Genetic Determinants of Poor Response to Treatment in Severe Asthma
Abstract
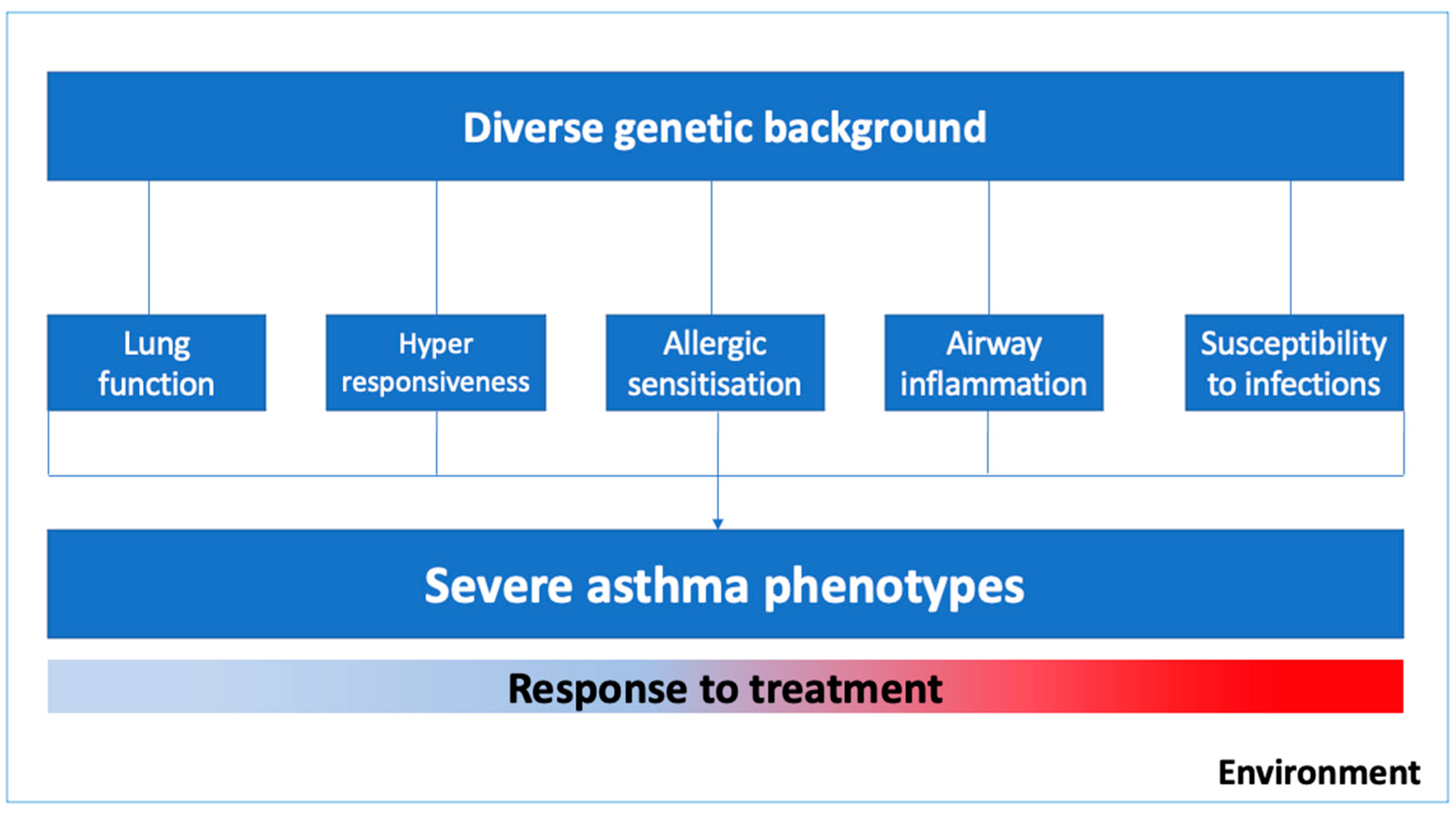
1. Introduction
2. Pharmacogenomics of the Therapeutic Response of Asthma
3. Candidate Genes Studies Linked to Therapeutic Response of Asthma
3.1. Influence of Genetic Variant in ADRB2 on Response to the Bronchodilator
3.2. Variants in GLCCI1 Associated with Response to Corticosteroids
3.3. Genetic Variants in the Vitamin D Pathway and the Therapeutic Response on Asthma
4. Interaction between Genetics and Age in Response to Asthma Treatment
5. Epigenetic Mechanisms Involved in the Lack of Therapeutic Control of Asthma
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Global Asthma Report 2018; Global Asthma Network: Auckland, New Zealand, 2018; ISBN 9780473465230.
- Moffatt, M.F.; Kabesch, M.; Liang, L.; Dixon, A.L.; Strachan, D.; Heath, S.; Depner, M.; Von Berg, A.; Bufe, A.; Rietschel, E.; et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 2007, 448, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S.F. Genetics of asthma: An introduction for the clinician. Eur. Clin. Respir. J. 2015, 2, 24643. [Google Scholar] [CrossRef]
- Samedy-Bates, L.A.; Oh, S.S.; Nuckton, T.J.; Elhawary, J.R.; White, M.; Elliot, T.; Zeiger, A.M.; Eng, C.; Salazar, S.; LeNoir, M.A.; et al. Racial/Ethnic-Specific Differences in the Effects of Inhaled Corticosteroid Use on Bronchodilator Response in Patients with Asthma. Clin. Pharmacol. Ther. 2019, 106, 1133–1140. [Google Scholar] [CrossRef]
- Sadeghnejad, A.; Karmaus, W.; Arshad, S.H.; Kurukulaaratchy, R.; Huebner, M.; Ewart, S. IL13 gene polymorphisms modify the effect of exposure to tobacco smoke on persistent wheeze and asthma in childhood, a longitudinal study. Respir. Res. 2008, 9, 2. [Google Scholar] [CrossRef]
- Sly, P.D.; Holt, P.G. Role of innate immunity in the development of allergy and asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 127–131. [Google Scholar] [CrossRef]
- Keskin, O.; Farzan, N.; Birben, E.; Akel, H.; Karaaslan, C.; Maitland-Van Der Zee, A.H.; Wechsler, M.E.; Vijverberg, S.J.; Kalayci, O. Genetic associations of the response to inhaled corticosteroids in asthma: A systematic review. Clin. Transl. Allergy 2019, 9, 1–25. [Google Scholar] [CrossRef]
- Keskin, O.; Uluca, Ü.; Birben, E.; Coşkun, Y.; Ozkars, M.Y.; Keskin, M.; Kucukosmanoglu, E.; Kalayci, O. Genetic associations of the response to inhaled corticosteroids in children during an asthma exacerbation. Pediatr. Allergy Immunol. 2016, 27, 507–513. [Google Scholar] [CrossRef]
- Hu, C.; Xun, Q.; Li, X.; He, R.; Lu, R.; Zhang, S.; Hu, X.; Feng, J. GLCCI1 Variation Is Associated with Asthma Susceptibility and Inhaled Corticosteroid Response in a Chinese Han Population. Arch. Med. Res. 2016, 47, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, H.; Wu, X.; Xu, Y.; Zhao, J.; Xie, J.; Yu, J. GLCCI1 rs37973: A potential genetic predictor of therapeutic response to inhaled corticosteroids in Chinese asthma patients. Medicine 2017, 96, e9442. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.M.; Lee, H.Y.; Kim, S.H.; Jee, Y.K.; Lee, S.K.; Lee, S.H.; Park, H.S. Pharmacogenetic study of the effects of NK2R G231E G > A and TBX21 H33Q C > G polymorphisms on asthma control with inhaled corticosteroid treatment. J. Clin. Pharm. Ther. 2009, 34, 693–701. [Google Scholar] [CrossRef]
- Rogers, A.J.; Tantisira, K.G.; Fuhlbrigge, A.L.; Litonjua, A.A.; Lasky-Su, J.A.; Szefler, S.J.; Strunk, R.C.; Zeiger, R.S.; Weiss, S.T. Predictors of poor response during asthma therapy differ with definition of outcome. Pharmacogenomics 2009, 10, 1231–1242. [Google Scholar] [CrossRef]
- Tantisira, K.G.; Hwang, E.S.; Raby, B.A.; Silverman, E.S.; Lake, S.L.; Richter, B.G.; Peng, S.L.; Drazen, J.M.; Glimcher, L.H.; Weiss, S.T. TBX21: A functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc. Natl. Acad. Sci. USA 2004, 101, 18099–18104. [Google Scholar] [CrossRef]
- Tantisira, K.G.; Silverman, E.S.; Mariani, T.J.; Xu, J.; Richter, B.G.; Klanderman, B.J.; Litonjua, A.A.; Lazarus, R.; Rosenwasser, L.J.; Fuhlbrigge, A.L.; et al. FCER2: A pharmacogenetic basis for severe exacerbations in children with asthma. J. Allergy Clin. Immunol. 2007, 120, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Koster, E.S.; Maitland-Van Der Zee, A.H.; Tavendale, R.; Mukhopadhyay, S.; Vijverberg, S.J.H.; Raaijmakers, J.A.M.; Palmer, C.N.A. FCER2 T2206C variant associated with chronic symptoms and exacerbations in steroid-treated asthmatic children. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 1546–1552. [Google Scholar] [CrossRef]
- Farzan, N.; Vijverberg, S.J.; Hernandez-Pacheco, N.; Bel, E.H.D.; Berce, V.; Bønnelykke, K.; Bisgaard, H.; Burchard, E.G.; Canino, G.; Celedón, J.C.; et al. 17Q21 Variant Increases the Risk of Exacerbations in Asthmatic Children Despite Inhaled Corticosteroids Use. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pacheco, N.; Farzan, N.; Francis, B.; Karimi, L.; Repnik, K.; Vijverberg, S.J.; Soares, P.; Schieck, M.; Gorenjak, M.; Forno, E.; et al. Genome-wide association study of inhaled corticosteroid response in admixed children with asthma. Clin. Exp. Allergy 2019, 49, 789–798. [Google Scholar] [CrossRef]
- Hernandez-Pacheco, N.; Vijverberg, S.J.; Herrera-Luis, E.; Li, J.; Sio, Y.Y.; Granell, R.; Corrales, A.; Maroteau, C.; Lethem, R.; Perez-Garcia, J.; et al. Genome-wide association study of asthma exacerbations despite inhaled corticosteroids use. Eur. Respir. J. 2020, 2003388. [Google Scholar] [CrossRef]
- Hernandez-Pacheco, N.; Gorenjak, M.; Jurgec, S.; Corrales, A.; Jorgensen, A.; Karimi, L.; Vijverberg, S.J.; Berce, V.; Schieck, M.; Acosta-Herrera, M.; et al. Combined analysis of transcriptomic and genetic data for the identification of loci involved in glucocorticosteroid response in asthma. J. Allergy Clin. Immunol. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spear, M.L.; Hu, D.; Pino-Yanes, M.; Huntsman, S.; Eng, C.; Levin, A.M.; Ortega, V.E.; White, M.J.; McGarry, M.E.; Thakur, N.; et al. A genome-wide association and admixture mapping study of bronchodilator drug response in African Americans with asthma. Pharm. J. 2019, 19, 249–259. [Google Scholar] [CrossRef]
- Slob, M.A.; Richards, L.B.; Vijverberg, S.J.H.; Longo, S.; Koppelman, G.H.; Pijnenburg, M.W.H.; Bel, E.H.D.; Neerincx, A.H.; Luis, E.H.; Perez-Garcia, J.; et al. Pediatric allergy and immunology Genome—wide association studies of exacerbations in children using long—acting beta2—agonists. Pediatr. Allergy Immunol. 2021, 13494. [Google Scholar] [CrossRef]
- Slob, E.M.A.; Vijverberg, S.J.H.; Palmer, C.N.A.; Zazuli, Z.; Farzan, N.; Oliveri, N.M.B.; Pijnenburg, M.W.; Koppelman, G.H.; Maitland-van der Zee, A.H. Pharmacogenetics of inhaled long-acting beta2-agonists in asthma: A systematic review. Pediatr. Allergy Immunol. 2018, 29, 705–714. [Google Scholar] [CrossRef]
- Martinez, F.D.; Graves, P.E.; Baldini, M.; Solomon, S.; Erickson, R. Association between genetic polymorphisms of the β2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J. Clin. Investig. 1997, 100, 3184–3188. [Google Scholar] [CrossRef]
- Sahi, P.K.; Shastri, S.; Lodha, R.; Gupta, N.; Pandey, R.M.; Kabra, S.K.; Kabra, M. ADRB2 polymorphism and salbutamol responsiveness in Northern Indian children with mild to moderate exacerbation of asthma. Indian Pediatr. 2016, 53, 211–215. [Google Scholar] [CrossRef]
- Scaparrotta, A.; Franzago, M.; Marcovecchio, M.L.; Di Pillo, S.; Chiarelli, F.; Mohn, A.; Stuppia, L. Role of THRB, ARG1, and ADRB2 Genetic Variants on Bronchodilators Response in Asthmatic Children. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 164–173. [Google Scholar] [CrossRef]
- Shah, N.J.; Vinod Kumar, S.; Gurusamy, U.; Annan Sudarsan, A.K.; Shewade, D.G. Effect of ADRB2 (adrenergic receptor β2) gene polymorphisms on the occurrence of asthma and on the response to nebulized salbutamol in South Indian patients with bronchial asthma. J. Asthma 2015, 52, 755–762. [Google Scholar] [CrossRef]
- Ortega, V.E.; Hawkins, G.A.; Moore, W.C.; Hastie, A.T.; Ampleford, E.J.; Busse, W.W.; Castro, M.; Chardon, D.; Erzurum, S.C.; Israel, E.; et al. Effect of rare variants in ADRB2 on risk of severe exacerbations and symptom control during longacting β agonist treatment in a multiethnic asthma population: A genetic study. Lancet Respir. Med. 2014, 2, 204–213. [Google Scholar] [CrossRef]
- Almomani, B.A.; Al-Eitan, L.N.; Al-Sawalha, N.A.; Samrah, S.M.; Al-Quasmi, M.N. Association of genetic variants with level of asthma control in the arab population. J. Asthma Allergy 2019, 12, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Bournissen, F.G.; Hutson, J.R.; Shannon, M. Polymorphism of the ADRB2 gene and response to inhaled beta- agonists in children with asthma: A meta-analysis. J. Asthma 2009, 46, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; Nelson, H.S.; Kraft, M.; Corren, J.; Meyers, D.A.; Yancey, S.W.; Anderson, W.H.; Emmett, A.H.; Ortega, H.G. 2-Receptor Polymorphisms in Patients Receiving Salmeterol with or without Fluticasone Propionate. Am. J. Respir. Crit. Care Med. 2010, 181, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Jerome, R.N.; Pulley, J.M.; Sathe, N.A.; Krishnaswami, S.; Dickerson, A.B.; Worley, K.J.; Lima, M.F.; Wilkins, C.H. Uncovering Outcome Disparities of β2 Adrenergic Agonists in Blacks: A Systematic Review. J. Natl. Med. Assoc. 2020, 113, 2021. [Google Scholar] [CrossRef]
- Hikino, K.; Kobayashi, S.; Ota, E.; Mushiroda, T.; Urayama, K.Y.; Kobayashi, T. A meta-analysis of the influence of ADRB2 genetic polymorphisms on albuterol (salbutamol) therapy in patients with asthma. Br. J. Clin. Pharmacol. 2021, 87, 1708–1716. [Google Scholar] [CrossRef]
- Toraih, E.A.; Hussein, M.H.; Ibrahim, A.; AbdAllah, N.B.; Mohammad, E.; Kishk, A.M.; Fawzy, M.S. Beta2-adrenergic receptor variants in children and adolescents with bronchial asthma. Front. Biosci. 2019, 11, 61–68. [Google Scholar]
- Bhosale, S.; Nikte, S.V.; Sengupta, D.; Joshi, M. Differential Dynamics Underlying the Gln27Glu Population Variant of the β2-Adrenergic Receptor. J. Membr. Biol. 2019, 252, 499–507. [Google Scholar] [CrossRef]
- McDonagh, E.M.; Whirl-Carrillo, M.; Garten, Y.; Altman, R.B.; Klein, T.E. From pharmacogenomic knowledge acquisition to clinical applications: The PharmGKB as a clinical pharmacogenomic biomarker resource. Clin. Transl. Sci. 2012, 5, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; de Roos, E.W.; McGeachie, M.J.; Verhamme, K.M.C.; Brusselle, G.G.; Tantisira, K.G.; Iribarren, C.; Lu, M.; Wu, A.C.; Stricker, B.H.; et al. Pharmacogenetics of inhaled corticosteroids and exacerbation risk in adults with asthma. Clin. Exp. Allergy 2021, 13829. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma Amira. Clin Chest Med. 2020, 40, 163–177. [Google Scholar] [CrossRef]
- Izuhara, Y.; Matsumoto, H.; Kanemitsu, Y.; Izuhara, K.; Tohda, Y.; Horiguchi, T.; Kita, H.; Kuwabara, K.; Tomii, K.; Otsuka, K.; et al. GLCCI1 variant accelerates pulmonary function decline in patients with asthma receiving inhaled corticosteroids. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 668–673. [Google Scholar] [CrossRef]
- Tantisira, K.G.; Lasky-Su, J.; Harada, M.; Murphy, A.; Litonjua, A.A.; Himes, B.E.; Lange, C.; Lazarus, R.; Sylvia, J.; Klanderman, B.; et al. Genomewide Association between GLCCI1 and Response to Glucocorticoid Therapy in Asthma. N. Engl. J. Med 2011, 365, 1173–1183. [Google Scholar] [CrossRef]
- Rijavec, M.; Žavbi, M.; Lopert, A.; Fležar, M.; Korošec, P. GLCCI1 polymorphism rs37973 and response to treatment of asthma with inhaled corticosteroids. J. Investig. Allergol. Clin. Immunol. 2018, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.A.; De Araújo Sena, F.; de Andrade Belitardo, E.M.M.; De Santana, M.B.R.; Costa, G.N.D.O.; Cruz, Á.A.; Barreto, M.L.; Costa, R.D.S.; Alcantara-Neves, N.M.; Figueiredo, C.A. Genetic polymorphisms in vitamin D pathway influence 25(OH)D levels and are associated with atopy and asthma. Allergy Asthma Clin. Immunol. 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Nanzer, A.M.; Pfeffer, P.E.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high)immunophenotypes: Potential benefits of calcitriol. J. Allergy Clin. Immunol. 2015, 136, 628–637.e4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, J.; Yu, Z. Budesonide up-regulates vitamin D receptor expression in human bronchial fibroblasts and enhances the inhibitory effect of calcitriol on airway remodeling. Allergol. Immunopathol. 2019, 47, 585–590. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abdel-Rehim, A.S. Influence of vitamin D receptor gene FokI and ApaI polymorphisms on glucocorticoid response in patients with asthma. Int. Forum Allergy Rhinol. 2019, 10, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Leiter, K.; Franks, K.; Borland, M.L.; Coleman, L.; Harris, L.; Le Souëf, P.N.; Laing, I.A. Vitamin D receptor polymorphisms are associated with severity of wheezing illnesses and asthma exacerbations in children. J. Steroid Biochem. Mol. Biol. 2020, 201, 105692. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, A.; Sordillo, J.E.; McGeachie, M.; Kelly, R.S.; Tantisira, K.G.; Lutz, S.M.; Lasky-Su, J.; Wu, A.C. Genome-wide interaction study reveals agedependent determinants of responsiveness to inhaled corticosteroids in individuals with asthma. PLoS ONE 2020, 15, e0229241. [Google Scholar] [CrossRef]
- Sordillo, J.E.; McGeachie, M.; Lutz, S.M.; Lasky-Su, J.; Tantisira, K.; Tsai, C.H.; Dahlin, A.; Kelly, R.; Wu, A.C. Longitudinal Analysis of Bronchodilator Response in Asthmatics and Effect Modification of Age-related trends by Genotype. Physiol. Behav. 2019, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kabesch, M.; Michel, S.; Tost, J. Epigenetic mechanisms and the relationship to childhood asthma. Eur. Respir. J. 2010, 36, 950–961. [Google Scholar] [CrossRef]
- Li, J.; Panganiban, R.; Kho, A.T.; McGeachie, M.J.; Farnam, L.; Chase, R.P.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Circulating micrornas and treatment response in childhood asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 65–72. [Google Scholar] [CrossRef]
- Yu, B.; Yao, L.; Liu, C.; Tang, L.; Xing, T. Upregulation of microRNA-16 alters the response to inhaled β-agonists in patients with asthma though modulating expression of ADRB2. Mol. Med. Rep. 2019, 49, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Qiu, W.; Demeo, D.L.; Raby, B.A.; Weiss, S.T.; Tantisira, K. DNA methylation is associated with improvement in lung function on inhaled corticosteroids in pediatric asthmatics. Pharmacogenetics Genom. 2019, 29, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Nafea, O.E.; El-Korashi, L.A.; Gehad, M.H.; Yousif, Y.M.; Zake, L.G. Association between blood aluminum and beta-2 receptor gene methylation with childhood asthma control. Hum. Exp. Toxicol. 2020, 39, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
| SNP | Score PharmGKB | Gene | Outcome | Reference |
|---|---|---|---|---|
| rs11681246 | - | LTBP1 | Negatively associated with asthma exacerbations regardless of ICS use | [20] |
| rs76390075 | - | LTBP1 | [20] | |
| rs73650726 | - | LOC105376110 | Negatively associated with BDR | [21] |
| rs7903366 | - | PRKG1 | Positively associated with the BDR | [21] |
| rs7081864 | - | PRKG1 | Positively associated with the BDR | [21] |
| rs7070958 | - | PRKG1 | Negatively associated with the BDR | [21] |
| rs1042713 | 2A | ADRB2 | Better response to treatment with A allele | [24,30] |
| Worst response to treatment with A allele | [29] | |||
| No association with therapeutic response | [25,32,34] | |||
| rs1042714 | - | ADRB2 | Better response to treatment | [23,29] |
| No association with therapeutic response | [24,27,30,34] | |||
| rs1042713/rs1042714 | - | ADRB2 | Better response to treatment | [35] |
| rs180888 | - | ADRB2 | Uncontrolled asthma during LABA treatment | [28] |
| rs37972 | - | GLCCI1 | Attenuation of the response to ICS treatment | [41] |
| rs37973 | 3 | GLCCI1 | Worst response to treatment with G Allele | [38,40,41] |
| Better response to treatment with G allele | [42] | |||
| rs2228570 | - | VDR | Risk of resistance to inhaled glucocorticoids | [46] |
| Higher exacerbation severity scores and poorer β2-agonist treatment response | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, R.G.; Costa, R.S.; Figueiredo, C.A.; Cruz, A.A. Genetic Determinants of Poor Response to Treatment in Severe Asthma. Int. J. Mol. Sci. 2021, 22, 4251. https://doi.org/10.3390/ijms22084251
Figueiredo RG, Costa RS, Figueiredo CA, Cruz AA. Genetic Determinants of Poor Response to Treatment in Severe Asthma. International Journal of Molecular Sciences. 2021; 22(8):4251. https://doi.org/10.3390/ijms22084251
Chicago/Turabian StyleFigueiredo, Ricardo G., Ryan S. Costa, Camila A. Figueiredo, and Alvaro A. Cruz. 2021. "Genetic Determinants of Poor Response to Treatment in Severe Asthma" International Journal of Molecular Sciences 22, no. 8: 4251. https://doi.org/10.3390/ijms22084251
APA StyleFigueiredo, R. G., Costa, R. S., Figueiredo, C. A., & Cruz, A. A. (2021). Genetic Determinants of Poor Response to Treatment in Severe Asthma. International Journal of Molecular Sciences, 22(8), 4251. https://doi.org/10.3390/ijms22084251






