Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Ethylene–norbornene Copolymer (Topas) and Polylactide (PLA) Composites with Cannabidiol (CBD) Extract
2.2. Thermal Analysis of Ethylene-norbornene Copolymer (Topas) and Polylactide (PLA) Composites with Cannabidiol (CBD) Extract
3. Materials and Methods
3.1. Reagents
3.2. Methods of Samples Preparation
3.3. Weather-Aging
3.4. Measurement Methods
3.4.1. Surface Free Energy (SFE)
3.4.2. Surface Morphology Analysis
3.4.3. Fourier-Transform Infrared Spectroscopy
3.4.4. Change in Color Measurements
3.4.5. Thermogravimetric Analysis (TGA)
3.4.6. Oxidative-Induction Time (OIT)
- Heating from 0 to 200 °C for 15 min under an argon atmosphere at a flow rate of 50 mL/min;
- Heating in the investigation temperature, 200 °C for 10 min under an argon atmosphere at a flow rate of 50 mL/min;
- Cooling from 200 to 0 °C for 20 min under an argon atmosphere at a flow rate of 50 mL/min;
- Heating from 0 to 350 °C for 30 min under an air atmosphere at a flow rate of 50 mL/min.
4. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol extract obtained from Cannabis Plants |
| PLA | Polylactide |
| COCs | Cyclic Olefin Copolymers |
| Topas | Ethylene–Norbornene Copolymer |
| PS | Polystyrene |
| PP | Polypropylene |
| PE | Polyethylene |
| PHAs | Polyhydroxyalkanoates from aliphatic polyesters group |
| BHA | Butylated Hydroxyanisole |
| BHT | Butylated Hydroxytoluene |
| THC | Tetrahydrocannabinol |
| FTIR | Fourier-transform Infrared Spectroscopy |
| OIT | Oxidation Induction Time/Temperature |
| SFE | Surface Free Energy [mJ/m2] |
| CI | Carbonyl Index [–] |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sangroniz, A.; Zhu, J.B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Kurtela, A.; Antolović, N. The problem of plastic waste and microplastics in the seas and oceans: Impact on marine organisms. Ribar. Croat. J. Fish. 2019, 77, 51–56. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Khare, A.; Deshmukh, S. Studies toward producing eco-friendly plastics. J. Plast. Film Sheeting 2006, 22, 193–211. [Google Scholar] [CrossRef]
- Sudesh, K.; Iwata, T. Sustainability of biobased and biodegradable plastics. CLEAN—Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Nkwachukwu, O.; Chima, C.; Ikenna, A.; Albert, L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int. J. Ind. Chem. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Haider, T.P.; Vçlker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and fossil-based polymeric blends and nanocomposites for packaging: Structure-property relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, H.; Liu, R.; Wu, C. Preparation of Long Sisal Fiber-Reinforced Polylactic Acid Biocomposites with Highly Improved Mechanical Performance. Polymers 2021, 13, 1124. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Jawaid, M.; Parveez, B. Bamboo Fiber Based Cellulose Nanocrystals/Poly(Lactic Acid)/Poly(Butylene Succinate) Nanocomposites: Morphological, Mechanical and Thermal Properties. Polymers 2021, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life 2014, 1, 140–150. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic profile and antioxidant activity of Juglans regia L. leaves and husk extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Flamminii, F.; Pittia, P.; Caponio, F.; Di Mattia, C. Radical Scavenging Activity of Olive Oil Phenolic Antioxidants in Oil or Water Phase during the Oxidation of O/W Emulsions: An Oxidomics Approach. Antioxidants 2020, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Emad, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration dependence of the antioxidant and prooxidant activity of trolox in hela cells: Involvement in the induction of apoptotic volume decrease. Antioxidants 2020, 9, 1058. [Google Scholar] [CrossRef]
- Lukanina, Y.K.; Kolesnikova, N.N.; Popov, A.A.; Khvatov, A.V. Oxo-degradation of LDPE with pro-oxidant additive. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 22–23 August 2019; Volume 525, p. 012104. [Google Scholar]
- Dintcheva, N.T.; Gennaro, D.; Teresi, R.; Baiamonte, M. Pro-degradant activity of naturally occurring compounds on polyethylene in accelerate weathering conditions. Materials 2019, 12, 195. [Google Scholar] [CrossRef]
- Lukanina, Y.K.; Popov, A.A.; Khvatov, A.V. Biodegradation of polymer compositions with pro-oxidants. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Karnataka, India, 17–18 July 2020; Volume 921, p. 012016. [Google Scholar]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A.; Zawadziłło, J. Processability and Mechanical Properties of Thermoplastic Polylactide/Polyhydroxybutyrate (PLA/PHB) Bioblends. Materials 2021, 14, 898. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Rybiński, P.; Zukowski, W.; Marzec, A. Application of earth pigments in cycloolefin copolymer: Protection against combustion and accelerated aging in the full sunlight spectrum. Materials 2020, 13, 3381. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Toschi, T.G. Preliminary study: Comparison of antioxidant activity of cannabidiol (CBD) and α-tocopherol added to refined olive and sunflower oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.V.; Shah, S.; Albornoz, C.A.; Saedi, N. Consumer interest in topical cannabidiol (CBD): An examination of online search trends from 2015–2019. Clin. Dermatol. 2020. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of cannabinoid delivery: How can nanomedicine help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Cannabinoid Pharmacology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 80, pp. 73–115. [Google Scholar]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Hauser, C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.M.B.; Tomé, L.C.; Garcia, H.; Brandão, L.; Mendes, A.M.; Marrucho, I.M. Effect of natural and synthetic antioxidants incorporation on the gas permeation properties of poly(lactic acid) films. J. Food Eng. 2013, 116, 562–571. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Kloziński, A. Accelerated weathering of polylactide-based composites filled with linseed cake: The influence of time and oil content within the filler. Polymers 2019, 11, 1495. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Islam, M.R.; Beg, M.D.H.; Pickering, K.L. Effect of accelerated weathering on physico-mechanical properties of polylactide bio-composites. J. Polym. Environ. 2019, 27, 942–955. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Morin hydrate as pro-ecological antioxidant and pigment for polyolefin polymers. C. R. Chim. 2013, 16, 990–996. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Anthocyanin—A Natural Dye for Smart Food Packaging Systems. Korean J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of impregnation of biodegradable polyesters with polyphenols from cistus linnaeus and Juglans regia Linnaeus walnut green husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of (+)-catechin and polydatin as functional additives for biodegradable polyesters. Int. J. Mol. Sci. 2020, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M. The effect of substances of plant origin on the thermal and thermo-oxidative ageing of aliphatic polyesters (PLA, PHA). Polymers 2018, 10, 1252. [Google Scholar] [CrossRef]
- Krehula, L.K.; Katancǐć, Z.; Siročić, A.P.; Hrnjak-Murgić, Z. Weathering of high-density polyethylene-wood plastic composites. J. Wood Chem. Technol. 2014, 34, 39–54. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Wolski, K. Innovative cellulose fibres reinforced ethylene-norbornene copolymer composites of an increased degradation potential. Polym. Degrad. Stab. 2019, 159, 174–183. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; da Silva, C.F.; de Moraes, M.A. Chapter 3—Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. In Biopolymer Membranes and Films; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 67–95. ISBN 9780128181348. [Google Scholar]
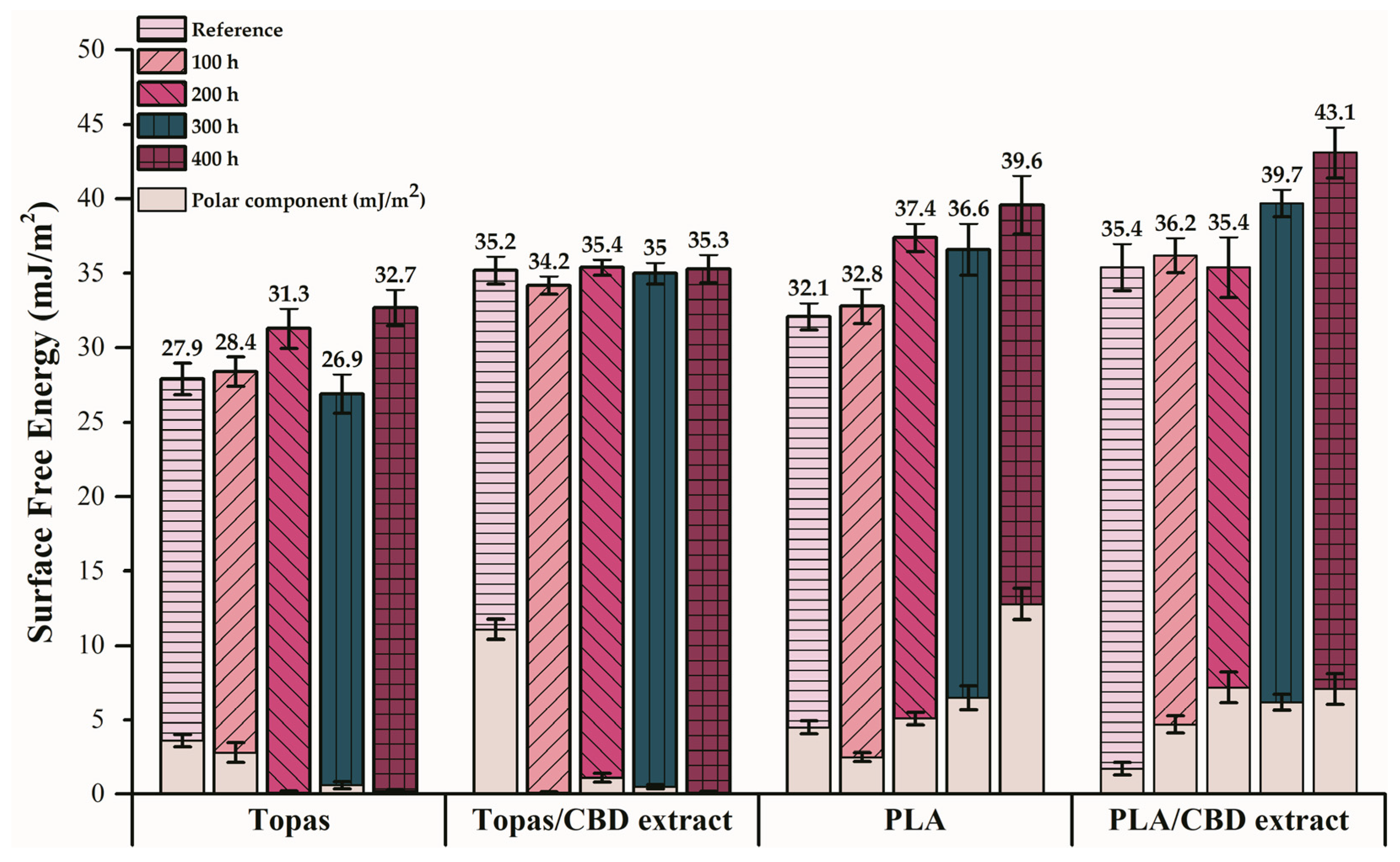

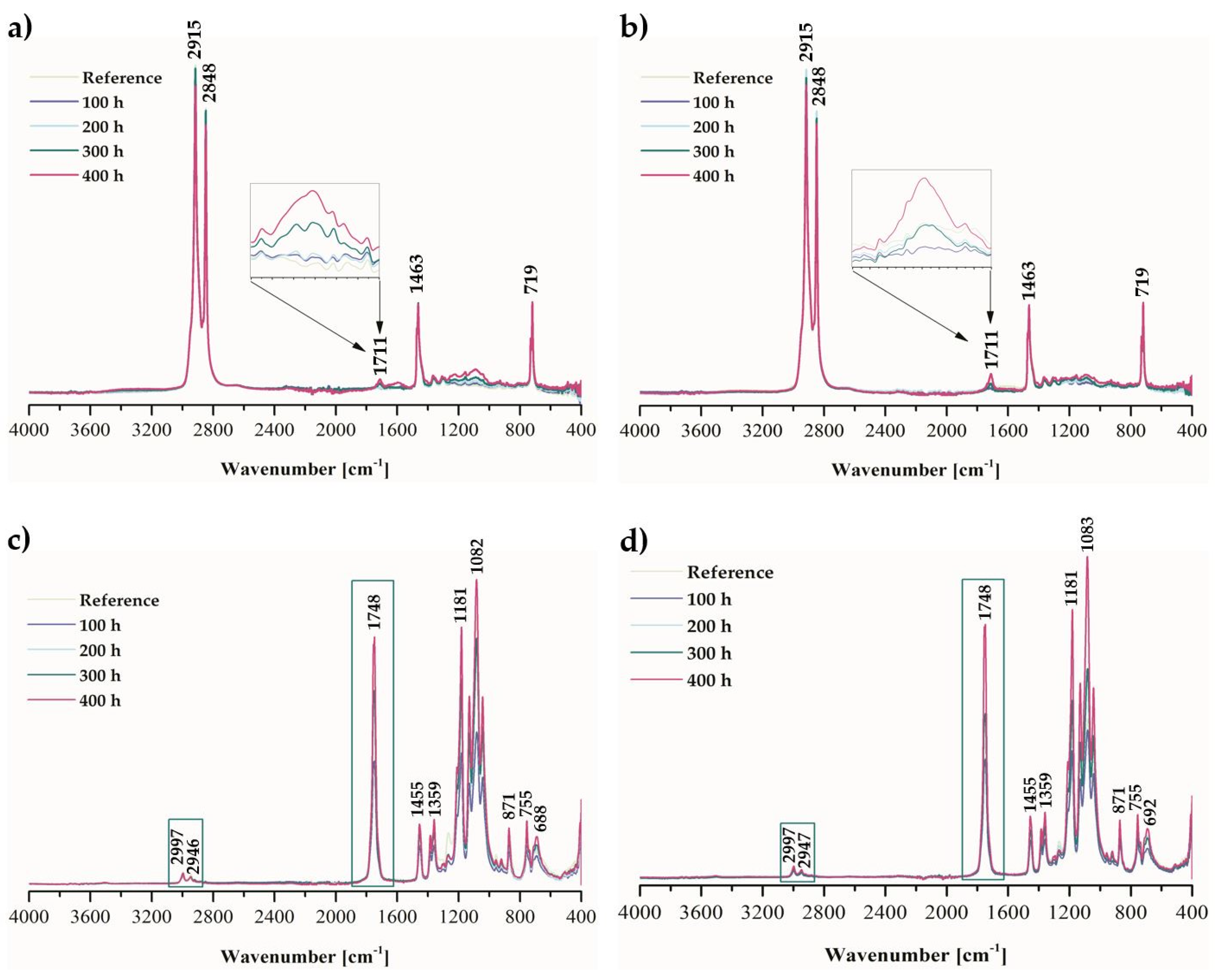
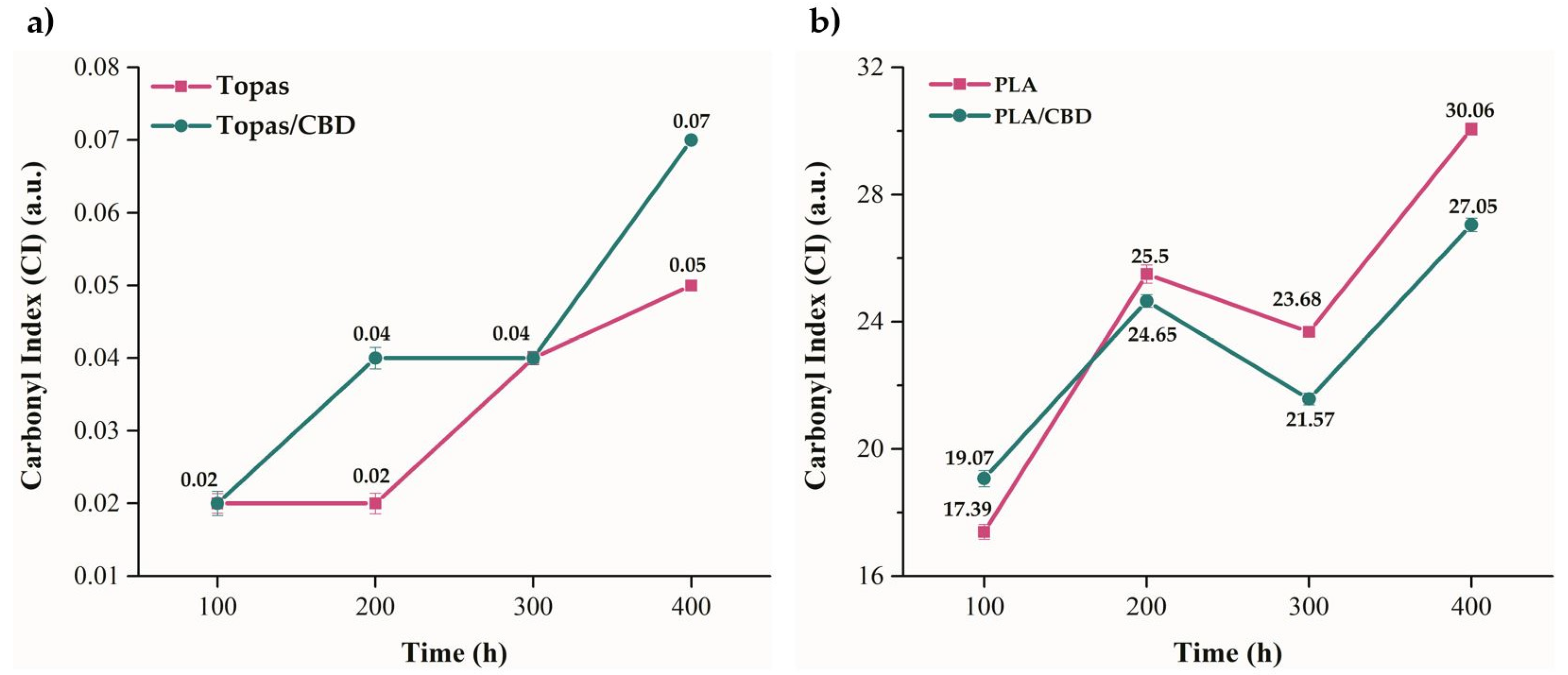


| Liquid | Contact Angle after Weathering Aging (°) | ||||
|---|---|---|---|---|---|
| Reference | 100 h | 200 h | 300 h | 400 h | |
| Topas | |||||
| Water | 87.6 ± 0.79 | 90.6 ± 6.61 | 98.2 ± 1.01 | 97.8 ± 1.73 | 97.6 ± 0.97 |
| Diiodomethane | 61.6 ± 1.43 | 59.9 ± 0.74 | 55.4 ± 1.89 | 62.5 ± 1.73 | 53.8 ± 1.68 |
| Ethylene glycol | 63.3 ± 1.72 | 65.4 ± 1.79 | 74.9 ± 1.04 | 75.0 ± 0.66 | 69.5 ± 2.05 |
| Topas/CBD | |||||
| Water | 71.2 ± 0.92 | 97.3 ± 1.08 | 85.9 ± 1.08 | 92.9 ± 1.04 | 97.1 ± 0.99 |
| Diiodomethane | 55.4 ± 0.95 | 51.1 ± 0.74 | 47.0 ± 0.56 | 48.6 ± 0.96 | 49.5 ± 1.28 |
| Ethylene glycol | 48.1 ± 2.29 | 71.9 ± 1.88 | 58.0 ± 1.32 | 65.9 ± 1.17 | 69.4 ± 1.09 |
| PLA | |||||
| Water | 82.1 ± 0.84 | 86.1 ± 0.54 | 76.0 ± 0.95 | 75.0 ± 1.25 | 65.1 ± 0.73 |
| Diiodomethane | 54.2 ± 1.17 | 51.6 ± 1.74 | 44.0 ± 1.32 | 46.9 ± 2.50 | 41.3 ± 3.50 |
| Ethylene glycol | 57.8 ± 1.47 | 61.2 ± 1.45 | 49.5 ± 0.85 | 48.0 ± 1.10 | 42.7 ± 1.65 |
| PLA/CBD | |||||
| Water | 87.8 ± 1.44 | 78.9 ± 1.62 | 76.8 ± 1.76 | 71.6 ± 1.01 | 67.9 ± 2.04 |
| Diiodomethane | 47.6 ± 2.23 | 46.8 ± 1.46 | 51.4 ± 2.68 | 40.3 ± 1.23 | 33.8 ± 2.43 |
| Ethylene glycol | 58.9 ± 1.34 | 51.1 ± 1.28 | 48.2 ± 0.84 | 50.3 ± 1.84 | 40.7 ± 3.32 |
| Mixture | Temperatures of Mass Change (°C) | ||||||
|---|---|---|---|---|---|---|---|
| T2% | T5% | T10% | T20% | T50% | T70% | T90% | |
| Topas | 432.5 | 449.2 | 456.7 | 464.2 | 475.8 | 480.8 | 487.5 |
| Topas/CBD | 429.2 | 448.3 | 456.7 | 464.2 | 475.0 | 480.8 | 487.5 |
| PLA | 320.0 | 330.0 | 338.3 | 347.5 | 360.8 | 367.5 | 375.0 |
| PLA/CBD | 314.2 | 325.0 | 334.2 | 344.2 | 358.3 | 365.0 | 373.3 |
| Mixture | Oxidative-Induction Time (min) | Energy of Oxidation (J/g) |
|---|---|---|
| Topas | 5.09 | 170 |
| Topas/CBD | 8.49 | 223 |
| Mixture | Oxidative-Induction Temperatures (°C) | Energy of Oxidation (J/g) | |
|---|---|---|---|
| Onset (°C) | Endset (°C) | ||
| PLA | 222.0 | 261.3 | 11 |
| PLA/CBD | 276.1 | 305.9 | 8 |
| Mixture | Weight Composition (phr) | ||
|---|---|---|---|
| Topas | PLA | CBD Extract | |
| 1 | 100 | - | - |
| 2 | 100 | - | 1 |
| 3 | - | 100 | - |
| 4 | - | 100 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plota, A.; Masek, A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. Int. J. Mol. Sci. 2021, 22, 4012. https://doi.org/10.3390/ijms22084012
Plota A, Masek A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. International Journal of Molecular Sciences. 2021; 22(8):4012. https://doi.org/10.3390/ijms22084012
Chicago/Turabian StylePlota, Angelika, and Anna Masek. 2021. "Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials" International Journal of Molecular Sciences 22, no. 8: 4012. https://doi.org/10.3390/ijms22084012
APA StylePlota, A., & Masek, A. (2021). Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. International Journal of Molecular Sciences, 22(8), 4012. https://doi.org/10.3390/ijms22084012







