Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Effect of Bee Bread on Lee Obesity Index, Lipid Profile and Atherogenic Ratios in Obese Rats
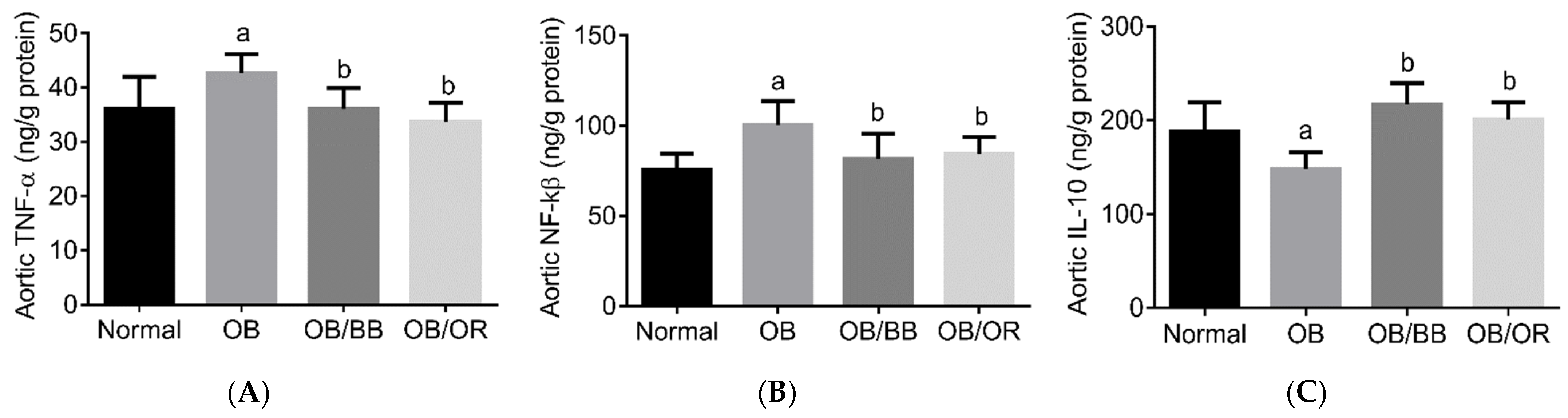
2.2. Effect of Bee Bread on Aortic Inflammatory Markers in Obese Rats
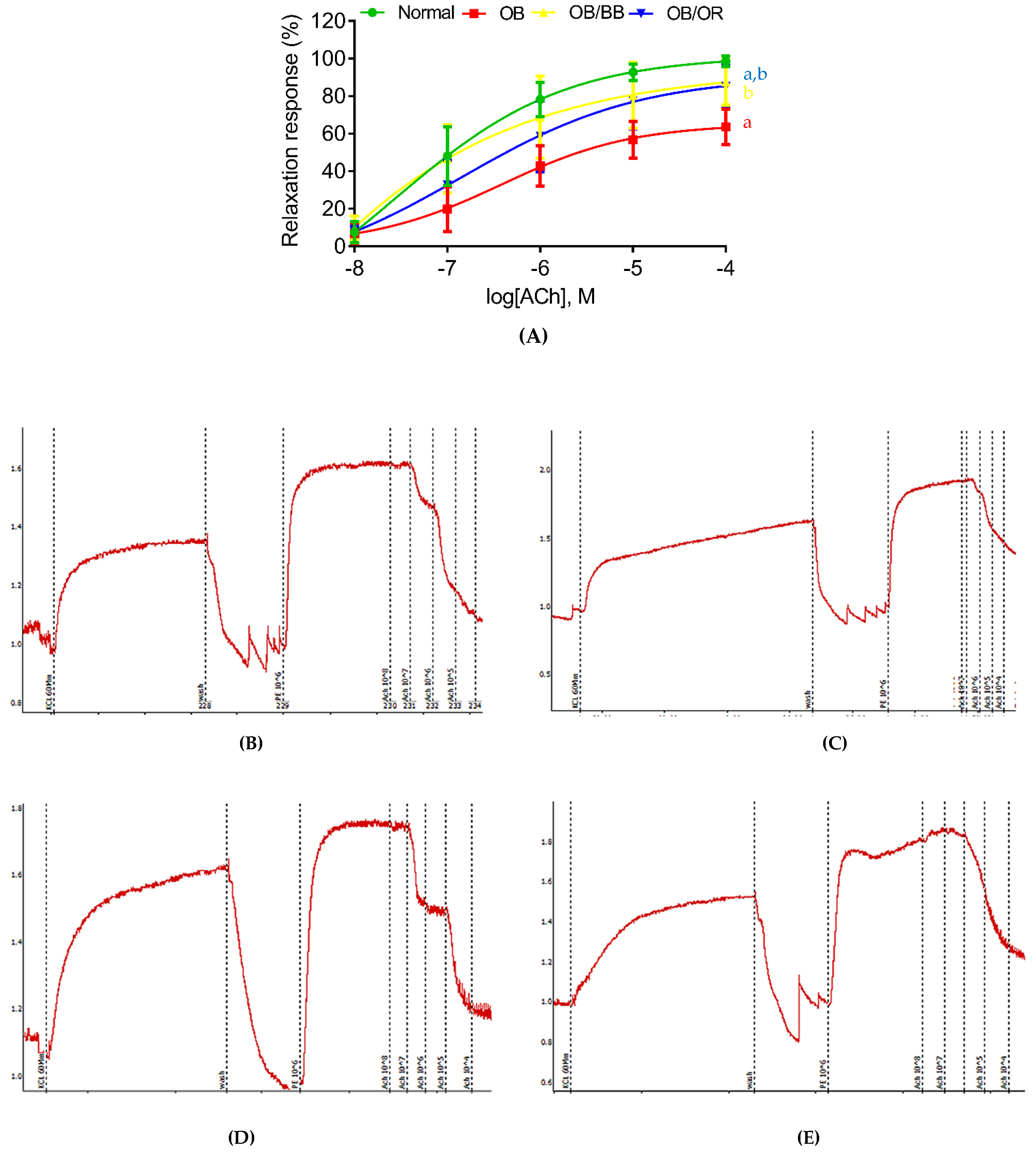
2.3. Effect of Bee Bread on Endothelium-Dependent Vascular Reactivity in Obese Rats
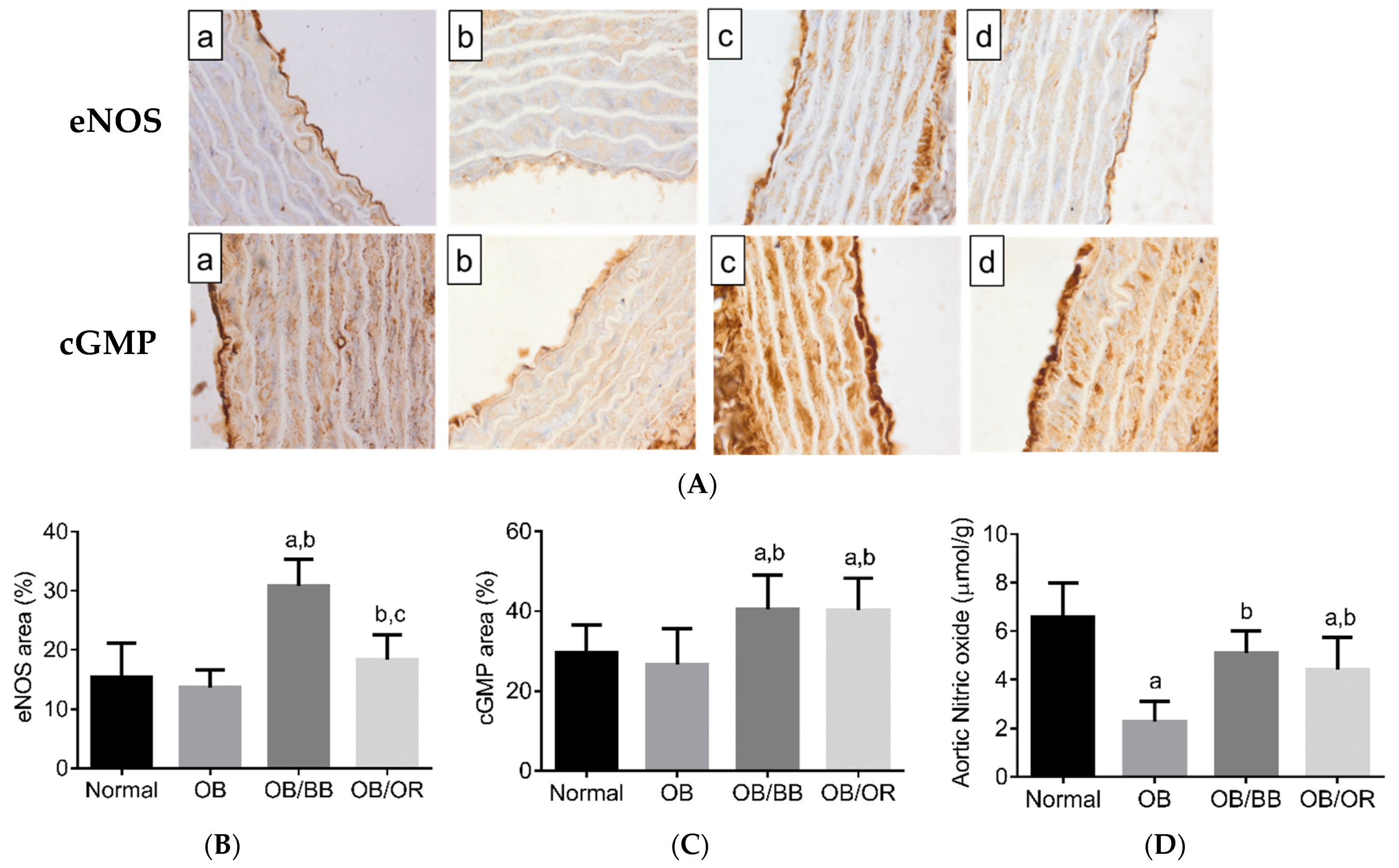
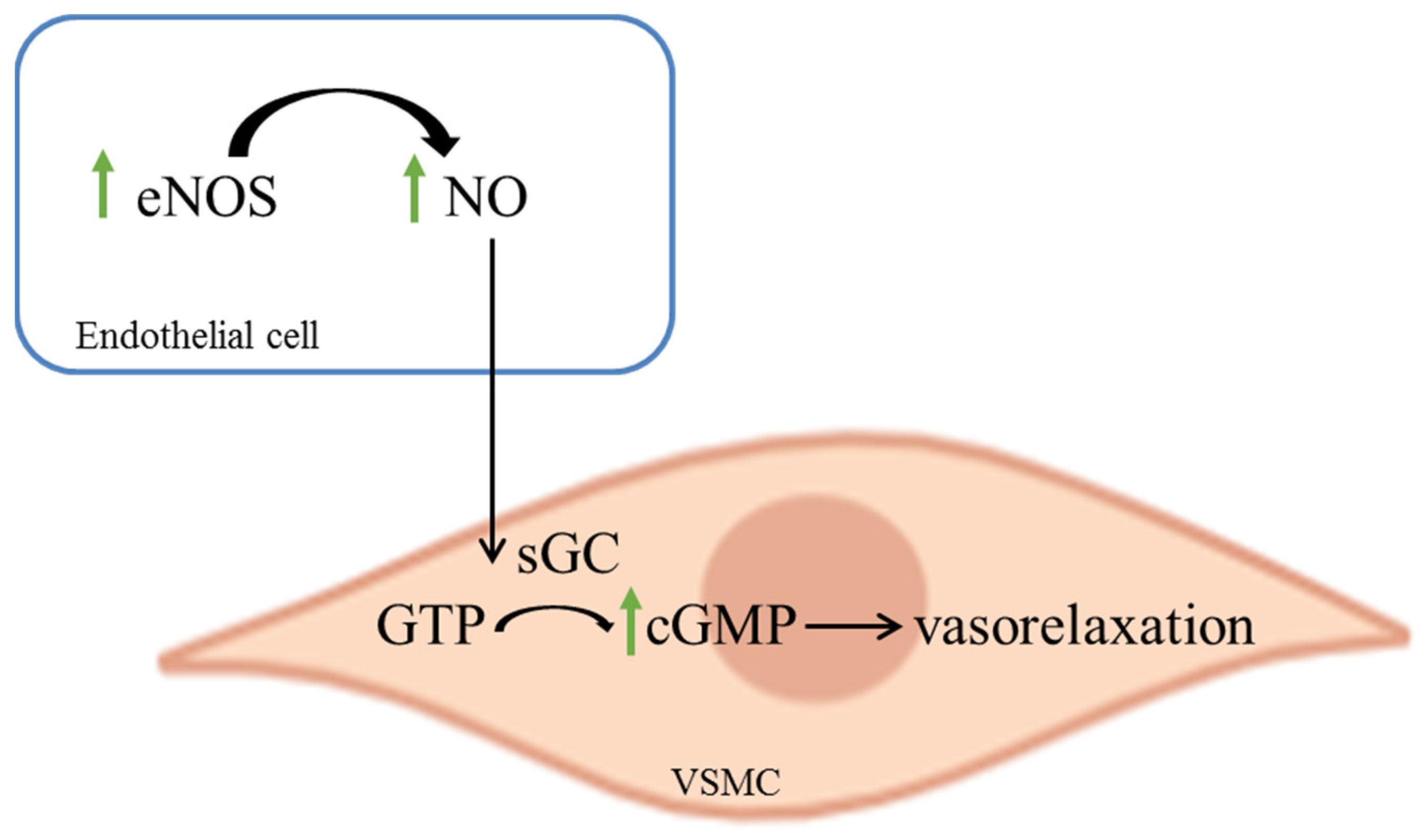
2.4. Effect of Bee Bread on Immunoexpression of eNOS and cGMP, and NO Level in the Aorta of Obese Rats
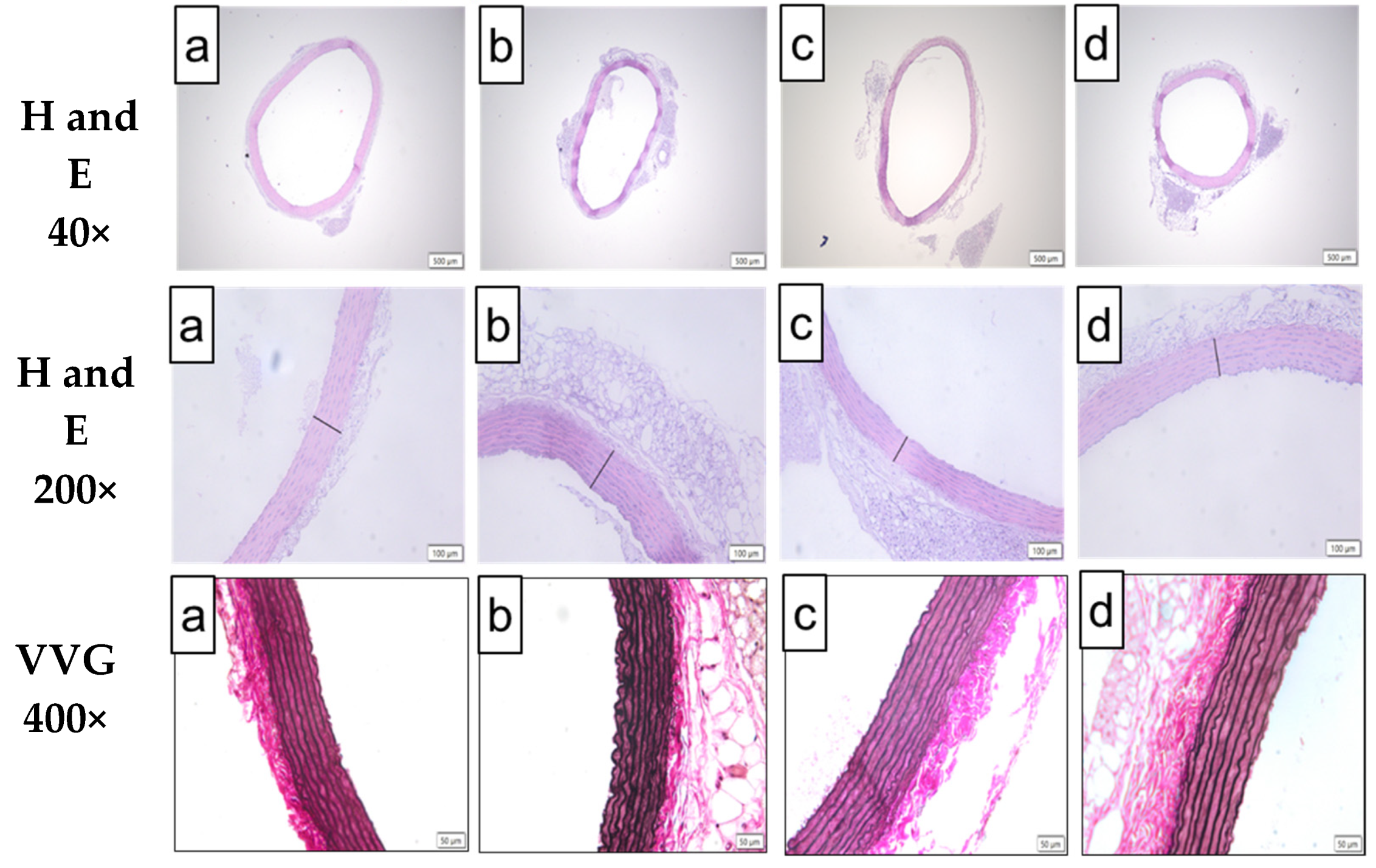
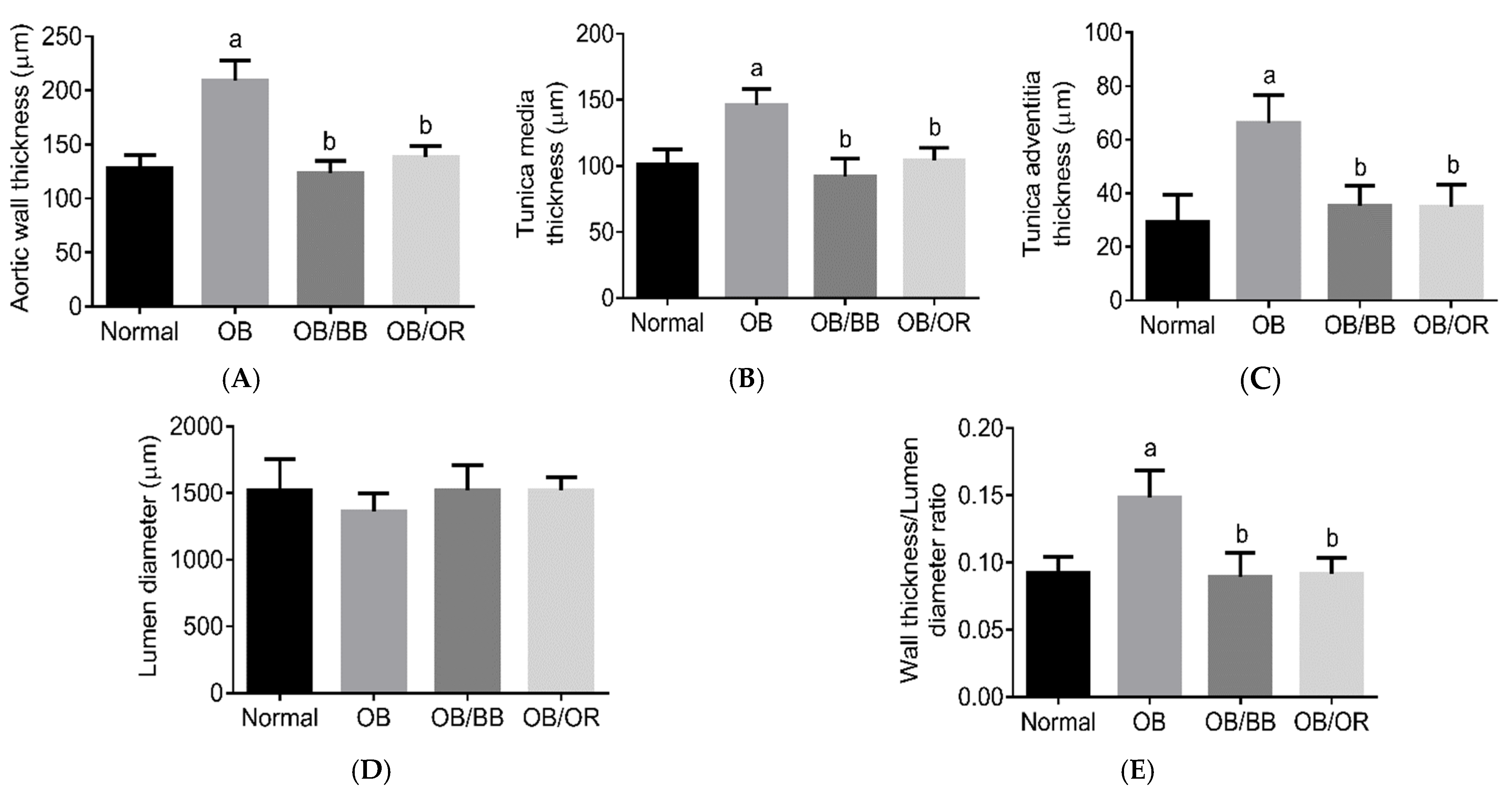
2.5. Effect of Bee Bread on Morphology of Aorta in Obese Rats
2.6. Mineral Composition of Bee Bread
3. Discussion
4. Materials and Methods
4.1. Chemical and Drugs
4.2. Preparation of Bee Bread
4.3. Animals and Diet
4.4. Experimental Design
4.5. Measurement of Obesity Parameters
4.6. Measurement of Lipid Profile and Atherogenic Ratios
4.7. Determination of Aortic Inflammatory Markers
4.8. Determination of Aortic NO Level
4.9. Preparation of Aorta and Measurement of Vascular Reactivity
4.10. Immunohistochemistry
4.11. Aorta histological Analysis
4.12. Mineral Analysis of Bee Bread
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). World Health Organization, 2009. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed on 12 January 2021).
- Amiri, M.; Majid, H.A.; Hairi, F.; Thangiah, N.; Bulgiba, A.; Su, T.T. Prevalence and determinants of cardiovascular disease risk factors among the residents of urban community housing projects in Malaysia. BMC Public Health 2014, 14 (Suppl 3), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, F.F.; Moreno, B.; Rodríguez-Azeredo, R.; Massien, C.; Conthe, P.; Formiguera, X.; Barrios, V.; Balkau, B. Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clin. Endocrinol. 2010, 73, 35–40. [Google Scholar] [CrossRef]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wang, Q.; Zhou, X.; Li, Y. Endothelial dysfunction in renal arcuate arteries of obese Zucker rats: The roles of nitric oxide, endothelium-derived hyperpolarizing factors, and calcium-activated K+ channels. PLoS ONE 2017, 12, e0183124. [Google Scholar] [CrossRef]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Malyszko, J. Endothelial Dysfunction and nitric oxide: Albuminuria as a central marker. In Cardio-Nephrology; Springer: Cham, Switzerland, 2017; pp. 3–9. [Google Scholar]
- Cabrera-Pastor, A.; Malaguarnera, M.; Taoro-Gonzalez, L.; Llansola, M.; Felipo, V. Extracellular cGMP modulates learning biphasically by modulating glycine receptors, CaMKII and glutamate-nitric oxide-cGMP pathway. Sci. Rep. 2016, 6, 33124. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, Y.J.; Han, B.H.; Yoon, J.J.; Ahn, Y.M.; Hong, M.H.; Tan, R.; Kang, D.G.; Lee, H.S. Mantidis ootheca induces vascular relaxation through PI3K/AKT-mediated nitric oxide-cyclic GMP-protein kinase G signaling in endothelial cells. J. Physiol. Pharmacol. 2017, 68, 215–221. [Google Scholar]
- Alexandre, E.C.; Leiria, L.O.; Silva, F.H.; Mendes-Silvério, C.B.; Calmasini, F.B.; Davel, A.P.C.; Mónica, F.Z.; De Nucci, G.; Antunes, E. Soluble guanylyl cyclase (sGC) degradation and impairment of nitric oxide-mediated responses in urethra from obese mice: Reversal by the sGC activator BAY 60-2770. J. Pharmacol. Exp. Ther. 2014, 349, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Cynanchum wilfordii ameliorates hypertension and endothelial dysfunction in rats fed with high fat/cholesterol diets. Immunopharmacol. Immunotoxicol. 2012, 34, 4–11. [Google Scholar] [CrossRef]
- Gulhan, M.F. Therapeutic potentials of propolis and pollen on biochemical changes in reproductive function of L-NAME induced hypertensive male rats. Clin. Exp. Hypertens. 2019, 41, 292–298. [Google Scholar] [CrossRef]
- Liang, Y.; Kagota, S.; Maruyama, K.; Oonishi, Y.; Miyauchi-Wakuda, S.; Ito, Y.; Yamada, S.; Shinozuka, K. Royal jelly increases peripheral circulation by inducing vasorelaxation through nitric oxide production under healthy conditions. Biomed. Pharmacother. 2018, 106, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, C.M.; Serrato, J.C.; Quicazan, M.C. Chemical, nutritional and bioactive characterization of Colombian bee-bread. Chem. Eng. Trans. 2015, 43, 175–180. [Google Scholar]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbettia, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Akhir, R.A.M.; Bakar, M.F.A.; Sanusi, S.B. Antioxidant and antimicrobial activity of stingless bee bread and propolis extracts. In Proceedings of the 2nd International Conference on Applied Science and Technology 2017 (ICAST’17), Kedah, Malaysia, 3–5 April 2017; AIP Publishing: Melville, NY, USA, 2017; p. 020090. [Google Scholar]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Duenas, M.; Tomas, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid composition and antitumour activity of bee bread collected in northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Ceksteryte, V.; Balzekas, J. The use of beebread—Honey mixture in the treatment of liver diseases in alcohol-dependent patients. J. Chem. Technol. 2012, 2, 62–66. [Google Scholar]
- Othman, Z.A.; Ghazali, W.S.W.; Noordin, L.; Yusof, N.A.M.; Mohamed, M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants 2019, 9, 33. [Google Scholar] [CrossRef]
- Zhou, B.; Ren, C.; Zu, L.; Zheng, L.; Guo, L.; Gao, W. Elevated plasma migration inhibitory factor in hypertension–hyperlipidemia patients correlates with impaired endothelial function. Medicine 2016, 95, e5207. [Google Scholar] [CrossRef]
- Bellinger, L.L.; Bernardis, L.L. Effect of dorsomedial hypothalamic nuclei knife cuts on ingestive behavior. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R1772–R1779. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.K.; Tyagi, S. Atherogenic index of plasma, castelli risk index and atherogenic coefficient—New parameters in assessing cardiovascular risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364. [Google Scholar]
- Prince, P.S.M.; Kumaran, K.S. Preventive effects of caffeic acid on lipids, lipoproteins and glycoproteins in isoproterenol induced myocardial infarcted rats. Food Res. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pingeesakikul, T.; Tirawanchai, N.; Tuntipopipat, S.; Lilitchan, S.; Komindr, S. Effects of Ferulic Acid Supplementation on Lipid Profiles, Oxidative Stress and Inflammatory Status in Hypercholesterolemic Subjects. FASEB J. 2012, 1, 263–267. [Google Scholar]
- Jeepipalli, S.P.K.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Gomaa, A.A.; El-Sers, D.A.; Al-Zokeim, N.I.; Gomaa, M.A. Amelioration of experimental metabolic syndrome induced in rats by orlistat and Corchorus olitorius leaf extract; role of adipocytokines. J. Pharm. Pharmacol. 2018, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Audikovszky, M.; Pados, G.; Seres, I.; Harangi, M.; Fülöp, P.; Katona, E.; Winkler, G.; Paragh, G. Changes in lipid profile and paraoxonase activity in obese patients as a result of orlistat treatment. Orv. Hetil. 2001, 142, 2779–2783. [Google Scholar] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, X.; Liu, X.; Liu, Y.; Zhang, W.; Huang, Q.; Liu, W.; Xiong, L.; Tan, R.; Wang, H.; et al. Weighted gene co-expression network analysis identifies FKBP11 as a key regulator in acute aortic dissection through a NF-kB dependent pathway. Front. Physiol. 2017, 8, 1010. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Nna, V.U.; Zakaria, Z.; Othman, Z.A.; Bakar, A.B.A.; Mohamed, M. Obesity-induced testicular oxidative stress, inflammation and apoptosis: Protective and therapeutic effects of orlistat. Reprod. Toxicol. 2020, 95, 113–122. [Google Scholar] [CrossRef]
- Eleazu, C.; Suleiman, J.B.; Othman, Z.A.; Nna, V.U.; Hussain, N.; Mohamed, M. Bee bread attenuates high fat diet induced renal pathology in obese rats via modulation of oxidative stress, downregulation of NF-kB mediated inflammation and Bax signalling. Arch. Physiol. Biochem. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringeni, and pelargonidin inhibit only NF-κB activation along with their inhibitory ef. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar] [CrossRef]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Ghazali, W.S.W.; Mohamed, M. Anti-atherogenic effects of orlistat on obesity-induced vascular oxidative stress rat model. Antioxidants 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Mallat, R.K.; John, C.M.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Cai, Y.; He, C.; Li, Z. Coupled Modeling of Lipid Deposition, Inflammatory Response and Intraplaque Angiogenesis in Atherosclerotic Plaque. Ann. Biomed. Eng. 2019, 47, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Lastra, G.; Manrique, C.; Jia, G.; Aroor, A.R.; Hayden, M.R.; Barron, B.J.; Niles, B.; Padilla, J.; Sowers, J.R. Xanthine oxidase inhibition protects against Western diet-induced aortic stiffness and impaired vasorelaxation in female mice. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.M.; Abou-Nazel, M.W.; Karawya, F.S.; Omar, A.M. The Possible Protective Effects of Inegy versus Cinnamon Oil on the Aorta of Albino Rats with Experimentally Induced Hyperlipidemia. Int. J. Clin. Exp. Med. Sci. 2016, 1, 78–91. [Google Scholar]
- Godo, S.; Shimokawa, H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic. Biol. Med. 2017, 109, 4–10. [Google Scholar] [CrossRef]
- Anwar, M.A.; Samaha, A.A.; Ballan, S.; Saleh, A.I.; Iratni, R.; Eid, A.H. Salvia fruticosa Induces vasorelaxation in rat isolated thoracic aorta: Role of the PI3K/Akt/eNOS/NO/cGMP signaling pathway. Sci. Rep. 2017, 7, 686. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Kim, S.; Kim, J.D.; Lee, J.J.; Baek, Y.Y.; Jeoung, D.; Lee, H.; Jongseon, C.; Ha, K.-S.; Won, M.-H.; et al. Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase. Exp. Mol. Med. 2012, 44, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Shamsaldeen, Y.A.; Ugur, R.; Benham, C.D.; Lione, L.A. Diabetic dyslipidaemia is associated with alterations in eNOS, caveolin-1, and endothelial dysfunction in streptozotocin treated rats. Diabetes Metab. Res. Rev. 2018, 34, e2995. [Google Scholar] [CrossRef] [PubMed]
- Schirra, C.; Xia, N.; Schuffler, A.; Heck, A.; Hasselwander, S.; Forstermann, U.; Li, H. Phosphorylation and activation of endothelial nitric oxide synthase by red fruit (Pandanus conoideus Lam) oil and its fractions. J. Ethnopharmacol. 2019, 251, 112534. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; Seiringer, G.; Sykora, C.; Dirsch, V.M.; Zehl, M.; Kopp, B. Evaluation of Apricot, Bilberry, and Elderberry Pomace Constituents and Their Potential To Enhance the Endothelial Nitric Oxide Synthase (eNOS) Activity. ACS Omega 2018, 3, 10545–10553. [Google Scholar] [CrossRef] [PubMed]
- Fetherolf, M.M.; Boyd, S.D.; Taylor, A.B.; Kim, H.J.; Wohlschlegel, J.A.; Blackburn, N.J.; Hart, P.J.; Winge, D.R.; Winkler, D.D. Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site. J. Biol. Chem. 2017, 292, 12025–12040. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Friedewald, W.; Levy, R.; Fredrickson, D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Jeevangi, S.; Manjunath, S.; Sakhare, P. A study of anti-hyperlipidemia, hypolipedimic and anti-atherogenic activity of fruit of emblica officinalis (Amla) in high fat fed albino rats. Int. J. Med. Res. Heal. Sci. 2013, 2, 70–77. [Google Scholar]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels: The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Lee, K.; Ham, I.; Yang, G.; Lee, M.; Bu, Y.; Kim, H.; Choi, H.Y. Vasorelaxant effect of Prunus yedoensis bark. BMC Complement. Altern. Med. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Normal | OB | OB/BB | OB/OR |
|---|---|---|---|---|
| Lee obesity index | 304.85 (7.20) | 332.13 (7.15) a | 311.42 (6.94) b | 316.70 (7.81) b |
| TC (mmol/L) | 1.61 (0.16) | 2.63 (0.65) a | 1.99 (0.15) b | 2.05 (0.20) b |
| TG (mmol/L) | 0.50 (0.08) | 0.92 (0.10) a | 0.59 (0.16) b | 0.66 (0.14) b |
| LDL (mmol/L) | 0.93 (0.19) | 1.65 (0.57) a | 1.10 (0.35) b | 1.08 (0.24) b |
| HDL (mmol/L) | 0.41 (0.10) | 0.40 (0.05) | 0.49 (0.10) | 0.66 (0.09) a,b,c |
| Atherogenic index | 2.361 (0.57) | 4.24 (0.73) a | 3.18 (0.52) b | 2.49 (0.62) b |
| Castelli risk index 1 | 3.75 (0.96) | 5.63 (0.33) a | 4.07 (0.47) b | 3.49 (0.62) b |
| Castelli risk index 11 | 2.04 (0.56) | 3.53 (0.69) a | 2.53 (0.33) b | 2.36 (0.51) b |
| Parameters | Normal | OB | OB/BB | OB/OR |
|---|---|---|---|---|
| Emax KCl (g) | 0.44 (0.08) | 0.47 (0.18) | 0.55 (0.13) | 0.44 (0.1) |
| Emax PE (g) | 0.60 (0.14) | 0.66 (0.15) | 0.71 (0.13) | 0.64 (0.14) |
| Emax ACh (%) | 98.43 (3.01) | 65.71 (11.14) a | 87.50 (12.38) b | 82.91 (12.28) a,b |
| pEC50 | 6.77 (0.07) | 6.28 (0.10) a | 6.77 (0.15) b | 6.43 (0.12) |
| Minerals | Composition (mg/kg) |
|---|---|
| Calcium | 1108.48 |
| Copper | 11.05 |
| Iron | 56.58 |
| Magnesium | 1530.87 |
| Potassium | 7323.04 |
| Selenium | 0.13 |
| Sodium | 252.73 |
| Zinc | 42.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Nna, V.U.; Che Romli, A.; Wan Ghazali, W.S.; Mohamed, M. Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4225. https://doi.org/10.3390/ijms22084225
Othman ZA, Zakaria Z, Suleiman JB, Nna VU, Che Romli A, Wan Ghazali WS, Mohamed M. Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(8):4225. https://doi.org/10.3390/ijms22084225
Chicago/Turabian StyleOthman, Zaidatul Akmal, Zaida Zakaria, Joseph Bagi Suleiman, Victor Udo Nna, Aminah Che Romli, Wan Syaheedah Wan Ghazali, and Mahaneem Mohamed. 2021. "Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway" International Journal of Molecular Sciences 22, no. 8: 4225. https://doi.org/10.3390/ijms22084225
APA StyleOthman, Z. A., Zakaria, Z., Suleiman, J. B., Nna, V. U., Che Romli, A., Wan Ghazali, W. S., & Mohamed, M. (2021). Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway. International Journal of Molecular Sciences, 22(8), 4225. https://doi.org/10.3390/ijms22084225










