Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model
Abstract
1. Introduction
2. Results
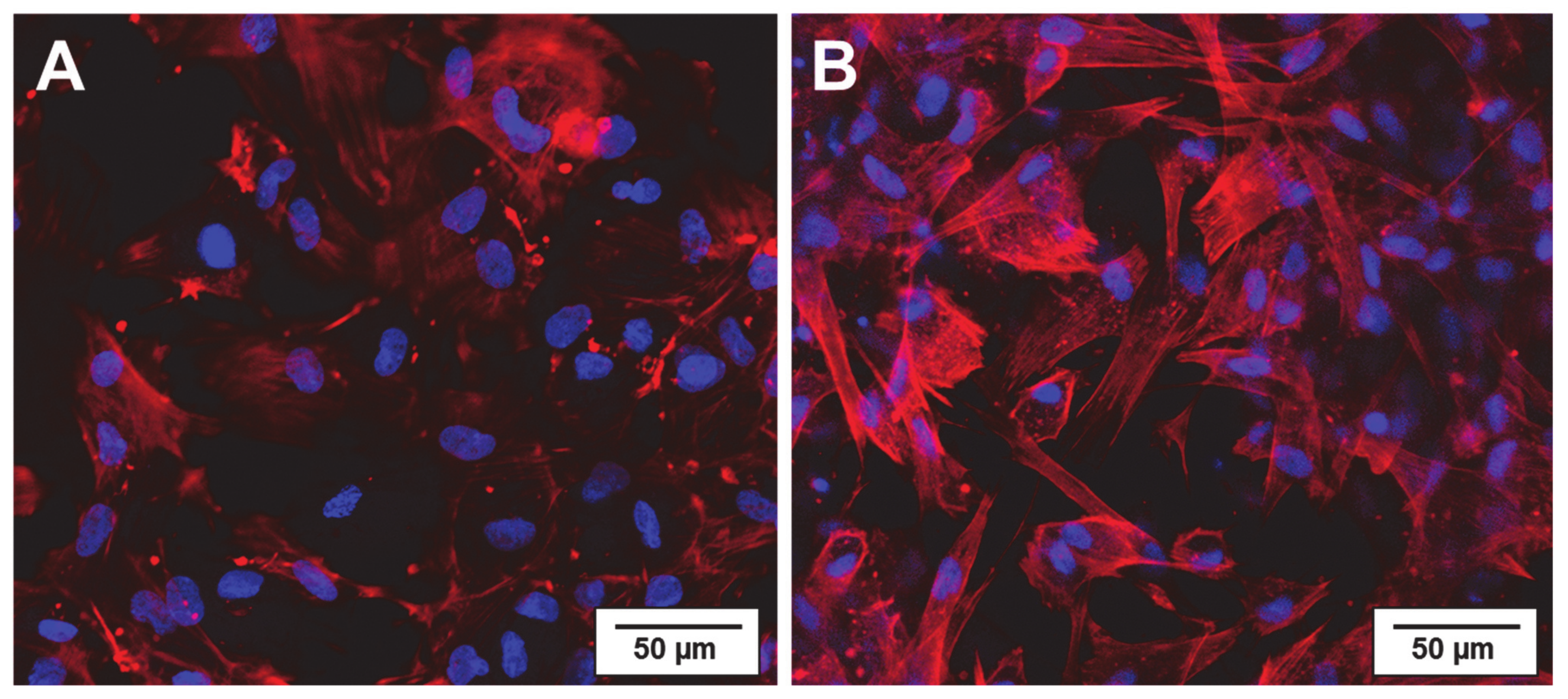
2.1. Physiological Compatibility of Collagen Hydrogel
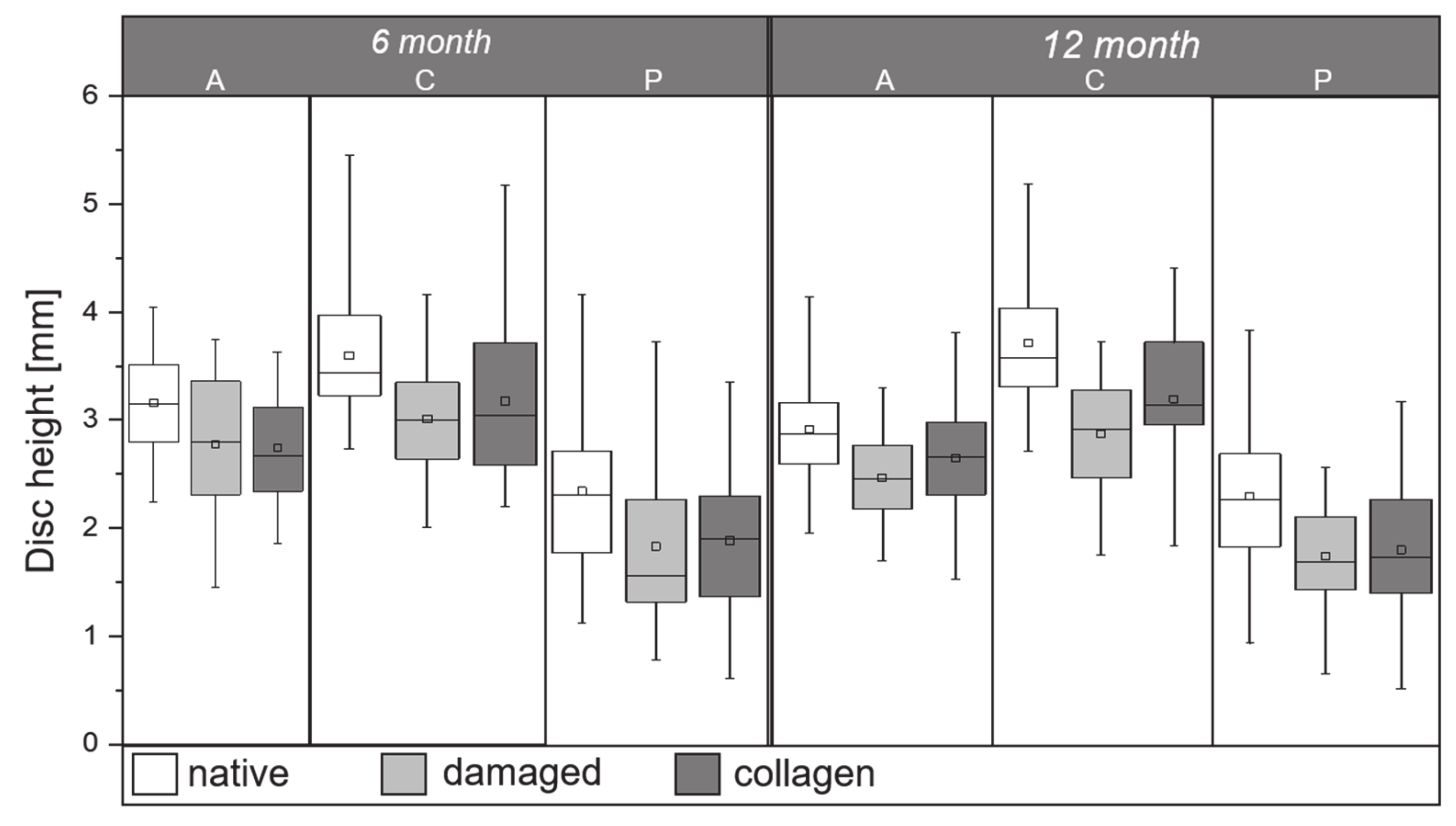
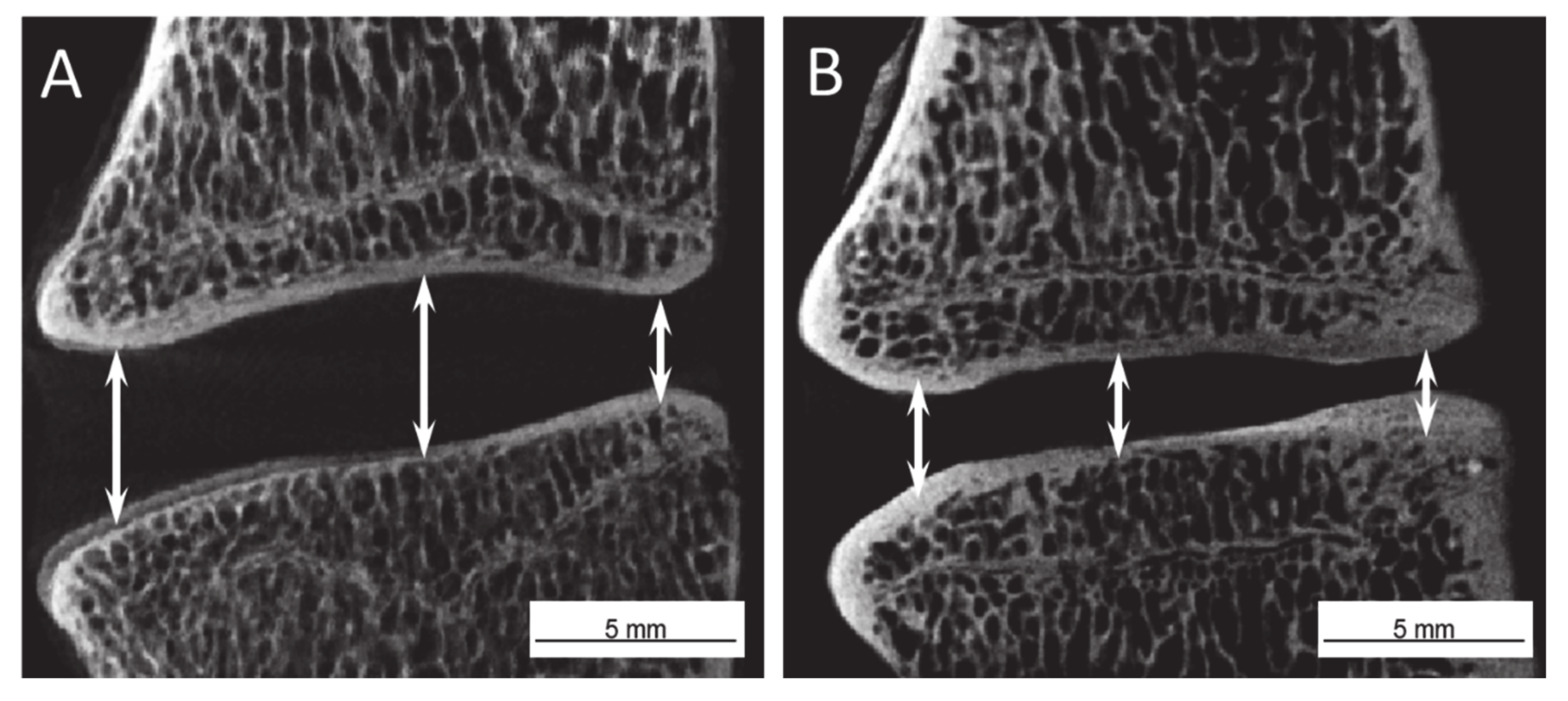
2.2. Disc Height Analysis
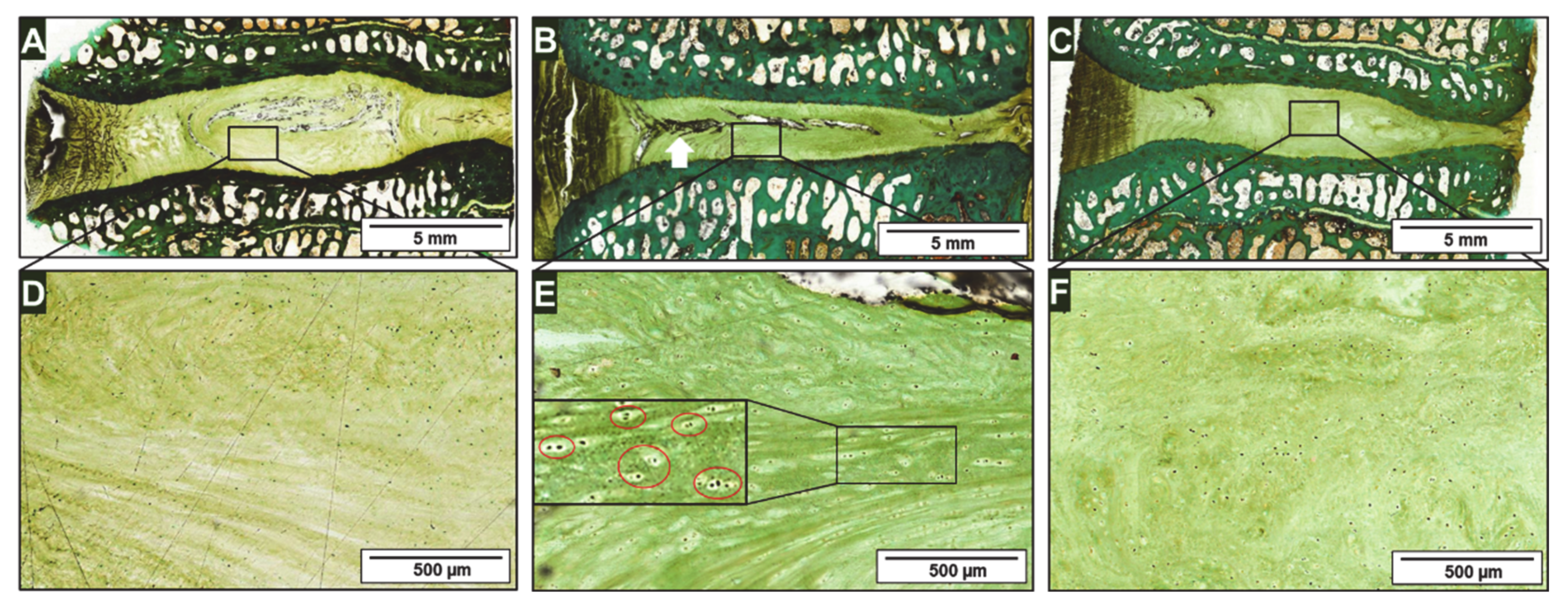
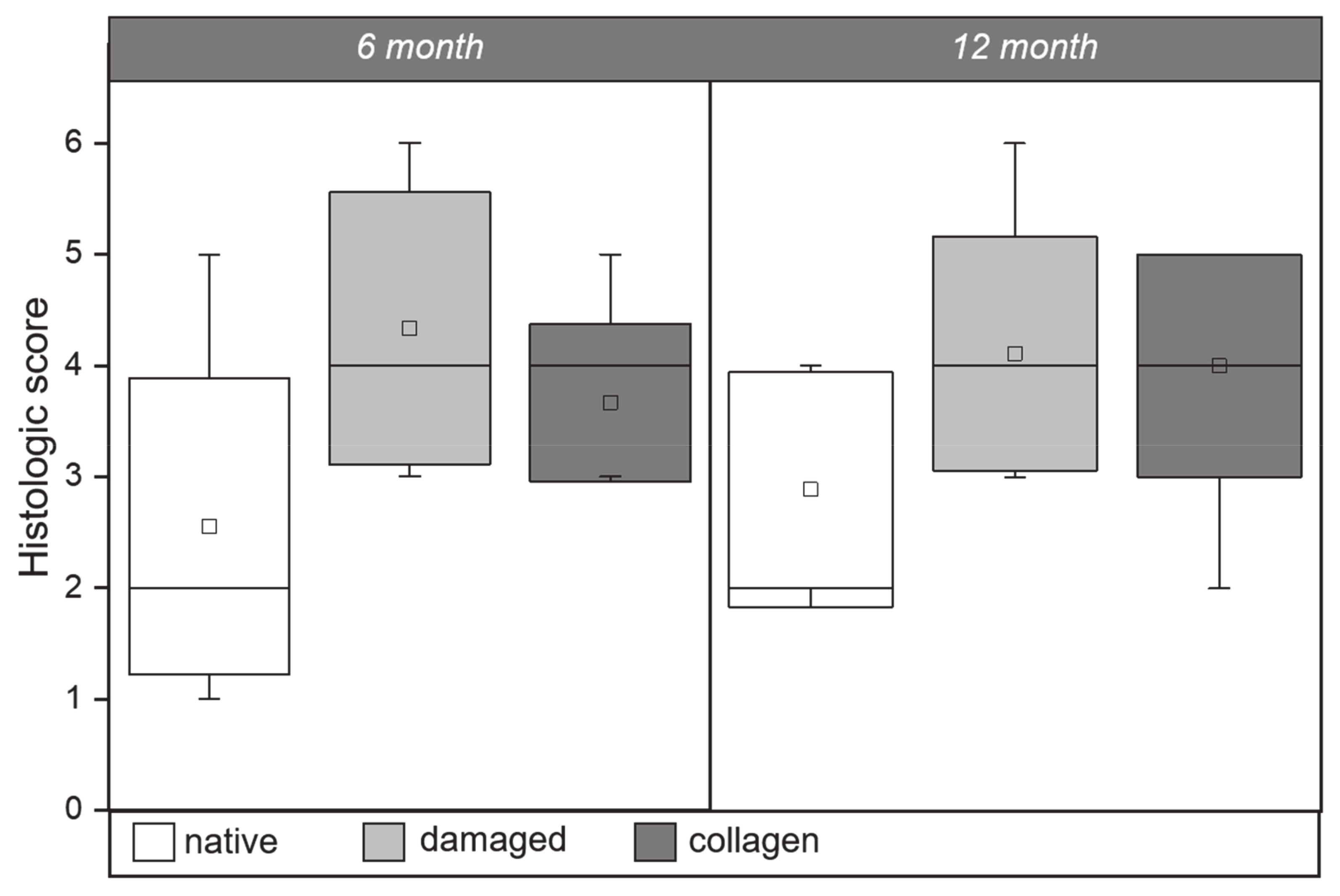
2.3. Histology
3. Discussion
4. Materials and Methods
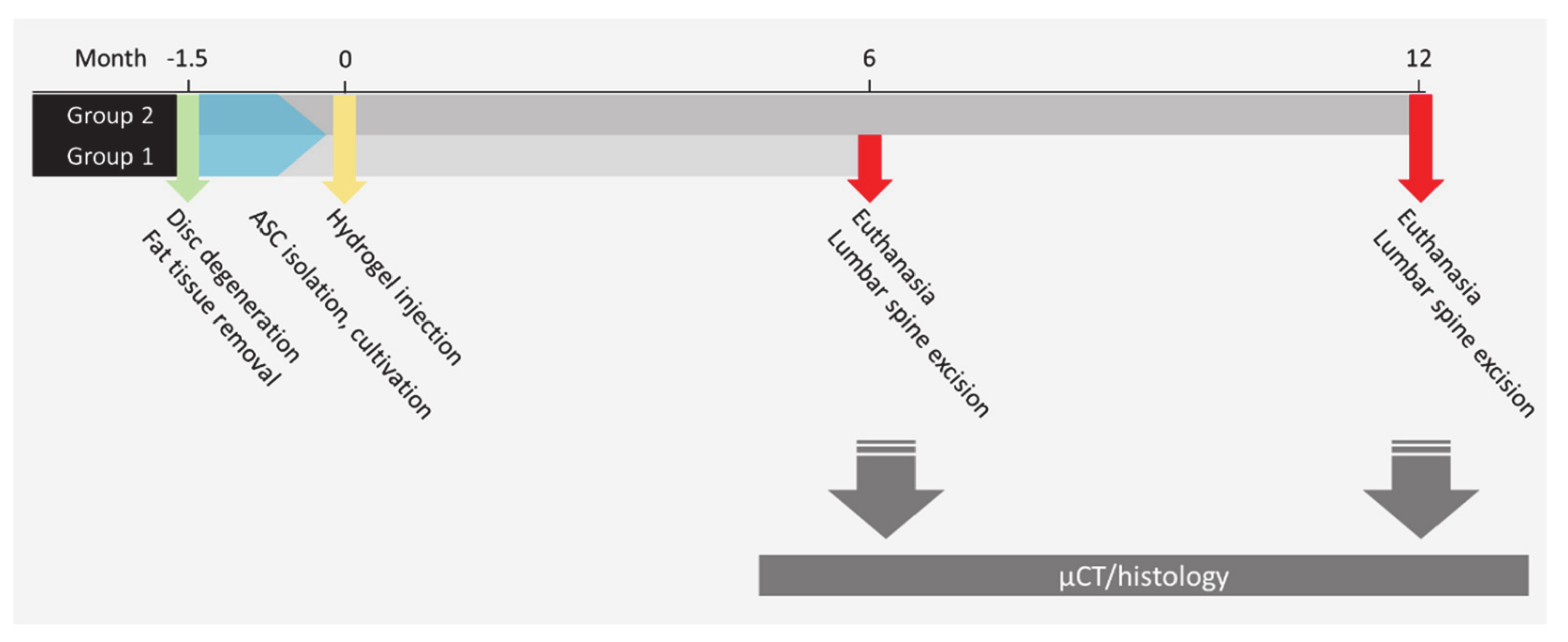
4.1. Animal Model
4.2. Cell Isolation and Cultivation
4.3. Collagen Hydrogel Fabrication
4.4. Physiological Compatibility of Collagen Hydrogel
4.5. Injection of Cell Loaded Collagen Hydrogel into IVDs
4.6. Postoperative Protocol
4.7. µ-CT Analysis
4.8. Histology and Light Microscopy
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giselli, G.; Wang, J.C.; Bahita, N.N.; Hsu, W.K.; Dawson, E.G. Adjacent segment degeneration in the lumbar spine. J. Bone Jt. Surg. Am. Vol. 2004, 86, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Lu, Z.; Qin, L.; Mauck, R.L.; Smith, H.E.; Smith, L.J.; Malhotra, N.R.; Heyworth, M.F.; Caldera, F.; Enomoto-Iwamoto, M.; et al. Cell therapy for the degenerating intervertebral disc. Transl. Res. 2017, 181, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tschugg, A.; Michnacs, F.; Strowitzki, M.; Meisel, H.J.; Thommé, C. A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: Study protocol. Trials 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vedicherla, S.; Buckley, C.T. Cell-Based Therapies for Intervertebral Disc and Cartilage Regeneration—Current Concepts, Parallels, and Perspectives. J. Orthop. Res. 2017, 35, 8–22. [Google Scholar] [CrossRef]
- Coric, D.; Pettine, K.; Sumich, A.; Boltes, M.O. Prospective study of disc repair with allogeneic chondrocytes. J. Neurosurg. Spine 2013, 18, 85–95. [Google Scholar] [CrossRef]
- Mochida, J.; Sakai, D.; Nakamura, Y.; Watanabe, T.; Yamamoto, Y.; Kato, S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: A three-year, prospective clinical study of its safety. Eur. Cells Mater. 2015, 29, 202–212. [Google Scholar] [CrossRef]
- Wuest, S.L.; Caliò, M.; Wernas, T.; Tanner, S.; Giger-Lange, C.; Wyss, F.; Ille, F.; Gantenbein, B.; Egli, M. Influence of Mechanical Unloading on Articular Chondrocyte Dedifferentiation. Int. J. Mol. Sci. 2018, 19, 1289. [Google Scholar] [CrossRef]
- Clouet, J.; Fusellier, M.; Camus, A.; Le Visage, C.; Guicheux, J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv. Drug Deliv. Rev. 2019, 146, 306–324. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, W.D.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Hiyama, A.; Mochida, J.; Sakai, D. Stem cell applications in intervertebral disc repair. Cell. Mol. Biol. 2008, 54, 24–32. [Google Scholar]
- Vadala, G.; Russo, F.; Ambrosio, L.; Loppini, M.; Denaro, V. Stem cells sources for intervertebral disc regeneration. World J. Stem Cells 2016, 8, 185–201. [Google Scholar] [CrossRef]
- Ludtka, C.; Schwan, S.; Friedmann, A.; Brehm, W.; Wiesner, I.; Goehre, F. Micro-CT evaluation of asymmetrical ovine intervertebral disc height loss from surgical approach. Eur. Spine J. 2017, 26, 2031–2037. [Google Scholar] [CrossRef]
- Mageed, M.; Berner, D.; Jülke, H.; Hohaus, C.; Brehm, W.; Gerlach, K. Morphometrical dimensions of the sheep thoracolumbar vertebrae as seen on digitised CT images. Lab. Anim. Res. 2013, 29, 138–147. [Google Scholar] [CrossRef]
- Reitmaier, S.; Schmidt, H.; Ihler, R.; Kocak, T.; Graf, N.; Ignatius, A.; Wilke, H.J. Preliminary Investigations on Intradiscal Pressures during Daily Activities: An In Vivo Study Using the Merino Sheep. PLoS ONE 2013, 8, e69610. [Google Scholar] [CrossRef]
- Wilke, H.J.; Kettler, A.; Wenger, K.H.; Claes, L.E. Anatomy of the sheep spine and its comparison to the human spine. Anat. Rec. 1997, 247, 542–555. [Google Scholar] [CrossRef]
- Benz, K.; Stippich, C.; Fischer, L.; Möhl, K.; Werber, K.; Lang, J.; Steffen, F.; Beintner, B.; Gaissmaier, C.; Mollenhauer, J.A. Intervertebral disc cell- and hydrogel-supported and spontaneous intervertebral disc repair in nucleotomized sheep. Eur. Spine J. 2012, 21, 1758–1768. [Google Scholar] [CrossRef][Green Version]
- Masuda, K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008, 17, 441–451. [Google Scholar] [CrossRef]
- Smith, L.J.; Gorth, D.J.; Showalter, B.L.; Chairo, J.A.; Beattie, E.E.; Elliott, D.W.; Mauck, R.L.; Chen, W.; Malhotra, N.R. In Vitro Characterization of a Stem-Cell-Seeded Triple-Interpenetrating-Network Hydrogel for Functional Regeneration of the Nucleus Pulposus. Tissue Eng. Part A 2014, 20, 1841–1849. [Google Scholar] [CrossRef]
- Uysal, O.; Arslan, E.; Gulseren, G.; Kilinc, M.C.; Dogan, I.; Ozalp, H.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Collagen Peptide Presenting Nanofibrous Scaffold for Intervertebral Disc Regeneration. Acs Appl. Bio. Mater. 2019, 2, 1686–1695. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Henry, N.; Clouet, J.; Le Bideau, J.; Le Visage, C.; Guicheux, J. Innovative strategies for intervertebral disc regenerative medicine: From cell therapies to multiscale delivery systems. Biotechnol. Adv. 2018, 36, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal. Transduct. Target. Ther. 2021, 6. [Google Scholar] [CrossRef]
- Lee, C.K.; Heo, D.H.; Chung, H.; Roh, E.J.; Darai, A.; Kyung, J.W.; Choi, H.; Kwon, S.Y.; Bhujel, B.; Han, I. Advances in tissue engineering for disc repair. Appl. Sci. 2021, 11, 1919. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Semino, C.E. Self-assembling peptides: From bio-inspired materials to bone regeneration. Crit. Rev. Oral Biol. Med. 2008, 7, 606–616. [Google Scholar] [CrossRef]
- Hoogendoorn, R.J.; Wuisman, P.I.; Smit, T.H.; Everts, V.E.; Helder, M.N. Experimental Intervertebral Disc Degeneration Induced by Chondroitinase ABC in the Goat. Spine 2007, 32, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. Biomed Res. Int. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schwan, S.; Ludtka, C.; Wiesner, I.; Bearthel, A.; Friedmann, A.; Goehre, F. Percutaneous posterolateral approach for the simulation of a far-lateral disc herniation in an ovine model. Eur. Spine J. 2018, 1, 222–230. [Google Scholar] [CrossRef]
- Schwan, S.; Ludtka, C.; Friedmann, A.; Heilmann, A.; Bearthel, A.; Brehm, W.; Wiesner, I.; Meisel, H.J.; Goehre, F. Long-Term Pathology of Ovine Lumbar Spine Degeneration Following Injury Via Percutaneous Minimally Invasive Partial Nucleotomy. J. Orthop. Res. 2019, 37, 2367–2375. [Google Scholar] [CrossRef]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Ganey, T.; Hutton, W.C.; Moseley, T.; Hedrick, M.; Meisel, H.J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine 2009, 34, 2297–2304. [Google Scholar] [CrossRef]
- Kroeze, R.J.; Smit, T.H.; Vergroesen, P.P.; Bank, R.A.; Stoop, R.; Rietbergen, B. van; Royen, B.J. van; Helder, M.N. Spinal fusion using adipose stem cells seeded on a radiolucent cage filler: A feasibility study of a single surgical procedure in goats. Eur. Spine J. 2015, 24, 1031–1042. [Google Scholar] [CrossRef]
- Ying, J.; Han, Z.; Zeng, Y.; Du, S. Y Pei; Su, L.; Ruan, D.; Chen, C. Evaluation of intervertebral disc regeneration with injection of mesenchymal stem cells encapsulated in PEGDA-microcryogel delivery system using quantitative T2 mapping: A study in canines. Am. J. Transl. Res. 2019, 11, 2028–2041. [Google Scholar]
- Bach, F.C.; Tellegen, A.R.; Beukers, M.; Miranda-Bedate, A.; Teunissen, M.; de Jong, W.A.M.; de Vries, S.A.H.; Creemers, L.B.; Benz, K.; Meij, B.P.; et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: From bench towards bedside. Oncotarget 2018, 9, 26507–26526. [Google Scholar] [CrossRef]
- Bach, F.C.; Willems, N.; Penning, L.C.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef]
- Tschugg, A.; Diepers, M.; Simone, S.; Michnacs, F.; Quirbach, S.; Strowitzki, M.; Meisel, H.J.; Thomé, C. A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: Safety results of Phase I-a short report. Neurosurg. Rev. 2017, 40, 155–162. [Google Scholar]
- Gullbrand, S.E.; Smith, L.J.; Smith, H.E.; Mauck, R.L. Promise, progress, and problems in whole disc tissue engineering. JOR Spine 2018, 1, e1015. [Google Scholar] [CrossRef]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation: A treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef]
- Schwan, S.; Ludtka, C.; Friedmann, A.; Mendel, T.; Meisel, H.J.; Heilmann, A.; Kaden, I.; Goehre, F. Calcium Microcrystal Formation in Recurrent Herniation Patients After Autologous Disc Cell Transplantation. Tissue Eng. Regen. Med. 2017, 14, 803–814. [Google Scholar] [CrossRef]
- Ding, F.; Shao, Z.W.; Xiong, L.X. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef]
- Friedmann, A.; Goehre, F.; Ludtka, C.; Mendel, T.; Meisel, H.J.; Heilmann, A.; Schwan, S. Microstructure analysis method for evaluating degenerated intervertebral disc tissue. Micron 2017, 92, 51–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoogendoorn, R.J.W.; Lu, Z.F.; Kroeze, R.J.; Bank, R.A.; Wuisman, P.I.; Helder, M.N. Adipose stem cells for intervertebral disc regeneration: Current status and concepts for the future. J. Cell. Mol. Med. 2008, 12, 2205–2216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Leung, V.Y.L.; Lu, W.W.; Luk, K.D.K. The effects of microenvironment in mesenchymal stem cell–based regeneration of intervertebral disc. Spine J. 2013, 13, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Goldschlager, T.; Ghosh, P.; Rosenfeld, J.V.; Jenkin, G. Cell-Based Therapies Used to Treat Lumbar Degenerative Disc Disease: A Systematic Review of Animal Studies and Human Clinical Trials. Stem Cells Int. 2015, 946031. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Smith, L.J.; Silverman, L.; Sakai, D.; Le Maitre, C.L.; Mauck, R.L.; Malhotra, N.R.; Lotz, J.C.; Buckley, C.T. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. J. Orthopeadic Res. Soc. Spine 2018, 1, 1036–1050. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kaito, T.; Yarimitsu, S.; Hashimoto, K.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Takenaka, S.; Makino, T.; Sakai, Y.; et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019, 87, 118–129. [Google Scholar] [CrossRef]
- Hussain, I.; Sloan, S.R., Jr.; Wipplinger, C.; Navarro-Ramirez, R.; Zubkov, M.; Kim, E.; Kirnaz, S.; Bonassar, L.J.; Härtl, R. Mesenchymal Stem Cell-Seeded High-Density Collagen Gel for Annular Repair: 6-Week Results From In Vivo Sheep Models. Neurosurgery 2019, 85, 350–359. [Google Scholar] [CrossRef]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar]
- Tomatszewski, K.; Waloche, A.J.; Mizia, E.; Gladysz, T.; Glowacki, R.; Tomaszewska, R. Age- and degeneration-related variations in cell density and glycosaminoglycan content in the human cervical intervertebral disc and its endplates. Pol. J. Pathol. 2019, 66, 296–300. [Google Scholar]
- Chen, Y.; Ouyang, X.; Wu, Y.; Guo, S.; Xie, Y.; Wang, G. Co-culture and Mechanical Stimulation on Mesenchymal Stem Cells and Chondrocytes for Cartilage Tissue Engineering. Curr. Stem Cell Res. Ther. 2020, 15, 54–60. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Kim, K. Engineered Co-culture Strategies Using Stem Cells for Facilitated Chondrogenic Differentiation and Cartilage Repair. Biotechnol. Bioprocess. Eng. 2018, 23, 261–270. [Google Scholar] [CrossRef]
- Huazheng, Q.; Yang, G.; Zhao, Y.; Wang, J.; Li, T. Chondrogenic Differentiation of Adipose-Derived Stem Cells Induced by Co-Culture with Chondrocytes and Synoviocytes. J. Biomater. Tissue Eng. 2017, 7, 1021–1027. [Google Scholar]
- Liu, X.; Souzandeh, H.; Xie, Y.; Zhong, W.H.; Wang, C. Soy protein isolate/bacterial cellulose composite membranes for high efficiency particulate air filtration. Compos. Sci. Technol. 2017, 138, 124–133. [Google Scholar] [CrossRef]
- Chowghury, T.T.; Schulz, R.M.; Rai, S.S.; Thuemmler, C.B.; Wuestneck, N.; Bader, A.; Homandberg, G.A. RBeiseoarmch aertciclhe anical modulation of collagen fragment-induced anabolic and catabolic activities in chondrocyte/agarose constructs. Arthritis Res. Ther. 2010, 12. [Google Scholar]
- Dabbs, V.M.; Dabbs, L. Correlation Between Disc Height Narrowing and Low-Back Pain. Spine 1990, 15, 1366–1368. [Google Scholar] [CrossRef]
- Mulisch, M.; Welsch, U. Romei-Mikroskopische Technik; Springer Spektrum: Berlin/Heidelberg, Germany, 2010. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedmann, A.; Baertel, A.; Schmitt, C.; Ludtka, C.; Milosevic, J.; Meisel, H.-J.; Goehre, F.; Schwan, S. Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. Int. J. Mol. Sci. 2021, 22, 4248. https://doi.org/10.3390/ijms22084248
Friedmann A, Baertel A, Schmitt C, Ludtka C, Milosevic J, Meisel H-J, Goehre F, Schwan S. Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. International Journal of Molecular Sciences. 2021; 22(8):4248. https://doi.org/10.3390/ijms22084248
Chicago/Turabian StyleFriedmann, Andrea, Andre Baertel, Christine Schmitt, Christopher Ludtka, Javorina Milosevic, Hans-Joerg Meisel, Felix Goehre, and Stefan Schwan. 2021. "Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model" International Journal of Molecular Sciences 22, no. 8: 4248. https://doi.org/10.3390/ijms22084248
APA StyleFriedmann, A., Baertel, A., Schmitt, C., Ludtka, C., Milosevic, J., Meisel, H.-J., Goehre, F., & Schwan, S. (2021). Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. International Journal of Molecular Sciences, 22(8), 4248. https://doi.org/10.3390/ijms22084248





