Characterization of Dog Glutathione Transferase P1-1, an Enzyme Relevant to Veterinary Medicine
Abstract
1. Introduction
2. Results
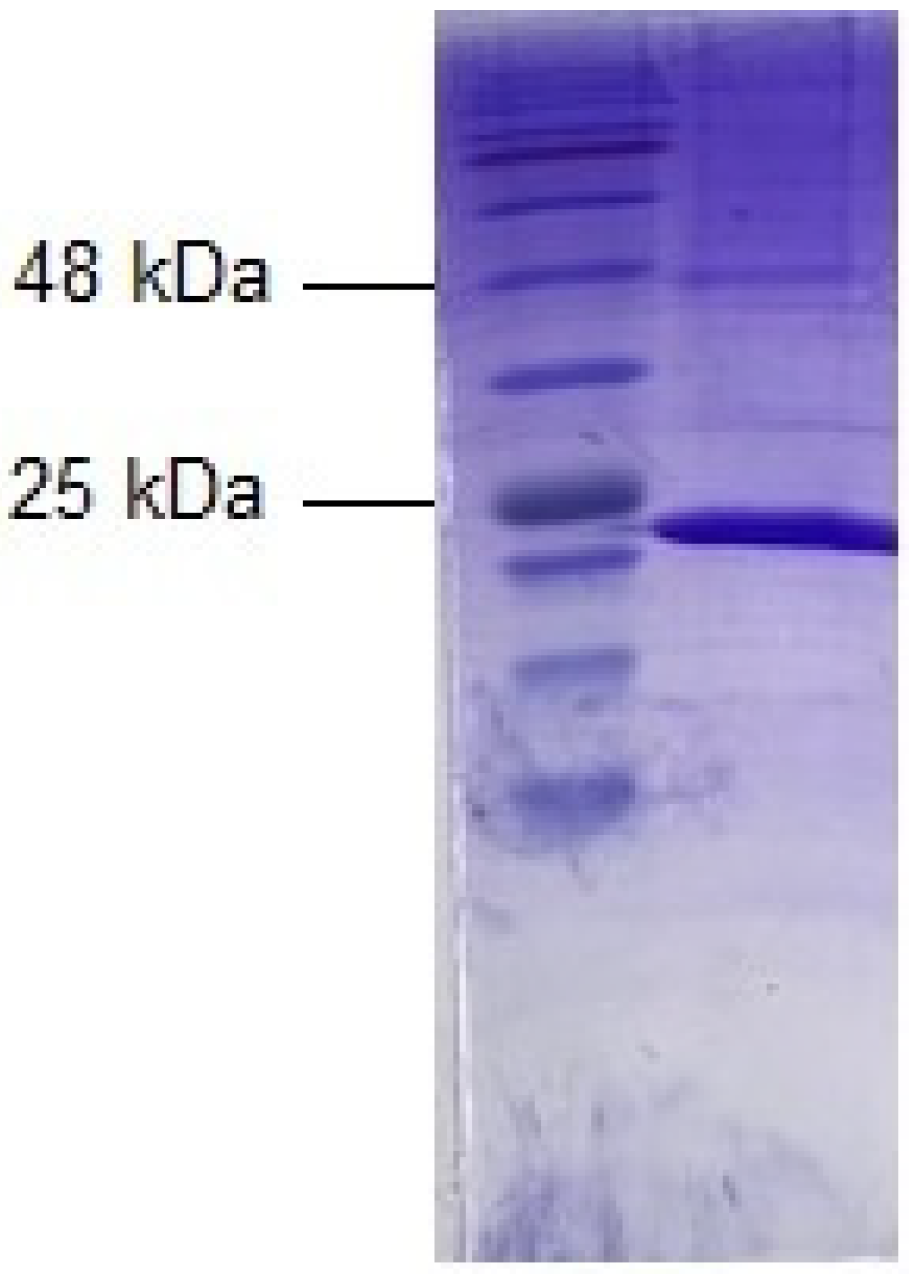
2.1. Expression, Purification, and Characterization of Dog GST P1-1
2.2. Primary Structure
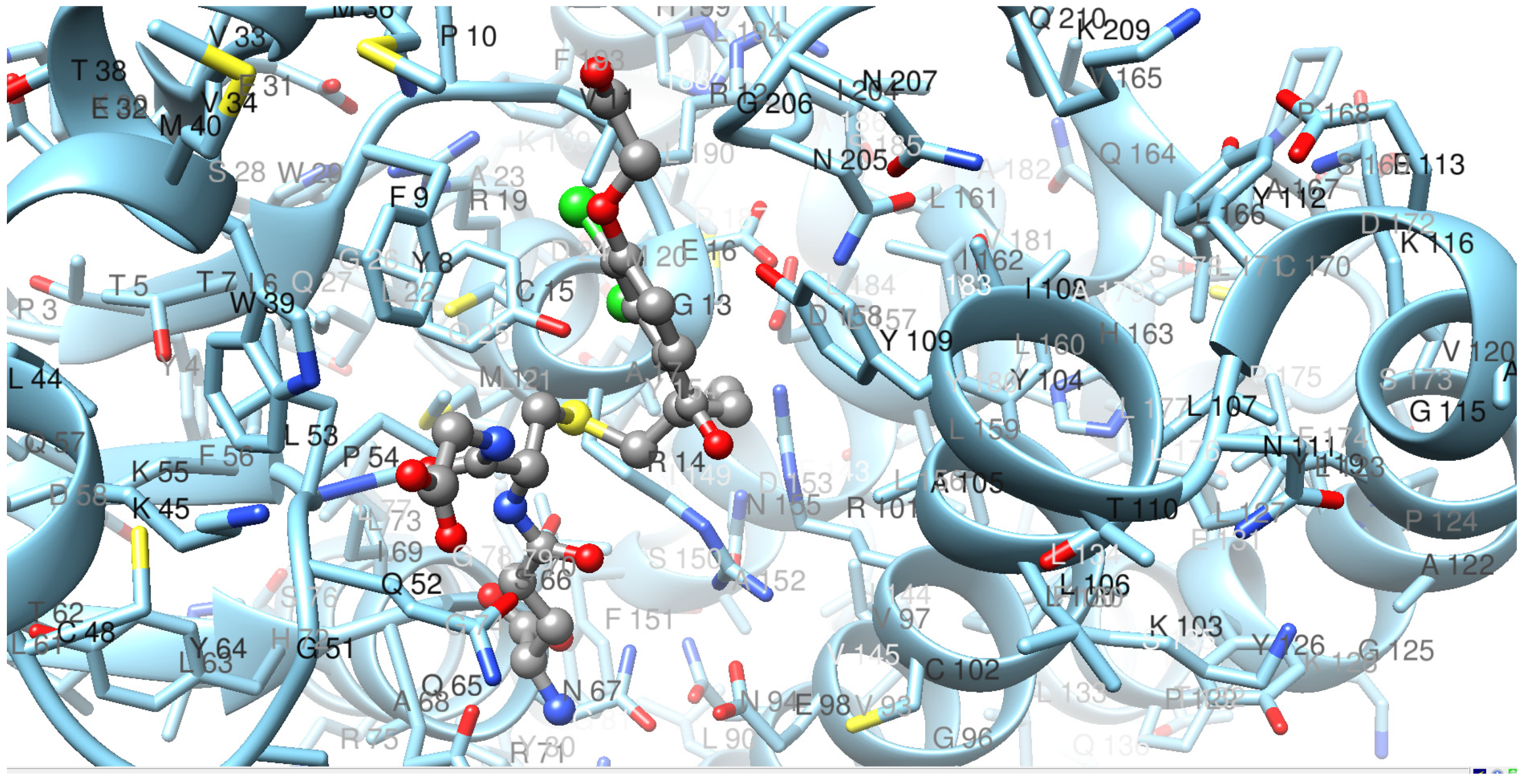
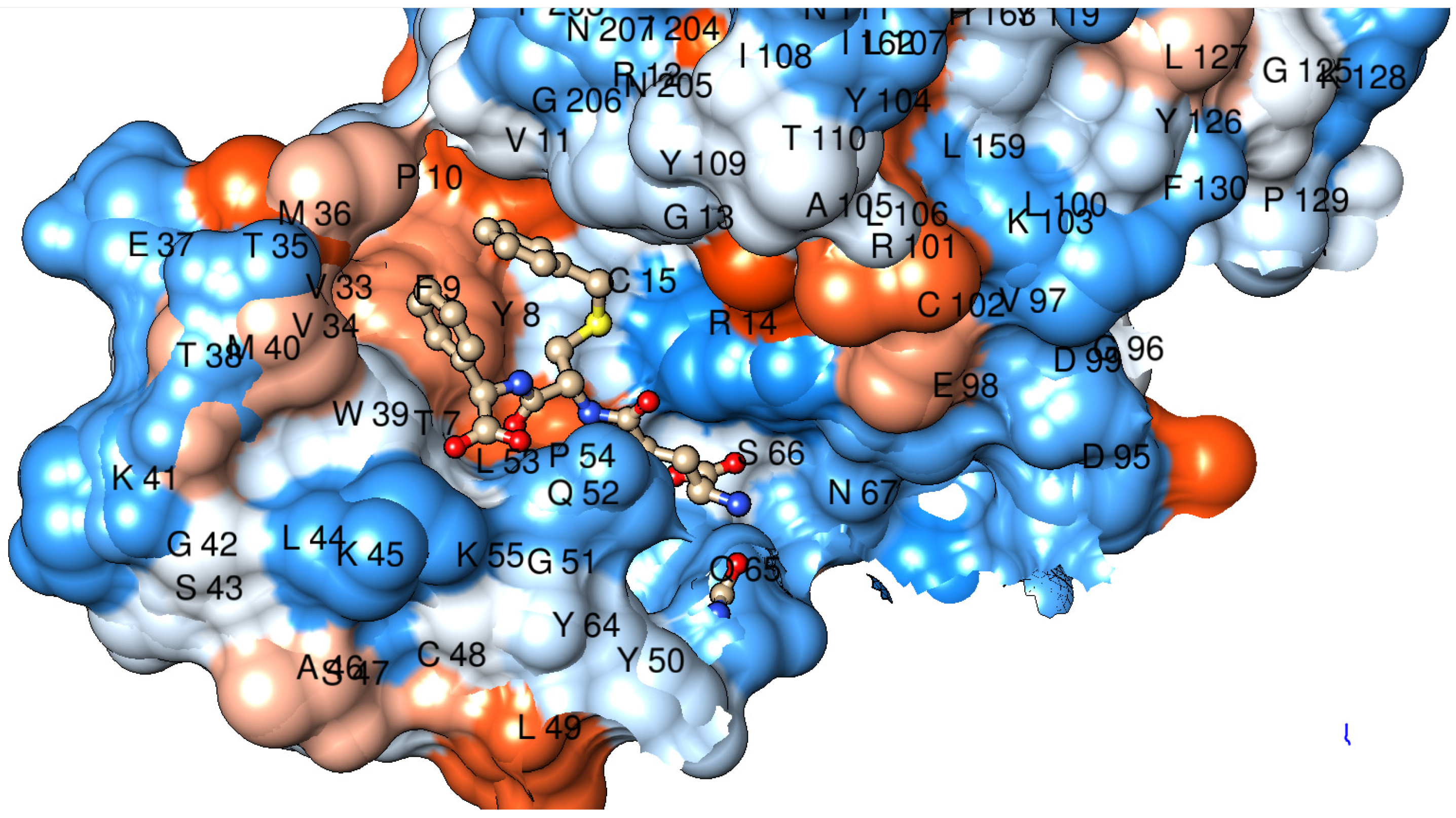
2.3. Tertiary Structure
2.4. Activities with Alternative Substrates
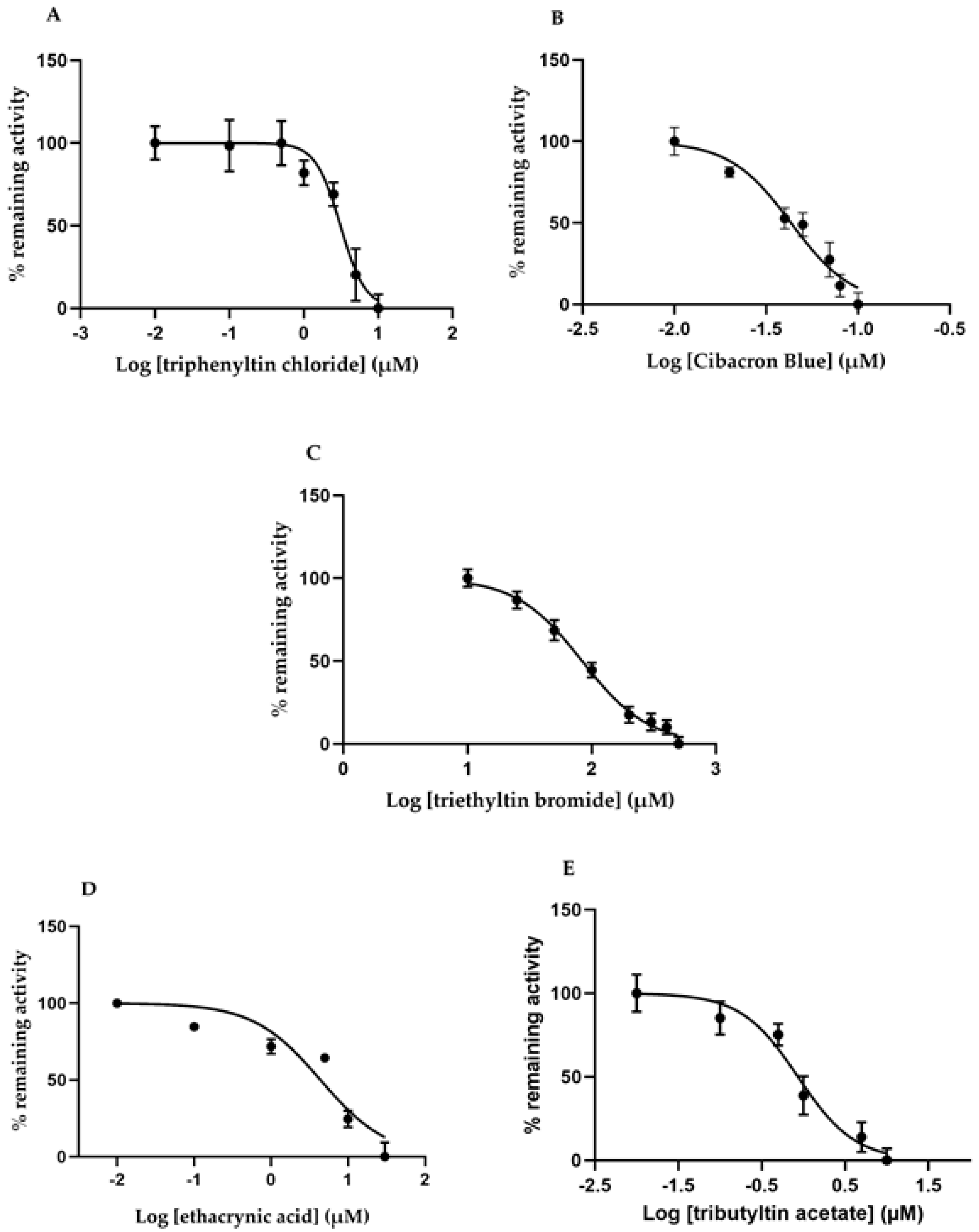
2.5. Effects of Different GST Inhibitors
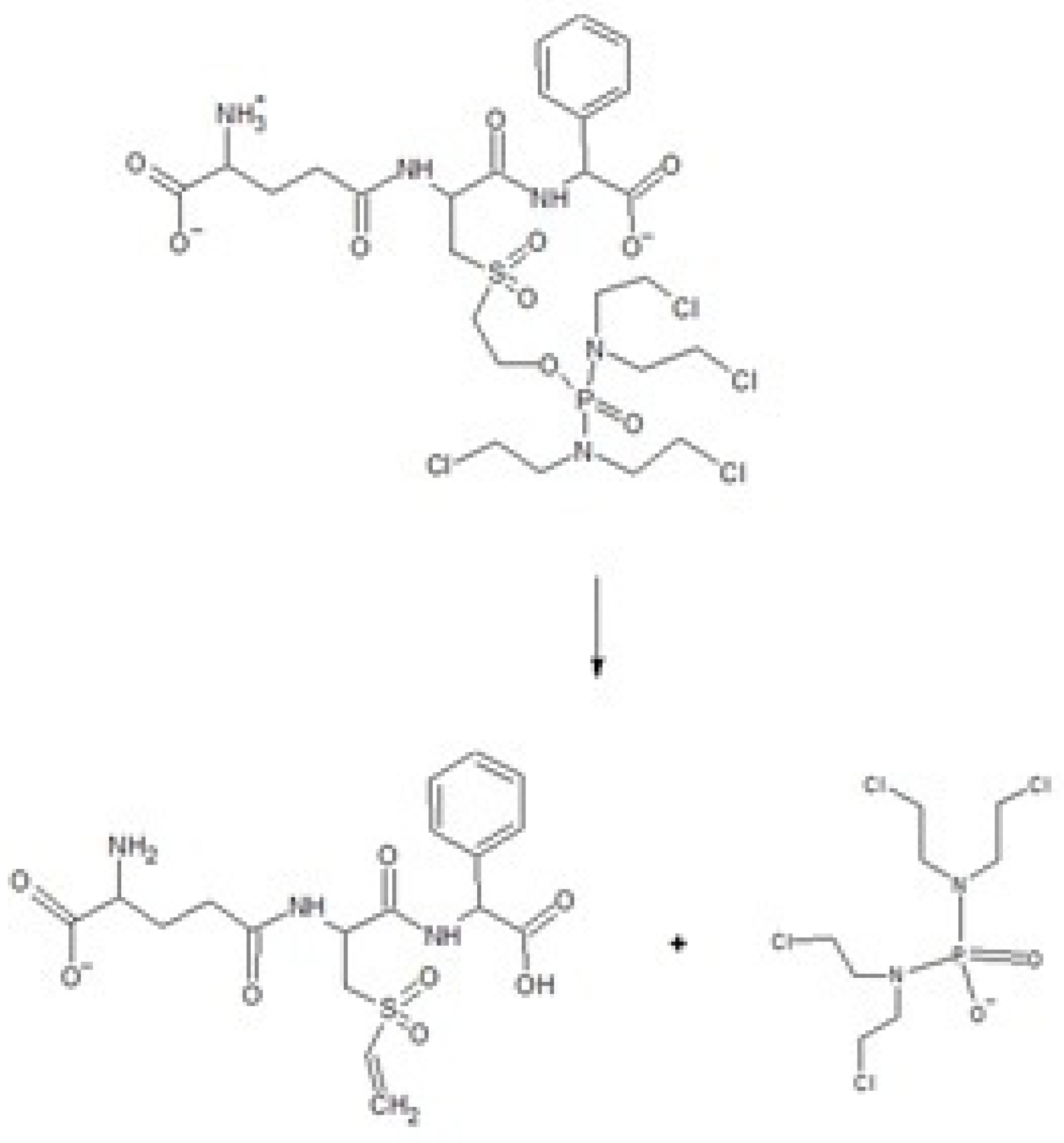
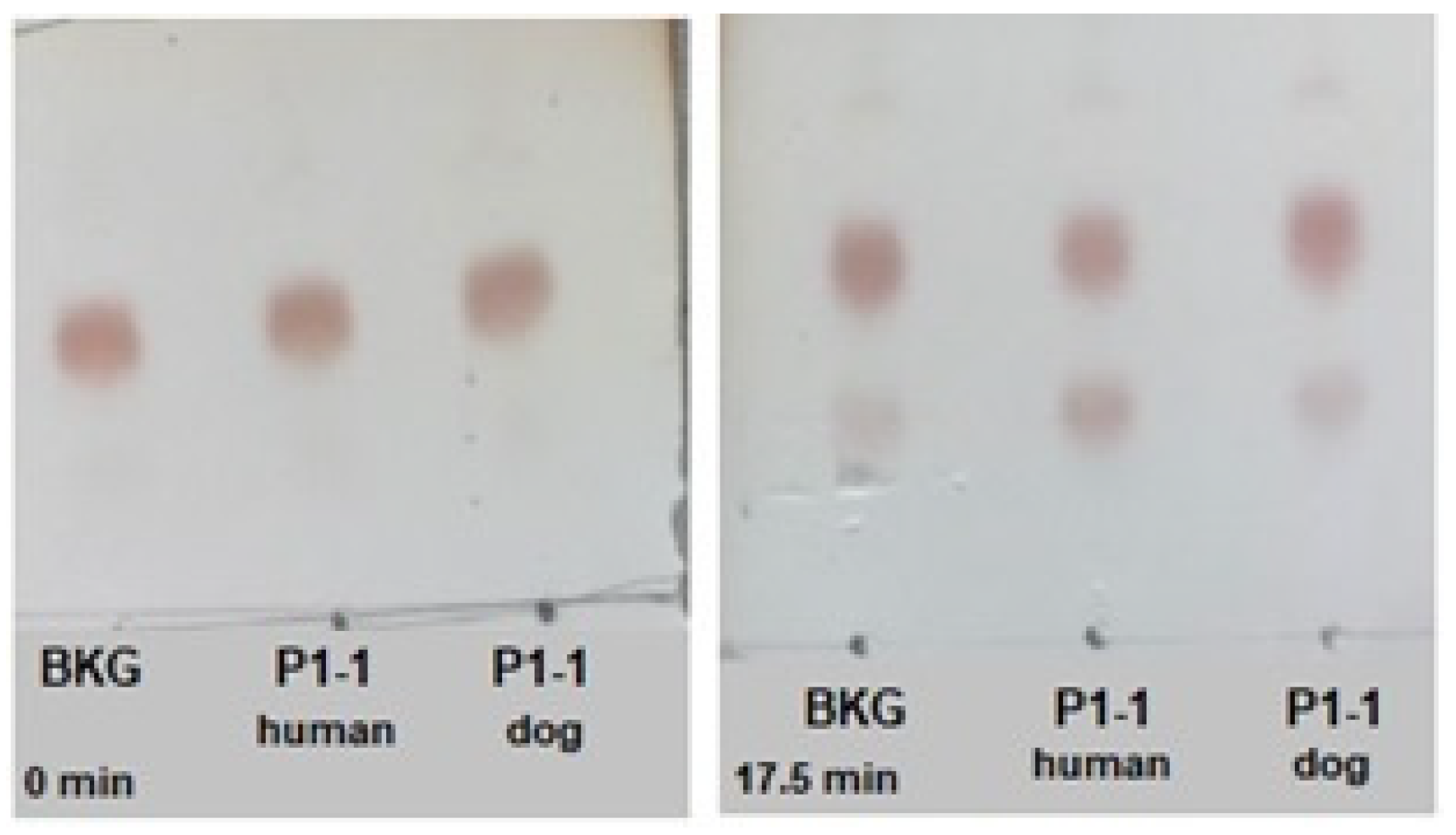
2.6. Activation of the Prodrug Telcyta
3. Discussion
4. Materials and Methods
4.1. Heterologous Expression and Purification of Dog GST P1-1
4.2. SDS-PAGE
4.3. Enzyme Activity Measurements
4.4. Thin-Layer Chromatography to Assay Activity with the Prodrug Telcyta
4.5. Modeling of Protein Structures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GSH | Glutathione |
| GST | Glutathione transferases |
| Clu | Canis lupus familiaris |
| CDNB | 1-Chloro-2,4-dinitrobenzene |
| PEITC | Phenethyl Isothiocyanate |
| BITC | Benzyl isothiocyanate |
| AITC | Allyl isothiocyanate |
| PITC | Propyl isothiocyanate |
| cHITC | Cyclohexyl isothiocyanate |
| CuOOH | Cumene hydroperoxide |
| EA | Ethacrynic acid |
| AD | Δ5-Androstene-3,17-dione |
| Telcyta (TLK286) | γ-l-Glutamyl-α-amino-β-(2-ethyl-N,N,N′,N′-tetrakis(2-chloroethyl)phosphorodiamidate)sulfonylpropionyl-R(−)phenylglycine |
| TLK117 | γ-l-Glutamyl-S-(benzyl)-l-cysteinyl-R(−)-phenylglycine |
References
- Josephy, P.D.; Mannervik, B. Molecular Toxicology, 2nd ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; pp. 333–364. [Google Scholar]
- Boyland, E.; Chasseaud, L.F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 173–219. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.E.; Anders, M.W. The mercapturic acid pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef]
- Mannervik, B. Glutathione and the Evolution of Enzymes for Detoxication of Products of Oxygen-Metabolism. Chem. Scr. 1986, 26B, 281–284. [Google Scholar]
- Jakobsson, P.J.; Morgenstern, R.; Mancini, J.; Ford-Hutchinson, A.; Persson, B. Common structural features of MAPEG—A widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 1999, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Board, P.G.; Hayes, J.D.; Listowsky, I.; Pearson, W.R. Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol. 2005, 401, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.M.; Low, W.Y. Rapid birth-death evolution and positive selection in detoxification-type glutathione S-transferases in mammals. PLoS ONE 2018, 13, e209336. [Google Scholar] [CrossRef]
- Mannervik, B.; Ålin, P.; Guthenberg, C.; Jensson, H.; Tahir, M.K.; Warholm, M.; Jörnvall, H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: Correlation between structural data and enzymatic properties. Proc. Natl. Acad. Sci. USA 1985, 82, 7202–7206. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Guthenberg, C.; Jensson, H.; Warholm, M.; Ålin, P. Isozymes of Glutathione S-Transferases in Rat and Human Tissues. In Functions of Glutathione: Biochemical, Physiological, Toxicological, and Clinical Aspects; Larsson, A., Orrenius, S., Holmgren, A., Mannervik, B., Eds.; Raven Press: New York, NY, USA, 1983; pp. 75–88. ISBN 0-89004-908-4. [Google Scholar]
- Mannervik, B.; Guthenberg, C.; Jensson, H.; Tahir, M.K.; Warholm, M.; Ålin, P. Species and Tissue Differences in the Occurrence of Multiple Forms of Rat and Human Glutathione Transferases. In Proceedings of the IUPHAR 9th International Congress of Pharmacology; Paton, W., Mitchell, J.F., Turner, P., Eds.; Macmillan Press: London, UK, 1984; Volume 3, pp. 225–230. [Google Scholar]
- Guthenberg, C.; Åkerfeldt, K.; Mannervik, B. Purification of glutathione-S-transferase from human placenta. Acta Chem. Scand. B 1979, 33, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Bammler, T.K.; Smith, C.A.; Wolf, C.R. Isolation and characterization of two mouse Pi-class glutathione S-transferase genes. Biochem. J. 1994, 298, 385–390. [Google Scholar] [CrossRef]
- Mannervik, B.; Castro, V.M.; Danielson, U.H.; Tahir, M.K.; Hansson, J.; Ringborg, U. Expression of class Pi glutathione transferase in human malignant melanoma cells. Carcinogenesis 1987, 8, 1929–1932. [Google Scholar] [CrossRef]
- Sato, K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv. Cancer Res. 1989, 52, 205–255. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, M.H.; Hocker, M.D.; Hui, H.C.; Caldwell, C.G.; Aaron, D.T.; Engqvist-Goldstein, A.; Flatgaard, J.E.; Bauer, K.E. Isozyme-specific glutathione-S-transferase inhibitors: Design and synthesis. J. Med. Chem. 1994, 37, 189–194. [Google Scholar] [CrossRef]
- Igarashi, T.; Kohara, A.; Shikata, Y.; Sagami, F.; Sonoda, J.; Horie, T.; Satoh, T. The unique feature of dog liver cytosolic glutathione S-transferases. An isozyme not retained on the affinity column has the highest activity toward 1,2-dichloro-4-nitrobenzene. J. Biol. Chem. 1991, 266, 21709–21717. [Google Scholar] [CrossRef]
- Bohets, H.H.; Nouwen, E.J.; De Broe, M.E.; Dierickx, P.J. The cytosolic glutathione S-transferase isoenzymes in the dog kidney cortex as compared with the corresponding MDCK renal cell line. Biochim. Biophys. Acta 1996, 1311, 93–101. [Google Scholar] [CrossRef]
- Nishinaka, T.; Kodaka, R.; Nanjo, H.; Terada, T.; Mizoguchi, T.; Nishihara, T. Purification and characterization of glutathione S-transferase isozymes in dog lens. Int. J. Biochem. 1992, 24, 1737–1742. [Google Scholar] [CrossRef]
- Gerardi, D.G.; Tinucci-Costa, M.; Silveira, A.C.T.; Moro, J.V. Expression of P-glycoprotein, multidrug resistance-associated protein, glutathione-S-transferase pi and p53 in canine transmissible venereal tumor. Pesqui. Vet. Bras. 2014, 34, 71–78. [Google Scholar] [CrossRef]
- Hao, X.Y.; Castro, V.M.; Bergh, J.; Sundström, B.; Mannervik, B. Isoenzyme-specific quantitative immunoassays for cytosolic glutathione transferases and measurement of the enzymes in blood plasma from cancer patients and in tumor cell lines. Biochim. Biophys. Acta 1994, 1225, 223–230. [Google Scholar] [CrossRef]
- Mannervik, B.; Danielson, U.H. Glutathione transferases--structure and catalytic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef] [PubMed]
- Reinemer, P.; Dirr, H.W.; Ladenstein, R.; Huber, R.; Lo Bello, M.; Federici, G.; Parker, M.W. Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J. Mol. Biol. 1992, 227, 214–226. [Google Scholar] [CrossRef]
- Dragani, B.; Stenberg, G.; Melino, S.; Petruzzelli, R.; Mannervik, B.; Aceto, A. The conserved N-capping box in the hydrophobic core of glutathione S-transferase P1-1 is essential for refolding. Identification of a buried and conserved hydrogen bond important for protein stability. J. Biol. Chem. 1997, 272, 25518–25523. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, G.; Dragani, B.; Cocco, R.; Mannervik, B.; Aceto, A. A conserved “hydrophobic staple motif” plays a crucial role in the refolding of human glutathione transferase P1-1. J. Biol. Chem. 2000, 275, 10421–10428. [Google Scholar] [CrossRef]
- Hegazy, U.M.; Mannervik, B.; Stenberg, G. Functional role of the lock and key motif at the subunit interface of glutathione transferase p1-1. J. Biol. Chem. 2004, 279, 9586–9596. [Google Scholar] [CrossRef]
- Kolm, R.H.; Sroga, G.E.; Mannervik, B. Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase P1-1. Biochem. J. 1992, 285, 537–540. [Google Scholar] [CrossRef]
- Widersten, M.; Kolm, R.H.; Björnestedt, R.; Mannervik, B. Contribution of five amino acid residues in the glutathione-binding site to the function of human glutathione transferase P1-1. Biochem. J. 1992, 285, 377–381. [Google Scholar] [CrossRef]
- Johansson, A.S.; Stenberg, G.; Widersten, M.; Mannervik, B. Structure-activity relationships and thermal stability of human glutathione transferase P1-1 governed by the H-site residue 105. J. Mol. Biol. 1998, 278, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lindström, H.; Mazari, A.M.A.; Musdal, Y.; Mannervik, B. Potent inhibitors of equine steroid isomerase EcaGST A3-3. PLoS ONE 2019, 14, e214160. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, M.H.; Satyam, A.; Hocker, M.D.; Bauer, K.E.; Caldwell, C.G.; Hui, H.C.; Morgan, A.S.; Mergia, A.; Kauvar, L.M. Glutathione-S-transferase activates novel alkylating agents. J. Med. Chem. 1994, 37, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Fabrini, R.; De Luca, A.; Stella, L.; Mei, G.; Orioni, B.; Ciccone, S.; Federici, G.; Lo Bello, M.; Ricci, G. Monomer-dimer equilibrium in glutathione transferases: A critical re-examination. Biochemistry 2009, 48, 10473–10482. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Jensson, H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J. Biol. Chem. 1982, 257, 9909–9912. [Google Scholar] [CrossRef]
- Hegazy, U.M.; Tars, K.; Hellman, U.; Mannervik, B. Modulating catalytic activity by unnatural amino acid residues in a GSH-binding loop of GST P1-1. J. Mol. Biol. 2008, 376, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Lo Bello, M.; Caccurri, A.M.; Pastore, A.; Nuccetelli, M.; Parker, M.W.; Federici, G. Site-directed mutagenesis of human glutathione transferase P1-1. Mutation of Cys-47 induces a positive cooperativity in glutathione transferase P1-1. J. Biol. Chem. 1995, 270, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, G.; Abdalla, A.M.; Mannervik, B. Tyrosine 50 at the subunit interface of dimeric human glutathione transferase P1-1 is a structural key residue for modulating protein stability and catalytic function. Biochem. Biophys. Res. Commun. 2000, 271, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Reinemer, P.; Dirr, H.W.; Ladenstein, R.; Schäffer, J.; Gallay, O.; Huber, R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991, 10, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- García-Sáez, I.; Párraga, A.; Phillips, M.F.; Mantle, T.J.; Coll, M. Molecular structure at 1.8 A of mouse liver class pi glutathione S-transferase complexed with S-(p-nitrobenzyl)glutathione and other inhibitors. J. Mol. Biol. 1994, 237, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.J.; Lo Bello, M.; Nuccetelli, M.; Mazzetti, A.P.; Parker, M.W. The ligandin (non-substrate) binding site of human Pi class glutathione transferase is located in the electrophile binding site (H-site). J. Mol. Biol. 1999, 291, 913–926. [Google Scholar] [CrossRef]
- Al-Qattan, M.N.; Mordi, M.N.; Mansor, S.M. Assembly of ligands interaction models for glutathione-S-transferases from Plasmodium falciparum, human and mouse using enzyme kinetics and molecular docking. Comput. Biol. Chem. 2016, 64, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Škerlová, J.; Ismail, A.; Lindström, H.; Sjödin, B.; Mannervik, B.; Stenmark, P. Structural and functional analysis of the inhibition of equine glutathione transferase A3-3 by organotin endocrine disrupting pollutants. Environ. Pollut. 2021, 268, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Gershenson, D.M.; Choi, H.; Lewis, L.; Patel, K.; Brown, G.L.; Garcia, A.; Spriggs, D.R. Multi-institutional phase 2 study of TLK286 (TELCYTA, a glutathione S-transferase P1-1 activated glutathione analog prodrug) in patients with platinum and paclitaxel refractory or resistant ovarian cancer. Int. J. Gynecol. Cancer 2005, 15, 593–600. [Google Scholar] [CrossRef]
- Dourado, D.F.; Fernandes, P.A.; Ramos, M.J.; Mannervik, B. Mechanism of glutathione transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA). Biochemistry 2013, 52, 8069–8078. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.J.; Lo Bello, M.; Battistoni, A.; Ricci, G.; Rossjohn, J.; Villar, H.O.; Parker, M.W. The structures of human glutathione transferase P1-1 in complex with glutathione and various inhibitors at high resolution. J. Mol. Biol. 1997, 274, 84–100. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]

| Specific Activity (µmol/min/mg) | ||
|---|---|---|
| Substrate | Dog GST P1-1 | Human GST P1-1/Ile105 1 |
| 1-Chloro-2,4-dinitrobenzene (CDNB) | 23.0 ± 1.3 | 106 |
| Cumene hydroperoxide (CuOOH) | 0.07 ± 0.01 | 0.03 |
| Phenethyl isothiocyanate (PEITC) | 6.37 ± 1.03 | 60 |
| Benzyl isothiocyanate (BITC) | 39.0 ± 5.2 | 63 |
| Allyl isothiocyanate (AITC) | 1.94 ± 0.60 | 38 |
| Propyl isothiocyanate (PITC) | 2.55 ± 0.20 | 49 |
| Cyclohexyl isothiocyanate (cHITC) | 1.27 ± 0.08 | 11 |
| Ethacrynic acid (EA) | 2.05 ± 0.13 | 2.0 |
| trans-2-Pentenal | 0.100 ± 0.002 | N/A |
| trans-2-Nonenal | 0.124 ± 0.005 | N/A |
| trans-2-Decenal | 0.111 ± 0.005 | N/A |
| trans-2-Dodecenal | 0.046 ± 0.006 | N/A |
| Δ5-androstene-3,17-dione (AD) | 0.014 ± 0.005 | 0.01 |
| Inhibitor | IC50 (µM) |
|---|---|
| Ethacrynic acid | 4.38 ± 1.96 |
| Triethyltin bromide | 83.6 ± 1.9 |
| Triphenyltin chloride | 3.11 ± 0.38 |
| Tributyltin acetate | 0.85 ± 0.13 |
| Cibacron Blue | 0.043 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.; Lewis, E.; Sjödin, B.; Mannervik, B. Characterization of Dog Glutathione Transferase P1-1, an Enzyme Relevant to Veterinary Medicine. Int. J. Mol. Sci. 2021, 22, 4079. https://doi.org/10.3390/ijms22084079
Ismail A, Lewis E, Sjödin B, Mannervik B. Characterization of Dog Glutathione Transferase P1-1, an Enzyme Relevant to Veterinary Medicine. International Journal of Molecular Sciences. 2021; 22(8):4079. https://doi.org/10.3390/ijms22084079
Chicago/Turabian StyleIsmail, Aram, Elizabeth Lewis, Birgitta Sjödin, and Bengt Mannervik. 2021. "Characterization of Dog Glutathione Transferase P1-1, an Enzyme Relevant to Veterinary Medicine" International Journal of Molecular Sciences 22, no. 8: 4079. https://doi.org/10.3390/ijms22084079
APA StyleIsmail, A., Lewis, E., Sjödin, B., & Mannervik, B. (2021). Characterization of Dog Glutathione Transferase P1-1, an Enzyme Relevant to Veterinary Medicine. International Journal of Molecular Sciences, 22(8), 4079. https://doi.org/10.3390/ijms22084079







