Radiation on Earth or in Space: What Does It Change?
Abstract
1. Introduction
2. The Three Sources of Ionizing Radiation Emitted from Space
2.1. Historical Features
2.2. From the Cosmos to the Shielding: The Three Sources of IRS and Their Physical Features
- -
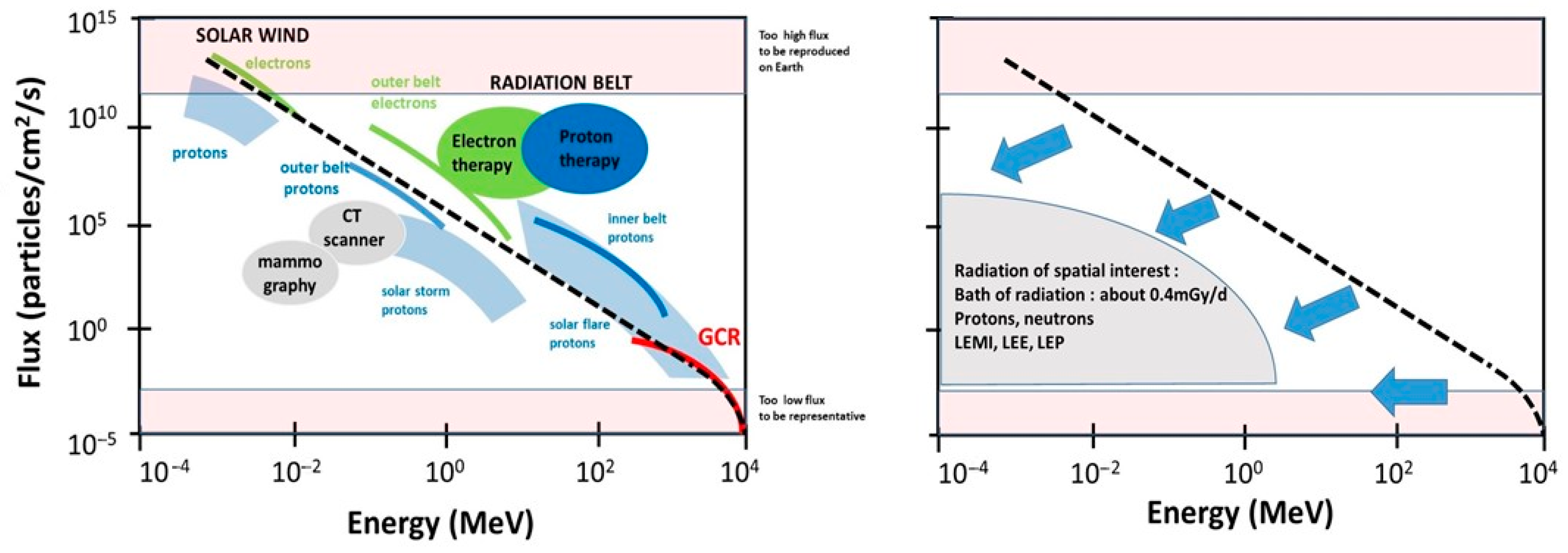
- The cosmos: Galactic cosmic radiation (GCR) is composed of 85% protons, 14% helium ions, and about 1% heavier elements, such as iron. In GCR, the corresponding fluxes are about four particles/cm2/s for protons, 0.4 for helium ions and 10−4 to 10−2 for heavier ions. Their energy can be very high (more than 1011 MeV). The probability of impact of the heaviest ions to astronauts can be considered as negligible in low Earth orbit (LEO) but not in deep space [19]. For quantifying the risks during LEO, GCR can be reasonably reduced as a flux of protons and helium ions [6].
- -
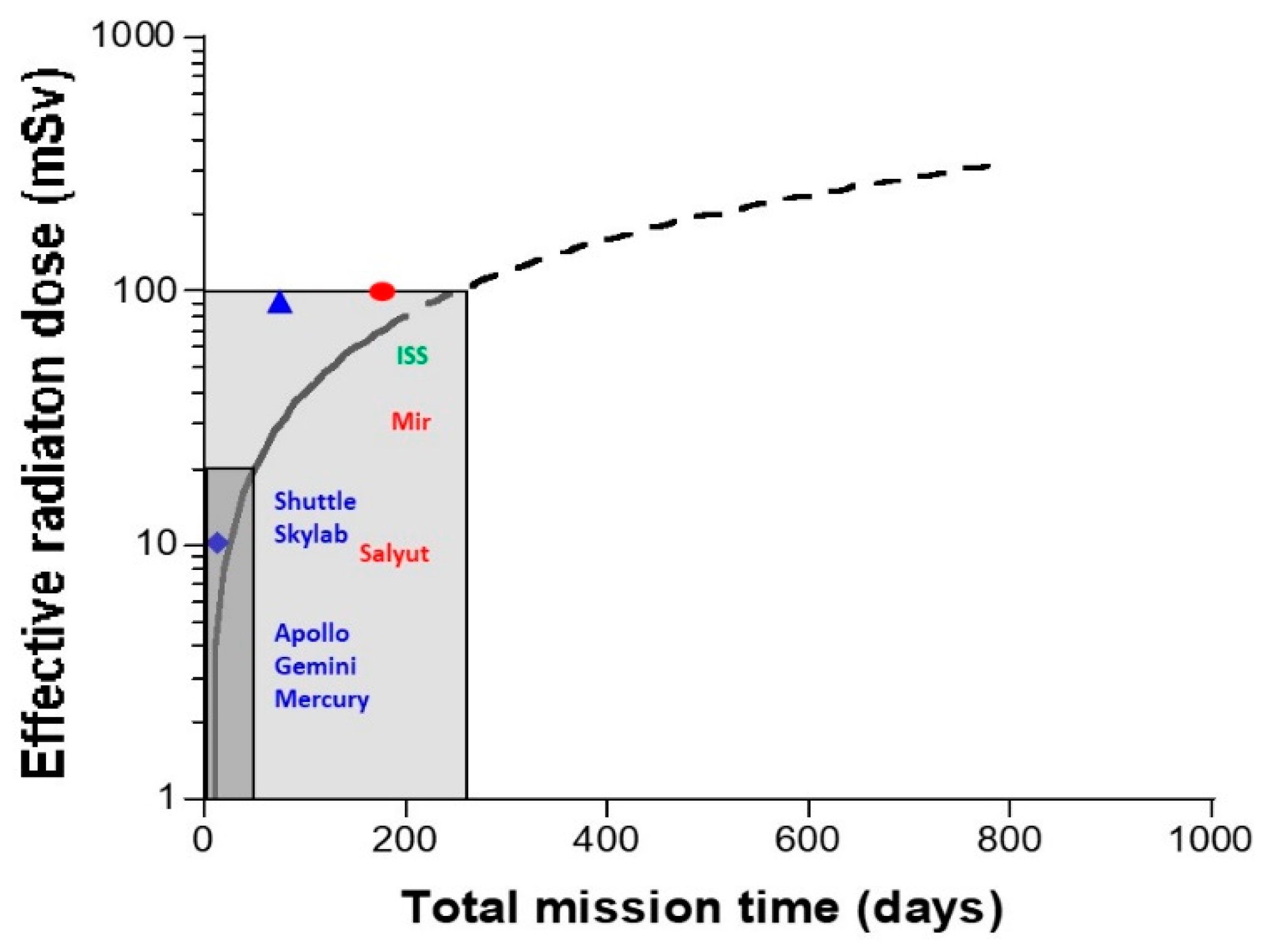
- The Sun: Solar flares generally consist of 92% protons, 6% helium ions and about 2% heavier elements at various MeV values. The solar wind is an intense (about 108 protons/cm2/s) flux of protons at various keV values. Solar flares, storms and winds are infrequent events (generally obeying a cycle of 11 years for solar flares) [20]. Even if space missions are scheduled to avoid such events, some missions, such as Apollo-XIV, Skylab-4, and Mir-15, have been exposed to some significant solar events, at least partially: The crews received 11.4 mGy in 9 days, 77 mGy in 90 days, and 92.9 mGy in 185 days. All these values were obtained from on-board dosimeters that included the GCR contribution [6] (Figure 2). However, no significant injury was reported to astronauts or electronics, probably because the duration of the mission was short and/or the excess of dose remained limited. Predicting solar events should, therefore, be an essential part of ensuring the radiological protection of astronauts. By excluding exceptional solar events, one can reasonably consider the Sun as a simple source of protons emitted at high flux but at a relatively low energy.
- -
- The Van Allen radiation belt is made of two layers: An “outer” belt composed of electrons, the energy of which ranges from 40 keV to 7 MeV (in electron radiotherapy, the energy of electrons is 6–25 MeV), and an “inner” belt, mostly composed of protons, the energy of which ranges from 100 keV and 400 MeV (in proton therapy, the energy of protons is 60–280 MeV). As described above, the particularity of the Van Allen belt is the excess of radiation observed in the SAA. At each passage above the SAA, electronic devices and astronauts receive a peak of dose that may represent a dose six-fold higher than the average one experienced outside the SAA [18]. Lastly, it is noteworthy that inside the Van Allen belt, the flux and the nature of the particles that impact the spacecraft are greatly conditioned by the flight parameters (notably, day/night and orbit inclination [21,22]. Hence, significant differences may appear when comparing data from the Apollo missions—that spent some time outside the Van Allen belt—and the ISS that remains in LEO, protected inside the magnetosphere [23]. The assessed and calculated doses are discussed below.
2.3. Inside the Shielding: Occurrence of a Variety of Electronic and Nuclear Reactions
- -
- A general decrease in the flux: The flux of the secondary particles provided from the spacecraft shielding must be lower than that of the incident particles. A decreased flux leads to a lower absorbed dose: By omitting exceptional solar events, the dose delivered to astronauts is generally in the order of mGy per day. The decrease in the flux by the shielding can be roughly represented by a vertical shift in the data plotted in Figure 1.
- -
- A general degradation of the energy combined with changes in the nature of radiation: The energy of the secondary particles provided from the spacecraft shielding must be lower than the incident particles and their nature can be changed. The decrease in energy can be roughly represented by a horizontal shift in the data plotted in Figure 1. High-energy particles interact with matter to produce atomic displacements and/or electronic ionizations and excitations. If the kinetic energy of the incident particle transferred to the nucleus of the atoms of the shielding is sufficient, the moving atom may serve as a projectile to produce secondary displacements or ionize or excite other atoms adjacent to its path [32]. For example, while electrons emitted from space may be stopped by shielding, they can produce a non-negligible effect through the bremsstrahlung phenomenon, which results in an intense ray build-up behind the shielding [21,22]. With regard to protons emitted from space, numerous excitations and ionizations are expected inside the shielding, together with the emission of low-energy metal ions (LEMI) [32,33]. In addition, secondary neutrons provided from the aluminum-containing shielding have also been detected in low earth orbiting spacecraft [34,35]. However, although their energy spectrum is difficult to measure, the long duration exposure facility (LDEF) mission has provided data suggesting that neutrons of more than 1 MeV are the most abundant and responsible for most of the total equivalent neutron dose [36]. At this stage, it is important to address whether neutrons may be responsible for some of the activation reactions in the spacecraft. In general, the mechanical properties of metals and ceramics that are present in the spacecraft do not significantly suffer from a flux that is lower than 1017/cm2 for protons > 1 MeV, 1017/cm2 for neutrons > 1 keV and 1018/cm2 for electrons > 1 MeV [32]. The neutron activation and the neutron photo-emission or spallation of oxygen inside the spacecraft may result in 19O and 15O, respectively. However, the lifetimes of these oxygen isotopes are too short to obtain reliable measurements. Conversely, 7Be, 22Na and 24Na, are the major activable gamma-ray-emitting radioisotopes present in the body, with half-lives that are long enough to be measurable. Additionally, in 1969, Brodzinski et al. proposed to quantify the radiation dose to Apollo astronauts from the assessment of such activated nuclides. Relevant dosimetry data were obtained, suggesting that measurable activation events occur in the spacecraft [37]. Unfortunately, to our knowledge, no data have been published in regard to this technique being applied in the ISS, maybe because the physical conditions of neutron activation are not so favorable at LEO. Further investigations are, however, needed to document the impact of this particular process on the dose.
2.4. From the Shielding to Astronauts: The Contribution of Each IRF to the Radiation Dose
- -
- At the end of the 1960s, it was reported that helmets used during the Apollo missions were impacted by specific low-energy metal ions (LEMI). As described above, the probability of the impact of such GCR metal ions is approximately a few particles per km2 per century. Thus, the LEMIs detected in the helmets did not come from the cosmos, but were generated from the walls of the spacecraft made of iron, zinc, aluminum, nickel, and copper. These particles resulted from the interaction between the incident protons from space with the metal shielding. LEMIs were found to emit at a few keV to several MeV [33]. In addition to these LEMIs, some low-energy protons (LEP) and low-energy electrons (LEE) can be emitted from the shielding and may have a significant impact on electronics [38]. It is noteworthy that LEMI, LEP and LEE deliver nearly all their energy at the surface of matter (with an average track path of several mm to nm) [39,40]. Hence, these particles preferentially target the external parts of the human body, such as the skin and eyes (Figure 1). It is noteworthy that this low-energy particle component may be reduced in the ISS in comparison with deep space missions.
- -
- The other rays and particles directly or indirectly produced by these secondary particles represent a constant “bath of radiation”, notably made of the build-up of X-rays and gamma-rays. An overview of the dosimetry of all space missions, from that of Gagarin in 1962 to the most recent with the Space Shuttle, has shown that the dose received by astronauts is strongly time-dependent [6] (Figure 2). Whether the mission concerns LEO, the surface of the Moon or a trip from Earth to the Moon (Apollo missions only), the data review indicates a constant dose rate of about 0.4 mGy/d (Table 1). Such a “bath of radiation” is mainly made of X-rays and gamma-rays and should concern the human body as a whole, even at depth [6].
2.5. From the Assessment of the Absorbed Dose to the Calculation of the Effective Dose
2.6. Comparison with the Natural Radiation Backgrounds on Earth, the Moon and Mars
- -
- The telluric radiation component: The three major radioactive decay chains of natural radiation, such as uranium (235U or 238U) and thorium (232Th), are present on Earth and continuously provide several unstable and stable radionuclides. The major part of the telluric radiation component comes from radon, a naturally radioactive gas resulting from the decay of uranium and radium naturally present in the soil. The average effective dose inhaled in air produced by radon is about 1.3 mSv/y, while terrestrial radiation from the ground represents about 0.5 mSv/y.
- -
- The organic radiation component: An effective dose-rate of about 0.3 mSv/y comes from organic products that contain naturally radioactive substances such as potassium (40K) and carbon (14C).
- -
- The cosmic radiation component: At sea level, an effective dose-rate of 0.3 mSv/y comes from cosmic radiation.
3. The Potential Radiation-Induced Risks for the Astronauts: What Do We Expect?
3.1. Radiosensitivity in Space
3.2. Radiosusceptibility in Space
- -
- Recently, an increase in mortality from skin melanoma has been reported among astronauts. This increase was shown to be consistent with skin melanoma observed among aircraft pilots. Ultraviolet radiation and lifestyle were suggested as potential causes. However, it must be stressed that neutrons, LEMI, LEP and LEE may also present a cause of concern for aircraft pilots, similarly to astronauts. Conversely, ultraviolet radiation cannot be suggested as a cause in the case of astronauts [61,62]. Further investigations are, therefore, needed to document the potential physical causes of skin melanoma in both aircraft and spacecraft crews. It is noteworthy that the physical parameters of the flights (notably LEO or non-LEO) significantly affects the occurrence of such tumors [63].
- -
- With regard to radiation-induced eye tumors, although rare, choroid melanoma is the most frequent primarily adult tumor in the eye [64]. However, to our knowledge, no IR-induced choroid melanoma has been observed so far. No choroid melanoma has been reported in astronauts. Retinoblastoma, another common tumor of the ocular system can be evoked. However, the retina may be too deep in the eye to be reached by energy deposition from LEMI, LEP and LEE (the average distance between the iris and retina is about 2 cm). Again, the flight parameters and the relatively low duration of previous space missions may limit the occurrence of such specific events.
3.3. Radiodegeneration in Space
- -
- The loss of bone mass in astronauts, especially in weight-bearing bones, is a current observation performed after each space mission, that may have consequences for the immune system. The loss of bone mass was hypothesized to be similar to osteoporosis [73,74,75,76]. Although the inherent mechanisms of this loss of bone mass have been based on animal models data, it appears that a reduced osteoblast function leads to decreased bone formation, while bone resorption is unaltered or increased. The loss of bone mass has long been attributed to microgravity [73]. However, while the molecular and cellular pathways by which microgravity may act in the form of biochemical signals are still unknown, some emerging data suggest that radiation (and logically, the “bath of radiation”) may also affect bones [77]. Interestingly, by radiobiologically characterizing various genetic syndromes associated with facial dysmorphy, osteoblasts were shown to be more radiosensitive than the skin of the same donor, which may suggest that IR contribution to the loss of bone mass in astronauts has been underestimated [78]. Further studies are, therefore, needed to document the radiodegeneration of bone in response to IRS. In the following chapters, we discuss the advantages of anti-osteoporosis drugs as countermeasures.
- -
- Epidemiology data of women patients with breast cancer have shown that more than 50% of women are at risk of a heart attack for 10 years post-radiotherapy [79,80,81,82]. Such examples, suggest that the cardiovascular system may be mechanically affected by radiation and sensitive to low-dose. To date, there is no evidence of any cardiovascular disease in astronauts caused by exposure to IRS [62,83]. Again, the microgravity contribution should be separated from the radiation contribution [84]. However, even though the cohorts investigated have been too small, the potential radiodegeneration of heart tissue needs to be further investigated.
4. Radiation Protection Factors and Radiobiological Effects Specific to Low-Dose Radiation to Be Considered
4.1. Radiation Protection Factors to Be Investigated to Refine the Estimation of Risks
4.2. Radiobiological Effects Specific to Low-Dose Radiation to Be Investigated to Refine the Estimation of Risks
5. Countermeasures
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhmar, G.D.; Saganti, P.B.; Dicello, J.F. Space Radiation Cancer Risk Projections for Exploration Missions: Uncertainty Reduction and Mitigation; NASA: Hanover, MD, USA, 2002; JSC-29295.
- Cucinotta, F.A.; Kim, M.H.; Ren, L. Managing Luna and Mars Mission Radiation Risks. Part I; Cancer Risks, Uncertainties and Shielding Effectiveness; NASA: Hanover, MD, USA, 2005; TP-2005-213164.
- Durante, M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 2014, 87, 20130626. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Rev. 2016, 770, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Durante, M.; Foray, N. Biological effects of space radiation on human cells: History, advances and outcomes. J. Radiat. Res. 2011, 52, 126–146. [Google Scholar] [CrossRef][Green Version]
- Ferlazzo, M.L.; Foray, N. Space radiobiology needs realistic hypotheses and relevant methodology. Proc. Natl. Acad. Sci. USA 2017, 114, E6733. [Google Scholar] [CrossRef] [PubMed]
- Curie, P. Oeuvres de Pierre Curie; Editions des Archives Contemporaines: Paris, France, 1984. [Google Scholar]
- Todd, P.; Tobias, C.A.; Silver, I.L. Current topics in space radiation biology. In Space Radiation Biology and Related Topics; Tobias, C., Todd, P., Eds.; Academic Press: New York, NY, USA; London, UK, 1974; pp. 1–18. [Google Scholar]
- Solomon, J. Théorie du Passage des Rayons Cosmiques à Travers la Matière; Hermann: Paris, France, 1936. [Google Scholar]
- Millikan, R.A. Electrons (+ and -), Protons, Photons, Neutrons, Mesotrons and Cosmic Rays; University of Chicago Press: Chikago, IL, USA, 1947. [Google Scholar]
- Hess, V.F.; Eugster, J.A.G. Cosmic Radiation and Its Biological Effects; Fordham University Press: New York, NY, USA, 1949. [Google Scholar]
- Van Allen, J.A.; Franck, L.A. Radiation around the earth to a radial distance of 107,400 km. Nature 1959, 183, 430. [Google Scholar] [CrossRef]
- Van Allen, J.A.; Franck, L.A. Radiation measurements to 658,300 km with Pioneer IV. Nature 1959, 184, 219. [Google Scholar] [CrossRef]
- Neugebauer, M.; Snyder, C.W. Interplanetary solar wind measurements by Mariner II. In Space Research; Muller, P., Ed.; North-Holland Publications: Amsterdam, The Netherlands, 1964; Volume 4, pp. 89–113. [Google Scholar]
- Hellweg, C.E.; Matthiä, D.; Berger, T.; Baumstark-Khan, C. Radiation in Space: Relevance and Risk for Human Missions; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wilson, J.W. Environmental geophysics and SPS shielding. In Proceedings of the Workshop on the Radiation Environment of the Satellite Power System, Berkeley, CA, USA, 15 September 1978; Report LBL-8581. California Univ.: Berkeley, CA, USA, 1978. [Google Scholar]
- Bottollier-Depois, J.F.; Siegrist, M.; Petrov, V.M.; Shurshakov, V.V.; Bengin, V.; Koslova, S.B. TEPC measurements obtained on the Mir space station. Radiat. Meas. 2002, 35, 485–488. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Baumstark-Khan, C. Getting ready for the manned mission to Mars: The astronauts’ risk from space radiation. Naturwissenschaften 2007, 94, 517–526. [Google Scholar] [CrossRef]
- Dachev, T.P.; Tomov, B.T.; Matviichuk, Y.N.; Dimitrov, P.G.; Bankov, N.G. High dose rates obtained outside ISS in June 2015 during SEP event. Life Sci. Space Res. 2016, 9, 84–92. [Google Scholar] [CrossRef]
- NASA. Human Health and Performance Risks for Space Exploration Missions. NASA-SP-2009-3405; NASA: Houston, TX, USA, 2009.
- NASA. Second Symposium on Protection against Radiations in Space-SP71; NASA: Galtinburg, TE, USA, 1964.
- Reitz, G.; Beaujean, R.; Benton, E.; Burmeister, S.; Dachev, T.; Deme, S.; Luszik-Bhadra, M.; Olko, P. Space radiation measurements on-board ISS--the DOSMAP experiment. Radiat. Prot. Dosim. 2005, 116, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Haffner, J.W. (Ed.) Radiation and Shielding in Space; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Jorgensen, A.M.; Patamia, S.E.; Gassend, B. Passive radiation shielding considerations for the proposed space elevator. Acta Astronaut. 2007, 60, 198–209. [Google Scholar] [CrossRef]
- Parker, E.N. Shielding space travelers. Sci. Am. 2006, 294, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Bengin, V.; Casolino, M.; Roca, V.; Zanini, A.; Durante, M. Tests of shielding effectiveness of Kevlar and Nextel onboard the International Space Station and the Foton-M3 capsule. Radiat. Environ. Biophys. 2010, 49, 359–363. [Google Scholar] [CrossRef]
- Spillantini, P.; Casolino, M.; Durante, M.; Mueller-Mellin, R.; Reitz, G.; Rossi, L.; Shurshakov, V.; Sorbi, M. Shielding from cosmic radiation for interplanetary missions: Active and passive methods. Radiat. Meas. 2007, 42, 14–23. [Google Scholar] [CrossRef]
- Wilson, J.W.; Cucinotta, F.A.; Shinn, J.L.; Simonsen, L.C.; Dubey, R.R.; Jordan, W.R.; Jones, T.D.; Chang, C.K.; Kim, M.Y. Shielding from solar particle event exposures in deep space. Radiat. Meas. 1999, 30, 361–382. [Google Scholar] [CrossRef]
- Sihver, L.; Sato, T.; Puchalska, M.; Reitz, G. Simulations of the MATROSHKA experiment at the international space station using PHITS. Radiat. Environ. Biophys. 2010, 49, 351–357. [Google Scholar] [CrossRef]
- Villagrasa, C.; Bordage, M.C.; Bueno, M.; Bug, M.; Chiriotti, S.; Gargioni, E.; Heide, B.; Nettelbeck, H.; Parisi, A.; Rabus, H. Assessing the Contribution of Cross-Sections to the Uncertainty of Monte Carlo Calculations in Micro- and Nanodosimetry. Radiat. Prot. Dosim. 2019, 183, 11–16. [Google Scholar] [CrossRef] [PubMed]
- NASA. Nuclear ad Space Radiation Effects on Materials; NASA: Springfield, Virginia, 1970.
- Fleischer, R.L.; Hart, H.R., Jr.; Giard, W.R. Particle track identification: Application of a new technique to apollo helmets. Science 1970, 170, 1189–1191. [Google Scholar] [CrossRef]
- Badhwar, G.D.; Keith, J.E.; Cleghorn, T.F. Neutron measurements onboard the space shuttle. Radiat. Meas. 2001, 33, 235–241. [Google Scholar] [CrossRef]
- Smith, M.B.; Khulapko, S.; Andrews, H.R.; Arkhangelsky, V.; Ing, H.; Koslowksy, M.R.; Lewis, B.J.; Machrafi, R.; Nikolaev, I.; Shurshakov, V. Bubble-detector measurements of neutron radiation in the international space station: ISS-34 to ISS-37. Radiat. Prot. Dosim. 2016, 168, 154–166. [Google Scholar] [CrossRef]
- Benton, E.R.; Benton, E.V.; Frank, A.L. Neutron dosimetry in low-earth orbit using passive detectors. Radiat. Meas. 2001, 33, 255–263. [Google Scholar] [CrossRef]
- Brodzinski, R.L.; Wognan, N.A.; Perkins, R.W. Cosmic-rat-induced radioactivity in astronauts as a measure of radiation dose. Space Life Sci. 1969, 2, 69. [Google Scholar]
- Rodbell, K.P. Low-energy protons-Where and why “rare events” matter. IEEE Trans. Nucl. Sci. 2020, 67, 1204–1215. [Google Scholar] [CrossRef]
- Francis, Z.; Villagrasa, C.; Clairand, I. Simulation of DNA damage clustering after proton irradiation using an adapted DBSCAN algorithm. Comput. Methods Programs Biomed. 2011, 101, 265–270. [Google Scholar] [CrossRef]
- Uehara, S.; Toburen, L.H.; Nikjoo, H. Development of a Monte Carlo track structure code for low-energy protons in water. Int. J. Radiat. Biol. 2001, 77, 139–154. [Google Scholar] [CrossRef]
- Benton, E.R.; Benton, E.V. Space radiation dosimetry in low-Earth orbit and beyond. Nucl. Instrum. Methods Phys. Res. B 2001, 184, 255–294. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Kim, M.H.; Willingham, V.; George, K.A. Physical and biological organ dosimetry analysis for international space station astronauts. Radiat. Res. 2008, 170, 127–138. [Google Scholar] [CrossRef]
- Stricklin, D.; VanHorne-Sealy, J.; Rios, C.I.; Carnell, L.S.; Taliaferro, L.P. Neutron Radiobiology and Dosimetry. Radiat. Res. 2021. [Google Scholar] [CrossRef]
- Kohler, J.; Ehresmann, B.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Hassler, D.M.; Reitz, G.; Brinza, D.E.; Appel, J.; Bottcher, S.; Bohm, E.; et al. Measurements of the neutron spectrum in transit to Mars on the Mars Science Laboratory. Life Sci. Space Res. 2015, 5, 6–12. [Google Scholar] [CrossRef]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef]
- ICRP. Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Reitz, G.; Berger, T.; Mattiae, D. Radiation exposure to the Moon environment. Planet. Space Sci. 2012, 74, 78–83. [Google Scholar] [CrossRef]
- Zhang, S.; Wimmer-Schweingruber, R.F.; Yu, J.; Wang, C.; Fu, Q.; Zou, Y.; Sun, Y.; Wang, C.; Hou, D.; Bottcher, S.I.; et al. First measurements of the radiation dose on the lunar surface. Sci. Adv. 2020, 6, eaaz1334. [Google Scholar] [CrossRef]
- Straume, T.; Blattnig, S.; Zeitlin, C. Radiation Hazards and the Colonization of Mars. In The Human Mission to Mars: Colonizing the Red Planet; Levine, J.S., Schild, E.R.E., Eds.; Cosmology Science Publishers: Cambridge, MA, USA, 2010; pp. 803–850. [Google Scholar]
- Abbasi, S.; Mortazavi, S.A.R.; Mortazavi, S.M.J. Martian Residents: Mass Media and Ramsar High Background Radiation Areas. J. Biomed. Phys. Eng. 2019, 9, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Dartnell, L.R. Ionizing radiation and life. Astrobiology 2011, 11, 551–582. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, N.; Gent, R.N.; Carr, Z.; Schneider, R.; Bader, J.; Buglova, E.; Chao, N.; Coleman, C.N.; Ganser, A.; Gorin, C.; et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med. Public Health Prep. 2011, 5, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Alp, M.; Sulzman, F.M.; Wang, M. Space radiation risks to the central nervous system. Life Sci. Space Res. 2014, 2, 54–69. [Google Scholar] [CrossRef]
- Preston, D.L.; Shimizu, Y.; Pierce, D.A.; Suyama, A.; Mabuchi, K. Studies of Mortality of Atomic Bomb Survivors; Report 13: Solid Cancer and Noncancer Disease Mortality: 1950–1997. Radiat. Res. 2003, 160, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A. A new approach to reduce uncertainties in space radiation cancer risk predictions. PLoS ONE 2015, 10, e0120717. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef]
- Tang, F.R.; Loganovsky, K. Low dose or low dose rate ionizing radiation-induced health effect in the human. J. Environ. Radioact. 2018, 192, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J. Radiat. Res. 2017, 58, 165–182. [Google Scholar] [CrossRef]
- Di Trolio, R.; Di Lorenzo, G.; Fumo, B.; Ascierto, P.A. Cosmic radiation and cancer: Is there a link? Future Oncol. 2015, 11, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Little, M.P.; Day, S.M.; Charvat, J.; Blattnig, S.; Huff, J.L.; Patel, Z.S. Cancer Incidence and Mortality in the U.S. Astronaut Corps, 1959–2017. Res. Sq. 2020, in press. [Google Scholar]
- Meier, M.M.; Matthia, D. Assessment of the skin dose for aircrew. J. Radiol. Prot. 2017, 37, 321–328. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare and deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Aleci, C. From international ophthalmology to space ophthalmology: The threats to vision on the way to Moon and Mars colonization. Int. Ophthalmol. 2020, 40, 775–786. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Manuel, F.K.; Jones, J.; Iszard, G.; Murrey, J.; Djojonegro, B.; Wear, M. Space radiation and cataracts in astronauts. Radiat. Res. 2001, 156, 460–466. [Google Scholar] [CrossRef]
- Blakely, E.A.; Chang, P.Y. A review of ground-based heavy ion radiobiology relevant to space radiation risk assessment: Cataracts and CNS effects. Adv. Space Res. 2007, 40, 1307–1319. [Google Scholar] [CrossRef][Green Version]
- Witze, A. Astronaut twins study spots subtle genetic changes caused by space travel. Nature 2019. [Google Scholar] [CrossRef]
- Luxton, J.J.; Bailey, S.M. Twins, Telomeres, and Aging-in Space! Plast. Reconstr. Surg. 2021, 147, 7S–14S. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364. [Google Scholar] [CrossRef]
- Dai, Z.; Lei, X.; Yang, C.; Zhao, L.; Lu, L.; Li, Y. Systematic biomedical research of the NASA Twins Study facilitates the hazard risk assessment of long-term spaceflight missions. Protein Cell 2019, 10, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Meydan, C.; Schmidt, C.M.; Afshinnekoo, E.; Mason, C.E. The NASA Twins Study: The Effect of One Year in Space on Long-Chain Fatty Acid Desaturases and Elongases. Lifestyle Genom. 2020, 13, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Abegaz, M.F.; Schreurs, A.S.; Alwood, J.S.; Globus, R.K.; Appel, E.A. A human mission to Mars: Predicting the bone mineral density loss of astronauts. PLoS ONE 2020, 15, e0226434. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.D.; Hays, S.M.; Tsuji, J.S. Modeling of blood lead levels in astronauts exposed to lead from microgravity-accelerated bone loss. Aviat. Space Environ. Med. 2013, 84, 1229–1234. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef]
- Farley, A.; Gnyubkin, V.; Vanden-Bossche, A.; Laroche, N.; Neefs, M.; Baatout, S.; Baselet, B.; Vico, L.; Mastrandrea, C. Unloading-Induced Cortical Bone Loss is Exacerbated by Low-Dose Irradiation During a Simulated Deep Space Exploration Mission. Calcif. Tissue Int. 2020, 107, 170–179. [Google Scholar] [CrossRef]
- Bachelet, J.T.; Granzotto, A.; Ferlazzo, M.L.; Sonzogni, L.; Berthel, E.; Devic, C.; Foray, N. First Radiobiological Characterization of Skin and Bone Cells from A Patient Suffering from the PI3KCA-Related Overgrowth Spectrum (PROS) Syndrome. Arch. Med. Clin. Case Rep. 2020, 4, 1052–1066. [Google Scholar] [CrossRef]
- Seddon, B.; Cook, A.; Gothard, L.; Salmon, E.; Latus, K.; Underwood, S.R.; Yarnold, J. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2002, 64, 53–63. [Google Scholar] [CrossRef]
- Tang, S.; Otton, J.; Holloway, L.; Delaney, G.P.; Liney, G.; George, A.; Jameson, M.; Tran, D.; Batumalai, V.; Thomas, L.; et al. Quantification of cardiac subvolume dosimetry using a 17 segment model of the left ventricle in breast cancer patients receiving tangential beam radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 132, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; McGale, P.; Bronnum, D.; Correa, C.; Cutter, D.; Duane, F.K.; Gigante, B.; Jensen, M.B.; Lorenzen, E.; Rahimi, K.; et al. Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study With Individual Patient Data. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2288–2296. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; Hall, P. Ischemic heart disease after breast cancer radiotherapy. N. Engl. J. Med. 2013, 368, 2527. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.J.; Day, S.M. Mortality due to cardiovascular disease among Apollo Lunar astronauts. Aerosp. Med. Hum. Perform. 2017, 88, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef]
- Nikjoo, H.; Lindborg, L. RBE of low energy electrons and photons. Phys. Med. Biol. 2010, 55, R65–R109. [Google Scholar] [CrossRef] [PubMed]
- Bianco, D.; Villagrasa, C.; Dos Santos, M. Multi-Scale Analysis of Simulated Proton and Alpha Irradiation. Radiat. Prot. Dosim. 2014. [Google Scholar] [CrossRef][Green Version]
- Britel, M.; Bourguignon, M.; Foray, N. Radiosensitivity: A term with various meanings at the origin of numerous confusions. A semantic analysis. Int. J. Radiat. Biol. 2018, 94, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Marples, B.; Fertil, B.; Malaise, E.P.; Joiner, M.C. Hypersensitivity of a human tumour cell line to very low radiation doses. Int. J. Radiat. Biol. 1993, 63, 639–650. [Google Scholar] [CrossRef]
- Marples, B.; Joiner, M.C. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 1993, 133, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Martin, J.; Devic, C.; Diserbo, M.; Thariat, J.; Foray, N. Impact of dose-rate on the low-dose hyper-radiosensitivity and induced radioresistance (HRS/IRR) response. Int. J. Radiat. Biol. 2013, 89, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Slonina, D.; Kowalczyk, A.; Janecka-Widla, A.; Kabat, D.; Szatkowski, W.; Biesaga, B. Low-Dose Hypersensitive Response for Residual pATM and gammaH2AX Foci in Normal Fibroblasts of Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef]
- Devic, C.; Ferlazzo, M.L.; Berthel, E.; Foray, N. Influence of Individual Radiosensitivity on the Hormesis Phenomenon: Toward a Mechanistic Explanation Based on the Nucleoshuttling of ATM Protein. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820913784. [Google Scholar] [CrossRef]
- Luckey, T.D. Hormesis with Ionizing Radiation; CRC Press: New York, NY, USA, 1980. [Google Scholar]
- Calabrese, E.J. Hormesis is central to toxicology, pharmacology and risk assessment. Hum. Exp. Toxicol. 2008, 29, 249–261. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: A fundamental concept in biology. Microb. Cell 2014, 1, 145–149. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C.B. Radiation-induced bystander effects--implications for cancer. Nat. Rev. Cancer 2004, 4, 158–164. [Google Scholar]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects, carcinogenesis and models. Oncogene 2003, 22, 7028–7033. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Krog, R.L.; Feiveson, A.H. Extravehicular mobility unit training and astronaut injuries. Aviat. Space Environ. Med. 2005, 76, 469–474. [Google Scholar]
- Beggs, J.C. Design and development of the Apollo extravehicular mobility unit. Ann. N. Y. Acad. Sci. 1965, 134, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Cooke, W.H.; Lukie, K.G. Insipratory resistance as a potnetial treatment for orthostatic intolerance and hemorrhagic shock. Aviat. Space Environ. Med. 2005, 76, 319–325. [Google Scholar] [PubMed]
- Baiocco, G.; Giraudo, M.; Bocchini, L.; Barbieri, S.; Locantore, I.; Brussolo, E.; Giacosa, D.; Meucci, L.; Steffenino, S.; Ballario, A.; et al. A water-filled garment to protect astronauts during interplanetary missions tested on board the ISS. Life Sci. Space Res. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Phillips, T.L. Rationale for initial clinical trials and future development of radioprotectors. Cancer Clin. Trials 1980, 3, 165–173. [Google Scholar]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef]
- Langell, J.; Jennings, R.; Clarck, J.; Ward Jr, J.B. Pharmacological agents for the prevention and treatment of toxic radiation exposure in spaceflight. Aviat. Space Environ. Med. 2008, 79, 651–660. [Google Scholar] [CrossRef]
- Berthel, E.; Foray, N.; Ferlazzo, M.L. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 2019, 11, 905. [Google Scholar] [CrossRef] [PubMed]
| Missions | References | Absorbed Dose/Day (mGy/d) | Effective Dose/Day (mSv/d) |
|---|---|---|---|
| Missions before ISS | [41] | Salyut: 0.1–0.3 Apollo: 0.22–1.27 | |
| Missions from Gagarin to Space Shuttle | [6] | 0.2–1.26 Average: 0.4 | |
| ISS | [23] | 0.153–0.231 | |
| ISS during Solar event | [23] | 0.535 | |
| ISS | [35] | 0.15 | |
| ISS during Solar event | [20] | 10.48 | |
| Moon surface | [19] | 0.37–0.97 | |
| [47] | 0.3–1 | ||
| [48] | 0.31 | ||
| Mars surface | [19] | 0.17–0.325 | |
| [49] | 0.002–0.9 | ||
| [52] | 0.15 | ||
| [51] | 0.33 |
| On Earth | In Space | ||
|---|---|---|---|
| Effective dose (mSv) | 700–12,000 | Acute radiation syndrome (irradiation accident) | ARS during solar events? |
| 2000 | Tumor dose per fraction in radiotherapy Dose per fraction in total body irradiation | ||
| 200 | Threshold dose for solid cancer | 500 days in ISS | |
| 100 | Threshold dose for leukemia | ||
| 20 | Coronarography | 50 days in ISS | |
| 0.3–0.5 | 2 mammography views | 1 day in ISS | |
| 0.01 | 1 day for a nuclear worker | ||
| Effective dose-rate (mSv/y) | 0.5 | Radiation background in Japan | |
| 2.4 | Worldwide radiation background | ||
| 70 | Radiation background in Ramsar | ||
| 110–180 | In LEO (ISS) | ||
| 130–260 | At the surface of Mars | ||
| 110–300 | At the surface of the Moon | ||
| 500–700 | In deep space |
| Radiation-Induced Consequences | Type of Space Radiation | Type of Tissues | Clinical Consequences | Countermeasures |
|---|---|---|---|---|
| Radiosusceptibility The 100–200 mGy thresholds for cancer risks derived from Hiroshima survivors are the only consensual series of data. | Low energy ions/particles including neutrons | “surface tissues”: Eye Skin | Eye melanoma Skin melanoma | Glasses? Water-filled worksuits? |
| “Bath of radiation” including fast neutrons | “deep tissues”: Cardiovascular System Bones General | No cancer observed yet Osteosarcoma Leukemia | Radioprotective drugs | |
| Radiodegeneration There are no well-defined dose thresholds (>0.1 Gy?) nor biomarkers that are specific to aging yet. | Low energy ions/particles including neutrons | “surface tissues”: Eye Skin | Cataracts Aging | Glasses? Water-filled worksuits? |
| “Bath of radiation” including fast neutrons | “deep tissues”: Cardiovascular System Bones General | Heart attacks Loss of bone mass To be investigated | Radioprotective drugs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restier-Verlet, J.; El-Nachef, L.; Ferlazzo, M.L.; Al-Choboq, J.; Granzotto, A.; Bouchet, A.; Foray, N. Radiation on Earth or in Space: What Does It Change? Int. J. Mol. Sci. 2021, 22, 3739. https://doi.org/10.3390/ijms22073739
Restier-Verlet J, El-Nachef L, Ferlazzo ML, Al-Choboq J, Granzotto A, Bouchet A, Foray N. Radiation on Earth or in Space: What Does It Change? International Journal of Molecular Sciences. 2021; 22(7):3739. https://doi.org/10.3390/ijms22073739
Chicago/Turabian StyleRestier-Verlet, Juliette, Laura El-Nachef, Mélanie L. Ferlazzo, Joëlle Al-Choboq, Adeline Granzotto, Audrey Bouchet, and Nicolas Foray. 2021. "Radiation on Earth or in Space: What Does It Change?" International Journal of Molecular Sciences 22, no. 7: 3739. https://doi.org/10.3390/ijms22073739
APA StyleRestier-Verlet, J., El-Nachef, L., Ferlazzo, M. L., Al-Choboq, J., Granzotto, A., Bouchet, A., & Foray, N. (2021). Radiation on Earth or in Space: What Does It Change? International Journal of Molecular Sciences, 22(7), 3739. https://doi.org/10.3390/ijms22073739







