Marine Collagen Hydrolysates Promote Collagen Synthesis, Viability and Proliferation While Downregulating the Synthesis of Pro-Catabolic Markers in Human Articular Chondrocytes
Abstract
:1. Introduction
2. Results
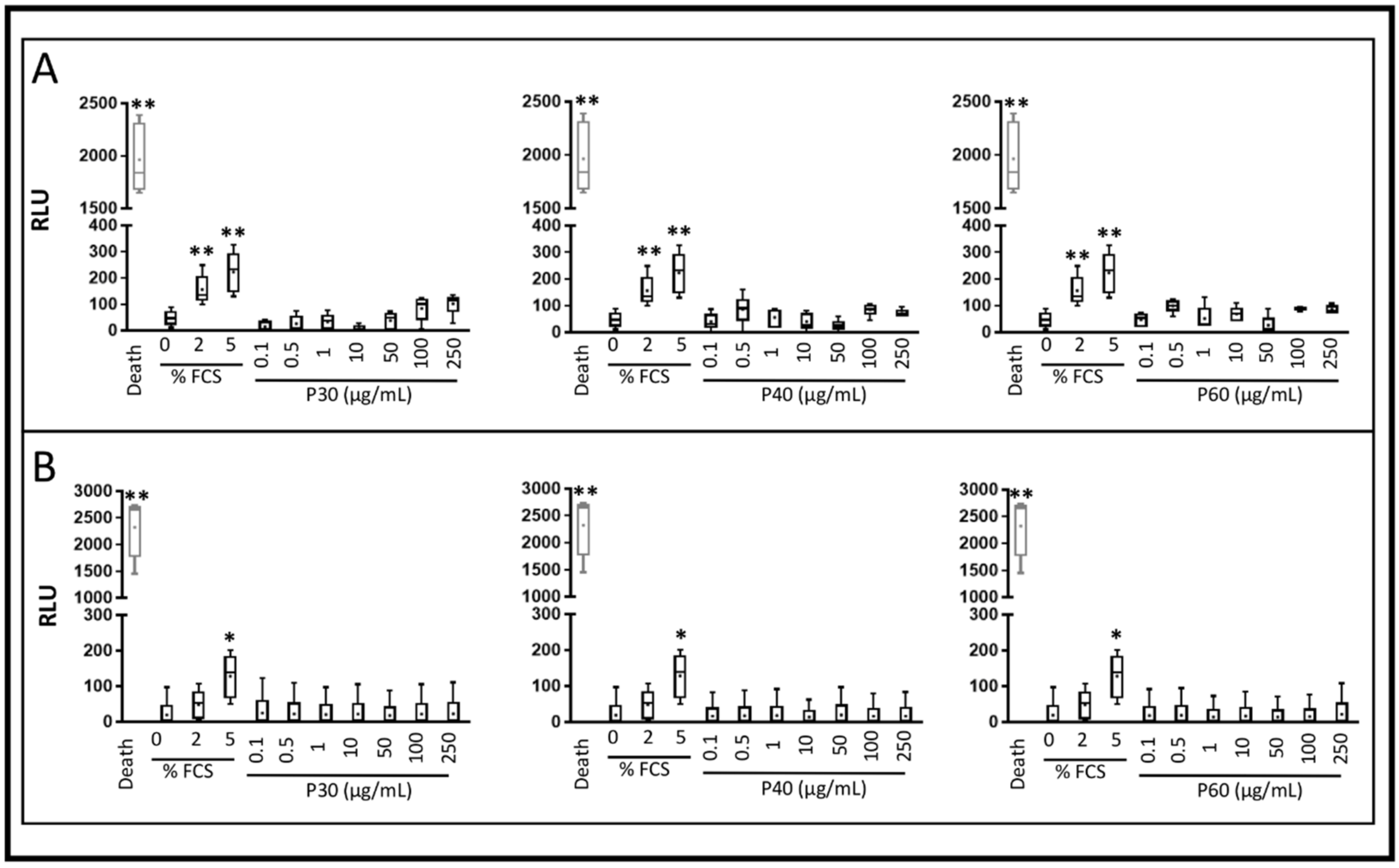
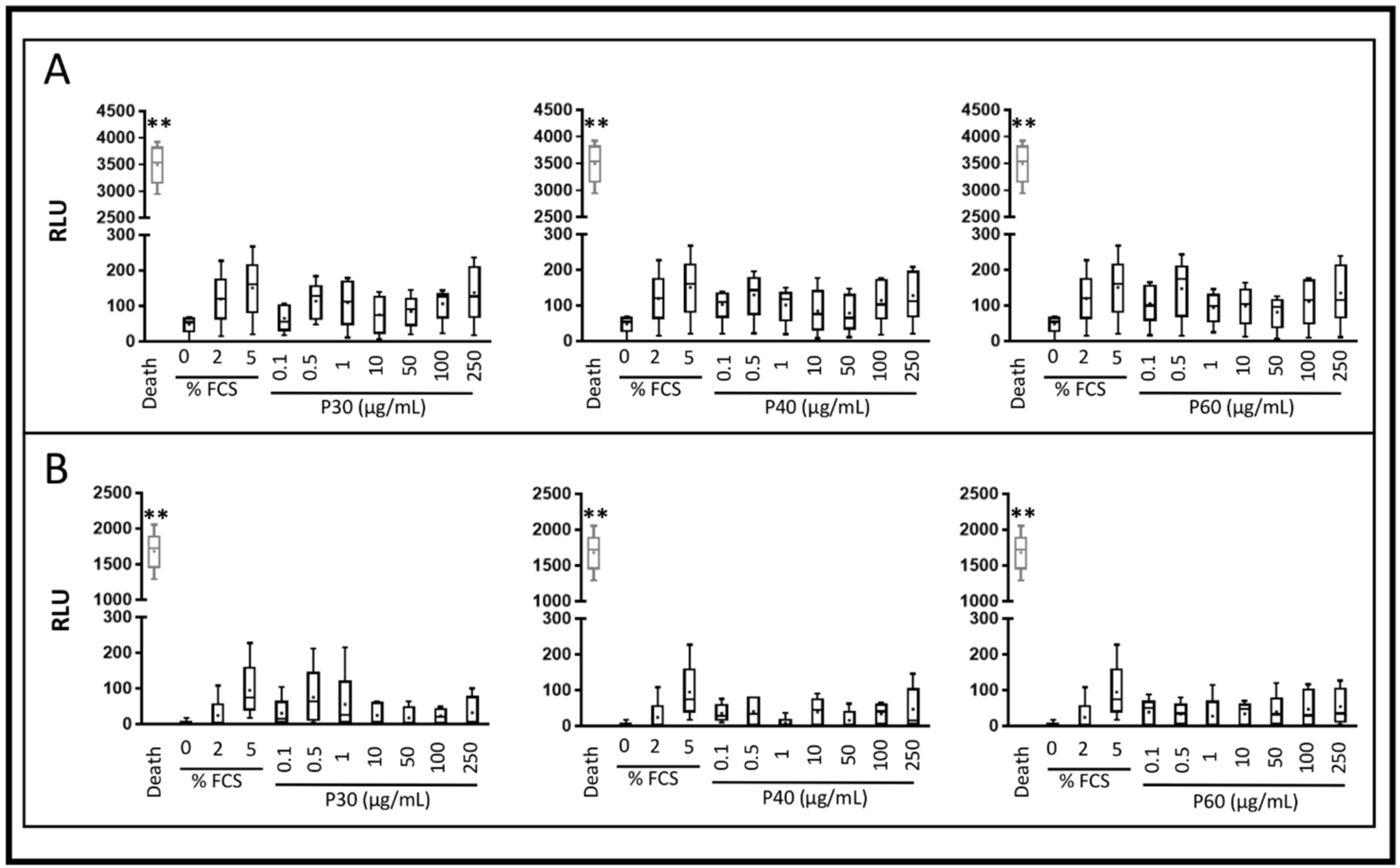
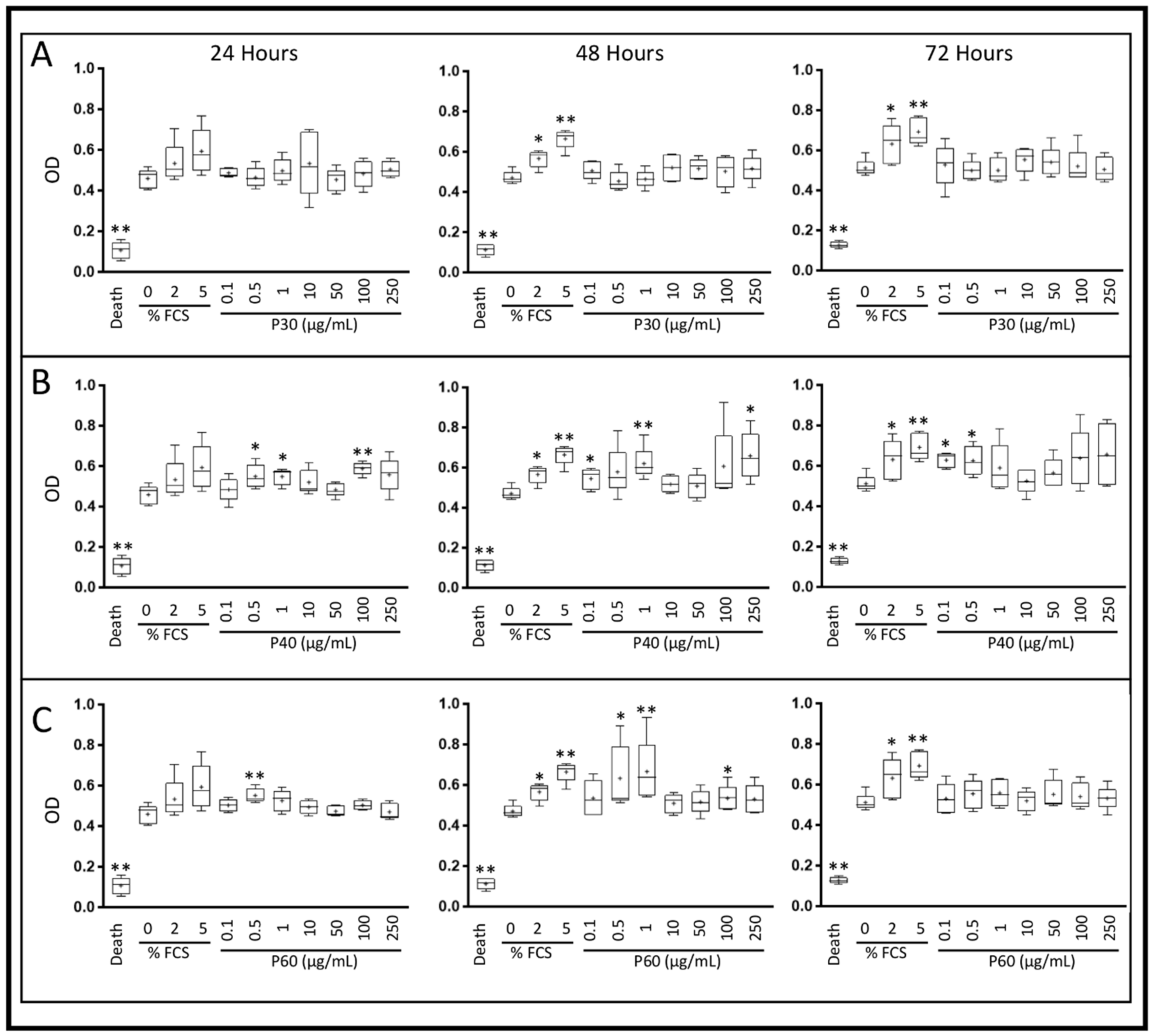
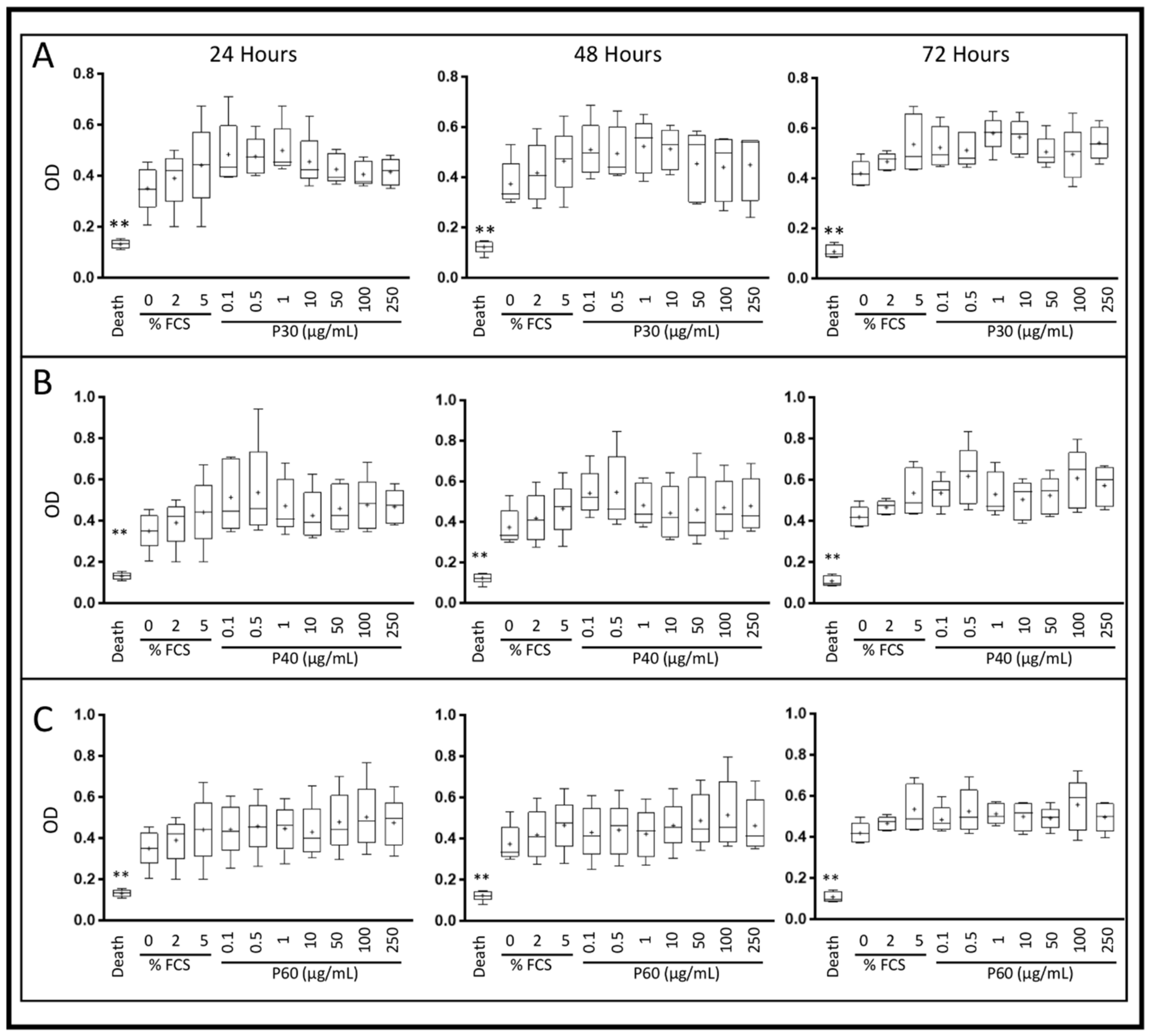
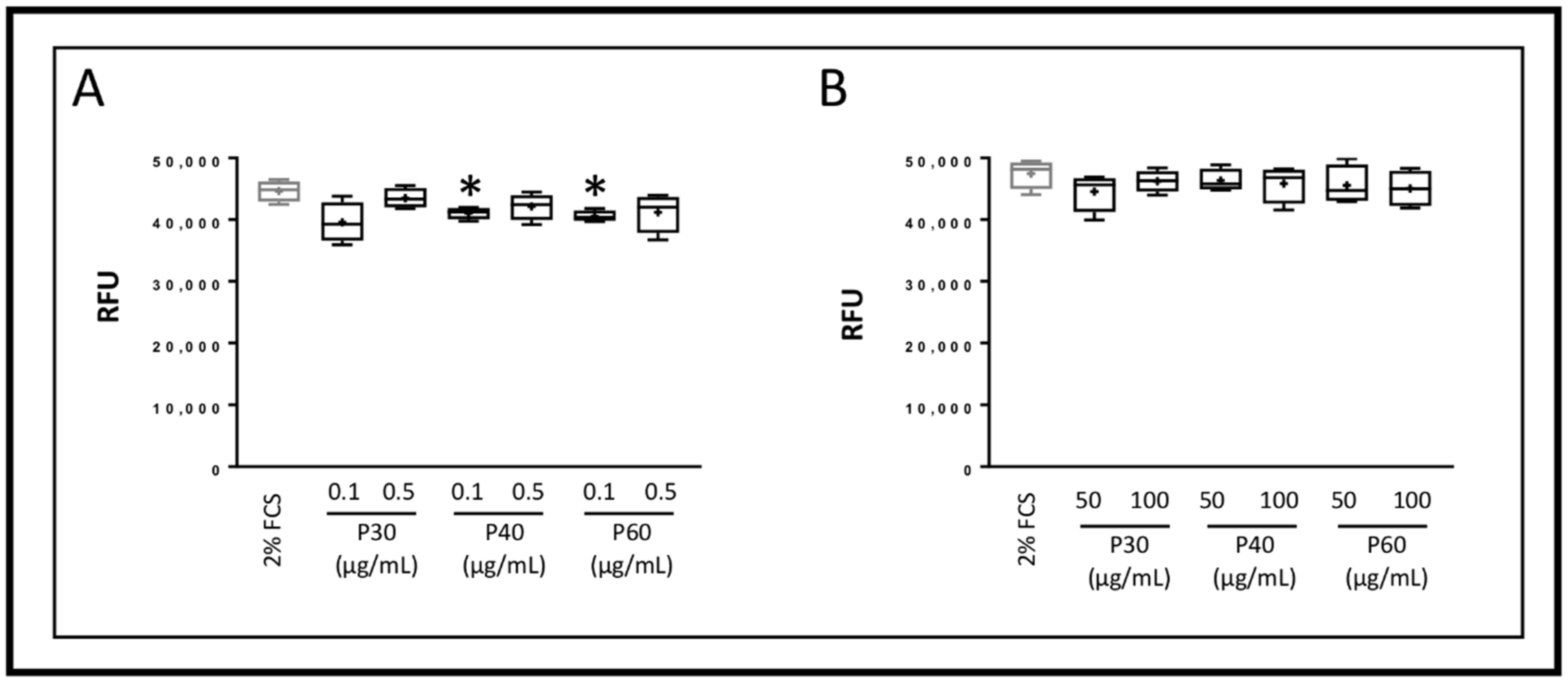
2.1. Promerim®30, 40 and 60 Show No Cytotoxic Effects on HACs, Promote Their Viability, Proliferation and Inhibit Cell Senescence
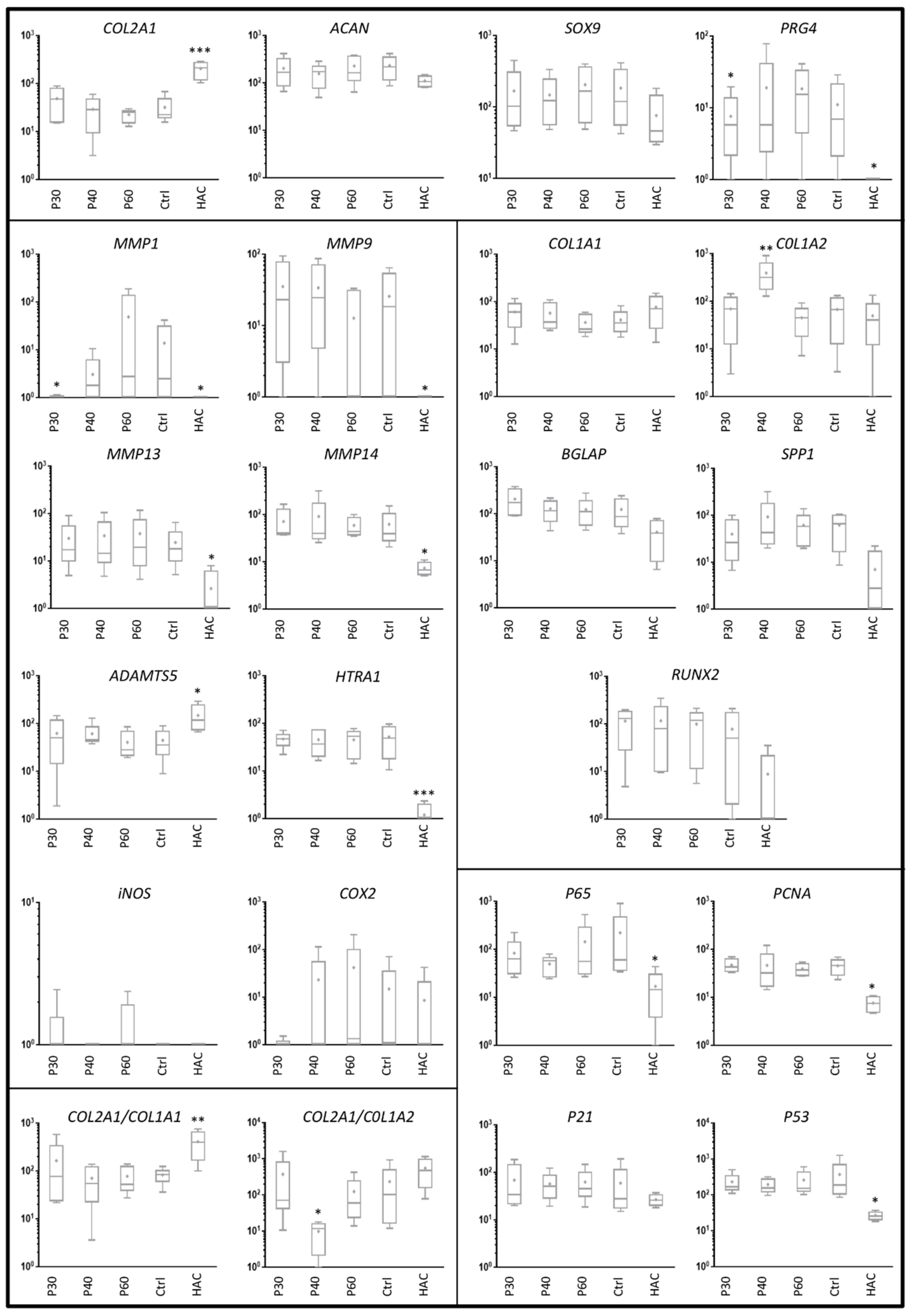
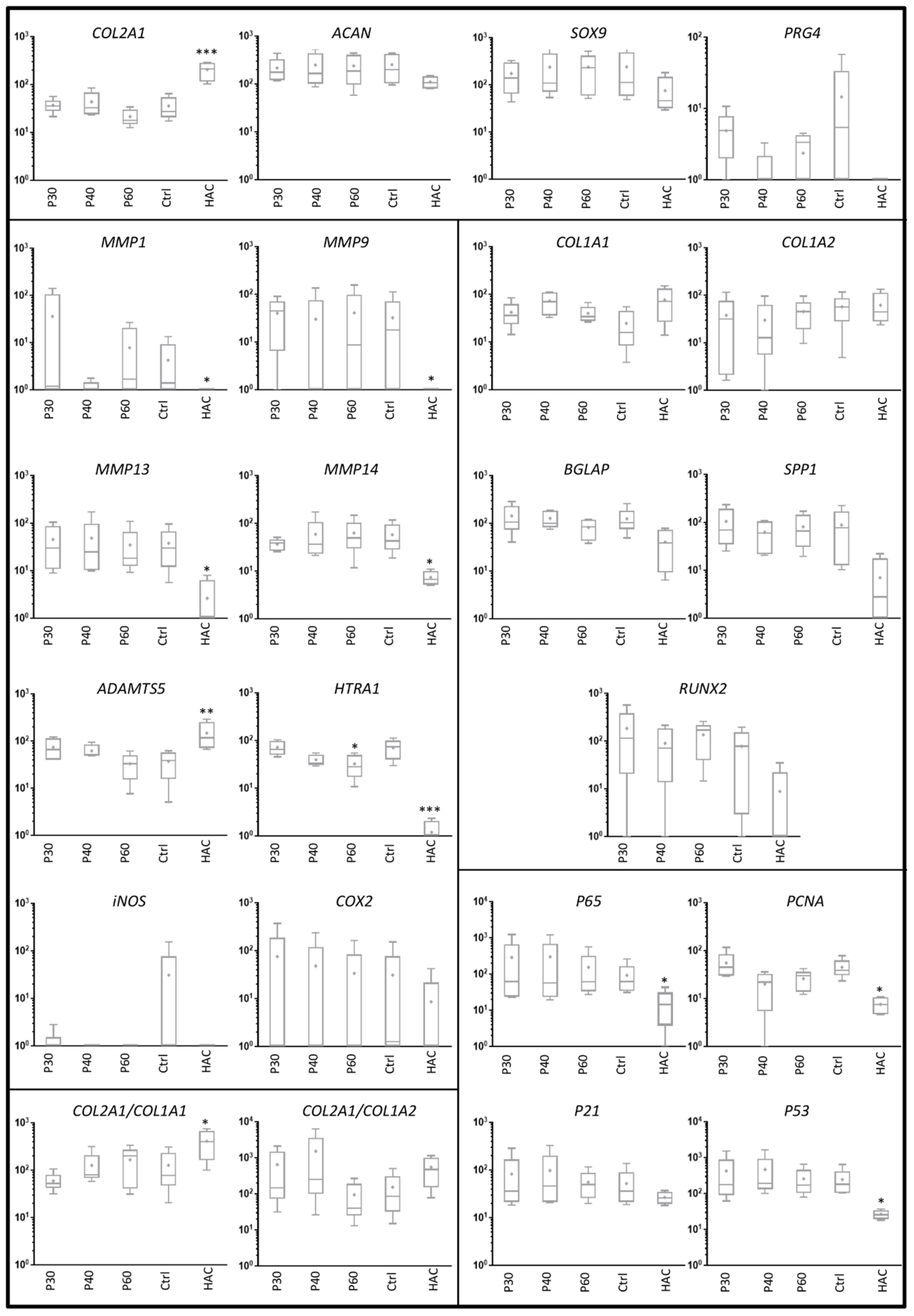
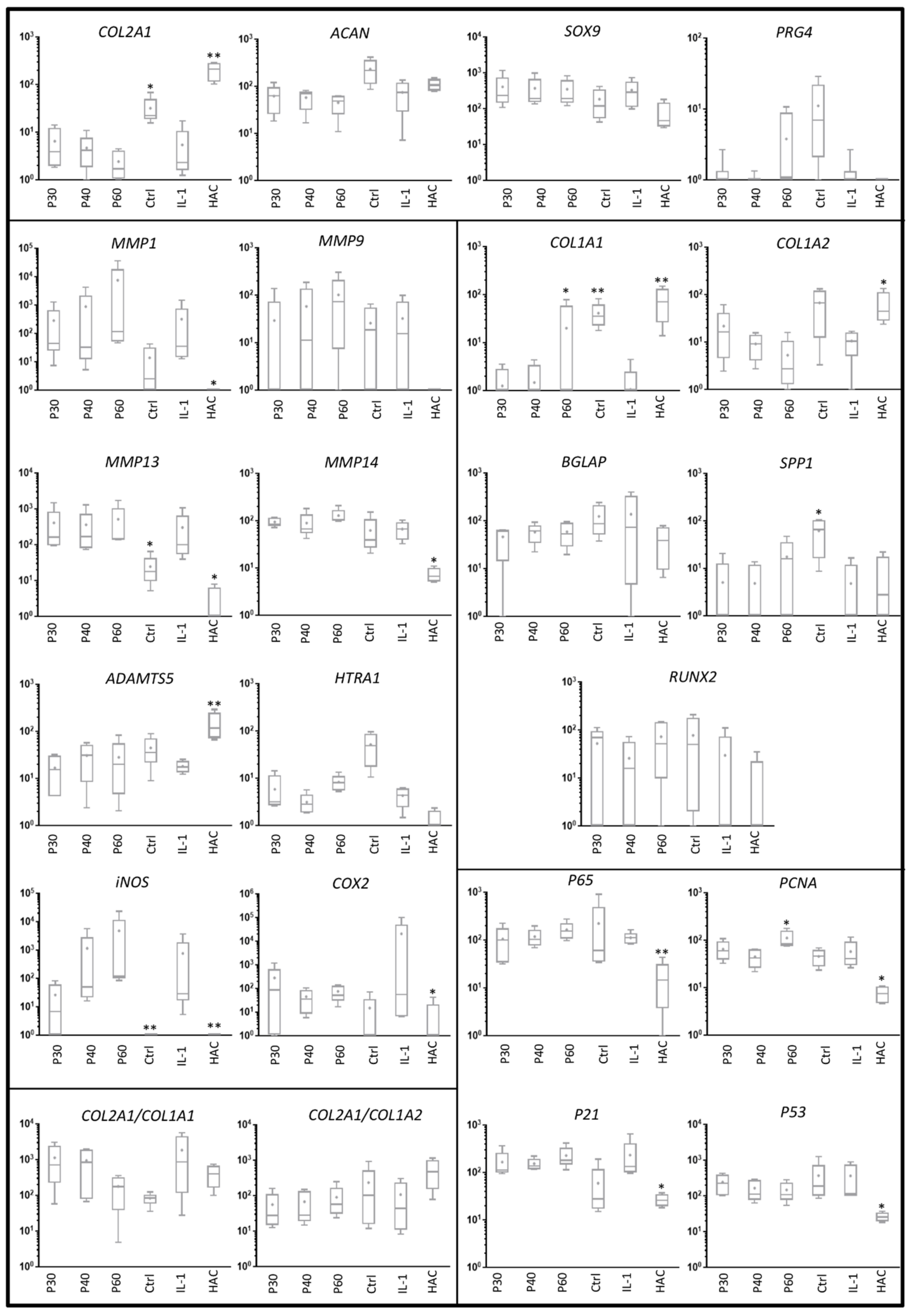
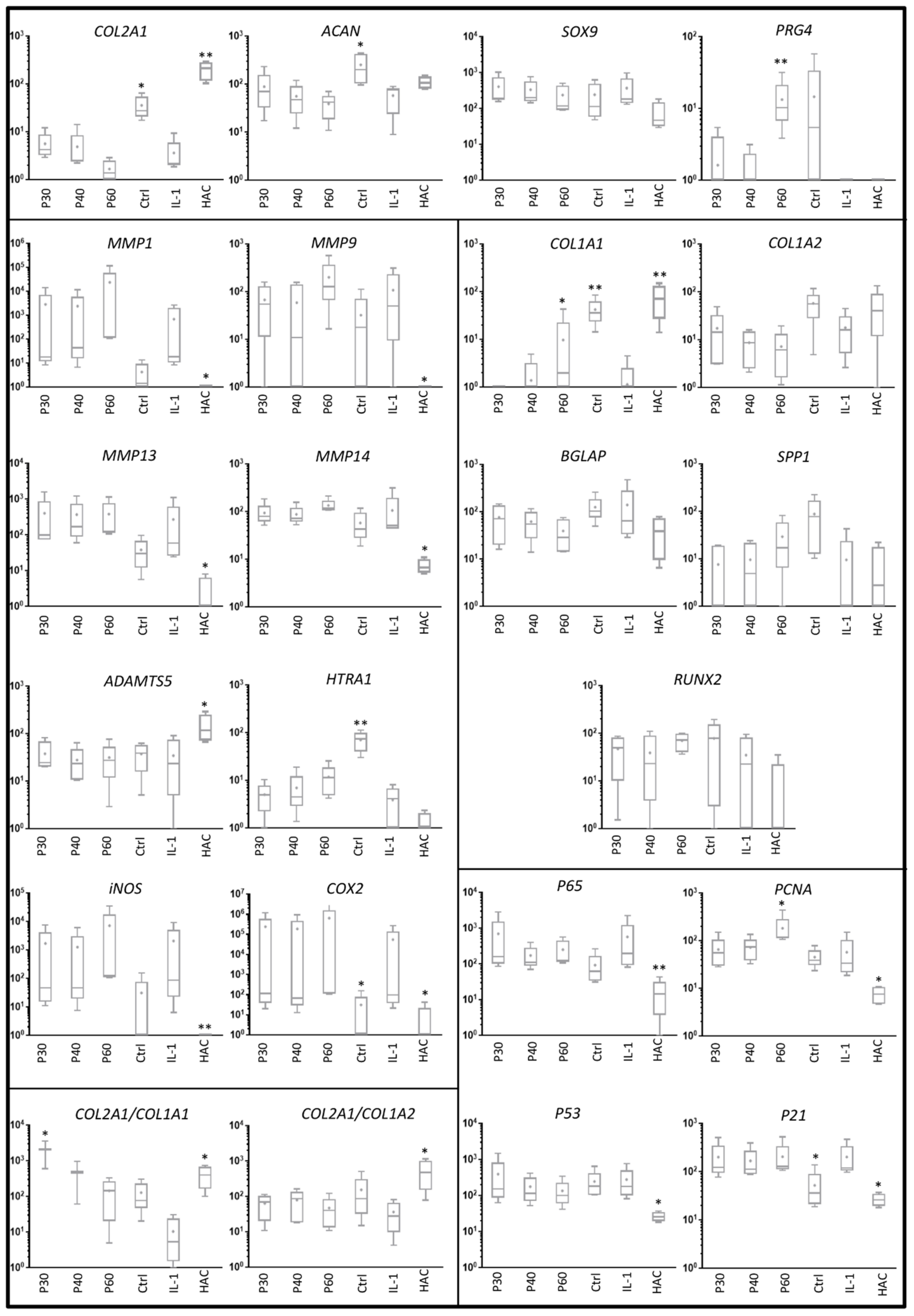
2.2. Effect of Promerim® on the Expression of mRNAs Typical and Atypical of Articular Chondrocytes
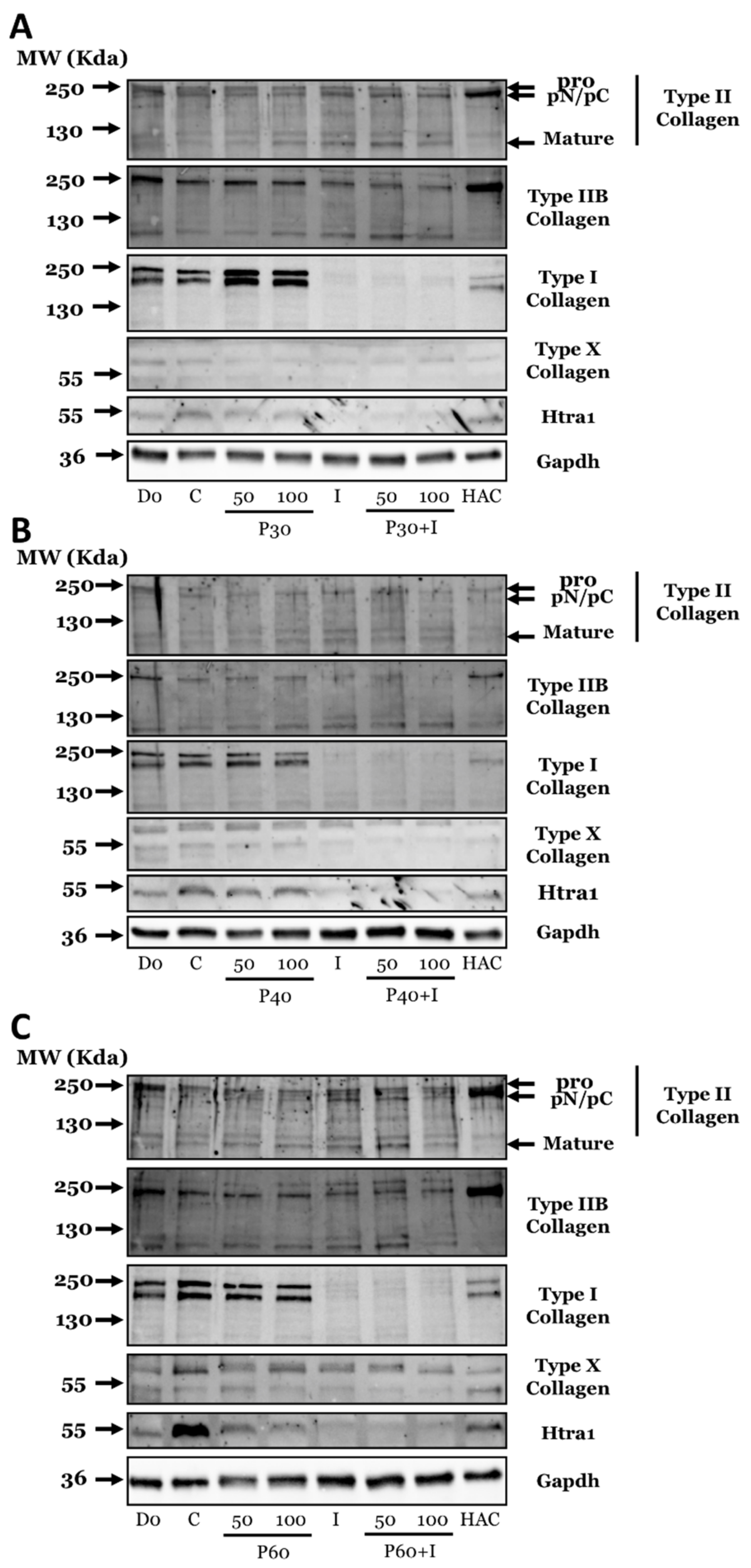
2.3. Effect of Promerim® Hydrolysates on the Expression of Type II and I Collagen Proteins and Htra1
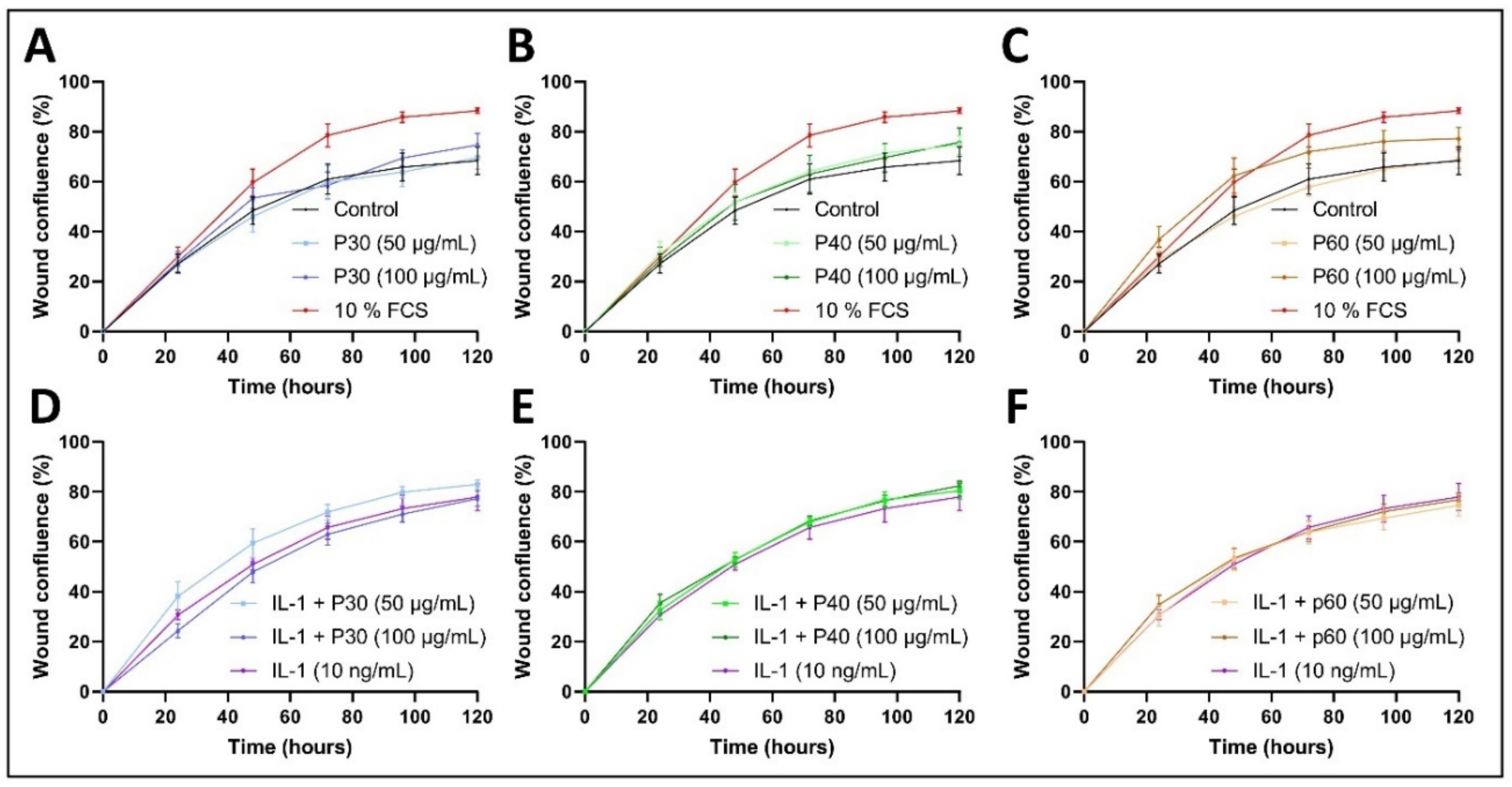
2.4. Promerim®30 and 40 Promote Proliferation, Whereas Promerim®60 Promotes Migration
3. Discussion
4. Materials and Methods
4.1. Collagen Hydrolysates
4.2. Human Articular Chondrocyte Culture
4.3. 3D Model for Chondrocyte Redifferentiation
4.4. Chondrocyte Toxicity Assay
4.5. HAC Viability and Proliferation Measurement
4.6. Senescence-Associated β-Galactosidase Quantification
4.7. RNA Extraction and Quantitative Real-Time PCR
4.8. Protein Extractions
4.9. Western-Blot
4.10. Scratch Wound Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aigner, T.; Cook, N.; Gerwin, S.S.; Glasson, S.; Laverty, C.B.; Little, W.; McIlwraith, C.W.; Kraus, V.B. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarth. Cartil. 2010, 18, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Stöve, J. Collagens major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug Deliv. Rev. 2005, 55, 1569–1593. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, P.; van der Rest, M. Structure and function of cartilage collagens. Microsc. Res. Tech. 1994, 28, 378–384. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 159–161. [Google Scholar] [CrossRef]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Sharma, L.; Kapoor, D.; Issa, S. Epidemiology of osteoarthritis: An update. Curr. Opin. Rheumatol. 2006, 18, 147–156. [Google Scholar] [CrossRef]
- Qin, B.; Yang, M.; Fu, H.; Ma, N.; Wei, T.; Tang, Q.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Body mass index and the risk of rheumatoid arthritis: A systematic review and dose-response meta-analysis. Arthritis Res. Ther. 2015, 17, 86. [Google Scholar] [CrossRef] [Green Version]
- Sparks, J.A.; Barbhaiya, M.; Tedeschi, S.K.; Leatherwood, C.L.; Tabung, F.K.; Speyer, C.B.; Malspeis, S.; Costenbader, K.H.; Karlson, E.W.; Lu, B. Inflammatory dietary pattern and risk of developing rheumatoid arthritis in women. Clin. Rheumatol. 2019, 38, 243–250. [Google Scholar] [CrossRef]
- Cisternas, M.G.; Murphy, L.; Sacks, J.J.; Solomon, D.H.; Pasta, D.J.; Helmick, C.G. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res. 2016, 68, 574–580. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, e44. [Google Scholar] [CrossRef] [Green Version]
- Pujol, J.P.; Galera, P.; Redini, F.; Mauviel, A.; Loyau, G. Role of cytokines in osteoarthritis: Comparative effects of interleukin 1 and transforming growth factor-beta on cultured rabbit articular chondrocytes. J. Rheumatol. Suppl. 1991, 27, 76–79. [Google Scholar]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Pujol, J.P.; Chadjichristos, C.; Legendre, F.; Bauge, C.; Beauchef, G.; Andriamanalijaona, R.; Galera, P.; Boumediene, K. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect. Tissue Res. 2008, 49, 293–297. [Google Scholar] [CrossRef]
- Schett, G.; Elewaut, D.; McInnes, I.B.; Dayer, J.M.; Neurath, M.F. How cytokine networks fuel inflammation: Toward a cytokine based disease taxonomy. Nat. Med. 2013, 19, 822–824. [Google Scholar] [CrossRef]
- Pham, T. Les injections intra-articulaires de hanche dans la coxarthrose: Corticoïdes, hyaluronan. Rev. Rhum. 2009, 76, 356–360. [Google Scholar] [CrossRef]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef]
- Grunke, M.; Schulze-Koops, H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis. 2006, 65, 555–556. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarth. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Zegels, B.; Leonori, L.; Rabenda, V.; Janssen, A.; Bourges, C.; Reginster, G.Y. Effect of collagen hydrolysate in articular pain: A 6-month randomized, double-blind, placebo controlled study. Complement. Ther. Med. 2012, 20, 124–130. [Google Scholar] [CrossRef]
- Fujita, T.; Ohue, M.; Fujii, Y.; Miyauchi, A.; Takagi, Y. The effect of active absorbable algal calcium (AAA Ca) with collagen and other matrix components on back and joint pain and skin impedance. J. Bone Miner. Metab. 2002, 20, 298–302. [Google Scholar] [CrossRef]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis?—A narrative review from the lessons taken with five products. Osteoarth. Cartil. 2011, 19, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef]
- Götz, B. Well nourished cartilage does not grind. Ärztliche Prax. 1982, 34, 3130–3134. [Google Scholar]
- Jiang, J.X.; Yu, S.; Huang, Q.R.; Zhang, X.L.; Zhang, C.Q.; Zhou, J.L.; Prawitt, J. Collagen peptides improve knee osteoarthritis in elderly women: A 6-month randomized, double blind, placebo-controlled study. Agro Food Ind. Hi Tech 2014, 25, 19–23. [Google Scholar]
- Galéra, P.; Ollitrault, D.; Legendre, F.; Demoor, M.; Mallein-Gerin, F.; Boumediene, K.; Herbage, B.; Duterque-Coquillaud, M.; Damour, O. Brevet Francais FR 2965278 du 23 September 2010. Method for Obtaining Differentiated Articular Chondrocytes in vitro or ex vivo and Uses of Same. WO 2012/038668, 29 March 2012. [Google Scholar]
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Benateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Legendre, F.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Gruchy, N.; Mallein-Gerin, F.; Leclercq, S.; Demoor, M.; Galera, P. Enhanced chondrogenesis of bone marrow-derived stem cells by using a combinatory cell therapy strategy with BMP-2/TGF-β1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci. Rep. 2017, 7, 3406. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.; Marshall, M.; Rathod, T.; Bowen, C.J.; Menz, H.B.; Roddy, E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS ONE 2018, 13, e0193662. [Google Scholar] [CrossRef]
- De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; De Girolamo, L.; Volpi, P. Intra-articular injection of hydrolyzed collagen to treat symptoms of knee osteoarthritis. A functional in vitro investigation and a pilot retrospective clinical study. J. Clin. Med. 2019, 8, 975. [Google Scholar] [CrossRef] [Green Version]
- Kizhakkedath, R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol. Med. Rep. 2013, 8, 1542–1548. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Kaur, J.; Shishodia, S.; Aggarwal, B.B.; Ralhan, R. Curcumin down regulates smokeless tobacco-induced NF-kappaB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology 2006, 228, 1–15. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [Green Version]
- DiNubile, N.A. A potential role for avocado- and soybean-based nutritional supplements in the management of osteoarthritis: A review. Phys. Sports Med. 2010, 38, 71–81. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.A.; Noorbaloochi, S.; MacDonald, R.; Maxwell, L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015, 1. [Google Scholar]
- Dahmer, S.; Schiller, R.M. Glucosamine. Am. Fam. Physician 2008, 78, 471–476. [Google Scholar]
- DiNubile, N.A. Glucosamine and Chondroitin Sulfate: What has been learned since the glucosamine/chondroitin arthritis intervention trial. Orthopedics 2018, 41, 200–207. [Google Scholar] [CrossRef]
- De Almagro, C. The use of collagen hydrolysates and native collagen in osteoarthritis. Am. J. Biomed. Sci. Res. 2020, 7. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarth. Cartil. 2011, 19, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Lotz, M.F. Effects of aging on articular cartilage homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Maneiro, E.; Martín, M.A.; de Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003, 48, 700–708. [Google Scholar] [CrossRef]
- Blanco, F.J.; López-Armada, M.J.; Maneiro, E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion 2004, 4, 715–728. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; Riveiro-Naveira, R.R.; Valcárcel-Ares, M.N.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012, 64, 2927–2936. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, T.; Fukuda, K.; Yamazaki, K.; Hashimoto, K.; Ueda, H.; Mori, S.; Hamanishi, C. Cyclic compression loaded on cartilage explants enhances the production of reactive oxygen species. J. Rheumatol. 2007, 34, 556–562. [Google Scholar] [PubMed]
- Cha, B.H.; Lee, J.S.; Kim, S.W.; Cha, H.J.; Lee, S.H. The modulation of the oxidative stress response in chondrocytes by Wip1 and its effect on senescence and dedifferentiation during in vitro expansion. Biomaterials 2013, 34, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarth. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.G.; Zeng, C.; Li, L.J.; Luo, W.; Zhang, F.J.; Tian, J.; Cheng, C.; Tu, M.; Xiong, Y.L.; Jiang, W.; et al. Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int. J. Rheum. Dis. 2016, 19, 226–232. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.I.; Carozza, M.; Klein, M.; Nantermet, P.; Luk, D.; Crowl, R.M. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J. Biol. Chem. 1998, 273, 34406–34412. [Google Scholar] [CrossRef] [Green Version]
- Oka, C.; Tsujimoto, R.; Kajikawa, M.; Koshiba-Takeuchi, K.; Ina, J.; Yano, M.; Tsuchiya, A.; Ueta, Y.; Soma, A.; Kanda, H.; et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004, 131, 1041–1053. [Google Scholar] [CrossRef] [Green Version]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Bouyoucef, M.; Rakic, R.; Gómez-Leduc, T.; Latire, T.; Marin, F.; Leclercq, S.; Carreiras, F.; Serpentini, A.; Lebel, J.M.; Galéra, P.; et al. Regulation of Extracellular Matrix Synthesis by Shell Extracts from the Marine Bivalve Pecten maximus in Human Articular Chondrocytes—Application for Cartilage Engineering. Mar. Biotechnol. 2018, 20, 436–450. [Google Scholar] [CrossRef]
- Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galera, P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. Int. J. Mol. Sci. 2021, 22, 580. [Google Scholar]
- Latire, T.; Legendre, F.; Bigot, N.; Carduner, L.; Kellouche, S.; Bouyoucef, M.; Carreiras, F.; Marin, F.; Lebel, J.M.; Galéra, P.; et al. Shell extracts from the marine bivalve Pecten maximus regulate the synthesis of extracellular matrix in primary cultured human skin fibroblasts. PLoS ONE 2014, 9, e99931. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.Z. Boswellia Serrata, A potential anti-inflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Boe, C.; Vangsness, C.T. Fish Oil and Osteoarthritis: Current Evidence. Am. J. Orthop. 2015, 44, 302–305. [Google Scholar]
- Latire, T.; Legendre, F.; Bouyoucef, M.; Marin, F.; Carreiras, F.; Rigot-Jolivet, M.; Lebel, J.M.; Galéra, P.; Serpentini, A. Shell extracts of the edible mussel and oyster induce an enhancement of the catabolic pathway of human skin fibroblasts, in vitro. Cytotechnology 2017, 69, 815–829. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Matrikines for therapeutic and biomedical applications. Life Sci. 2018, 214, 22–33. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourdon, B.; Cassé, F.; Gruchy, N.; Cambier, P.; Leclercq, S.; Oddoux, S.; Noël, A.; Lafont, J.E.; Contentin, R.; Galéra, P. Marine Collagen Hydrolysates Promote Collagen Synthesis, Viability and Proliferation While Downregulating the Synthesis of Pro-Catabolic Markers in Human Articular Chondrocytes. Int. J. Mol. Sci. 2021, 22, 3693. https://doi.org/10.3390/ijms22073693
Bourdon B, Cassé F, Gruchy N, Cambier P, Leclercq S, Oddoux S, Noël A, Lafont JE, Contentin R, Galéra P. Marine Collagen Hydrolysates Promote Collagen Synthesis, Viability and Proliferation While Downregulating the Synthesis of Pro-Catabolic Markers in Human Articular Chondrocytes. International Journal of Molecular Sciences. 2021; 22(7):3693. https://doi.org/10.3390/ijms22073693
Chicago/Turabian StyleBourdon, Bastien, Frédéric Cassé, Nicolas Gruchy, Pierre Cambier, Sylvain Leclercq, Sarah Oddoux, Antoine Noël, Jérôme E. Lafont, Romain Contentin, and Philippe Galéra. 2021. "Marine Collagen Hydrolysates Promote Collagen Synthesis, Viability and Proliferation While Downregulating the Synthesis of Pro-Catabolic Markers in Human Articular Chondrocytes" International Journal of Molecular Sciences 22, no. 7: 3693. https://doi.org/10.3390/ijms22073693
APA StyleBourdon, B., Cassé, F., Gruchy, N., Cambier, P., Leclercq, S., Oddoux, S., Noël, A., Lafont, J. E., Contentin, R., & Galéra, P. (2021). Marine Collagen Hydrolysates Promote Collagen Synthesis, Viability and Proliferation While Downregulating the Synthesis of Pro-Catabolic Markers in Human Articular Chondrocytes. International Journal of Molecular Sciences, 22(7), 3693. https://doi.org/10.3390/ijms22073693





