Genetic Labeling of Cells Allows Identification and Tracking of Transgenic Platelets in Mice
Abstract
1. Introduction
2. Results
2.1. Main Text
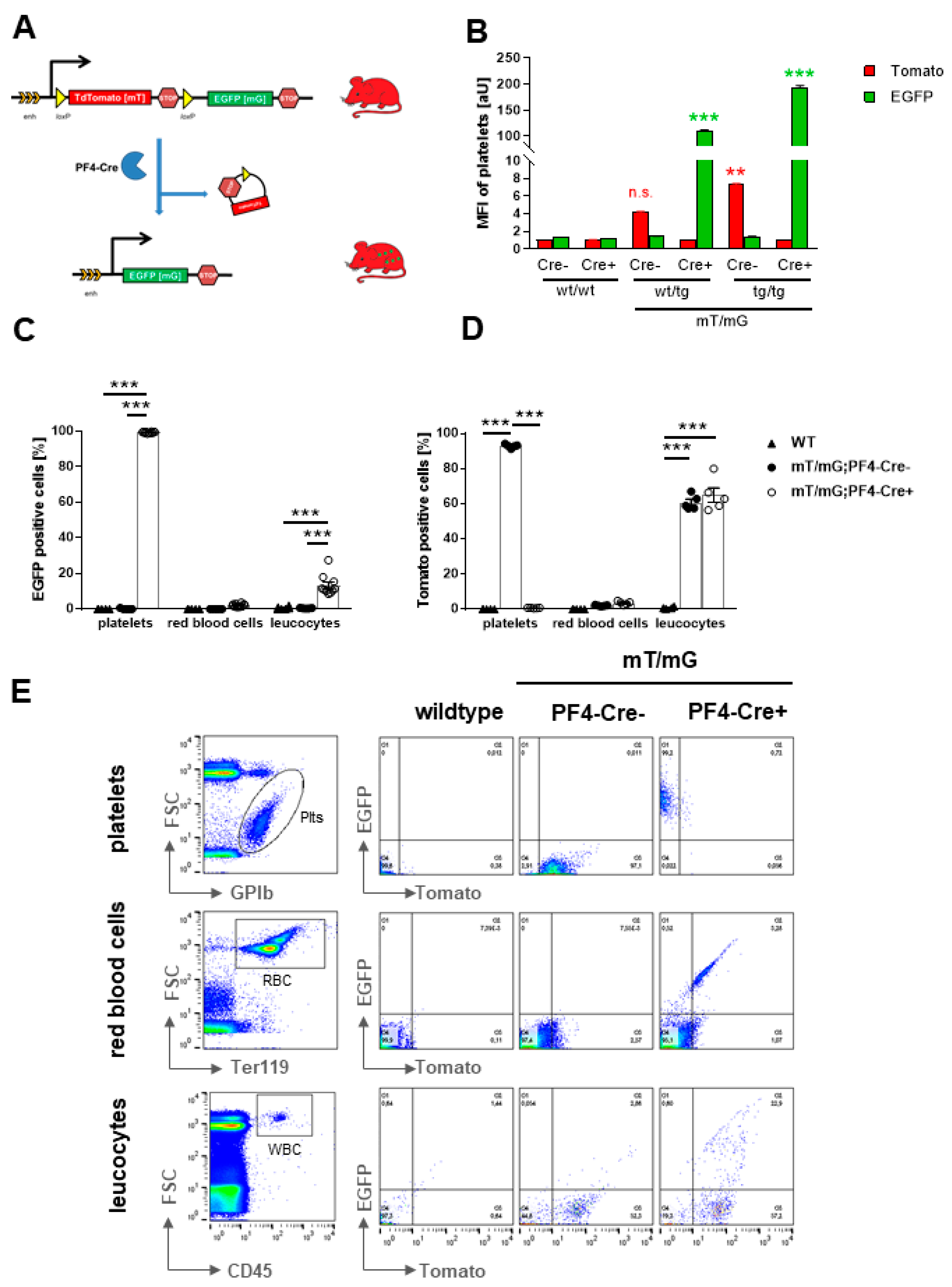
2.1.1. Autofluorescence of Platelets in mT/mG;PF4-Cre transgenic Mice
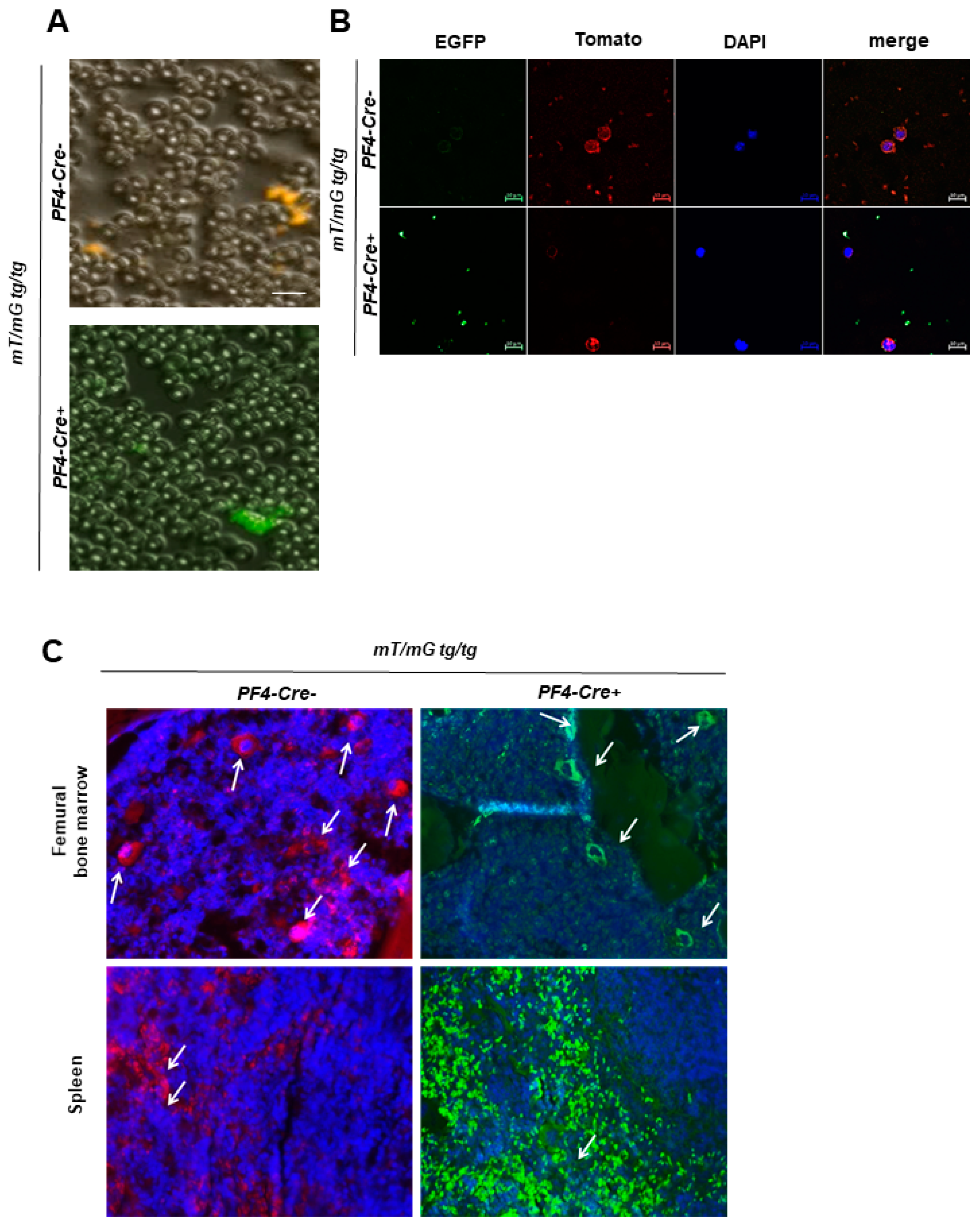
2.1.2. Fluorescently labeled Platelets and Megakaryocytes in Blood Smear, Spleen, and Bone Marrow of Transgenic Mice
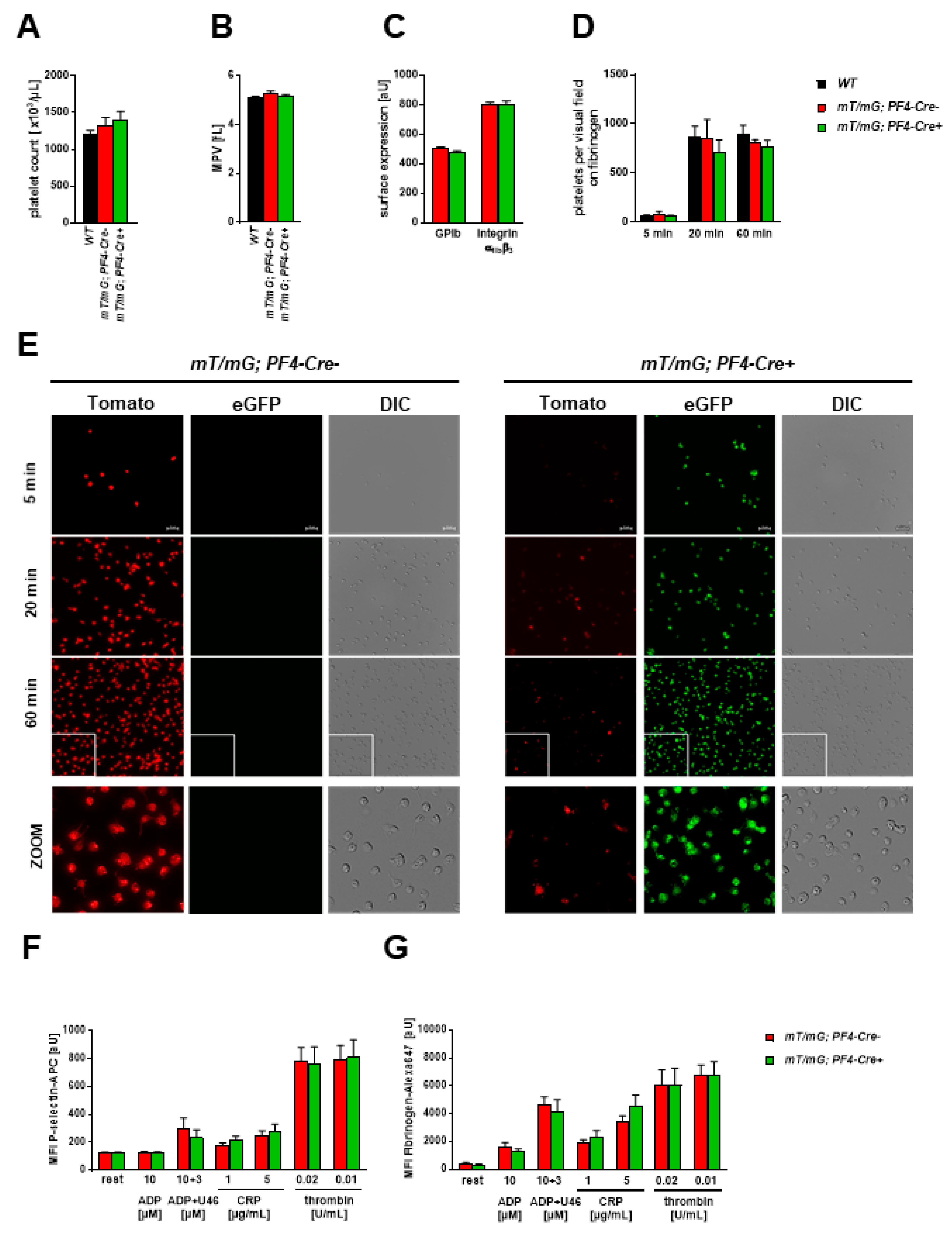
2.1.3. Unaltered Platelet Activation and Receptor Surface Expression in PF4-Cre negative and PF4-Cre positive Mice
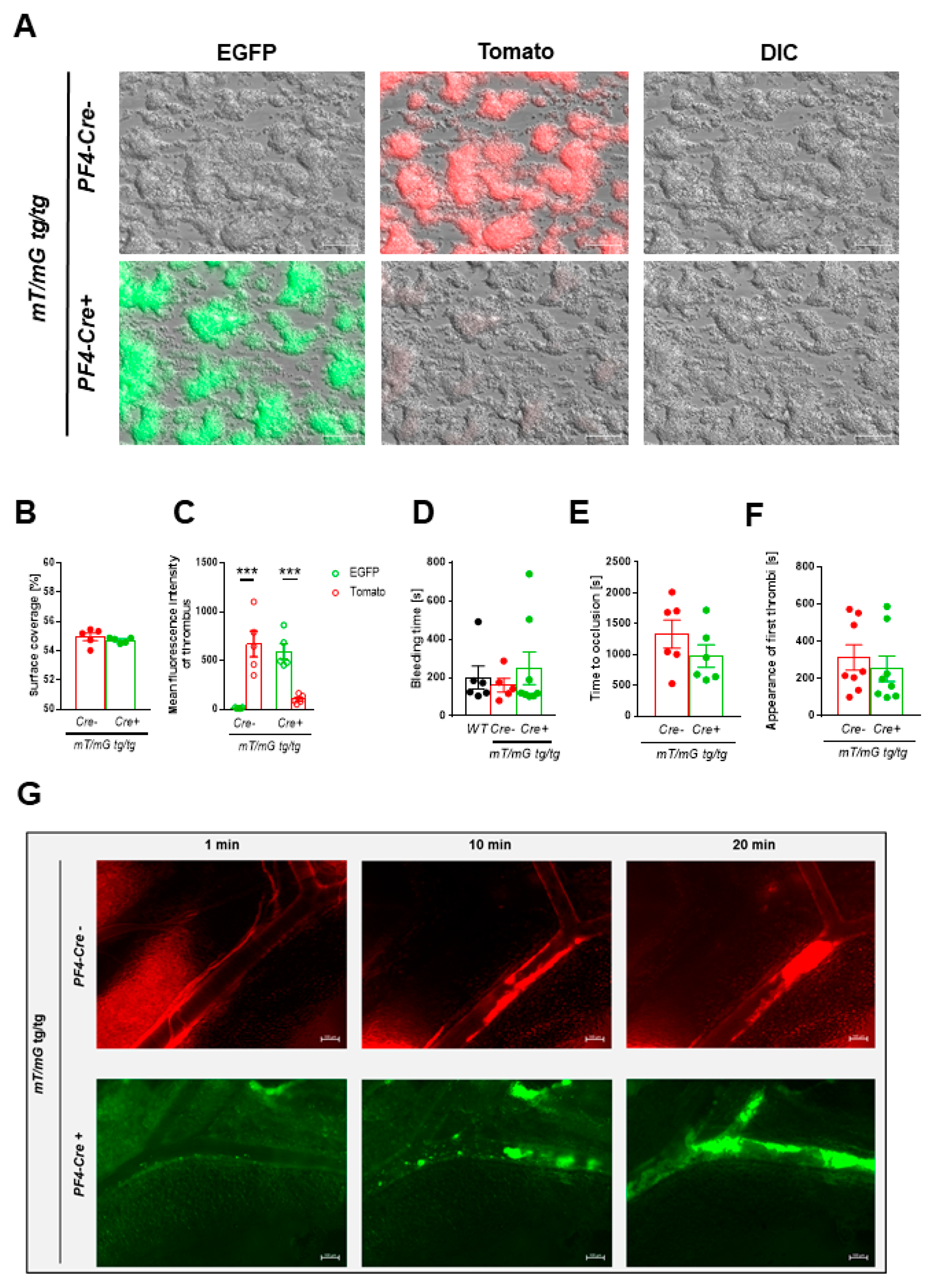
2.1.4. Comparable Thrombus Formation in PF4-Cre negative and positive mT/mG transgenic Mice
2.1.5. Tracking of mT/mG positive Platelets via Intravital Microscopy
2.1.6. Transfer of Bone Marrow from mT/mG;PF4-Cre positive Mice to C57BL/6 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Murine Platelet Preparation and Determination of Cell Count
4.4. Flow Cytometry
4.5. Fluorescence Microscopy
4.6. Flow Chamber Experiments to Analyze Thrombus Formation under Flow Conditions
4.7. Platelet Adhesion and Spreading
4.8. Intravital Microscopy of Arterial Thrombosis in Mesenteric Arterioles Following Injury with FeCl3
4.9. Determination of the Bleeding Time to Investigate Hemostasis in Transgenic Mice
4.10. Generation of Bone Marrow Chimeric Mice
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphophate |
| BSA | bovine serum albumine |
| CaCl2 | calcium chloride |
| CLEC-2 | C-type lectin-like receptor 2 |
| Cre | cyclization recombination or causes recombination |
| CRP | collagen-related peptide |
| DIC | differential interference contrast |
| EDTA | ethylenediaminetetraacetic acid |
| FeCl3 | iron(III) chloride |
| GPIb/VI | glycoprotein (GP) Ib / VI |
| HE | hematoxylin/eosin |
| KCl | potassium chloride |
| KHCO3 | potassium bicarbonate |
| loxP | locus of X-over P1, recognition site for cre to excision |
| MFI | mean fluorescence intensity |
| mG | membrane-targeted enhanced green fluorescent protein (EGFP) |
| min | minute |
| mT | membrane-targeted tandem dimer Tomato (red fluorescent protein) |
| NaCl | sodium chloride |
| NaHCO3 | sodium bicarbonate |
| Na2HPO4 | disodium phosphate |
| NH4Cl | ammonium chloride |
| PLD1 | phospholipase D1 |
| PF4 | platelet factor 4 |
| PFA | paraformaldehyde |
| PRP | platelet-rich plasma |
| rest | resting |
| Thr | thrombin |
| U46 | U46619, thromboxane analogue |
| vWF | von Willebrand Factor |
References
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: A Major Contributor to the Global Disease Burden. J. Thromb. Haemost. 2014, 12, 1580–1590.
- Mackman, N. Triggers, Targets and Treatments for Thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef]
- Gowert, N.S.; Kruger, I. Loss of Reelin protects mice against arterial thrombosis by impairing integrin activation and thrombus formation under high shear conditions. Cell Signal 2017, 40, 210–221. [Google Scholar] [CrossRef]
- Elvers, M.; Stegner, D. Impaired Alpha(IIb)beta(3) Integrin Activation and Shear-dependent Thrombus Formation in Mice Lacking Phospholipase D1. Sci. Signal 2010, 3, ra1. [Google Scholar] [CrossRef]
- Miura, Y.; Takahashi, T. Analysis of the Interaction of Platelet Collagen Receptor Glycoprotein VI (GPVI) with Collagen. J. Biol. Chem. 2002, 277, 46197–46204. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ehrenberg, A. Molecular Drivers of Platelet Activation: Unraveling Novel Targets for Anti-Thrombotic and Anti-Thrombo-Inflammatory Therapy. Int. J. Mol. Sci. 2020, 21, 7906. [Google Scholar] [CrossRef]
- Nieswandt, B.; Watson, S.K. Platelet-collagen Interaction: Is GPVI the Central Receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef]
- Krueger, I.; Gremer, L. Reelin Amplifies Glycoprotein VI Activation and AlphaIIb Beta3 Integrin Outside-In Signaling via PLC Gamma 2 and Rho GTPases. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2391–2403. [Google Scholar] [CrossRef]
- Jagadeeswaran, P.; Cooley, B.C. Animal Models of Thrombosis from Zebrafish to Nonhuman Primates. Circ. Res. 2016, 118, 1363–1379. [Google Scholar] [CrossRef]
- Thijs, T.; Deckmyn, H. Model Systems of Genetically Modified Platelets. Blood 2012, 119, 1634–1642. [Google Scholar] [CrossRef]
- Hart, A.W.; Morgan, J.E. Cardiac Malformations and Midline Skeletal Defects in Mice Lacking Filamin A. Hum. Mol. Gen. 2006, 15, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Monkley, S.J.; Zhou, X.-H. Disruption of the Talin Gene Arrests Mouse Development at the Gastrulation Stage. Dev. Dyn. 2000, 219, 560–574. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Inoue, O. Essential in Vivo Roles of the C-type Lectin Receptor CLEC-2. J Biol Chem 2010, 285, 24494–24507. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Guimarães, M.J. Mammalian Genomes Contain Active Recombinase Recognition Sites. Gene 2000, 244, 47–54. [Google Scholar] [CrossRef]
- Lepage, A.; Leboeuf, M.n. The αIIbβ3 Integrin and GPIb-V-IX Complex Identify Distinct Stages in the Maturation of CD34+cord Blood Cells to Megakaryocytes. Blood 2000, 96, 4169–4177. [Google Scholar] [CrossRef]
- Szalai, G.; LaRue, A.C. Molecular Mechanisms of Megakaryopoiesis. Cell. Mol. Life Sci. 2006, 63, 2460–2476. [Google Scholar] [CrossRef]
- Falet, H.; Pollitt, A.Y. A Novel Interaction between FlnA and Syk Regulates Platelet ITAM-mediated Receptor Signaling and Function. J. Exp. Med. 2010, 207, 1967–1979. [Google Scholar] [CrossRef]
- Tiedt, R.; Schomber, T. PF4-Cre Transgenic Mice Allow the Generation of Lineage-restricted Gene Knockouts for Studying Megakaryocyte and Platelet Function in Vivo. Blood 2007, 109, 1503–1506. [Google Scholar] [CrossRef]
- Klatt, C.; Kruger, I. Platelet-RBC Interaction Mediated by FasL-FasR Induces Procoagulant Activity Important for Thrombosis. J. Clin. Investig. 2018, 128, 3906–3925. [Google Scholar] [CrossRef]
- Gowert, N.S.; Klier, M. Defective Platelet Activation and Bleeding Complications upon Cholestasis in Mice. Cell Physiol Biochem 2017, 41, 2133–2149. [Google Scholar] [CrossRef]
- Muzumdar, M.D.; Tasic, B. A Global Double-fluorescent Cre Reporter Mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V. Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef]
- Konig, K. Multiphoton Microscopy in Life Sciences. J Microsc 2000, 200, 83–104. [Google Scholar] [CrossRef]
- Calaminus, S.D.J.; Guitart, A.V. Correction: Lineage Tracing of PF4-Cre Marks Hematopoietic Stem Cells and Their Progeny. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Pertuy, F.; Aguilar, A. Broader Expression of the Mouse Platelet Factor 4-cre Transgene beyond the Megakaryocyte Lineage. J. Thromb. Haemost. 2015, 13, 115–125. [Google Scholar] [CrossRef]
- Rudolph, J.M.; Guttek, K. Characterization of Mice with a Platelet-Specific Deletion of the Adapter Molecule ADAP. Mol. Cell. Biol. 2019, 39, e00365-18. [Google Scholar] [CrossRef]
- Donner, L.; Falker, K. Platelets Contribute to Amyloid-beta Aggregation in Cerebral Vessels through Integrin AlphaIIbbeta3-induced outside-in Signaling and Clusterin Release. Sci. Signal 2016, 9, ra52. [Google Scholar] [CrossRef]
- Elvers, M.; Pozgaj, R. Platelet Hyperreactivity and a Prothrombotic Phenotype in Mice with a Gain-of-function Mutation in Phospholipase Cgamma2. J. Thromb. Haemost. 2010, 8, 1353–1363. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, I.; Reusswig, F.; Krott, K.J.; Lersch, C.F.; Spelleken, M.; Elvers, M. Genetic Labeling of Cells Allows Identification and Tracking of Transgenic Platelets in Mice. Int. J. Mol. Sci. 2021, 22, 3710. https://doi.org/10.3390/ijms22073710
Krüger I, Reusswig F, Krott KJ, Lersch CF, Spelleken M, Elvers M. Genetic Labeling of Cells Allows Identification and Tracking of Transgenic Platelets in Mice. International Journal of Molecular Sciences. 2021; 22(7):3710. https://doi.org/10.3390/ijms22073710
Chicago/Turabian StyleKrüger, Irena, Friedrich Reusswig, Kim Jürgen Krott, Celina Fabienne Lersch, Martina Spelleken, and Margitta Elvers. 2021. "Genetic Labeling of Cells Allows Identification and Tracking of Transgenic Platelets in Mice" International Journal of Molecular Sciences 22, no. 7: 3710. https://doi.org/10.3390/ijms22073710
APA StyleKrüger, I., Reusswig, F., Krott, K. J., Lersch, C. F., Spelleken, M., & Elvers, M. (2021). Genetic Labeling of Cells Allows Identification and Tracking of Transgenic Platelets in Mice. International Journal of Molecular Sciences, 22(7), 3710. https://doi.org/10.3390/ijms22073710






