Abstract
The regulation of infection and inflammation by a variety of host peptides may represent an evolutionary failsafe in terms of functional degeneracy and it emphasizes the significance of host defense in survival. Neuropeptides have been demonstrated to have similar antimicrobial activities to conventional antimicrobial peptides with broad-spectrum action against a variety of microorganisms. Neuropeptides display indirect anti-infective capacity via enhancement of the host’s innate and adaptive immune defense mechanisms. However, more recently concerns have been raised that some neuropeptides may have the potential to augment microbial virulence. In this review we discuss the dual role of neuropeptides, perceived as a double-edged sword, with antimicrobial activity against bacteria, fungi, and protozoa but also capable of enhancing virulence and pathogenicity. We review the different ways by which neuropeptides modulate crucial stages of microbial pathogenesis such as adhesion, biofilm formation, invasion, intracellular lifestyle, dissemination, etc., including their anti-infective properties but also detrimental effects. Finally, we provide an overview of the efficacy and therapeutic potential of neuropeptides in murine models of infectious diseases and outline the intrinsic host factors as well as factors related to pathogen adaptation that may influence efficacy.
1. Introduction
Neuropeptides are a large group of peptides that play a key role in the dialogue between the central nervous system (CNS), peripheral nervous system (PNS), and the immune system. Thus, they can be perceived not only as neurotransmitters but also as hormones or effector compounds that interact with the immune system. Neuropeptides are found in Homo sapiens and almost all animal phyla. Since the first discovery of Substance P (SP) by von Euler and Gaddum in 1931, approximately 100 different human neuropeptides and more than 80 genes encoding for neuropeptides in the H. sapiens genome have been described [1,2]. Neuropeptides are generally described as macromolecular peptides that contain between 3 and 100 amino acids in their active forms and act via G protein-coupled receptors (GPCR) [3,4,5]. It is acknowledged that neuropeptides have characteristics that are common to peptides, including post translational processing, which may influence their biological activity. Furthermore, depending on receptor expression, they can trigger cell activation at multiple locations, which in the case of neuropeptides may involve both CNS and PNS sites [2,6,7]. Neuropeptides are primarily synthesized in neuronal and glial cells [4,8]. Nevertheless, some neuropeptides are produced by non-neuronal cells, including cells of the immune system. Immune cells such as B- and T-lymphocytes, monocytes, macrophages, dendritic and mast cells as well as polymorphonuclear leukocytes have all been reported to produce these macromolecules [9,10,11]. Fibroblasts have also been shown to synthesize selected neuropeptides and neuropeptide receptors [12,13]. Since immune cells participate in a bidirectional conversation with the nervous system and other stromal cells, they act not only as producers of neuropeptides but also detect neuropeptides by neuropeptide-specific GPCRs, or by mannose receptors via a so-called “non-specific” receptor mechanism. Likewise, receptor-independent cellular activation is also possible [11,14]. Considering the interaction of neuropeptides with their specific GPCR receptors, most neuropeptides can induce modulatory actions in the cell cytoplasm via GPCR-linked pathways. The quantity of neuropeptide that is required to trigger such action is significantly lower than required for other neuro-signaling molecules [4,15]. This is perhaps related to the affinity and bond strength between the neuropeptide and the GPCR, which appears to be enhanced compared with the classical neurotransmitter-receptor linkage. In addition to their interaction with their cognate receptors, neuropeptides have also been reported to trigger ionotropic reactions [16].
Beyond the traditional role of neuropeptides as neurotransmitters in the central and peripheral neural systems, they have also antimicrobial activity contributing to the formation of local barriers of defense against a variety of pathogens. However, recent data also indicate that neuropeptides can contribute to modulating vulnerability to infection. Thus, in addition to their anti-infective action, some neuropeptides may have a completely different, unfavorable role to play in augmenting bacterial virulence. In this review, we will summarize current data on the dual modulatory role of neuropeptides in infectious diseases caused by bacteria, fungi, and protozoan parasites, including their impact on virulence, pathogenesis, and possibility of resistance development. We also critically discuss attempts to date to employ neuropeptides in animal models of infections, together with future perspectives for these important molecules. This review mainly focuses on the following neuropeptides: substance P (SP), neuropeptide Y (NPY), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), pituitary andenylate cyclase-activating polypetide (PACAP), adrenomedullin (ADM), and somatostatin (SST). We also discuss the peptide hormones such as α-melanocyte stimulating hormone (α-MSH), atrial natriuretic peptide (ANP), C-type natriuretic peptide (CNP), and corticotropin-releasing hormone (CRH) as well as dynorphin as examples of opioid peptides.
2. Neuropeptides as Direct and Indirect Anti-Microbial Factors
The antimicrobial properties of human neuropeptides such as SP, NPY, CGRP, VIP, ADM, and α-MSH against a variety of microorganisms are widely documented. Their direct antimicrobial activities have been confirmed to date against Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Haemophilus influenzae, Moraxella catarrhalis, Aeromonas caviae) and Gram-positive bacteria (Staphylococcus aureus, Enterococcus faecalis, Streptococcus mutans, Nocardia brasiliensis), fungi (Candida albicans, Candida tropicalis, Candida krusei, Candidia utilis, Cryptococcus neoformans, Arthroderma simii), and protozoan parasites (Trypanosoma brucei, Leishmania major) [11,17,18]. Interestingly, some highly proteolytic oral anaerobic bacteria such as Porphyromonas gingivalis and Prevotella spp have been reported to be resistant to the direct action of CGRP and ADM [19,20]. The direct action of neuropeptides encompasses both microbicidal and anti-virulent effects. The microbicidal action of neuropeptides is generally attributed to targeting the microbial membrane and causing either discrete pore formation or more extensive detergent-like disruption of the membrane. In either case, the effect is rapid, with leakage of microbial cytoplasmic contents, leading to cell death within minutes [21]. Less common, non-membrane-disruptive mechanisms of direct activity and killing have also been reported, including (i) Abnormal septum formation (scaffolding for peptidoglycan) during cell division by S. aureus in the presence of ADM [22] and (ii) ADM- and VIP-mediated disruption of intracellular endosome-lysosomal vesicles, resulting in metabolic failure in T. brucei [23]. Of note, the anti-virulent action of neuropeptides may be exemplified by the anti-proliferative (bacteriostatic) activity of somatostatin against H. pylori [24].
Considering membrane-disruptive mechanisms, an advantage of neuropeptides, like other AMPs, is their efficacy against both metabolically active and metabolically repressed microbial cells. Conversely, their significant drawback is generally rather weak microbicidal effects at physiological doses. Readers are referred to previous reviews by Augustyniak et al. and Sanz et al. [11,25] for deeper insights into the mechanisms of direct antimicrobial activities of neuropeptides.
However, in addition to direct action, neuropeptides may also display antimicrobial activity via indirect immunomodulatory effects on the innate and adaptive immune systems during the course of infection [6,26]. This humoral and cell-mediated immune modulation may be either stimulatory or inhibitory, implicating that neuropeptides have a dual role. For immunomodulating purposes, neuropeptides exploit specific receptors and also, paradoxically, utilize alternative non-cognate receptors or non-receptor-mediated mechanisms. During certain receptor–neuropeptide interactions, dual biological effects, such as for example stimulation versus inhibition of critical phagocytic events have previously been documented [11]. The stimulating potential of neuropeptides is essentially based on the intensification of mechanisms of innate defense. Modulation of critical phagocytic or mast cell function as well as triggering of proinflammatory mediators is worth mentioning in this context [6,27,28]. Conversely, a common example of inhibitory action is neuropeptide-mediated inhibition of pathogen-induced inflammation or reducing endotoxin-induced inflammatory responses. For more comprehensive information on these, we refer the reader to the previous reports [10,21,29].
It is important to note that the direct and indirect antimicrobial activities of neuropeptides presented above, are derived from in vitro studies, conducted under strictly controlled laboratory conditions, and therefore may not necessarily reflect the in vivo efficacy of the neuropeptides.
3. Neuropeptides as Direct Potentiators or Amplifiers of Microbial Virulence
Virulence is defined as the ability of a pathogen to cause disease. Virulence factors are secretory, membrane-associated, or cytosolic molecules produced by bacteria, fungi, and protozoa that enable them to colonize the host at a cellular level. These factors include, among others, adhesins, toxins, enzymes, as well as factors involved in biofilm formation. Despite the role of neuropeptides in the host’s intricate defense mechanisms to control microbial invasion [11,25], these pleiotropic peptides may under certain circumstances be considered to have virulence-enhancing features [30]. The potential virulence-enhancing roles of neuropeptides can be summarized by their ability to (1) increase microbial growth; (2) increase thickness and density of microbial biofilm; (3) increase exotoxin production or bacterial cytotoxicity; (4) interfere with quorum-sensing that may control expression of virulence factors. Previous reports demonstrating the detrimental effects of neuropeptides on virulence traits are summarized in Table 1 and Figure 1 and discussed in further detail below.
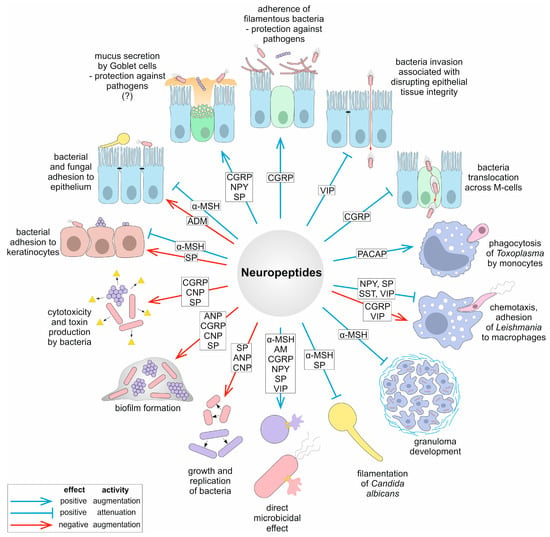
Figure 1.
Overview of mechanisms of pathogenesis and virulence traits modulated by neuropeptides. The question mark indicates that the given mechanism is putative.
3.1. Influence on Microbial Growth and Virulence
Some neuropeptides may exert virulence-enhancing action while inducing cellular stress in host microenvironment. Good examples include epinephrine (EPI), norepinephrine (NE), and SP that can trigger commensal-to-pathogen transition of Mennheimia haemolytica in the nasopharyngeal milieu. This Gram-negative bacterium is an important constituent within commensal biofilm communities in the bovine nasopharynx and can, under certain circumstances, play a key role in bovine respiratory disease. EPI, NE, and SP have been shown to significantly increase the growth and replication of M. haemolytica but also to trigger its transition from natural biofilm-former to pulmonary planktonic pathogen. The higher rate of cell division is generally thought to be connected to changes in bacterial virulence including production of the important cytotoxic virulence factor leukotoxin Lkt, which causes lysis of erythrocytes and induces apoptosis in macrophages [31,32]. The typically harmless bacterium M. haemolytica is therefore released from the biofilm community in the nasopharynx and spreads to a new site of infection in the lungs, causing bronchopneumonia [33]. In contrast, SP was shown not to affect the growth of selected Gram-positive (Bacillus cereus, S. aureus, and Staphylococcus epidermidis) and Gram-negative (Pseudomonas fluorescens) bacteria, although it was reported to enhance the virulence of Bacillus and Staphylococci [34], Table 1. Interestingly, enhancing fungal virulence has previously been exploited for human advantage. A mycoinsecticidal strategy has been used in the treatment and killing or fire ants, which are destructive insects that can easily spread to new ecosystems. It was shown that the expression of a fire ant pyrokinin β neuropeptide in the entomopathogenic fungus Beauveria bassiana strain improved the virulence of this fungus. As a result, the more virulent mycoinsecticidal fungal strain was able to kill fire ants and alter their behavior [35].
3.2. Influence on Biofilm Formation and Quorum Sensing
To date, the most intensely studied virulence process modulated by neuropeptides is biofilm formation [30] (Table 1, Figure 1). Biofilms facilitate colonization of the host by a wide range of microorganisms and constitute a barrier designed to counteract host defense and therapeutic intervention. Microorganisms within biofilms favor the survival of the entire community, which is achieved through subsequent stages of biofilm formation such as adherence, aggregation, and matrix formation, maturation, and dispersion (release of microcolonies) [36]. It has been shown that Gram-positive bacteria such as B. cereus, which causes food poisoning, and the opportunistic bacterium S. epidermidis, which inhabits the skin, develop significantly thicker biofilms after exposure to SP [37,38,39]. The population density in a biofilm is controlled through a complex cell-to-cell signaling mechanism known as quorum sensing (QS) [40,41]. During QS, bacteria produce, detect, and respond to extracellular signaling molecules called autoinducers and processes controlled by QS include, but are not limited to, biofilm formation and virulence factor secretion [42,43]. SP and also CGRP hypothetically may affect S. epiderimidis factors responsible for QS signaling which in turn affect biofilm formation. Following a mechanism potentially close to that of bacterial QS factors, but not yet identified precisely, for a factor known as ribosomal elongation factor thermo-unstable (Ef-Tu), which is engaged in translation, it has been shown that after binding to SP or when chaperone DnaK binds to CGRP, bacterial response such as increased adhesion and biofilm formation should be triggered [34].

Table 1.
Overview of detrimental effects of neuropeptides on virulence traits.
Table 1.
Overview of detrimental effects of neuropeptides on virulence traits.
| Bacteria | NP | Type of Virulence Modulation | Field of Action | Ref. |
|---|---|---|---|---|
| M. haemolytica | SP | Dispersion from biofilm ↑ Growth and replication ↑ Lkt protein production ↑ | Adherence, biofilm formation, spreading Direct damage | [31] |
| B. cereus | SP | Biofilm thickness ↑ Cytotoxic effect ↑ | Host immune response evasion, adherence Direct damage | [37] |
| S. epidermidis | SP | Biofilm thickness and density ↑ | Host immune response evasion, adherence | [38] |
| S. aureus | SP | SEC2 superantigen production ↑ | Acute host immune response | [38] |
| S. pneumoniae | CRH | Capsule thickness ↑ Growth ↑ Pneumolysin Ply production ↑ pavA production ↑ | Adherence, host immune response evasion Proliferation tempo Direct damage Adherence, invasion | [44,45] |
| S. epidermidis | CGRP | Cytotoxic effect ↑ Cationic potential of surface ↑ IsaB production ↑ | Direct damage Adherence Host immune response evasion | [46] |
| C. acnes | ANP, CNP | Growth ↑ Biofilm formation ↑ | Proliferation tempo Host immune response evasion, adherence | [47] |
| S. epidermidis | ANP, CNP | Biofilm formation ↑ | Host immune response evasion, adherence | [48] |
| P. aeruginosa | Dynorphin | HHQ and PQS molecules production ↑ pyocyanin production ↑ PA-I lectin/adhesin ↑ | Quorum sensing Direct damage, competition with other microorganisms Host immune response evasion Adherence | [49] |
↑—increase; NP—neuropeptide; HHQ—4−hydroxy-2-heptylquinoline; PQS—3,4-dihydroxy-2-heptylquinoline; SEC2—enterotoxin C2.
3.3. Influence on Microbial Toxin Release and Strain Cytotoxicity
Toxin production represents an efficient way to alter specific functions of target cells, enabling pathogenic effects. After exposure to CGRP, S. aureus increased production of enterotoxin C2 (SEC2) [46]. This virulence factor is a member of the super-antigen group, which possesses the ability to activate high numbers of host T-cells by crosslinking with Major Histocompatibility Class II (MHCII) molecules, thereby inducing profound and rapid cell activation, which can result in a generation of a cascade of cytokines, leading to toxic shock syndrome [50,51]. Another sensory neuropeptide, SP, has also been shown to affect bacterial cytotoxicity. This effect was confirmed for B. cereus against a cell line of human keratinocytes [37] as well as for S. aureus and S. epidermidis against a reconstructed human epidermis model [38,46]. SP also stimulate aggregation, hydrophobicity, lactic acid and tyramine production in E. faecalis, an important nosocomial pathogen causing severe urinary tract infections, surgical wound infections, bacteraemia, and bacterial endocarditis. SP was also shown to accelerate the cytotoxicity and translocation of this bacterium during infection of intestinal Caco-2/TC7 cells [52]. Another interesting example in this matter is CNP that modulates QS molecule and exotoxin A production in P. aeruginosa through a cyclic nucleotide-dependent sensor system. As a result, a toxin-dependent capacity of P. aeruginosa to kill nematode Caenorhabditis elegans was increased markedly. This activity was related to activation of bacterial regulators of virulence such as Vfr and PtxR, as well as the virulence activation cascade involved in production of acylhomoserine lactone, hydrogen cyanide, and aforementioned exotoxin [53].
3.4. Potential Influence on Immune Evasion
Pathogens utilize a vast array of sophisticated strategies to evade the host immune response. Generally, pathogens actively block or degrade immune components as well as modulate the cellular machinery of infected cells on various levels to allow cellular invasion and then transmission. Surface components of pathogens often play an important passive role in immune evasion. One of such components in Streptococcus pneumoniae is a capsule, a polysaccharide layer that surrounds a cell. After exposure of S. pneumoniae to CRH, the thickness of the capsule was markedly enhanced. This was evident for serotypes 1, 3, 19A and 23F, among the major causes of invasive pneumococcal disease. Furthermore, CRH-treated bacteria presented 3-fold greater resistivity for penicillin/streptomycin cocktail compared to control [45]. Since capsules generally act as an armor, that prevents complement-dependent cell lysis and C3b- or IgG-dependent opsonization that leads to phagocytosis enhancement [54], it is reasonable to suggest that CRH may make it harder for the phagocytic cell to recognize and phagocytize this encapsulated pathogen. Likewise, S. aureus immunodominant surface antigen B (IsaB) and precursor protein were shown to be increased during CGRP exposure in vitro [46]. The IsaB protein appears to be expressed during infection but not colonization and is increased in phagocytosis [55]. Since IsaB diminishes autophagic flux, to promote bacterial survival and host transmission, allowing Methicillin-Resistant S. aureus (MRSA) to evade host degradation [56], it is reasonable to suggest that a CGRP-dependent increase in expression of this surface antigen may favor bacterial survival and dissemination.
3.5. Pathogen Sensing of Neuropeptides
During the course of an infection, microorganisms depend on their ability to sense and respond to a myriad of environmental host factors and the activation of specific virulence traits that are needed to establish an infection successfully. Because the expression and/or activation of these virulence traits is metabolically expensive, it should occur only at sites that are appropriate for colonization [57]. Assuming that various antimicrobials, including neuropeptides, significantly alter the metabolic state of microorganisms, it seems that the microbes per se may also affect their intrinsic susceptibility to neuropeptides via moonlighting proteins. Indeed, bacteria and other pathogens have the ability to sense neuropeptides via highly conserved proteins, termed moonlighting proteins, which exhibit more than one unrelated function within the cell. Since the novel biological function of the moonlighting protein is usually connected with a change in its localization, moonlighting proteins often perform their canonical and moonlighting functions in separate cell compartments. Pathogens commonly use moonlighting cytosolic proteins engaged essentially in cellular processes (i.e., glycolysis, protein synthesis, chaperone activity, and nucleic acid stability) on their cell surface for forming and maintaining interactions with the host epithelial cells, mucus, and extracellular matrix (ECM) components [58,59]. A well-characterized example is the enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Streptococcus pyogenes or S. aureus. This enzyme is involved in adherence and internalization and thus directly serves as a virulence determinant for adhesion [60,61]. There is also an array of moonlighting proteins, with adhesive properties, in the yeast C. albicans, including enolase (ENO1), phosphoglycerate mutase 1 (GPM1), Ssa1 chaperoning, and GAPDH [62,63,64]. Another unique function of microbial moonlighting proteins involves the evasion of the innate immune system, in particular by binding of serum complement components or complement regulators (observed for the moonlighting proteins dihydrolipoamide dehydrogenase LpD, factor EfTu, GAPDH) [65,66,67]. Moonlighting proteins have also been shown to bind and potentially neutralize host antibodies (LpD, GroEL chaperone) [68]. Scientists have long been puzzled by the question of whether, analogous to other components of the humoral immune response, neuropeptides (which could be considered as part of this response) could also be sensed by moonlighting proteins. To our knowledge, this has only been confirmed in a few cases. One of them is the aforementioned bacterial environmental sensor Ef-Tu, which is as a molecule involved in the recognition of SP in S. aureus, S. epidermidis, and B. cereus. Interaction between Ef-Tu and SP led to increased bacterial adhesion potential and biofilm formation in a keratinocyte model of infection [38,39]. The same group of scientists identified DnaK chaperone as the S. epidermidis CGRP-binding protein. Following exposure to CGRP, the adherence of S. epidermidis to keratinocytes, as well as the cytotoxicity to these cells, was increased, whereas its internalization and biofilm formation activity was reduced. Of note, the CGRP-mediated increase in virulence was not observed for S. aureus [46], indicating therefore that even in closely related bacteria, the effects of the same neuropeptide can be quite different. Another well-documented example is the P. aeruginosa kinase (ParS) involved in the sensing machinery to defend against the host in response to the opioid neuropeptide dynorphin [69]. Host dynorphins, released from the intestinal mucosa, can activate quorum sensing quinolone signaling in P. aeruginosa using transcriptional regulator MvfR, and enhance the virulence of P. aeruginosa against Lactobacillus spp. and nematode C. elegans. These data demonstrate that P. aeruginosa can intercept opioid compounds released during host stress and integrate them into core elements of quorum sensing circuitry leading to enhanced virulence [49]. In other cases, the involvement of bacteria in recognizing certain neural peptides has been documented, but without identifying the bacterial molecular targets engaged in this recognition [30]. The lack of other experimentally documented examples of neuropeptide- or antimicrobial peptide-binding by bacterial moonlighting proteins seems to indicate that this virulence trait is unlikely to be generally preferred by bacterial pathogens, excluding those already mentioned above. It may be assumed that from a pathogen’s perspective, the virulence-enhancing benefits that the bacterium derives from using moonlighting proteins for adhesion or evasion of immune response far outweigh the other benefits following neuropeptide binding by these proteins. In this aspect, one can speculate that the direct sensing of neuropeptides by bacteria appears to be a dead end from evolutionary perspective.
4. Neuropeptides Modulate Crucial Stages of Microbial Pathogenesis
The ability of a pathogen to infect is called its pathogenicity. Microorganisms express their pathogenicity by means of their virulence, a term that refers to the relative, quantitative degree of pathogenicity [70,71]. Different microbial pathogens use various common strategies to cause infection and disease. These strategies enable them to attach, invade, and disseminate in the host, as well as acquire nutrients for their growth and multiplication within the host. In vivo, a pathogen must also evade continuous attacks from the host immune response and actively survive host defense mechanisms. Some pathogens from the lower Eukaryota have developed other mechanisms of pathogenesis such as yeast-to-hyphae transition (dimorphism); a mechanism exhibited by pathogenic yeasts such as C. albicans [72]. For protozoan parasites, it involves several different stages during their life cycle and a remarkable ability to manipulate host immunity [73,74]. Numerous pathogenic effects can be attenuated or enhanced in the presence of neuropeptides, placing these molecules amongst the important modulators of microbial pathogenesis. The involvement of neuropeptides as modulators of crucial stages of microbial pathogenesis has allowed the host to develop intricate and overlapping mechanisms to control microbial invasion. To date, the most intensely studied stages regulated by neuropeptides involve their interplay with microbial colonization and adhesion, invasion, intracellular lifestyle, and growth or multiplication (Figure 1). The dual role in exerting either beneficial or detrimental effects is outlined below with recent examples.
4.1. Impact of Neuropeptides on Microbial Colonization and Adhesion
The ability of microbial pathogens to successfully colonize the host relies initially on their ability to break down physical and chemical barriers. Following this, pathogens must be able to attach to host cells. Depending on the pathogen, attachment may be mediated by surface-associated protein structures such as fimbriae or pili, numerous surface proteins, and the capsule (if present). Since adhesion is the first step in the process of colonization and subsequent infection, inhibition of adhesion should be expected to limit the pathogenicity of at least some microorganisms. Evidence of a negative role for neuropeptides in bacterial adhesion has come from several approaches, including studies on cutaneous pathogens. It has been shown that SP increases adhesion of Gram-positive S. aureus and S. epidermidis to skin keratinocytes several times over control. Surprisingly, although adhesive potential was increased, invasive activities expressed by internalization of staphylococci by these cells were unchanged during experimental conditions [38]. As documented elsewhere, keratinocytes generally had a much lower uptake of S. aureus than, for example, the endothelial or epithelial cells [75]. Besides staphylococci, SP also increased the adhesion and invasive potential of another cutaneous bacterium, P. fluorescence in human keratinocytes [76]. Similarly, the negative effect of the neuropeptide ADM on colonization has been documented for invasive, facultative intracellular bacterium Helicobacter pylori, a leading mucosal pathogen of chronic gastritis and other digestive system diseases which colonize the stomach of its host [77]. In this case, ADM expression was positively correlated with H. pylori colonization in gastric mucosa [78]. Generally, the adhesion of H. pylori to gastric mucosa epithelial cells is mediated by outer membrane proteins and the colonization is expedited in a favorable inflammatory environment. This is facilitated by the delivery of H. pylori CagA effector protein, encoded by genes located within pathogenicity islands, to the mucosal epithelium, via interaction of CagA with integrin β1 in a type IV secretion (T4SS)-dependent manner. Another model for T4SS-dependent CagA translocation involves binding of pilus-exposed CagA to a host membrane phospholipid, phosphatidylserine. Both models permit CagA translocation in host epithelial target cells [79,80]. Apparently, ADM released from the gastric epithelium via cagA-dependent activation of PI3K-AKT signaling pathway, initiated inappropriate immune response and inflammation, creating a favorable environment for pathogen survival and colonization. This was partially due to direct induction of T cell response as well as indirect activation of T cells via IL-12, produced by ADM-activated macrophages. In both cases, Th1 lymphocytes release IFN-γ that exerts a pro-inflammatory effect within the gastric microenvironment, thus contributing to gastritis during H. pylori infection. It is worth adding that ADM levels have been shown to be increased in the gastric mucosa of H. pylori-infected patients and mice [78]. Interestingly, when the external conditions become unfavorable for bacteria to establish successful colonization, adhered H. pylori invade epithelial cells and multiply within double-layer membrane vesicles present either on the cell membrane or in the cell cytoplasm. Once the external environment conditions improve, H. pylori can be released from host cells for recolonization. In this way, bacterial numbers are maintained through a dynamic process involving invasion, proliferation, apoptosis, and release [79].
In contrast to the aforementioned examples of adverse effects of some neuropeptides, which are based on increasing adhesion, the effect of α-MSH on the initial stages of infections caused by S. aureus may be positive for the host. In the case of α-MSH, its antibacterial effects are connected with inhibition of adhesion and penetration during the early stages of infection. For example, in human keratinocytes α-MSH down-regulates β-1 integrins and heat shock surface protein 70, both of which are essential molecules for the invasion of keratinocytes by S. aureus [81].
Furthermore, more complicated effects of neuropeptides have been reported for obligatory intracellular protozoan parasites from Leishmania genus. Leishmania exhibits two main distinct developmental stages: (i) the free-living flagellated and invasive promastigote and (ii) the obligate intracellular aflagellated amastigotes living in phagolysosomes of macrophages [82]. For this pathogen, which causes mucocutaneous leishmaniasis, active migration followed by adherence and invasion into skin macrophages are key steps for initiation of its successful replication. However, these resident cells are also the major effector cells to eliminate the parasite. This duality in pathogen-cell response depends on which subset of macrophages is activated. It therefore may lead to different disease outcomes. More precisely, macrophages can be activated by divergent signals into functionally distinct subsets such as Leishmania-killing M1 and Leishmania-tolerating M2 [83]. Interestingly, by acting as pathogen chemorepellents or chemoattractants, certain neuropeptides at physiological concentrations can either block chemotaxis followed by adhesion and thus prevent parasite invasion into macrophages or alternatively, attract the parasite. Neuropeptides released from the autonomic nervous system, such as NPY and VIP, were shown to act as direct chemorepellents against Leishmania brasiliensis flagellated promastigotes. In contrast, the chemoattractive sensory neuropeptide SP was shown to cause decreased promastigote adherence to macrophages, thus indicating that despite inducing parasite migration, SP impairs parasite adhesion potency [84]. The reasons for these discrepancies require further research. Likewise, Leishmania major promastigote-induced macrophage migration was shown to be modulated in a dual way by sensory and autonomic neuropeptides. In this case, physiological concentrations of SST, SP, and NPY inhibited L. major-induced chemotaxis of phagocytic macrophages, whereas VIP and CGRP stimulated the chemotactic activity of these cells [85]. Interestingly, the potency of neuropeptides to modulate chemotaxis (initial step in phagocytosis) did not always correlate with later stages of phagocytosis such as ingestion and killing. Accordingly, the autonomic NPY and VIP have been shown to suppress phagocytic and leishmanicidal capacities of macrophages, whereas inhibition of phagocytosis by sensory SP and CGRP was accompanied by an enhancement of parasite killing [86]. Since macrophages constitute a reservoir for these obligate intracellular parasites and are crucial in disease progression, by preventing Leishmania from accessing these cells, certain neuropeptides seem to exert potent anti-adhesive properties, contributing to protection against this pathogen. In turn, certain other neuropeptides are useful in enhancement of leishmanicidal properties of infected cells. Another example of the anti-adhesive action of neuropeptides is α-MSH- and galanin message-associated peptide (GMAP)-mediated inhibition of germ-tube formation in C. albicans, which in turn limits yeast filamentation [87,88]. Furthermore, during our recent studies, we have observed a clear effect of SP in decreasing both the number and length of hyphal filaments in the pathogenic yeast C. albicans in the presence of serum and fluconazole (unpublished data). Since filamentous hyphal forms of this pathogenic yeast adhere more robustly and efficiently to host cells than yeast cells, and hyphae are considered dominant invasive forms of the fungus [89,90], it is reasonable to suggest that α-MSH, GMAP, and potentially also SP may have a role in blocking Candida adhesion.
4.2. Impact of Neuropeptides on Mucus Secretion
The adherence of pathogens to the epithelium of the respiratory, gastrointestinal, and urogenital tracts prevents their mechanical clearing. Fortunately, adherence is usually impaired in the presence of a physiologically produced mucus—a thick and viscous colloid hydrogel. The major sources of mucus are the numerous mucous cells located in the surface epithelium (called goblet cells) and in submucosal glands [91,92]. The ability of normal mucus to suppress pathogen growth depends on synergistic actions between its complex components (water, electrolytes, mucins) with antimicrobial humoral and cellular immune constituents. The protective role of the gel-forming, glycoprotein mucin is to trap bacteria that possess protein and carbohydrate ligands for mucin and block their access to other mucous cells. It has been shown that in normal conditions, neuropeptides such as SP, CGRP, NPY may exert a modulatory role in mucus secretion primarily through stimulation of goblet cells and stimulation of airway submucosal glands [93]. Therefore, by inducing favorable mucus secretion, key neuropeptides may indirectly interfere with microbial colonization. It is worth adding that mucus also plays an important role in the differentiation and behavior of microbial phenotypes of both commensals and pathogens [94].
Knowing the numerous benefits of the protective antibacterial effect of mucus, one cannot forget that mucus obstruction is an important feature of some chronic respiratory diseases including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and asthma. Since mucus is an important defensive mechanism, its increased or defective secretion, together with improper composition, can alter its functionality. Poor mucus clearance can contribute to airway inflammation and persistent bacterial infections. In CF, the defective secretion of mucus by submucosal glands is a consequence of lack of functional CF transmembrane conductance regulator (CFTR) chloride channels. Furthermore, in CF airways, mucus glands form tethered mucus plaques in which various antimicrobials become ineffective [95]. Moreover, conditions within the plaques, such as lower O2 tension, contribute to the formation of a mucoid and more resistant phenotype among residing bacterial pathogens such as P. aeruginosa [95]. It was demonstrated that CFTR of submucosal glands in CF patients may be abnormally activated in a CGRP-dependent manner. As a consequence, mucus production and proliferation of glandular progenitor cells may occur [96]. Under these circumstances, the aforementioned CGRP-mediated stimulation of mucus secretion will certainly be detrimental. Likewise, chronic bronchitis and asthma exacerbation are associated with elevated SP in sputum and SP-mediated neurogenic inflammation, contributing to airway narrowing [97]. Although the increase in SP levels in mucus is equivocal in COPD [93], it has previously been reported that this neuropeptide may be involved in the pathogenesis of some of the changes observed in COPD, as evidenced by increased SP- and VIP-immunoreactivity reported in epithelium and glands from COPD patients [98].
4.3. Impact of Neuropeptides on Tissue Integrity
Many bacterial pathogens can break epithelial integrity using an array of toxins that target occludin, a protein that plays a crucial role in tight junction structure and contributes to maintaining adequate epithelial permeability. These include toxins that can cause redistribution of occludin, such as Clostridium difficile toxin, or EspF of enteropathogenic E. coli but also toxins that cause proteolytic degradation of occludin such as Vibrio cholera cytotoxin [99,100]. Neuropeptides may help to partially restore epithelial barrier integrity after its disruption caused by enterotoxigenic E. coli (ETEC). It has been shown that VIP administered intraperitoneally to piglets may improve epithelial barrier function by preventing the disadvantageous translocation of occludin and other tight junction proteins. This phenomenon was accompanied by a reduced expression of proinflammatory cytokines induced by ETEC, possibly through modulation of Toll-like receptors (TLRs), nuclear factor κB (NF-κB), and mitogen-activated protein kinase (MAPK) pathways. Furthermore, VIP has been shown to upregulate occludin expression in ileum mucosa [101]. Likewise, VIP can stimulate diverse microbial communities associated with increased ecosystem stability in the gastrointestinal tract and resistance to pathogen invasion [102]. In this way, by minimizing bacteria-induced redistribution of tight junction proteins, VIP restores the colonic epithelial barrier, thus contributing to its protection.
4.4. Impact of Neuropeptides on the Infectious Inflammatory Response
In addition to disrupting tissue integrity, some pathogens modulate the inflammatory response and neuropeptide release during infection. They often utilize for this purpose evolutionarily conserved and unique for them soluble or surface-associated pathogen-associated molecular patterns (PAMPs). PAMPs of certain bacteria and pathogenic yeasts were shown to directly activate nociceptor TRPV1-positive (transient receptor potential vanilloid 1) neurons through interaction with pattern recognition receptors (PRRs). For example, LPS from S. enteritidis can increase levels of dopamine in the brain and neuropeptides such as SP, NPY, VIP, and galanin in cervical lymph nodes [103]. S. aureus, a major cause of wound and surgical infections leading to painful abscesses, was shown to stimulate TRPV1-positive neurons by releasing N-formylated peptides, acting on the formyl peptide receptor 1 expressed on nociceptors [104]. In a similar manner, β-glucans from C. albicans cell wall directly activate TRPV1-positive neurons by binding to the dectin-1 receptor on nociceptors [105,106]. Other bacterial PAMPs such as peptidoglycan, lipopolysaccharide, flagellin, lipoteichoic acid, as well as the pore-forming toxin alpha-haemolysin can also directly activate TRPV1-postive neurons, through a variety of distinct mechanisms [104,107]. Once activated, this specialized population of pain-sensing (nociceptive) neurons release neuropeptides from peripheral afferent terminals, which in turn act on immune cells, epithelial cells, and the enteric nervous system to maintain homeostasis [108]. Chiu et al. reported that neuropeptides released from nociceptors, upon acute staphylococcal infection, can directly modulate innate immune activation. The most potent of these neuropeptides, namely CGRP, Gal, and SST, suppressed TNF-α release from macrophages, thus contributing to down-regulation of the local inflammatory response [104]. It can therefore be assumed that the direct interplay between staphylococci and nociceptors serves the former in creating a suitable environment for pathogen to survive. Sensory neurons are also engaged in effective anti-fungal responses. For example, C. albicans directly affects TRPV1-postive nociceptive sensory neurons, causing release of CGRP. Then neuropeptide drives IL-23 production from dermal CD301b+ dendritic cells. IL-23 elicits IL-17 production from dermal lymphocytes Tγδ, thus inhibiting cutaneous C. albicans infection [109]. Therefore, in the skin, sensory neurons following direct sensing of yeast enhance host resistance to this pathogen via secretion of neuropeptide. This eminent cooperation between nervous and immune components provides critical host defense against fungal pathogens during the early stages of skin infection. Sometimes the same sensory neurons may exert opposing effects, which can lead to enhancement and survival of pathogens during infection. This occurs for example during aggressive bacterial subcutaneous infection of connective tissue leading to necrotizing fasciitis. In response to the S. pneumoniae toxin, streptolysin S, TRPV1-positive neurons produce pain and release CGRP locally. Local release of CGRP suppresses the recruitment and bactericidal activity of neutrophils. In this way, CGRP released by neurons into infected tissue blocks a pivotal cellular innate response against invasive bacteria [110].
4.5. Impact of Neuropeptides on Microbial Invasion
Once bacteria have successfully colonized their host, some bacterial species will invade the tissues and cells including monocytes or macrophages or epithelial cells whilst others are not capable of intracellular invasion. Extracellular pathogens multiply and colonize the surface of host cells and perhaps break down the barriers of tissue to proliferate and disseminate, but they remain outside of host cells (S. pyogenes, P. aeruginosa, E. coli). On the other hand, intracellular pathogens may actively penetrate host cells and survive within their environment. Intracellular pathogens are classified as: (i) obligate intracellular pathogens which cannot live outside host cell (certain bacteria: Chlamydia, Rickettsia, certain parasites: Toxoplasma spp.), (ii) facultative intracellular pathogens capable of living and reproducing either inside or outside host cells (Salmonella enterica serotype Typhimurium, Neisseria meningitidis, Mycobacterium spp.), and (iii) non-classical facultative intracellular pathogens, which are internalized by variety of cells and can survive within them (S. aureus) [92,111]. There are many infectious diseases where a dual extracellular/intracellular style of infectivity coexists including salmonellosis, tuberculosis, or plague [112]. Divergent intracellular strategies to invade the host can be modulated by certain neuropeptides as discussed below.
4.5.1. Impact of Neuropeptides on Facultative Intracellular Lifestyle
Recent evidence indicates that intracellular invasion to peculiar epithelial cells may be targeted by neuropeptides. For example, many enteric pathogens, including S. enterica serotype Typhimurium, utilize microfold (M)-cells to gain access to the host cell. M-cells represent a small proportion of the specialized follicular-associated epithelium (FAE) overlying mucosa-associated lymphoid tissues in the intestine. Under physiological conditions, M-cells are involved in lumen antigen sampling and transport via transcytosis to lymphocytes and macrophages, thereby generating an immune response [113]. However, these cells are also a highly efficient portal for pathogen entry as they possess many structural attributes relevant to bacterial infection [114,115]. S. enterica serotype Typhimurium uses two type-III secretion systems, encoded by genes located within pathogenicity islands to secrete effector proteins SopE, SopB, SipA, and SptP, directly into host epithelial cell including M-cells, where they participate in reorganization of the actin cytoskeleton [116]. Next, using M-cell during invasion, this intestinal pathogen can translocate across the gut epithelium to its deeper layers. Furthermore, to promote intestinal invasion, Salmonella is also capable of transforming new FAE epithelial cells into M-cells. Recent evidence indicates that Salmonella invasion may be diminished by neuropeptides. This assumption is supported by the fact that CGRP, released from gut-innervating nociceptive sensory neurons, inhibits Salmonella-mediated differentiation of M cells. This results in a decreased response to Salmonella infection [117]. Whilst a CGRP-dependent reduction in M-cell number seems to contribute to a lower vulnerability to invasion, this is not the sole determinant in decreasing Salmonella infection. The influence of CGRP on commensal microbiota involved in the mucosal immunity seems to be equally important. In fact, CGRP has been shown to facilitate the adherence and maintenance of relevant levels of segmental filamentous bacteria (SFB) to Peyer’s patch FAE and villi. These commensal gut residents, through adherence to the epithelium, form a mechanical barrier that is crucial in competing with enteric pathogens [117]. It is also worth adding that SFB commensals affect the priming and induction of Th-17 lymphocytes, which play key roles in mucosal defense not only against enteric bacteria but also against fungal pathogens essentially by induction of antimicrobial peptides production [118]. In conclusion, CGRP released from nociceptor neurons contributes directly and indirectly to defense against invasion of S. enterica.
4.5.2. Impact of Neuropeptides on the Obligate Intracellular Lifestyle
Sometimes to aid entry into a cell, intracellular protozoan pathogens, such as L. major, facilitate their engulfment by phagocytic cells. This allows the pathogen to find a safe niche in which to multiply. In such situations, SP- or NPY-mediated inhibition of Leishmania ingestion [86,119] can be proposed as defensive action to block intracellular invasion. An antiparasitic effect was also confirmed for neuropeptide PACAP against Toxoplasma gondii. T. gondii is an obligate intracellular parasite, which infects the small intestine and differentiates to its rapidly replicating stage (tachyzoite), which is able to infect all nucleated cells through active penetration. It has recently been reported that exogenous administration of PACAP in mice leads to decreased systemic parasitic burden as a result of PACAP-mediated phagocytic enhancement by mononuclear cells, contributing to the resolution of the infection [120].
4.5.3. Influence of Neuropeptides on Granulomatous Response
As outlined above, some neuropeptides participate in the pathogenesis of diseases caused by obligate intracellular pathogens, which have evolved to survive and replicate inside host cells after the invasion. To ensure an intracellular lifestyle, several pathogens also participate in granuloma formation. Granulomas are unique evolutionarily ancient structures that contain mainly macrophages and are formed in response to persistent particulate stimuli (infectious or noninfectious), that macrophages alone cannot eradicate. In the case of infectious stimuli, the granulomatous response is associated with bacterial, fungal, and parasitic infection. Currently, the formation of granulomas is understood to contribute to both protective and pathogenic consequences [121]. For example, for Mycobacterium tuberculosis early protective granulomas are responsible for eliminating bacteria, but late transmissive granulomas may facilitate bacterial growth and dissemination [122]. The role of neuropeptides in the formation of granulomas involves their immunomodulatory effects on the inflammatory response. The anti-inflammatory activity of α-MSH in an in vitro sarcoidosis-like granuloma model has been documented using peripheral blood mononuclear cells (monocytes and lymphocytes) from sarcoidosis patients challenged with cellular microparticles of Mycobacterium abscessus. The granuloma development in this model was reduced in the presence of α-MSH. This was accompanied by a significant decrease in production of pro-inflammatory cytokines and chemokines (comparison to non-treated granuloma controls), including those related to Th1 lymphocytes (IL-2R, IL-7, TNF-α, IFN-γ) and macrophages (CCL2 (MCP-1, CCL3 (MIP-α), CCL4 (MIP-1β), GM-CSF) and others typical of inflammation (IL-6, IL-8, IFN-α) [123]. It has been also shown that SP contributes to granuloma formation, in response to infection by the helminth cestode, Taenia crassiceps. Here, SP is involved in induction of proinflammatory cytokines IL-1 and TNF- and Il-6, within the granuloma. These cytokines are recognized to be key mediators of granuloma formation [124]. Likewise, granuloma formation during a normal granulomatous respons, observed in murine schistosomiasis requires the binding of SP to its specific NK1 receptor [125]. Initial granuloma formation by the host in response to agents causing chronic infections is thought to be essential for limiting and eventually clearing infection [124]. However, in some cases, late transmissive granuloma may contribute to the growth and dissemination of intracellular pathogens in the course of tuberculous infection [122,126].
5. Have Neuropeptides Met Their Expectations as Anti-Infective Agents?
The general principles of using novel peptides as anti-infective therapeutics could involve their use (i) as single agents with direct microbicidal (killing) or microbiostatic (inhibiting growth without killing) action; (ii) in combination with conventional antibiotics or other chemotherapeutics to promote additive or synergistic effects; (iii) as immunostimulatory agents that enhance natural innate or adaptive immunity; and (iv) as endotoxin-neutralizing agents [127,128]. These assumptions at least in part are fulfilled by neuropeptides. Furthermore, one of the beneficial actions that can be attributed to neuropeptides is undoubtedly their indirect effect on commensal microflora [25,129]. The discovery of the antimicrobial properties of neuropeptides placed high hopes for the possibility of their use in the treatment of infectious diseases. Recent examples from clinical trials highlight, however, the duality of action and therefore heterogeneous efficacy of neuropeptides as anti-infective compounds. On the one hand, beneficial health examples of neuropeptide action were reported, including (i) association between high levels of serum SP and lower mortality in severe septic patients [130], or (ii) association between high level of serum CGRP and lower mortality in children with severe pneumoniae [131]. On the other hand, the negative activity of neuropeptides was exemplified by the potential role of SP in severity of diarrhea in cryptosporidiosis in immunocompromised patients [132] or the well-documented detrimental role of SP in periodontal diseases [133]. The complexity of neuropeptide action in clinical trials is yet another example of their duality. After years of experimental research, as well as attempts to use neuropeptides or their analogs in approaches based on animal models, or in clinical trials it seems, however, that the aforementioned hopes have not been fulfilled in many cases. Accordingly, neuropeptides do not currently appear to be highly promising candidates for the treatment of microbial infections. Their therapeutic usefulness appears to be limited mainly because of their rather weak direct antimicrobial action at physiological concentrations and low stability in vivo, connected with the labile nature of peptides. However, it is important to note that many classical AMPs have suffered a similar fate. Currently, there are only a few AMPs in clinical development for the treatment of local skin (bovine indolicidin), nasal (synthetic antimicrobial peptidomimetic Lytixar (LTX-109)), or oral (LL-37) bacterial infections, all of which are intended for topical use at the site of infection. The only AMP in clinical trials for intravenous administration is human-derived Lactoferrin 1–11 (hLF1–11) for the treatment of life-threatening infections in patients after stem cell transplantation [134].
Regarding peptides derived strictly from the neuroendocrine system, to date, only two peptides, namely an α-MSH derivative and ghrelin have entered Phase II clinical trials for the treatment of vulvovaginal candidiasis and chronic respiratory infection, respectively. Encouragingly, positive anti-candidal efficacy has been reported for the α-MSH derivative and anti-inflammatory efficacy has been reported for both compounds [135].
Neuropeptides as naturally occurring protein molecules can be influenced by many factors in the host environment. In this aspect, both intrinsic factors (e.g., post-translational modifications) and acquired ones (e.g., pathogens and their released proteases) can potentially lead to disturbance or perturbation or modulation of neuropeptides activity. Some of these factors are discussed below.
5.1. Glycosylation of Neuropeptides and Their Receptors
Glycosylation, the enzymatic addition of monosaccharide or glycan, is the most common and complex post-translational modification of the polypeptide chain. Glycosylation adds increased structural diversity to proteins and peptides, impacting on their conformation and physicochemical properties [136,137,138] and in turn translating to their biological activities [139,140]. Changes in glycosylation profile are known to be associated with various physiological and pathological conditions and can modulate the biological recognition events, including inflammatory responses and host–microbe interactions [140,141,142]. Both linked glycans and free oligosaccharides are also a key factor in shaping the microbiome [143,144].
Recent studies have shown that the presence of glycans impacts the biological activity of neuropeptides. The neurokinin 1 receptor (NK1R) is a G protein-coupled receptor for SP, with two putative N-linked glycosylation sites, Asn14 and Asn18. This sugar moiety has been shown to be critical for receptor function, since ligand binding to the unglycosylated form of NK1R demonstrated reduced SP-induced IL-8 secretion [145]. Likewise, the double mutant Asn18Thr/Asn31Thr lacking both glycosylation sites for sst3 subtype of somatostatin receptor showed a significant reduction in high affinity binding of somatostatin [146]. The glycosylation of neuropeptide receptors therefore appears to have functional consequences on the biology of their ligands. Likewise, the importance of peptide glycosylation is demonstrated by the fact that one-third of the 279 classified peptide hormones carry O-glycans. Many of the well-characterized neuropeptides, such as NPY, VIP, CGRP, galanin, and secretin are glycosylated [147]. For example, the glucagon family members including VIP share a highly conserved O-glycan at Thr7, while the NPY family members share a conserved O-glycan at Thr32. Taking advantage of this, three glycovariants of VIP and NPY were chemoenzymatically synthesized to contain the most common O-GalNAc-type structures namely Tn (GalNAcα1-O-Ser/Thr), T (Galβ1-3GalNAcα1-O-Ser/Thr), and sialylated ST (NeuAcα2-3Galβ1-3GalNAcα1-O-Ser/Thr) at NPY Thr32 and VIP Thr7 to explore how O-glycans on these neuropeptides modulate receptor activation. Madsen et al. showed that: (i) O-linked VIP and NPY dampened their cognate receptor activation which was found to correlate positively with the size of the attached O-glycans, (ii) O-glycans located in the receptor-binding domain of the NPY had a greater impact on receptor activation than glycans in the receptor-binding domain of VIP, indicating that the nature of the entire peptide is also important [147]. Furthermore, the presence of O-glycans attached to atrial natriuretic peptide (ANP) attenuates its acute renal and cardiovascular actions in vivo, whereas in vitro O-glycosylation was shown to protect ANP from proteolytic cleavage and modulate interactions with its cognate receptor [148]. Recently, it has also been demonstrated that O-glycans within the receptor-binding motifs of members of the NPY and glucagon families substantially extend peptide half-life and modulate receptor activation properties [147]. Many of the identified O-glycosites are conserved and are predicted to serve roles in proprotein processing, receptor interaction, biodistribution, and biostability [Madsen, 2020]. For this reason, synthetic glycosylation via chemoenzymatic addition of N- or/and O-glycans may be an effective and promising strategy to enhance the bioavailability, half-life, and expected biological effects of therapeutic peptides [149,150,151].
5.2. Vulnerability of Neuropeptides to Host and Bacterial Proteases
Neuropeptides as others endogenous peptides can interact with various endogenous peptidases. It is important to note that levels of these proteins and enzymes can be regulated/altered by particular disease. At sites of infection in vivo, in patients suffering from periodontitis, specific CGRP degradation by carboxypeptidase present in the extracellular gingival milieu has been reported [152]. Similarly, peptide degradation by the classical AMP, cathelicidin LL-37 has also been reported in the same sites of infection [153], supporting the major role of proteases, including bacterial proteases [153] in resistance to the actions of antimicrobial peptides Although there are no studies on the mechanisms of neuropeptide resistance in microorganisms, by analogy with classical mammals AMPs such as LL-37 and defensins, it is assumed that they are similar. The induction of intrinsic adaptive resistance to AMPs in bacteria is tightly regulated in response to environmental pressure. This response includes: (i) the reduction of bacterial negative cell-surface charge through the incorporation of positively charged molecules into the cell membrane, (ii) efflux pumps to expel AMPs, (iii) proteolytic degradation of AMPs by microbial exotoxins/proteases, (iv) induction of AMP trapping mechanisms [134,154]. When comparing neuropeptides with AMPs in this respect, it should be borne in mind that due to the very low physiological concentration of neuropeptides in mammals, the emergence of selection pressure appears less likely. Proteolytic degradation of neuropeptides by microbial proteases, however, seems to be a different and more likely matter. The findings that various bacteria such as P. aeruginosa, E. faecalis, Proteus mirabilis, S. enterica, S. pyogenes, Burkholderia cenocepacia, Vibrio cholera and pathogenic yeasts such as C. albicans can cleave pivotal antimicrobial peptides including LL-37 appear to support this notion [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159]. Since many of the bacteria discussed produce exotoxins or proteases, we performed bioinformatic analyses to assess the potential sensitivity of the neuropeptides discussed in this paper to these enzymes (Appendix A, Figure 2). We considered the action of 12 proteolytic enzymes from 6 pathogenic bacteria (P. aeruginosa, P. gingivalis, S. typhimurium, S. aureus, S. pyogenes, L. longbeachae) on 11 neuropeptides. The cleavage sites were identified using PROSITE scanning program [160] and the cleavage patterns were created based on data available in MEROPS database for the individual peptidase [161]. The enzymes were chosen because they cleave the classical AMP, LL-37 and/or pivotal human interleukins [162]. The vast majority of neuropeptides seemed to be susceptible to the 6 out of 12 proteases studied using the in silico approach. CNP could be cleaved by six proteases, ADM, CGRP, and SST by five proteases, ANP, NPY, PACAP, and VIP by four, Dynorphin by three, whereas SP by one. α-MSH did not reveal cleavage sites recognized by any of the enzymes studied. For comparison, LL-37 was subject to the same in silico analysis. These results indicate that pathogenic bacteria can cleave many neuropeptides and as a result modulate the action of these compounds.
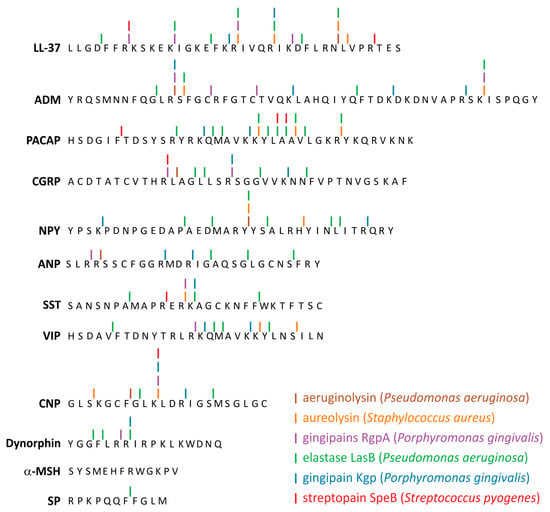
Figure 2.
Potential cleavage sites in neuropeptide sequences subjected to selected bacterial proteases. The sites were identified using PROSITE scanning program and cleavage patterns created based on data available in MEROPS database for the individual peptidases. In the pattern, we considered three positions before and after the cleavage site, i.e., P3 P2 P1 P1’ P2’ P3’. Antimicrobial peptide LL-37 was shown for comparison.
5.3. Duality of Action in Murine Models
Evidence for the dual role of neuropeptides in preclinical interventions in murine models of infectious diseases are summarized in Table 2 and Table 3. They involve (i) recent approaches for the use of exogenous neuropeptides as anti-infective drugs in murine models of diverse infections (Table 2); (ii) animal models of infectious disease aimed at modulating the expression of endogenous neuropeptides at the site of infection to boost innate mechanisms of immune response (Table 3). The two most commonly used neuropeptides in these studies were SP and VIP. Considering the results presented in Table 2 and Table 3 in more detail, striking similarities can be seen in the ineffectiveness of SP, regardless of the type of infectious disease or etiological factor or pathogen involved. This unexpected observation seems to be contradictory to common expectations. The duality of action of this neuropeptide is reflected by the fact that in some conditions, such as salmonellosis, healing was achieved. In contrast, in other disorders such as infectious keratitis, this neuropeptide has a completely different effect leading to worsening and disease exacerbation. Similar observations come from studies focused on endogenous expression of SP. In this case, in the course of various meningitis, irrespective of the bacterial pathogen involved, increased SP expression was associated with unsatisfactory clinical outcome. Alternatively, increase in SP during pneumonia corresponded with beneficial clinical parameters.

Table 2.
Exogenous application of neuropeptides as anti-infective drugs in murine models of diverse infections.

Table 3.
Endogenous expression of neuropeptides at the site of diverse infections in murine models.
In contrast to SP, administration of VIP has been shown to act beneficially in the course of the variety of infectious disorders, mainly by extinguishing inflammatory reactions. It essentially occurred through decrease in inflammatory cytokines and adhesion molecules of inflammatory cells as well as increase in growth factors for cells involved in tissue repair. Interestingly, VIP achieved healing in corneal infection models, and beneficial results in periodontal infection models and infectious keratitis model, and indeed was successful in all models studied, reflecting its well-recognized anti-inflammatory action. The immunosuppressive potential of neuropeptides such as VIP and PACAP could probably be exploited in future to restore homeostasis. These so-called anti-inflammatory neuropeptides could contribute directly to the later stages of inflammation, that require a dampening of the inflammatory response, in advance of healing and repair.
6. Conclusion and Future Prospects
In conclusion, the pleiotropic nature of neuropeptides indicates that they have pro-infective and anti-infective effects, as well as virulence-enhancing and virulence-attenuating properties. By having such a pleiotropic nature, which is largely dependent on specific virulence traits of pathogens, it is impossible to classify neuropeptides as compounds with a uniformly defined pattern of action. Considering their anti-infective benefits in vitro, neuropeptides have been shown to attenuate common stages of microbial pathogenesis such as adhesion, invasion, intracellular lifestyle. Nevertheless, these positive effects do not appear to be mirrored in preclinical interventions in murine in vivo models or in the relatively few clinical trials completed to date.
Evidence to date would suggest that the anti-infective activities of the majority of neuropeptides appear not to be as pronounced as their antibiotic counterparts, indicating that this aspect of their therapeutic effectiveness remains elusive. A notable exception is, however, VIP which has proved to be successful in murine models of certain infectious diseases.
In summary, although neuropeptides, as important components of innate immunity, are unlikely to be universally recognized as therapeutic antimicrobial agents, their value as natural factors involved in modulating host–pathogen interactions is currently being elucidated. In this respect, physiologically functional neuropeptides are important host constituents that contribute to general health by maintaining the balance between immunity to pathogens and their tolerance. Therefore, we are faced with the task of increasing research efforts in this field to fully recognize the immunomodulatory properties of neuropeptides as additional players in maintaining homeostasis.
Furthermore, the findings that neuropeptides have numerous anti-infective strategies but also virulence-enhancing properties raises future questions regarding (i) how microorganisms sense neuropeptides; (ii) how neuropeptides induce microbial virulence; (iii) whether bacteria can subvert action of neuropeptides. All these issues should be addressed in the future.
Funding
This study was supported by research grants of University of Wroclaw (1016/S/IGiM/T-20) and the National Science Centre Poland (Narodowe Centrum Nauki, Polska) Grant 2019/35/N/NZ8/03366. EK was supported by the Marie Skłodowska-Curie Action BactiVax, GA number 860325. Publication of this article was financially supported by the Excellence Initiative—Research University (IDUB) programme for the University of Wroclaw.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The bioinformatics data presented in this study are available on request from the corresponding author. The data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADM | Adrenomedullin |
| ALOX12 | Arachidonate 12-Lipoxygenase |
| AMPs | Antimicrobial Peptides |
| ANP | Atrial Natriuretic Peptide |
| Bcl-2 | B-cell Lymphoma 2 |
| CGRP | Calcitonin Gene-Related Peptide |
| CNP | C-type Natriuretic Peptide |
| COX-2 | Cyclooxygenase-2 |
| CRH | Corticotropin Releasing Hormone |
| Ef-Tu | Ribosomal Elongation Factor Thermo-Unstable |
| EGF | Epidermal Growth Factor |
| FGF | Fibroblast Growth Factors |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| GPCR | G Protein- Coupled Receptors |
| HGF | Hepatocyte Growth Factor |
| HHQ | 4-hydroxy-2-heptylquinoline |
| HIF-1α | Hypoxia-inducible factor 1 α |
| Hlf1–11 | Human-Derived Lactoferrin 1–11 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN-γ | Interferon-γ |
| IsaB | Immunodominant Staphylococcal Antigen B |
| LFA-1 | Lymphocyte Function-Associated Antigen 1 |
| Lkt | Leukotoxin |
| MIP-2 | Macrophage Inflammatory Protein 2 |
| MPO | Myeloperoxidase |
| NFκB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NPY | Neuropeptide Y |
| OPG | Osteoprotegerin |
| PACAP | Pituitary Andenylate Cyclase-Activating Polypetide |
| PA-I | Lectin/Adhesin PA-I |
| pavA | Pneumococcal Adherence and Virulence Factor A |
| PNS | Peripheral Nervous System |
| PQS | 2-heptyl-3,4-dihydroxyquinoline |
| PRRs | Pattern Recognition Receptors |
| PtxR | HTH-Type Transcriptional Regulator PtxR |
| QS | Quorum Sensing |
| SEC2 | Enterotoxin C2 |
| SFB | Segmental Filamentous Bacteria |
| SOCS3 | Suppressor of Cytokine Signaling 3 |
| SP | Substance P |
| sRANKL | Soluble Receptor Activator of Nuclear Factor-κB Ligand |
| SST | Somatostatin |
| TGF-β | Transforming Growth Factor β |
| TNF-α | Tumor Necrosis Factor α |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| Ucn | Urocortins |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF-A | Vascular Endothelial Growth Factor A |
| VIP | Vasoactive Intestinal Peptide |
| VLA-4 | Very Late Antigen-4 |
| α-MSH | α-Melanocyte Stimulating Hormone |
Appendix A
The number of potential cleavage sites in neuropeptides subjected to selected proteases. The sites were identified using PROSITE scanning program and cleavage patterns created based on data available in MEROPS database for the individual peptidases. In the pattern, we considered three positions before and after the cleavage site, i.e., P3 P2 P1 P1’ P2’ P3’. Antimicrobial peptide LL-37 was included for comparison.
| Name of Protease | Species | Accession Number | ADM | α-MSH | ANP | CGRP | CNP | Dynorphin | NPY | PACAP | SST | SP | VIP | LL-37 |
| Alkaline protease aeruginolysin | Pseudomonas aeruginosa | APRA_PSEAE | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Arg specific gingipains RgpA | Porphyromonas gingivalis | CPG1_PORGN | 4 | 1 | 2 | 1 | 1 | 1 | 1 | 4 | ||||
| Arg specific gingipains RgpB | Porphyromonas gingivalis | CPG2_PORGI | ||||||||||||
| Aureolysin | Staphylococcus aureus | AURE_STAAU | 2 | 2 | 2 | 3 | 1 | 2 | 4 | |||||
| C5a peptidase (ScpA) | Streptococcus pyogenes | C5AP_STRP6 | ||||||||||||
| Elastase (LasB, pseudolysin) | Pseudomonas aeruginosa | ELAS_PSEAE | 4 | 3 | 4 | 2 | 3 | 6 | 10 | 3 | 1 | 4 | 7 | |
| Gingipains [Lys-specific gingipain (Kgp) | Porphyromonas gingivalis | KGP83_PORGN | 4 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | ||
| LasA protease staphylolysin | Pseudomonas aeruginosa | LASA_PSEAE | ||||||||||||
| MspA | Legionella longbeachae | MSPA_LEGLO | ||||||||||||
| PgtE (OmpE, protein E) | Salmonella Typhimurium | PGTE_SALTY | ||||||||||||
| SlyCEP (ScpC) | Streptococcus pyogenes | Q3HV58_STRPY | ||||||||||||
| Streptopain (SpeB) | Streptococcus pyogenes | SPEB_STRPY | 1 | 1 | 3 | 1 | 2 |
References
- Larhammar, D. Neuropeptides Phylogeny and Evolution. In Encyclopedia of Neuroscience; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 873–880. [Google Scholar]
- Russo, A.F. Overview of Neuropeptides: Awakening the Senses? Headache J. Head Face Pain 2017, 57, 37–46. [Google Scholar] [CrossRef]
- Sobota, J.; Eipper, B.; Mains, R. Neuropeptide Synthesis and Storage. Encycl. Neurosci. 2009, 3, 829–836. [Google Scholar] [CrossRef]
- Merighi, A.; Salio, C.; Ferrini, F.; Lossi, L. Neuromodulatory function of neuropeptides in the normal CNS. J. Chem. Neuroanat. 2011, 42, 276–287. [Google Scholar] [CrossRef]
- Lundy, F.; Linden, G. Neuropeptides and Neurogenic Mechanisms in Oral and Periodontal Inflammation. Crit. Rev. Oral Biol. Med. 2004, 15, 82–98. [Google Scholar] [CrossRef]
- Brogden, K.A.; Guthmiller, J.M.; Salzet, M.; Zasloff, M.; Fauq, A.H.; Osborne, B.A.; Turley, D.M.; Shin, H.M.; Joshi, I.; Telfer, J.C.; et al. The nervous system and innate immunity: The neuropeptide connection. Nat. Immunol. 2005, 6, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A. Brain neuropeptide Y and corticotropin-releasing hormone in mediating stress and anxiety. Exp. Biol. Med. 2010, 235, 1163–1167. [Google Scholar] [CrossRef]
- Hökfelt, T.; Broberger, C.; Xu, Z.-Q.D.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—An overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Goetzl, E.J.T.; Turck, C.W.; Sreedharan, S.P. Production and Recognition of Neuropeptides by Cells of the Immune System. In Psychoneuroimmunology, 2nd ed.; Ader, R., Felten, D.L., Cohen, N., Eds.; Academic Press: Cambridge, MA, USA, 1991; pp. 263–282. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Ganea, D.; Delgado, M. Neuropeptides: Keeping the balance between pathogen immunity and immune tolerance. Curr. Opin. Pharmacol. 2010, 10, 473–481. [Google Scholar] [CrossRef]
- Augustyniak, D.; Nowak, J.; Lundy, F.T. Direct and Indirect Antimicrobial Activities of Neuropeptides and their Therapeutic Potential. Curr. Protein Pept. Sci. 2012, 13, 723–738. [Google Scholar] [CrossRef]
- Killough, S.A.; Lundy, F.T.; Irwin, C.R. Substance P Expression by Human Dental Pulp Fibroblasts: A Potential Role in Neurogenic Inflammation. J. Endod. 2009, 35, 73–77. [Google Scholar] [CrossRef]
- Killough, S.A.; Lundy, F.T.; Irwin, C.R. Dental pulp fibroblasts express neuropeptide Y Y1 receptor but not neuropeptide Y. Int. Endod. J. 2010, 43, 835–842. [Google Scholar] [CrossRef]
- Lambrecht, B.N. Immunologists getting nervous: Neuropeptides, dendritic cells and T cell activation. Respir. Res. 2001, 2, 133–138. [Google Scholar] [CrossRef]
- Hökfelt, T.; Bartfai, T.; Bloom, F. Neuropeptides: Opportunities for drug discovery. Lancet Neurol. 2003, 2, 463–472. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Marder, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017, 18, 389–403. [Google Scholar] [CrossRef]
- Cutuli, M.; Cristiani, S.; Lipton, J.M.; Catania, A. Antimicrobial effects of α-MSH peptides. J. Leukoc. Biol. 2000, 67, 233–239. [Google Scholar] [CrossRef]
- El Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Percival, R.S.; Rangarajan, M.; Curtis, M.A. Modulation of antibacterial peptide activity by products of Porphyromonas gingivalis and Prevotella spp. Microbiolohy 1999, 145, 965–971. [Google Scholar] [CrossRef]
- Allaker, R.P.; Sheehan, B.E.; McAnerney, D.C.; McKay, I.J. Interaction of adrenomedullin and calcitonin gene-related peptide with the periodontal pathogen Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2007, 49, 91–97. [Google Scholar] [CrossRef]
- Lundy, F.T.; Irwin, C.R.; McLean, D.F.; Linden, G.J.; El Karim, I.A. Natural Antimicrobials in the Dental Pulp. J. Endod. 2020, 46, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Grosvenor, P.W.; McAnerney, D.C.; Sheehan, B.E.; Srikanta, B.H.; Pell, K.; Kapas, S. Mechanisms of adrenomedullin antimicrobial action. Peptides 2006, 27, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Anderson, P.; Garcia-Salcedo, J.A.; Caro, M.; Gonzalez-Rey, E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2008, 16, 406–416. [Google Scholar] [CrossRef]
- Yamashita, K.; Kaneko, H.; Yamamoto, S.; Konagaya, T.; Kusugami, K.; Mitsuma, T. Inhibitory effect of somatostatin on proliferation in vitro. Gastroenterology 1998, 115, 1123–1130. [Google Scholar] [CrossRef]
- Sanz, J.A.; El Aidy, S. Microbiota and gut neuropeptides: A dual action of antimicrobial activity and neuroimmune response. Psychopharmacology 2019, 236, 1597–1609. [Google Scholar] [CrossRef]
- Souza-Moreira, L.; Campos-Salinas, J.; Caro, M.; Gonzalez-Rey, E. Neuropeptides as Pleiotropic Modulators of the Immune Response. Neuroendocrinology 2011, 94, 89–100. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Augustyniak, D.; Jankowski, A.; Mackiewicz, P.; Skowyra, A.; Gutowicz, J.; Drulis-Kawa, Z. Innate immune properties of selected human neuropeptides against Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Immunol. 2012, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Roszkowiak, J.; Wiśniewska, I.; Skała, J.; Gorczyca, D.; Drulis-Kawa, Z. Neuropeptides SP and CGRP Diminish the Moraxella catarrhalis Outer Membrane Vesicle- (OMV-) Triggered Inflammatory Response of Human A549 Epithelial Cells and Neutrophils. Mediat. Inflamm. 2018, 2018, 4847205. [Google Scholar] [CrossRef]
- Lesouhaitier, O.; Clamens, T.; Rosay, T.; Desriac, F.; Louis, M.; Rodrigues, S.; Gannesen, A.; Plakunov, V.K.; Bouffartigues, E.; Tahrioui, A.; et al. Host Peptidic Hormones Affecting Bacterial Biofilm Formation and Virulence. J. Innate Immun. 2018, 11, 227–241. [Google Scholar] [CrossRef]
- Rice, J.A.; Carrasco-Medina, L.; Hodgins, D.C.; Shewen, P.E. Mannheimia haemolyticaand bovine respiratory disease. Anim. Health Res. Rev. 2007, 8, 117–128. [Google Scholar] [CrossRef]
- Pillai, D.K.; Cha, E.; Mosier, D. Role of the stress-associated chemicals norepinephrine, epinephrine and substance P in dispersal of Mannheimia haemolytica from biofilms. Vet. Microbiol. 2018, 215, 11–17. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Genet. 2011, 10, 39–50. [Google Scholar] [CrossRef]
- N’Diaye, A.; Gannesen, A.; Borrel, V.; Maillot, O.; Enault, J.; Racine, P.-J.; Plakunov, V.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Substance P and Calcitonin Gene-Related Peptide: Key Regulators of Cutaneous Microbiota Homeostasis. Front. Endocrinol. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pereira, R.M.; Kilic, E.; Casella, G.; Keyhani, N.O. Pyrokinin β-Neuropeptide Affects Necrophoretic Behavior in Fire Ants (S. invicta), and Expression of β-NP in a Mycoinsecticide Increases Its Virulence. PLoS ONE 2012, 7, e26924. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Genet. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Mijouin, L.; Hillion, M.; Ramdani, Y.; Jaouen, T.; Duclairoir-Poc, C.; Follet-Gueye, M.-L.; Lati, E.; Yvergnaux, F.; Driouich, A.; Lefeuvre, L.; et al. Effects of a Skin Neuropeptide (Substance P) on Cutaneous Microflora. PLoS ONE 2013, 8, e78773. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.; Mijouin, L.; Hillion, M.; Diaz, S.; Konto-Ghiorghi, Y.; Percoco, G.; Chevalier, S.; Lefeuvre, L.; Harmer, N.J.; Lesouhaitier, O.; et al. Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis Virulence: Implication for Skin Homeostasis. Front. Microbiol. 2016, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.R.; Borrel, V.; Racine, P.-J.; Clamens, T.; Depayras, S.; Maillot, O.; Schaack, B.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Mechanism of action of the moonlighting protein EfTu as a Substance P sensor in Bacillus cereus. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The Evolution of Quorum Sensing in Bacterial Biofilms. PLoS Biol. 2008, 6, e14. [Google Scholar] [CrossRef]
- Guła, G.; Dorotkiewicz-Jach, A.; Korzekwa, K.; Valvano, M.A.; Drulis-Kawa, Z. Complex Signaling Networks Controlling Dynamic Molecular Changes in Pseudomonas aeruginosa Biofilm. Curr. Med. Chem. 2019, 26, 1979–1993. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Ndjom, C.G.N.; Jones, H.P. CRH promotes S. pneumoniae growth in vitro and increases lung carriage in mice. Front. Microbiol. 2015, 6, 279. [Google Scholar] [CrossRef] [PubMed]
- Ndjom, C.G.N.; Kantor, L.V.; Jones, H.P. CRH Affects the Phenotypic Expression of Sepsis-Associated Virulence Factors by Streptococcus pneumoniae Serotype 1 In vitro. Front. Cell. Infect. Microbiol. 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.R.; Leclerc, C.; Kentache, T.; Hardouin, J.; Poc, C.D.; Konto-Ghiorghi, Y.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Skin-bacteria communication: Involvement of the neurohormone Calcitonin Gene Related Peptide (CGRP) in the regulation of Staphylococcus epidermidis virulence. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Lesouhaitier, O.; Racine, P.-J.; Barreau, M.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G. Regulation of Monospecies and Mixed Biofilms Formation of Skin Staphylococcus aureus and Cutibacterium acnes by Human Natriuretic Peptides. Front. Microbiol. 2018, 9, 2912. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Lesouhaitier, O.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G.J. Regulation of Formation of Monospecies and Binary Biofilms by Human Skin Microbiota Components, Staphylococcus epidermidis and Staphylococcus aureus, by Human Natriuretic Peptides. Microbiology 2018, 87, 597–609. [Google Scholar] [CrossRef]
- Zaborina, O.; Lepine, F.; Xiao, G.; Valuckaite, V.; Chen, Y.; Li, T.; Ciancio, M.; Zaborin, A.; Petroff, E.; Turner, J.R.; et al. Dynorphin Activates Quorum Sensing Quinolone Signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007, 3, e35. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Wang, B.; Sun, Y.; Liu, Y.; Li, G. Staphylococcal enterotoxin C2 promotes osteogenesis of mesenchymal stem cells and accelerates fracture healing. Bone Jt. Res. 2018, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.D. Clarifying the Mechanism of Superantigen Toxicity. PLoS Biol. 2011, 9, e1001145. [Google Scholar] [CrossRef]
- Biaggini, K.; Borrel, V.; Szunerits, S.; Boukherroub, R.; N’Diaye, A.; Zébré, A.; Bonnin-Jusserand, M.; Duflos, G.; Feuilloley, M.; Drider, D.; et al. Substance P enhances lactic acid and tyramine production in Enterococcus faecalis V583 and promotes its cytotoxic effect on intestinal Caco-2/TC7 cells. Gut Pathog. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Blier, A.-S.; Veron, W.; Bazire, A.; Gerault, E.; Taupin, L.; Vieillard, J.; Rehel, K.; Dufour, A.; Le Derf, F.; Orange, N.; et al. C-type natriuretic peptide modulates quorum sensing molecule and toxin production in Pseudomonas aeruginosa. Microbiology 2011, 157, 1929–1944. [Google Scholar] [CrossRef]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcuspneumoniae Capsule Inhibits Complement Activity and Neutrophil Phagocytosis by Multiple Mechanisms. Infect. Immun. 2009, 78, 704–715. [Google Scholar] [CrossRef]
- Mackey-Lawrence, N.M.; Jefferson, K.K. Regulation of Staphylococcus aureus immunodominant antigen B (IsaB). Microbiol. Res. 2013, 168, 113–118. [Google Scholar] [CrossRef]
- Liu, P.-F.; Cheng, J.-S.; Sy, C.-L.; Huang, W.-C.; Yang, H.-C.; Gallo, R.L.; Huang, C.-M.; Shu, C.-W. IsaB Inhibits Autophagic Flux to Promote Host Transmission of Methicillin-Resistant Staphylococcus aureus. J. Investig. Dermatol. 2015, 135, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, V.; Korhonen, T.K. Dancing to Another Tune—Adhesive Moonlighting Proteins in Bacteria. Biology 2014, 3, 178–204. [Google Scholar] [CrossRef]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef] [PubMed]
- Boël, G.; Jin, H.; Pancholi, V. Inhibition of Cell Surface Export of Group A Streptococcal Anchorless Surface Dehydrogenase Affects Bacterial Adherence and Antiphagocytic Properties. Infect. Immun. 2005, 73, 6237–6248. [Google Scholar] [CrossRef] [PubMed]
- Hemmadi, V.; Biswas, M. An overview of moonlighting proteins in Staphylococcus aureus infection. Arch. Microbiol. 2021, 203, 481–498. [Google Scholar] [CrossRef]
- Silva, R.C.; Padovan, A.C.B.; Pimenta, D.C.; Ferreira, R.C.; Da Silva, C.V.; Briones, M.R.S. Extracellular enolase of Candida albicans is involved in colonization of mammalian intestinal epithelium. Front. Cell. Infect. Microbiol. 2014, 4, 66. [Google Scholar] [CrossRef]
- Lopez, C.M.; Wallich, R.; Riesbeck, K.; Skerka, C.; Zipfel, P.F. Candida albicans Uses the Surface Protein Gpm1 to Attach to Human Endothelial Cells and to Keratinocytes via the Adhesive Protein Vitronectin. PLoS ONE 2014, 9, e90796. [Google Scholar] [CrossRef] [PubMed]
- Satala, D.; Karkowska-Kuleta, J.; Zelazna, A.; Rapala-Kozik, M.; Kozik, A. Moonlighting Proteins at the Candidal Cell Surface. Microorganisms 2020, 8, 1046. [Google Scholar] [CrossRef]
- Hallström, T.; Uhde, M.; Singh, B.; Skerka, C.; Riesbeck, K.; Zipfel, P.F. Pseudomonas aeruginosa Uses Dihydrolipoamide Dehydrogenase (Lpd) to Bind to the Human Terminal Pathway Regulators Vitronectin and Clusterin to Inhibit Terminal Pathway Complement Attack. PLoS ONE 2015, 10, e0137630. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The Diverse Functional Roles of Elongation Factor Tu (EF-Tu) in Microbial Pathogenesis. Front. Microbiol. 2019, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Kopeckova, M.; Pavkova, I.; Stulik, J. Diverse Localization and Protein Binding Abilities of Glyceraldehyde-3-Phosphate Dehydrogenase in Pathogenic Bacteria: The Key to its Multifunctionality? Front. Cell. Infect. Microbiol. 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Seredyński, R.; McClean, S.; Roszkowiak, J.; Roszniowski, B.; Smith, D.L.; Drulis-Kawa, Z.; Mackiewicz, P. Virulence factors of Moraxella catarrhalis outer membrane vesicles are major targets for cross-reactive antibodies and have adapted during evolution. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Wright, M.H.; Fetzer, C.; Sieber, S.A. Chemical Probes Unravel an Antimicrobial Defense Response Triggered by Binding of the Human Opioid Dynorphin to a Bacterial Sensor Kinase. J. Am. Chem. Soc. 2017, 139, 6152–6159. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.-A. Host-Pathogen Interactions: Redefining the Basic Concepts of Virulence and Pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicanspathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A.; Schmid-Hempel, P. Mechanisms of pathogenesis and the evolution of parasite virulence. J. Evol. Biol. 2008, 21, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, N.; Kanzok, S.M.; Wek, R.C.; Sullivan, W.J., Jr. Stress response pathways in protozoan parasites. Cell. Microbiol. 2008, 10, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Strobel, M.; Pförtner, H.; Tuchscherr, L.; Völker, U.; Schmidt, F.; Kramko, N.; Schnittler, H.-J.; Fraunholz, M.; Löffler, B.; Peters, G.; et al. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin. Microbiol. Infect. 2016, 22, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Hillion, M.; Mijouin, L.; N’Diaye, A.; Percoco, G.; Enault, J.; Follet-Gueye, M.-L.; Yvergnaux, F.; Lefeuvre, L.; Feuilloley, M.G.J. Pseudomonas fluorescens, a forgotten member of the human cutaneous microflora sensible to skin communication and defense peptides. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 910–925. [Google Scholar]
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Kong, H.; You, N.; Chen, H.; Teng, Y.-S.; Liu, Y.-G.; Lv, Y.-P.; Mao, F.-Y.; Cheng, P.; Chen, W.; Zhao, Z.; et al. Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Q.-L.; Cheng, D.-D.; Xu, W.-T.; Lu, N.-H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N. Type IV Secretion and Signal Transduction of Helicobacter pylori CagA through Interactions with Host Cell Receptors. Toxins 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Paoletti, I.; Buommino, E.; Tufano, M.A.; Baroni, A. alpha-MSH reduces the internalization of Staphylococcus aureus and down-regulates HSP 70, integrins and cytokine expression in human keratinocyte cell lines. Exp. Dermatol. 2004, 13, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.D.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Giammarressi, M.; Vanegas, O.; Febres, A.; Silva-López, A.; López, E.D.; Ponte-Sucre, A. Chemotactic activities of vasoactive intestinal peptide, neuropeptide Y and substance P in Leishmania braziliensis. Exp. Parasitol. 2020, 219, 108009. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Wahbi, A.; Nordlind, K.; Kharazmi, A.; Sundqvist, K.G.; Mutt, V.; Lidén, S. In Vitro Leishmania majorPromastigote-Induced Macrophage Migration is Modulated by Sensory and Autonomic Neuropeptides. Scand. J. Immunol. 1998, 48, 79–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, A.A.; Wahbi, A.-H.; Nordlind, K. Neuropeptides Modulate a Murine Monocyte/Macrophage Cell Line Capacity for Phagocytosis and Killing Ofleishmania Majorparasites. Immunopharmacol. Immunotoxicol. 2001, 23, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Colombo, G.; Rossi, C.; Carlin, A.S.; Sordi, A.; Lonati, C.; Turcatti, F.; Leonardi, P.M.; Grieco, P.; Gatti, S. Antimicrobial Properties of α-MSH and Related Synthetic Melanocortins. Sci. World J. 2006, 6, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Rauch, I.; Lundström, L.; Hell, M.; Sperl, W.; Kofler, B. Galanin Message-Associated Peptide Suppresses Growth and the Budded-to-Hyphal-Form Transition of Candida albicans. Antimicrob. Agents Chemother. 2007, 51, 4167–4170. [Google Scholar] [CrossRef]
- Wilson, D.; Naglik, J.R.; Hube, B. The Missing Link between Candida albicans Hyphal Morphogenesis and Host Cell Damage. PLoS Pathog. 2016, 12, e1005867. [Google Scholar] [CrossRef]
- Murzyn, A.; Krasowska, A.; Augustyniak, D.; Majkowska-Skrobek, G.; Łukaszewicz, M.; Dziadkowiec, D. The effect of Saccharomyces boulardii on Candida albicans-infected human intestinal cell lines Caco-2 and Intestin. FEMS Microbiol. Lett. 2010, 310, 17–23. [Google Scholar] [CrossRef]
- Kurashima, Y.; Kiyono, H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu. Rev. Immunol. 2017, 35, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Reznikov, L.R. Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis. Respir. Res. 2018, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Werlang, C.; Cárcarmo-Oyarce, G.; Ribbeck, K. Engineering mucus to study and influence the microbiome. Nat. Rev. Mater. 2019, 4, 134–145. [Google Scholar] [CrossRef]
- Wine, J.J. Submucosal Glands and Airway Defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Fisher, J.T.; Lynch, T.J.; Luo, M.; Evans, T.I.; Neff, T.L.; Zhou, W.; Zhang, Y.; Ou, Y.; Bunnett, N.W.; et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J. Clin. Investig. 2011, 121, 3144–3158. [Google Scholar] [CrossRef] [PubMed]
- Tomaki, M.; Ichinose, M.; Miura, M.; Hirayama, Y.; Yamauchi, H.; Nakajima, N.; Shirato, K. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 1995, 151, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Vatrella, A.; Montagnani, S.; Calabrese, C.; Parrella, R.; Pelaia, G.; Biscione, G.L.; Corcione, N.; Marsico, S.A.; Guerra, G. Neuropeptide expression in the airways of COPD patients and smokers with normal lung function. J. Biol. Regul. Homeost. Agents 2010, 24, 425–432. [Google Scholar]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Conlin, V.S.; Wu, X.; Nguyen, C.; Dai, C.; Vallance, B.A.; Buchan, A.M.J.; Boyer, L.; Jacobson, K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am. J. Physiol. Liver Physiol. 2009, 297, G735–G750. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Sun, R.; Qiao, X.; Shang, X.; Niu, W. Modulatory Effects of Vasoactive Intestinal Peptide on Intestinal Mucosal Immunity and Microbial Community of Weaned Piglets Challenged by an Enterotoxigenic Escherichia coli (K88). PLoS ONE 2014, 9, e104183. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Złotkowska, D. Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis. Int. J. Mol. Sci. 2018, 19, 3274. [Google Scholar] [CrossRef]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nat. Cell Biol. 2013, 501, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Takayama, Y.; Kondo, T.; Ishibashi, K.-I.; Sahoo, B.R.; Kanemaru, H.; Kumagai, Y.; Martino, M.M.; Tanaka, H.; Ohno, N.; et al. Nociceptors Boost the Resolution of Fungal Osteoinflammation via the TRP Channel-CGRP-Jdp2 Axis. Cell Rep. 2017, 19, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Takayama, Y.; Sugisawa, E.; Yamanoi, Y.; Yokawa, T.; Kondo, T.; Ishibashi, K.-I.; Sahoo, B.R.; Takemura, N.; Mori, Y.; et al. The ATP Transporter VNUT Mediates Induction of Dectin-1-Triggered Candida Nociception. iScience 2018, 6, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.-Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural Transm. 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Defaye, M.; Altier, C. Neural control of gut homeostasis. Am. J. Physiol. Liver Physiol. 2020, 319, G718–G732. [Google Scholar] [CrossRef]
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015, 43, 515–526. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Baddal, B.; Haarsma, R.; O’Seaghdha, M.; Yang, N.J.; Blake, K.J.; Portley, M.; Verri, W.A.; Dale, J.B.; Wessels, M.R.; et al. Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 2018, 173, 1083–1097.e22. [Google Scholar] [CrossRef]
- Wilson, J.W.; Schurr, M.J.; Leblanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of bacterial pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef]
- Silva, M.T. Classical Labeling of Bacterial Pathogens According to Their Lifestyle in the Host: Inconsistencies and Alternatives. Front. Microbiol. 2012, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef]
- Jepson, M.A.; Clark, M. The role of M cells in Salmonella infection. Microbes Infect. 2001, 3, 1183–1190. [Google Scholar] [CrossRef]
- Hooper, L.V. Epithelial Cell Contributions to Intestinal Immunity. Adv. Immunol. 2015, 126, 129–172. [Google Scholar] [CrossRef] [PubMed]
- Raffatellu, M.; Wilson, R.P.; Chessa, D.; Andrews-Polymenis, H.; Tran, Q.T.; Lawhon, S.; Khare, S.; Adams, L.G.; Bäumler, A.J. SipA, SopA, SopB, SopD, and SopE2 Contribute to Salmonella enterica Serotype Typhimurium Invasion of Epithelial Cells. Infect. Immun. 2005, 73, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Panea, C.; Nakato, G.; Cebula, A.; Lee, C.; Diez, M.G.; Laufer, T.M.; Ignatowicz, L.; Ivanov, I.I. Segmented Filamentous Bacteria Antigens Presented by Intestinal Dendritic Cells Drive Mucosal Th17 Cell Differentiation. Immunity 2014, 40, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.J.; Olivier, M. Subversion of host cell signalling by the protozoan parasite Leishmania. Parasitology 2005, 130, S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.A.; Düsedau, H.P.; Steffen, J.; Gupta, N.; Dunay, M.P.; Toth, G.K.; Reglodi, D.; Heimesaat, M.M.; Dunay, I.R. Immunomodulatory Effects of the Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide in Acute Toxoplasmosis. Front. Cell. Infect. Microbiol. 2019, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Pagán, A.J.; Ramakrishnan, L. The Formation and Function of Granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The Granuloma in Tuberculosis: Dynamics of a Host–Pathogen Collusion. Front. Immunol. 2013, 3, 411. [Google Scholar] [CrossRef]
- Zhang, C.; Chery, S.; Lazerson, A.; Altman, N.H.; Jackson, R.; Holt, G.; Campos, M.; Schally, A.V.; Mirsaeidi, M. Anti-inflammatory effects of α-MSH through p-CREB expression in sarcoidosis like granuloma model. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Garza, A.; Tweardy, D.J.; Weinstock, J.; Viswanathan, B.; Robinson, P. Substance P Signaling Contributes to Granuloma Formation inTaenia crassicepsInfection, a Murine Model of Cysticercosis. J. Biomed. Biotechnol. 2010, 2010, 597086. [Google Scholar] [CrossRef]
- Blum, A.M.; Metwali, A.; Kim-Miller, M.; Li, J.; Qadir, K.; Elliott, D.E.; Lu, B.; Fabry, Z.; Gerard, N.; Weinstock, J.V. The substance P receptor is necessary for a normal granulomatous response in murine Schistosomiasis mansoni. J. Immunol. 1999, 162, 6080–6085. [Google Scholar]
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General Principles of Antimicrobial Therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exper. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Almeida, T.; Hernandez, M.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Jiménez, A. Association between serum substance P levels and mortality in patients with severe sepsis. J. Crit. Care 2015, 30, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Jiang, L.; Chen, L. Aberrant expression of calcitonin gene-related peptide and its correlation with prognosis in severe childhood pneumonia. Clinics 2020, 75, 131. [Google Scholar] [CrossRef]
- Robinson, P.; Okhuysen, P.C.; Chappell, C.L.; Weinstock, J.V.; Lewis, D.E.; Actor, J.K.; White, A.C., Jr. Substance P Expression Correlates with Severity of Diarrhea in Cryptosporidiosis. J. Infect. Dis. 2003, 188, 290–296. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Raj, S.; Aruna, G.; Chowdhry, S. Gingival crevicular fluid and plasma levels of neuropeptide Substance-P in periodontal health, disease and after nonsurgical therapy. J. Periodontal Res. 2009, 44, 232–237. [Google Scholar] [CrossRef]
- Andersson, D.; Hughes, D.; Kubicek-Sutherland, J. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the Immune System. Science 2001, 291, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr.; Cudic, M. Conformation of Glycopeptides. Mini-Rev. Med. Chem. 2003, 3, 703–711. [Google Scholar] [CrossRef]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1833, 2430–2437. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Orczyk-Pawiłowicz, M.; Augustyniak, D.; Hirnle, L.; Kątnik-Prastowska, I. Lectin-based analysis of fucose and sialic acid expressions on human amniotic IgA during normal pregnancy. Glycoconj. J. 2012, 30, 599–608. [Google Scholar] [CrossRef]
- Orczyk-Pawiłowicz, M.; Lis-Kuberka, J. The Impact of Dietary Fucosylated Oligosaccharides and Glycoproteins of Human Milk on Infant Well-Being. Nutrition 2020, 12, 1105. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.; Gajula, P. Recent advances in modulating the microbiome. F1000Research 2020, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Tansky, M.F.; Pothoulakis, C.; Leeman, S.E. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 10691–10696. [Google Scholar] [CrossRef] [PubMed]
- Nehring, R.B.; Richter, D.; Meyerhof, W. Glycosylation affects agonist binding and signal transduction of the rat somatostatin receptor subtype. J. Physiol. 2000, 94, 185–192. [Google Scholar] [CrossRef]
- Madsen, T.D.; Hansen, L.H.; Hintze, J.; Ye, Z.; Jebari, S.; Andersen, D.B.; Joshi, H.J.; Ju, T.; Goetze, J.P.; Martin, C.; et al. An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Hansen, L.H.; Madsen, T.D.; Goth, C.K.; Clausen, H.; Chen, Y.; Dzhoyashvili, N.; Iyer, S.R.; Sangaralingham, S.J.; Burnett, J.C., Jr.; Rehfeld, J.F.; et al. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J. Biol. Chem. 2019, 294, 12567–12578. [Google Scholar] [CrossRef]
- Hristodorov, D.; Fischer, R.; Linden, L. With or Without Sugar? (A)glycosylation of Therapeutic Antibodies. Mol. Biotechnol. 2012, 54, 1056–1068. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef]
- Borza, B.; Szigeti, M.; Szekrenyes, A.; Hajba, L.; Guttman, A. Glycosimilarity assessment of biotherapeutics 1: Quantitative comparison of the N -glycosylation of the innovator and a biosimilar version of etanercept. J. Pharm. Biomed. Anal. 2018, 153, 182–185. [Google Scholar] [CrossRef]
- Lundy, F.T.; Salmon, A.L.; Lamey, P.-J.; Shaw, C.; Linden, G.J. Carboxypeptidase-mediated metabolism of calcitonin gene-related peptide in human gingival crevicular fluid—A rôle in periodontal inflammation? J. Clin. Periodontol. 2000, 27, 499–505. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; Orr, D.F.; Yü, Y.; Coulter, W.A.; Manning, G.; Irwin, C.R.; Lundy, F.T. LL-37 in periodontal health and disease and its susceptibility to degradation by proteinases present in gingival crevicular fluid. J. Clin. Periodontol. 2013, 40, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Duperthuy, M. Antimicrobial Peptides: Virulence and Resistance Modulation in Gram-Negative Bacteria. Microorganisms 2020, 8, 280. [Google Scholar] [CrossRef]
- Ruissen, A.; Groenink, J.; Krijtenberg, P.; Walgreen-Weterings, E.; Hof, W.V.T.; Veerman, E.; Amerongen, A.N. Internalisation and Degradation of Histatin 5 by Candida albicans. Biol. Chem. 2003, 384, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Frick, I.-M.; Andersson, E.R.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Belas, R.; Manos, J.; Suvanasuthi, R. Proteus mirabilis ZapA Metalloprotease Degrades a Broad Spectrum of Substrates, Including Antimicrobial Peptides. Infect. Immun. 2004, 72, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Kooi, C.; Sokol, P.A. Burkholderia cenocepacia zinc metalloproteases influence resistance to antimicrobial peptides. Microbiology 2009, 155, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Rompikuntal, P.K.; Vdovikova, S.; Duperthuy, M.; Johnson, T.L.; Ahlund, M.K.; Lundmark, R.; Oscarsson, J.; Sandkvist, M.; Uhlin, B.E.; Wai, S.N. Outer Membrane Vesicle-Mediated Export of Processed PrtV Protease from Vibrio cholerae. PLoS ONE 2015, 10, e0134098. [Google Scholar] [CrossRef] [PubMed]
- Gattiker, A.; Gasteiger, E.; Bairoch, A. ScanProsite: A reference implementation of a PROSITE scanning tool. Appl. Bioinform. 2002, 1, 107–108. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Potempa, J.; Pike, R.N. Corruption of Innate Immunity by Bacterial Proteases. J. Innate Immun. 2008, 1, 70–87. [Google Scholar] [CrossRef]
- Kincy-Cain, T.; Bost, K.L. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J. Immunol. 1996, 157, 255–264. [Google Scholar] [PubMed]
- Foldenauer, M.E.B.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Substance P Affects Growth Factors in Pseudomonas aeruginosa–Infected Mouse Cornea. Cornea 2012, 31, 1176–1188. [Google Scholar] [CrossRef]
- McClellan, S.A.; Zhang, Y.; Barrett, R.P.; Hazlett, L.D. Substance P Promotes Susceptibility to Pseudomonas aeruginosa Keratitis in Resistant Mice: Anti-inflammatory Mediators Downregulated. Investig. Opthalmol. Vis. Sci. 2008, 49, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.A.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. VIP Promotes Resistance in the Pseudomonas aeruginosa–Infected Cornea by Modulating Adhesion Molecule Expression. Investig. Opthalmol. Vis. Sci. 2010, 51, 5776–5782. [Google Scholar] [CrossRef]
- Jiang, X.; McClellan, S.A.; Barrett, R.P.; Berger, E.A.; Zhang, Y.; Hazlett, L.D. VIP and Growth Factors in the Infected Cornea. Investig. Opthalmol. Vis. Sci. 2011, 52, 6154–6161. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, A.; Emingil, G.; Nizam, N.; Doğanavşargil, B.; Sezak, M.; Kutukculer, N.; Atilla, G. Therapeutic Efficacy of Vasoactive Intestinal Peptide in Escherichia coli Lipopolysaccharide-Induced Experimental Periodontitis in Rats. J. Periodontol. 2009, 80, 1655–1664. [Google Scholar] [CrossRef]
- Carion, T.W.; McWhirter, C.R.; Grewal, D.K.; Berger, E.A. Efficacy of VIP as Treatment for Bacteria-Induced Keratitis against Multiple Pseudomonas aeruginosa Strains. Investig. Opthalmol. Vis. Sci. 2015, 56, 6932–6940. [Google Scholar] [CrossRef]
- Bereswill, S.; Escher, U.; Grunau, A.; Kühl, A.A.; Dunay, I.R.; Tamas, A.; Reglodi, D.; Heimesaat, M.M. Pituitary Adenylate Cyclase-Activating Polypeptide—A Neuropeptide as Novel Treatment Option for Subacute Ileitis in Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Kluttz, J.M.; Bost, K.L.; Marriott, I. Prophylactic and Therapeutic Targeting of the Neurokinin-1 Receptor Limits Neuroinflammation in a Murine Model of Pneumococcal Meningitis. J. Immunol. 2011, 186, 7255–7263. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; McClellan, S.A.; Barrett, R.P.; Liu, J.; Zhang, Y.; Lighvani, S. Spantide I Decreases Type I Cytokines, Enhances IL-10, and Reduces Corneal Perforation in Susceptible Mice after Pseudomonas aeruginosa Infection. Investig. Opthalmol. Vis. Sci. 2007, 48, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lin, Q.; Tang, K.; Liu, S.; Du, Y.; Yu, X.; Li, S. Substance P participates in periodontitis by upregulating HIF-1α and RANKL/OPG ratio. BMC Oral Health 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lighvani, S.; Huang, X.; Trivedi, P.P.; Swanborg, R.H.; Hazlett, L.D. Substance P regulates natural killer cell interferon-γ production and resistance to Pseudomonas aeruginosa infection. Eur. J. Immunol. 2005, 35, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.; Vaickus, M.H.; Stein, T.D.; Lussier, B.L.; Kim, J.; Stepien, D.M.; Duffy, E.R.; Chiswick, E.L.; Remick, D.G. The Role of Substance P in Pulmonary Clearance of Bacteria in Comparative Injury Models. Am. J. Pathol. 2016, 186, 3236–3245. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Sterka, D.G.; Gray, D.L.; Bost, K.L.; Marriott, I. Neurogenic Exacerbation of Microglial and Astrocyte Responses to Neisseria meningitidis and Borrelia burgdorferi. J. Immunology 2008, 180, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.M.; Gay, J.; Mykoniatis, A.; Pan, A.; O’Brien, M.J.; Brown, D.; Karalis, K.; Pothoulakis, C. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc. Natl. Acad. Sci. USA 2004, 101, 8503–8508. [Google Scholar] [CrossRef] [PubMed]
- Kokkotou, E.; Torres, D.; Moss, A.C.; O’Brien, M.; Grigoriadis, D.E.; Karalis, K.; Pothoulakis, C. Corticotropin-Releasing Hormone Receptor 2-Deficient Mice Have Reduced Intestinal Inflammatory Responses. J. Immunol. 2006, 177, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, C.; Peng, X.-D.; Zhao, G.-Q.; Wu, Y.; Zheng, H.-R.; Wang, Q.; Xu, Q.; Jiang, N. Expression and role of calcitonin gene-related peptide in mouse Aspergillus fumigatus keratitis. Int. J. Ophthalmol. 2019, 12, 697–704. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).