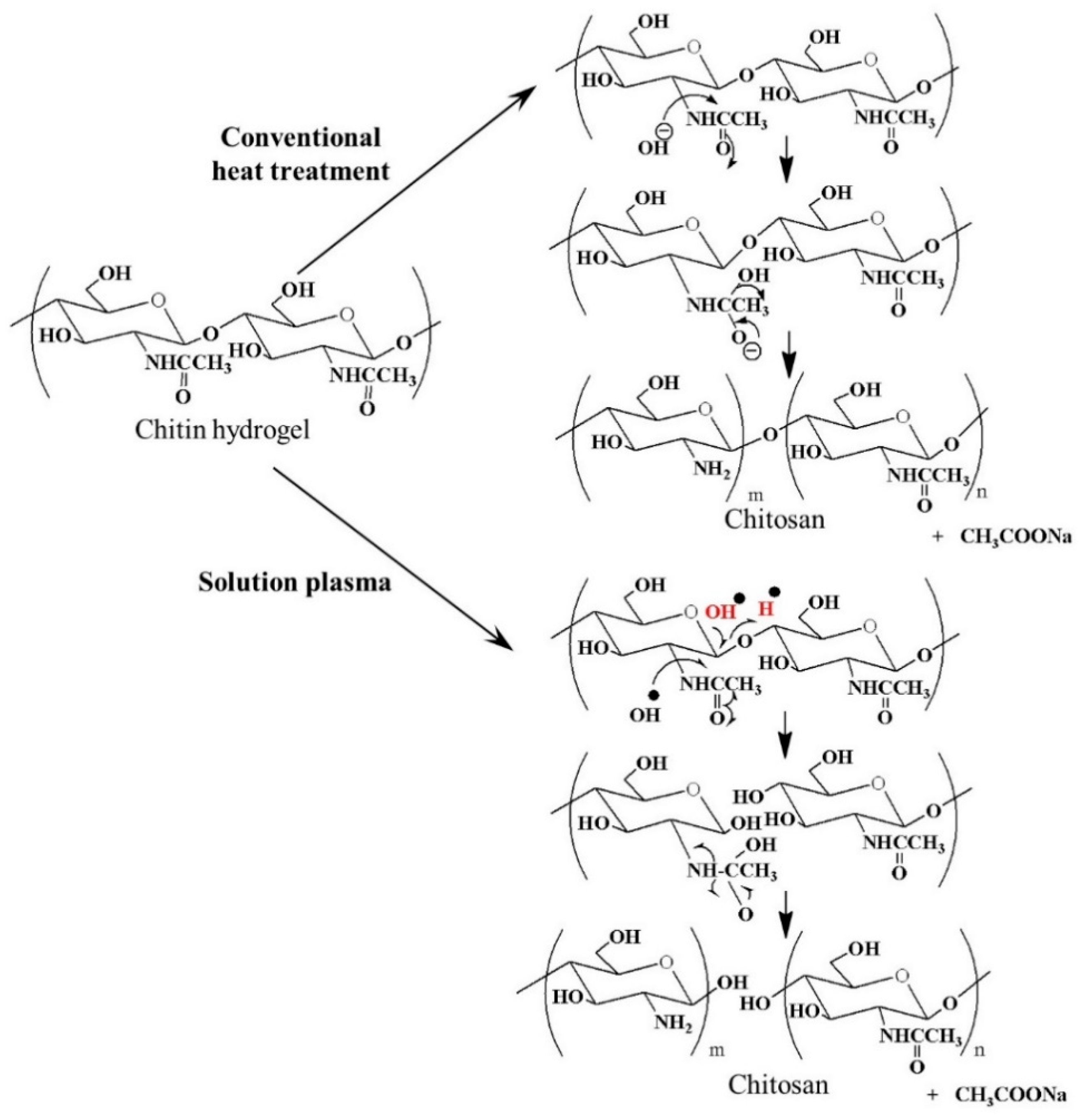
3.3. Deacetylation of Chitin Via SPP
4. Conclusions and Future Aspects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiede, R.; Hirschberg, J.; Daeschlein, G.; Von Woedtke, T.; Vioel, W.; Emmert, S. Plasma Applications: A Dermatological View. Contrib. Plasma Phys. 2014, 54, 118–130. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of Plasma-Liquid Systems: A Review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. In-liquid plasma: A novel tool in the fabrication of nanomaterials and in the treatment of wastewaters. RSC Adv. 2017, 7, 47196–47218. [Google Scholar] [CrossRef]
- Yui, H.; Banno, M. Microspectroscopic imaging of solution plasma: How do its physical properties and chemical species evolve in atmospheric-pressure water vapor bubbles? Jpn. J. Appl. Phys. 2017, 57, 0102A1. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B Atomic Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R. Cold plasma surface modification of biodegradable polymer biomaterials. In Biomaterials for Bone Regeneration; Dubruel, P., Van Vlierberghe, S., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 202–224. [Google Scholar]
- Taghvaei, H.; Kheirollahivash, M.; Ghasemi, M.; Rostami, P.; Rahimpour, M.R. Noncatalytic Upgrading of Anisole in an Atmospheric DBD Plasma Reactor: Effect of Carrier Gas Type, Voltage, and Frequency. Energy Fuels 2014, 28, 2535–2543. [Google Scholar] [CrossRef]
- Šunka, P. Pulse electrical discharges in water and their applications. Phys. Plasmas 2001, 8, 2587–2594. [Google Scholar] [CrossRef]
- Thagard, S.M.; Takashima, K.; Mizuno, A. Plasma Chemistry in Pulsed Electrical Discharge in Liquid. Trans. Mater. Res. Soc. Jpn. 2009, 34, 257–262. [Google Scholar] [CrossRef][Green Version]
- Sato, M.; Ohgiyama, T.; Clements, J.S. Formation of chemical species and their effects on microorganisms using a pulsed high-voltage discharge in water. IEEE Trans. Ind. Appl. 1996, 32, 106–112. [Google Scholar] [CrossRef]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water purification by electrical discharges. Plasma Sources Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Takai, O. Solution plasma processing (SPP). Pure Appl. Chem. 2008, 80, 2003–2011. [Google Scholar] [CrossRef]
- Hieda, J.; Saito, N.; Takai, O. Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J. Vac. Sci. Technol. A 2008, 26, 854. [Google Scholar] [CrossRef]
- Janpetch, N.; Saito, N.; Rujiravanit, R. Fabrication of bacterial cellulose-ZnO composite via solution plasma process for an-tibacterial applications. Carbohydr. Polym. 2016, 148, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Saqib, A.N.S.; Huong, N.T.T.; Kim, S.-W.; Jung, M.-H.; Lee, Y.H. Structural and magnetic properties of highly Fe-doped ZnO nanoparticles synthesized by one-step solution plasma process. J. Alloy. Compd. 2021, 853, 157153. [Google Scholar] [CrossRef]
- Kim, K.; Hashimi, K.; Bratescu, M.A.; Saito, N. The Initial Reactions from Pyridine to Hetero-Carbon Nanomaterials Through Solution Plasma. Nanosci. Nanotechnol. Lett. 2018, 10, 814–819. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Panomsuwan, G.; Ishizaki, T. Synthesis of graphitic-N and amino-N in nitrogen-doped carbon via a solution plasma process and exploration of their synergic effect for advanced oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 2073–2082. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Synthesis of structure-controlled carbon nano spheres by solution plasma process. Carbon 2013, 60, 292–298. [Google Scholar] [CrossRef]
- Prasertsung, I.; Chutinate, P.; Watthanaphanit, A.; Saito, N.; Damrongsakkul, S. Conversion of cellulose into reducing sugar by solution plasma process (SPP). Carbohydr. Polym. 2017, 172, 230–236. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Kim, S.-C.; Lee, S.-Y.; Kim, J.-W. The facile synthesis of chitosan-based silver nano-biocomposites via a solution plasma process and their potential antimicrobial efficacy. Arch. Biochem. Biophys. 2016, 605, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sulaimankulova, S.; Mametova, A.; Abdullaeva, Z. Fusiform gold nanoparticles by pulsed plasma in liquid method. Sn Appl. Sci. 2019, 1, 1427. [Google Scholar] [CrossRef]
- Yoshimura, M.; Senthilnathan, J. Submerged Liquid Plasma for the Formation of Nanostructured Carbon. In Carbon-related Materials in Recognition of Nobel Lectures by Prof. Akira Suzuki in ICCE; Kaneko, S., Mele, P., Endo, T., Tsuchiya, T., Tanaka, K., Yoshimura, M., Hui, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–78. [Google Scholar]
- Senthilnathan, J.; Rao, K.S.; Yoshimura, M. Submerged liquid plasma—Low energy synthesis of nitrogen-doped graphene for electrochemical applications. J. Mater. Chem. A 2014, 2, 3332–3337. [Google Scholar] [CrossRef]
- Yu, F.; Wang, C.; Li, Y.; Ma, H.; Wang, R.; Liu, Y.; Suzuki, N.; Terashima, C.; Ohtani, B.; Ochiai, T.; et al. Enhanced Solar Photothermal Catalysis over Solution Plasma Activated TiO2. Adv. Sci. 2020, 7, 2000204. [Google Scholar] [CrossRef] [PubMed]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Electrocatalytic oxygen reduction on nitrogen-doped carbon nanoparticles derived from cyano-aromatic molecules via a solution plasma approach. Carbon 2016, 98, 411–420. [Google Scholar] [CrossRef]
- Saito, N.; Hieda, J.; Takai, O. Synthesis process of gold nanoparticles in solution plasma. Thin Solid Film. 2009, 518, 912–917. [Google Scholar] [CrossRef]
- Pootawang, P.; Saito, N.; Takai, O. Ag nanoparticle incorporation in mesoporous silica synthesized by solution plasma and their catalysis for oleic acid hydrogenation. Mater. Lett. 2011, 65, 1037–1040. [Google Scholar] [CrossRef]
- Pootawang, P.; Saito, N.; Lee, S.Y. Discharge time dependence of a solution plasma process for colloidal copper nanoparticle synthesis and particle characteristics. Nanotechnology 2013, 24, 055604. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Takai, O.; Saito, N. Facile fabrication of PtAu alloy clusters using solution plasma sputtering and their electrocatalytic activity. J. Alloy. Compd. 2013, 552, 351–355. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Panomsuwan, G.; Saito, N. A novel one-step synthesis of gold nanoparticles in an alginate gel matrix by solution plasma sputtering. RSC Adv. 2014, 4, 1622–1629. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Kato, S.; Fujiwara, K.; Watanabe, H.; Ishii, T.; Ishizaki, T. A comparative study of undoped, boron-doped, and boron/fluorine dual-doped carbon nanoparticles obtained via solution plasma as catalysts for the oxygen reduction re-action. Sustain. Energy Fuels 2020, 4, 4570–4580. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Kaneko, Y.; Panomsuwan, G. Electrocatalytic activity for the oxygen reduction reaction of oxy-gen-containing nanocarbon synthesized by solution plasma. J. Mater. Chem. A 2014, 2, 10589–10598. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest Formation Routes of Nanocarbons in Solution Plasma Processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef] [PubMed]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chantaramethakul, J.; Chokradjaroen, C.; Ishizaki, T. In situ solution plasma synthesis of silver nanopar-ticles supported on nitrogen-doped carbons with enhanced oxygen reduction activity. Mater. Lett. 2019, 251, 135–139. [Google Scholar] [CrossRef]
- Phan, P.Q.; Naraprawatphong, R.; Pornaroontham, P.; Park, J.; Chokradjaroen, C.; Saito, N. N-Doped few-layer graphene encapsulated Pt-based bimetallic nanoparticles via solution plasma as an efficient oxygen catalyst for the oxygen reduction reaction. Mater. Adv. 2021, 2, 322–335. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Theeramunkong, S.; Yui, H.; Saito, N.; Rujiravanit, R. Cytotoxicity against cancer cells of chitosan oli-gosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohydr. Polym. 2018, 201, 20–30. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Saito, N. Degradation of β-chitosan by solution plasma process (SPP). Polym. Degrad. Stab. 2013, 98, 2089–2093. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Katepetch, C.; Vanichvattanadecha, C.; Saito, N.; Rujiravanit, R. Depolymerization of chitosan–metal complexes via a solution plasma technique. Carbohydr. Polym. 2014, 102, 504–512. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Saito, N. Effect of polymer concentration on the depolymerization of sodium alginate by the solution plasma process. Polym. Degrad. Stab. 2013, 98, 1072–1080. [Google Scholar] [CrossRef]
- Zalikhanov, B.Z. From an electron avalanche to the lightning discharge. Phys. Part Nucl. 2016, 47, 108–133. [Google Scholar] [CrossRef]
- Saito, N.; Bratescu, M.A.; Hashimi, K. Solution plasma: A new reaction field for nanomaterials synthesis. Jpn. J. Appl. Phys. 2017, 57, 0102A4. [Google Scholar] [CrossRef]
- Heo, Y.K.; Lee, S.H.; Bratescu, M.A.; Kim, S.M.; Lee, G.-J.; Saito, N. Generation of non-equilibrium condition in solution plasma discharge using low-pass filter circuit. Plasma Process. Polym. 2017, 14, 1600163. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Terashima, C.; Saito, N.; Takai, O. Preparation of low molecular weight chitosan using solution plasma system. Carbohydr. Polym. 2012, 87, 2745–2749. [Google Scholar] [CrossRef]
- Tantiplapol, T.; Singsawat, Y.; Narongsil, N.; Damrongsakkul, S.; Saito, N.; Prasertsung, I. Influences of solution plasma conditions on degradation rate and properties of chitosan. Innov. Food Sci. Emerg. Technol. 2015, 32, 116–120. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Lee, S.-Y.; Kim, J.-W. Solution plasma mediated formation of low molecular weight chitosan and its ap-plication as a biomaterial. Int. J. Biol. Macromol. 2018, 118, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Rujiravanit, R.; Theeramunkong, S.; Saito, N. Effect of electrical discharge plasma on cytotoxicity against cancer cells of N,O-carboxymethyl chitosan-stabilized gold nanoparticles. Carbohydr. Polym. 2020, 237, 116162. [Google Scholar] [CrossRef]
- Potocký, Š.; Saito, N.; Takai, O. Needle electrode erosion in water plasma discharge. Thin Solid Film. 2009, 518, 918–923. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takada, N.; Wahyudiono; Kanda, H.; Goto, M. Hydrogen Peroxide Formation by Electric Discharge with Fine Bubbles. Plasma Chem. Plasma Process. 2017, 37, 125–135. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms Microbiomes 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Tanioka, S.; Matsui, Y.; Irie, T.; Tanigawa, T.; Tanaka, Y.; Shibata, H.; Sawa, Y.; Kono, Y. Oxidative Depolymerization of Chitosan by Hydroxyl Radical. Biosci. Biotechnol. Biochem. 1996, 60, 2001–2004. [Google Scholar] [CrossRef]
- Miron, C.; Bratescu, M.A.; Saito, N.; Takai, O. Optical diagnostic of bipolar electrical discharges in HCl, KCl, and KOH solutions. J. Appl. Phys. 2011, 109, 123301. [Google Scholar] [CrossRef]
- Langendorf, S.; Walker, M. Effect of secondary electron emission on the plasma sheath. Phys. Plasmas 2015, 22, 033515. [Google Scholar] [CrossRef]
- Magnusson, J.M.; Collins, A.L.; Wirz, R.E.; Magnusson, J. Polyatomic Ion-Induced Electron Emission (IIEE) in Electrospray Thrusters. Aerospace 2020, 7, 153. [Google Scholar] [CrossRef]
- Phan, P.Q.; Chae, S.; Pornaroontham, P.; Muta, Y.; Kim, K.; Wang, X.; Saito, N. In situ synthesis of copper nanoparticles encapsulated by nitrogen-doped graphene at room temperature via solution plasma. RSC Adv. 2020, 10, 36627–36635. [Google Scholar] [CrossRef]
- Wang, H.; Wandell, R.J.; Tachibana, K.; Voráč, J.; Locke, B.R. The influence of liquid conductivity on electrical breakdown and hydrogen peroxide production in a nanosecond pulsed plasma discharge generated in a water-film plasma reactor. J. Phys. D Appl. Phys. 2018, 52, 075201. [Google Scholar] [CrossRef]
- Takeuchi, N.; Kawahara, K.; Gamou, F.; Li, O.L.H. Observation of solution plasma in water-ethanol mixed solution for reduction of graphene oxide. Int. J. Plasma Environ. Sci. Technol. 2020, 14, e01009. [Google Scholar]
- Schmieder, F. Neue Wirkungsquerschnittsmessungen an Gasen und Dämpfen. Z Elektrochem Angew P 1930, 36, 700–704. [Google Scholar]
- Szmytkowski, C.; Możejko, P. Electron-scattering total cross sections for triatomic molecules: NO2 and H2O. Opt. Appl. 2006, 36, 543–550. [Google Scholar]
- Panomsuwan, G.; Chokradjaroen, C.; Rujiravanit, R.; Ueno, T.; Saito, N. In vitro cytotoxicity of carbon black nanoparticles synthesized from solution plasma on human lung fibroblast cells. Jpn. J. Appl. Phys. 2018, 57, 0102BG. [Google Scholar] [CrossRef]
- Miron, C.; Bratescu, M.A.; Saito, N.; Takai, O.; Brǎtescu, M.A. Time-resolved Optical Emission Spectroscopy in Water Electrical Discharges. Plasma Chem. Plasma Process. 2010, 30, 619–631. [Google Scholar] [CrossRef]
- Miron, C.; Bratescu, M.; Saito, N.; Takai, O. Effect of the electrode work function on the water plasma breakdown voltage. Curr. Appl. Phys. 2011, 11, S154–S158. [Google Scholar] [CrossRef]
- Banno, M.; Kanno, K.; Yui, H. Development of direct gas injection system for atmospheric-pressure in-solution discharge plasma for plasma degradation and material syntheses. RSC Adv. 2016, 6, 16030–16036. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Tharanathan, R. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym. 2004, 58, 275–283. [Google Scholar]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Cohen, E. Chitin/Chitosan: Versatile Ecological, Industrial, and Biomedical Applications. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 541–624. [Google Scholar]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Rujiravanit, R.; Watthanaphanit, A.; Theeramunkong, S.; Saito, N.; Yamashita, K.; Arakawa, R. Enhanced degradation of chitosan by applying plasma treatment in combination with oxidizing agents for potential use as an anticancer agent. Carbohydr. Polym. 2017, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Xia, W. Hypocholesterolaemic effects of different chitosan samples in vitro and in vivo. Food Chem. 2008, 107, 419–425. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Lee, S.-H.; Senevirathne, M.; Ahn, C.-B.; Kim, S.-K.; Je, J.-Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R. Depolymerized products of chitosan as potent inhibitors of tumor-induced angiogenesis. Biochim. Biophys. Acta (Bba) Gen. Subj. 2005, 1722, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Kong, C.-S.; Kim, S.-K. Inhibitory effects of chitooligosaccharides on degranulation and cytokine generation in rat basophilic leukemia RBL-2H3 cells. Carbohydr. Polym. 2011, 84, 649–655. [Google Scholar] [CrossRef]
- Jung, W.-K.; Moon, S.-H.; Kim, S.-K. Effect of chitooligosaccharides on calcium bioavailability and bone strength in ovari-ectomized rats. Life Sci. 2006, 78, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Du, Y.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Einbu, A.; Grasdalen, H.; Vårum, K.M. Kinetics of hydrolysis of chitin/chitosan oligomers in concentrated hydrochloric acid. Carbohydr. Res. 2007, 342, 1055–1062. [Google Scholar] [CrossRef]
- Vårum, K.; Ottøy, M.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Kim, S.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Van Cutsem, P. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Shao, J.; Yang, Y.; Zhong, Q. Studies on preparation of oligoglucosamine by oxidative degradation under microwave irra-diation. Polym. Degrad. Stab. 2003, 82, 395–398. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yeates, S.G. “Green” molecular weight degradation of chitosan using microwave irradiation. Polym. Degrad. Stab. 2013, 98, 863–867. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of chitosan and sodium alginate by gamma radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Choi, W.-S.; Ahn, K.-J.; Lee, D.-W.; Byun, M.-W.; Park, H.-J. Preparation of chitosan oligomers by irradiation. Polym. Degrad. Stab. 2002, 78, 533–538. [Google Scholar] [CrossRef]
- Savitri, E.; Juliastuti, S.R.; Handaratri, A.; Sumarno; Roesyadi, A. Degradation of chitosan by sonication in very-low-concentration acetic acid. Polym. Degrad. Stab. 2014, 110, 344–352. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Qin, Y.; Meng, X.; Li, P. Microwave-assisted degradation of chitosan for a possible use in inhibiting crop pathogenic fungi. Int. J. Biol. Macromol. 2012, 51, 767–773. [Google Scholar] [CrossRef]
- Domard, A.; Cartier, N. Glucosamine oligomers: 1. Preparation and characterization. Int. J. Biol. Macromol. 1989, 11, 297–302. [Google Scholar] [CrossRef]
- Ngo, D.-N.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Production of chitin oligosaccharides with different molecular weights and their antioxidant effect in RAW 264.7 cells. J. Funct. Foods 2009, 1, 188–198. [Google Scholar] [CrossRef]
- Furusaki, E.; Ueno, Y.; Sakairi, N.; Nishi, N.; Tokura, S. Facile preparation and inclusion ability of a chitosan derivative bearing carboxymethyl-β-cyclodextrin. Carbohydr. Polym. 1996, 29, 29–34. [Google Scholar] [CrossRef]
- Defaye, J.; Gadelle, A.; Pedersen, C. A convenient access to β-(1 → 4)-linked 2-amino-2-deoxy-d-glucopyranosyl fluoride oligosaccharides and β-(1 → 4)-linked 2-amino-2-deoxy-d-glucopyranosyl oligosaccharides by fluorolysis and fluorohy-drolysis of chitosan. Carbohydr. Res. 1994, 261, 267–277. [Google Scholar] [CrossRef]
- Tian, F.; Liu, Y.; Hu, K.; Zhao, B. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr. Polym. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Fukamizo, T.; Honda, Y.; Goto, S.; Boucher, I.; Brzezinski, R. Reaction mechanism of chitosanase from Streptomyces sp. N174. Biochem. J. 1995, 311, 377–383. [Google Scholar] [CrossRef]
- Yoon, J.H. Enzymatic synthesis of chitooligosaccharides in organic cosolvents. Enzym. Microb. Technol. 2005, 37, 663–668. [Google Scholar] [CrossRef]
- Lin, H.; Wang, H.; Xue, C.; Ye, M. Preparation of chitosan oligomers by immobilized papain. Enzym. Microb. Technol. 2002, 31, 588–592. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Yu, H.; Guo, Z.; Wang, P.; Li, C.; Li, Z.; Li, P. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005, 340, 2150–2153. [Google Scholar] [CrossRef]
- Kang, B.; Dai, Y.-D.; Zhang, H.-Q.; Chen, D. Synergetic degradation of chitosan with gamma radiation and hydrogen peroxide. Polym. Degrad. Stab. 2007, 92, 359–362. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Yuan, Y.; Ren, X.; Yang, F. Synergistic degradation of chitosan by impinging stream and jet cavitation. Ultrason. Sonochem. 2015, 27, 592–601. [Google Scholar] [CrossRef]
- Yan, J.; Ai, S.; Yang, F.; Zhang, K.; Huang, Y. Study on mechanism of chitosan degradation with hydrodynamic cavitation. Ultrason. Sonochem. 2020, 64, 105046. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, Z.; Zhao, H.; Tian, S. Plasma Depolymerization of Chitosan in the Presence of Hydrogen Peroxide. Int. J. Mol. Sci. 2012, 13, 7788–7797. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Rujiravanit, R.; Theeramunkong, S.; Saito, N. Degradation of chitosan hydrogel dispersed in dilute carboxylic acids by solution plasma and evaluation of anticancer activity of degraded products. Jpn J. Appl. Phys. 2018, 57, 0102B5. [Google Scholar] [CrossRef]
- Ma, F.; Li, P.; Zhang, B.; Zhao, X.; Fu, Q.; Wang, Z.; Gu, C. Effect of solution plasma process with bubbling gas on physico-chemical properties of chitosan. Int. J. Biol. Macromol. 2017, 98, 201–207. [Google Scholar] [CrossRef]
- Skonberg, C.; Olsen, J.; Madsen, K.G.; Hansen, S.H.; Grillo, M.P.; Olsen, J. Metabolic activation of carboxylic acids. Expert Opin. Drug Metab. Toxicol. 2008, 4, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, S.; Li, P.; Sun, B.; Xu, Y.; Tao, D.; Zhao, H.; Cui, S.; Zhu, R.; Zhang, B. Investigation on the role of the free radicals and the controlled degradation of chitosan under solution plasma process based on radical scavengers. Carbohydr. Polym. 2021, 257, 117567. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Kamiya, M.; Nishimura, S.-I. Solubilization of a rigid polysaccharide: Controlled partial N-Acetylation of chitosan to develop solubility. Carbohydr. Polym. 1991, 16, 83–92. [Google Scholar] [CrossRef]
- Galed, G.; Miralles, B.; Paños, I.; Santiago, A.; Heras, Á. N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydr. Polym. 2005, 62, 316–320. [Google Scholar] [CrossRef]
- Rujiravanit, R.; Kantakanun, M.; Chokradjaroen, C.; Vanichvattanadecha, C.; Saito, N. Simultaneous deacetylation and degradation of chitin hydrogel by electrical discharge plasma using low sodium hydroxide concentrations. Carbohydr. Polym. 2020, 228, 115377. [Google Scholar] [CrossRef]



| Chemicals | Chemical Concentration | Temperature | Time | MW/ Products | Ref. |
|---|---|---|---|---|---|
| Hydrochloric acid | 35% | 80 °C | 1.4 h | 3–5 × 103 | [93] |
| Hydrochloric acid | 12 M | 40 °C | 8 h | 1–3 × 103 | [94] |
| Nitrous acid | 70 mM | 0 °C | 9 h | 2–6 × 103 | [95] |
| Hydrogen fluoride | 100% | 20 °C | 19h | ~2 × 103 | [96] |
| Hydrogen peroxide | 30% | 70 °C | 2 h | 1.7–3.81 × 103 | [97] |
| Chitosanase (Bacillus pumilus BN-262) | 0.1 M NaOAc buffer, pH 5.3 | 37 °C | 96 h | 2–3 oligomers | [98] |
| Chitinase (Vibrio furnissii) | Chitin in DMSO/LiCl, pH 7.9 | 37 °C | 24 h | 2 oligomers | [99] |
| Papain | NaOAc–AcOH buffer, pH 4.0 | 45 °C | 24 h | 2–50 oligomers | [100] |
| Methods | Chemical Concentration | T (°C) | Time | % MW Reduction | Ref. |
|---|---|---|---|---|---|
| Microwave 400 W | 2% acetic acid | N/A | 25 min | 79% | [101] |
| Microwave 100 W | 0.1 M acetic acid | N/A | 20 min | 92.5% | [87] |
| Ultraviolet 1 kW power | 0.1 M acetic acid | 25 | 15 min | 98.5% | [88] |
| Ultrasonication 200 W | 0.1 M acetic acid | N/A | 120 min | 52% | [88] |
| 60Co γ-rays Radiation | 2% acetic acid mixed 10 mL hydrogen peroxide | N/A | 7 h | 95% | [102] |
| Impinging stream and jet cavitation | acetic acid mixed with sodium acetate trihydrate | 40 | 30 min | 88% | [103] |
| Hydrodynamic cavitation | 0.2 acetic acid mixed with 0.1 sodium acetate | 40 | 30 min | 95% | [104] |
| Plasma 350 W | 1% acetic acid | N/A | 180 min | 83 % | [105] |
| SPP | 1 M acetic acid | 25–30 | 300 min | n/a | [46] |
| SPP | 1 M acetic acid | 25–30 | 300 min | 96% | [40] |
| SPP | 0.1 M acetic acid 4 M hydrogen peroxide | Room temperature | 60 min | 85% | [74] |
| SPP | 0.00155 mM carboxylic acids | Room temperature | 60 min | 86% | [106] |
| SPP | 0.02 M sodium chloride | Room temperature | 60 min | 96% | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokradjaroen, C.; Niu, J.; Panomsuwan, G.; Saito, N. Insight on Solution Plasma in Aqueous Solution and Their Application in Modification of Chitin and Chitosan. Int. J. Mol. Sci. 2021, 22, 4308. https://doi.org/10.3390/ijms22094308
Chokradjaroen C, Niu J, Panomsuwan G, Saito N. Insight on Solution Plasma in Aqueous Solution and Their Application in Modification of Chitin and Chitosan. International Journal of Molecular Sciences. 2021; 22(9):4308. https://doi.org/10.3390/ijms22094308
Chicago/Turabian StyleChokradjaroen, Chayanaphat, Jiangqi Niu, Gasidit Panomsuwan, and Nagahiro Saito. 2021. "Insight on Solution Plasma in Aqueous Solution and Their Application in Modification of Chitin and Chitosan" International Journal of Molecular Sciences 22, no. 9: 4308. https://doi.org/10.3390/ijms22094308
APA StyleChokradjaroen, C., Niu, J., Panomsuwan, G., & Saito, N. (2021). Insight on Solution Plasma in Aqueous Solution and Their Application in Modification of Chitin and Chitosan. International Journal of Molecular Sciences, 22(9), 4308. https://doi.org/10.3390/ijms22094308