Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1
Abstract
1. Introduction
2. Results
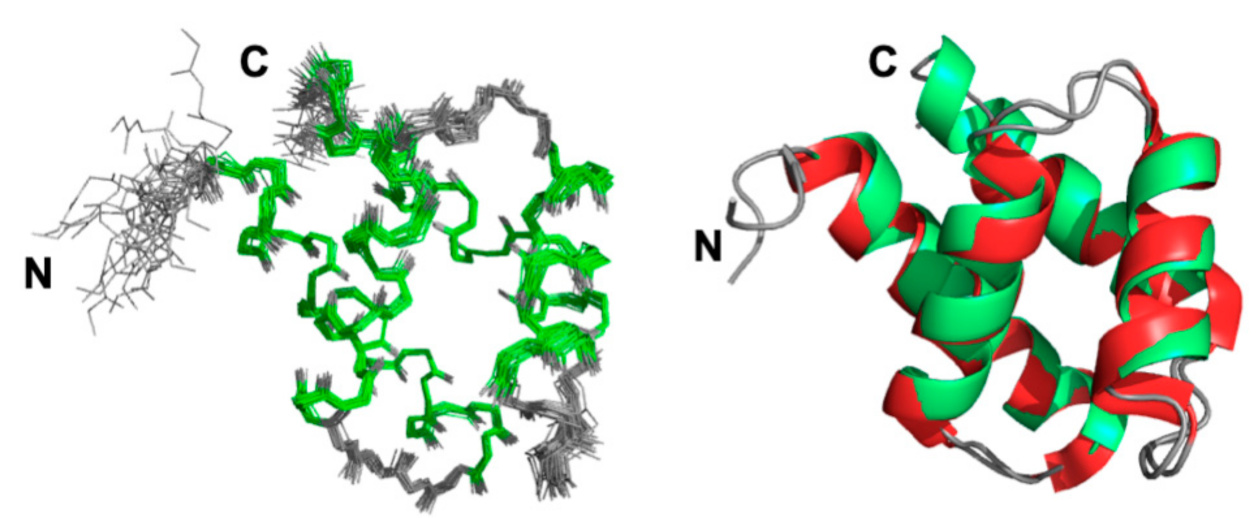
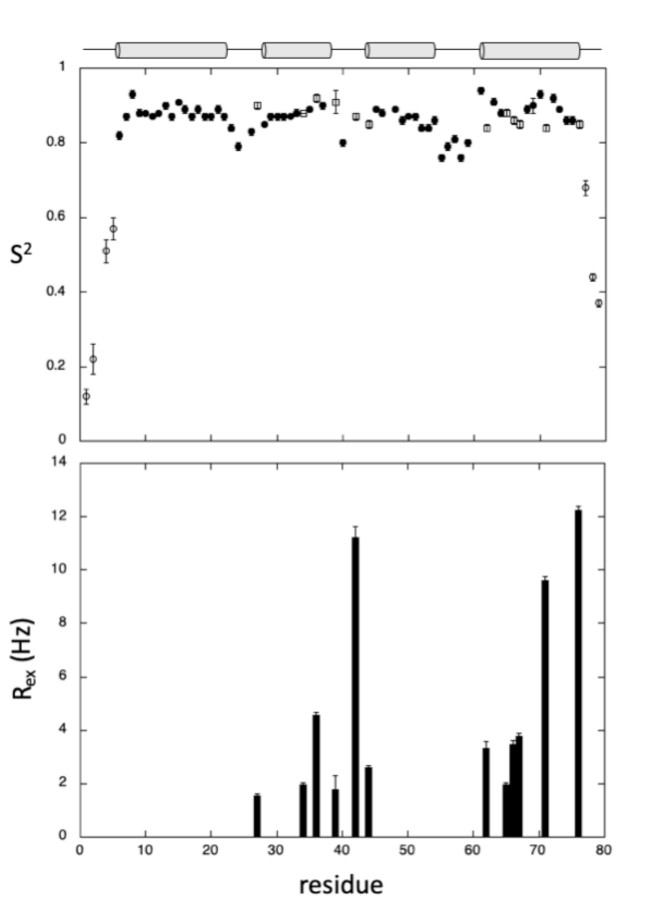
2.1. NMR Resonance Assignments and Solution Structure of GIPC1-GH2
2.2. GIPC1-GH2 Denaturation Studies
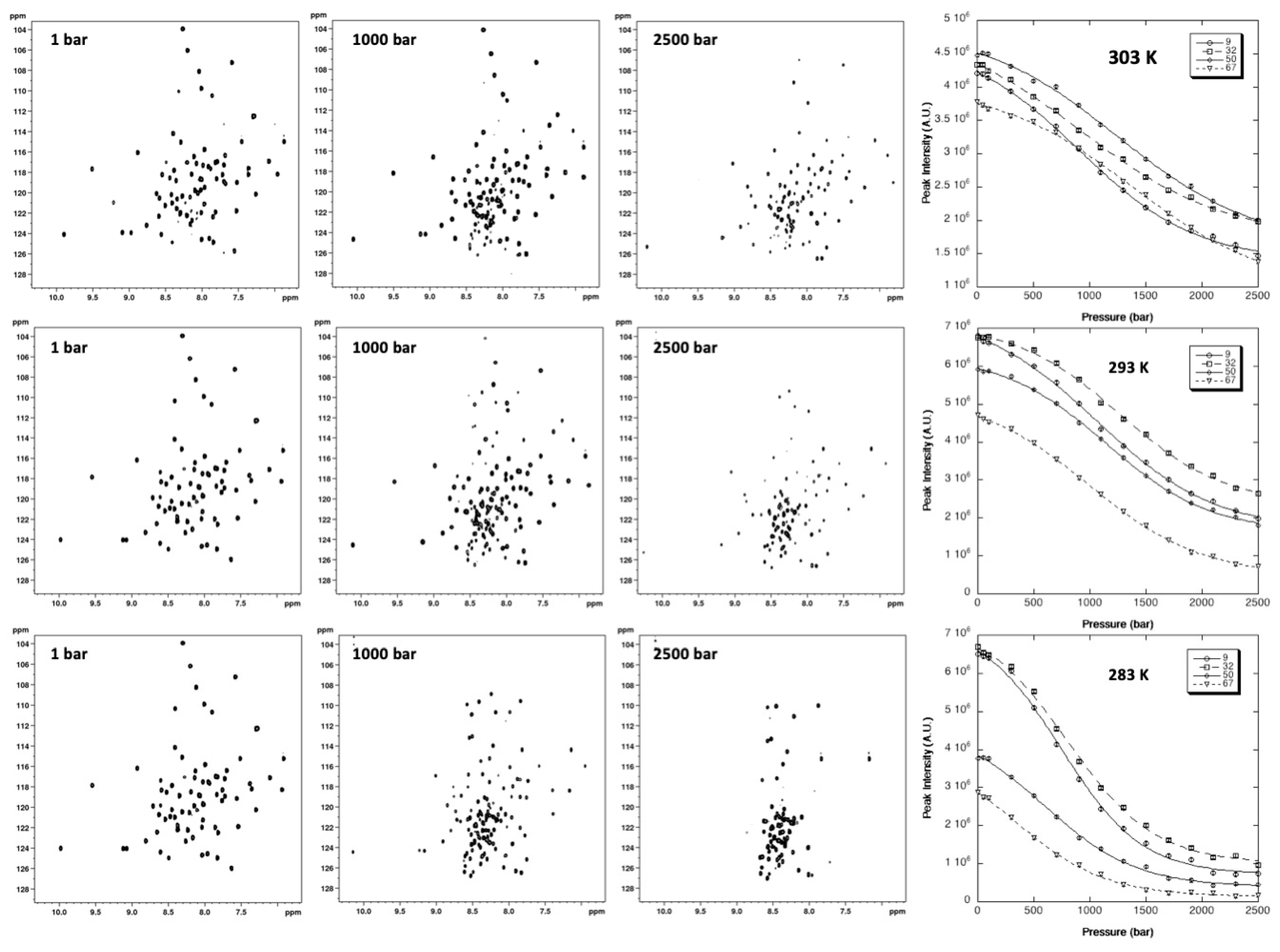
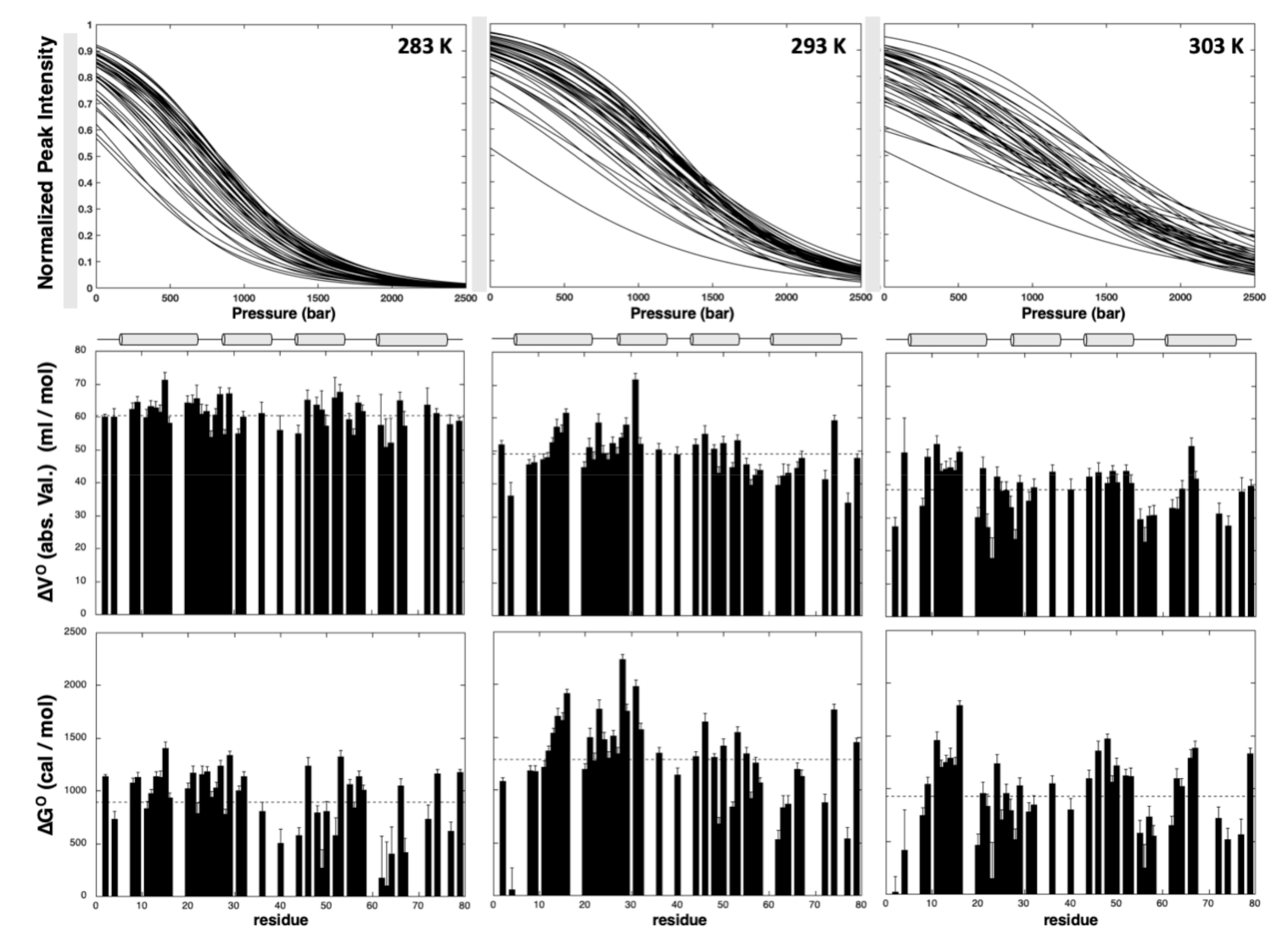
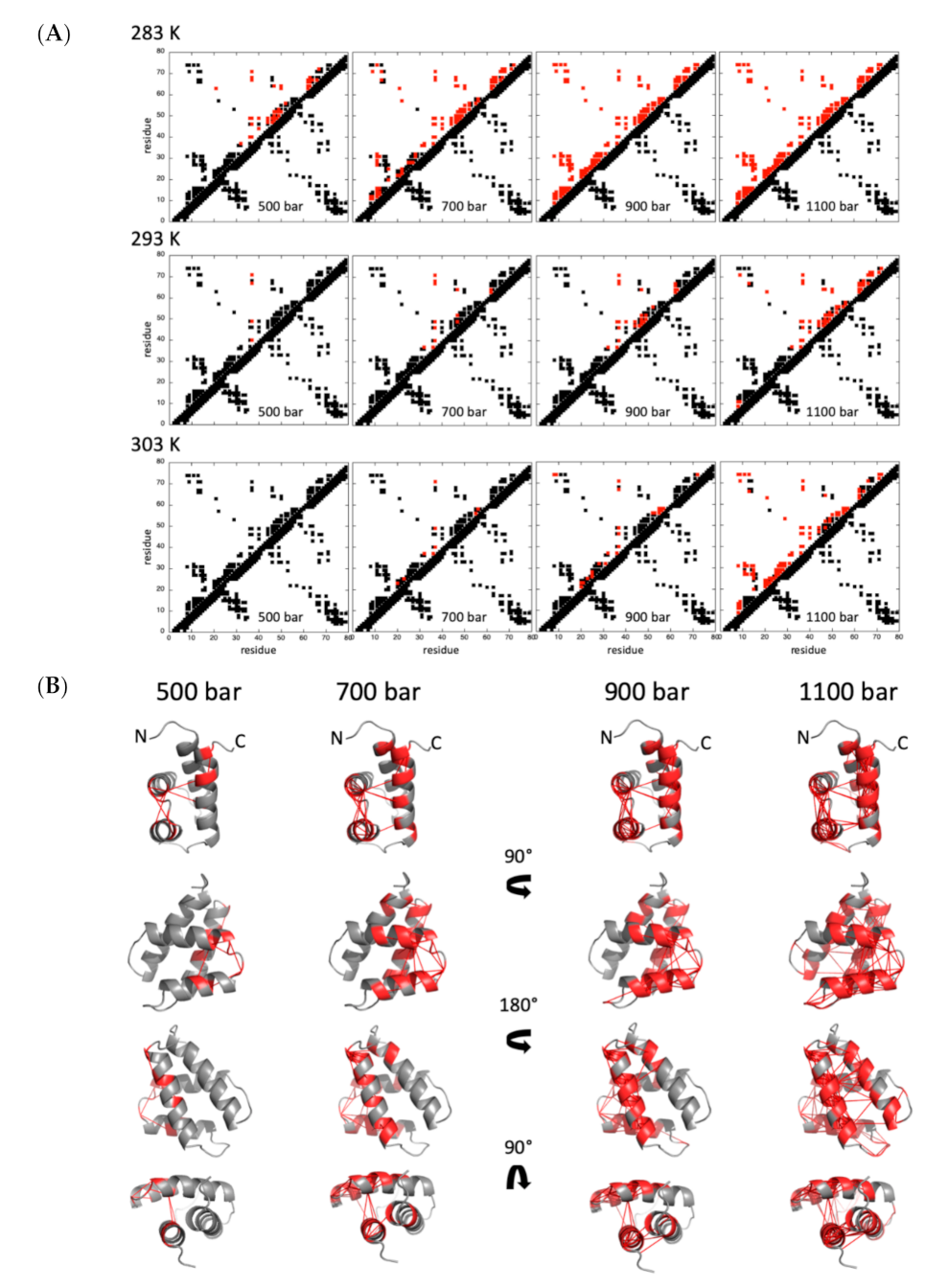
2.2.1. Pressure Denaturation
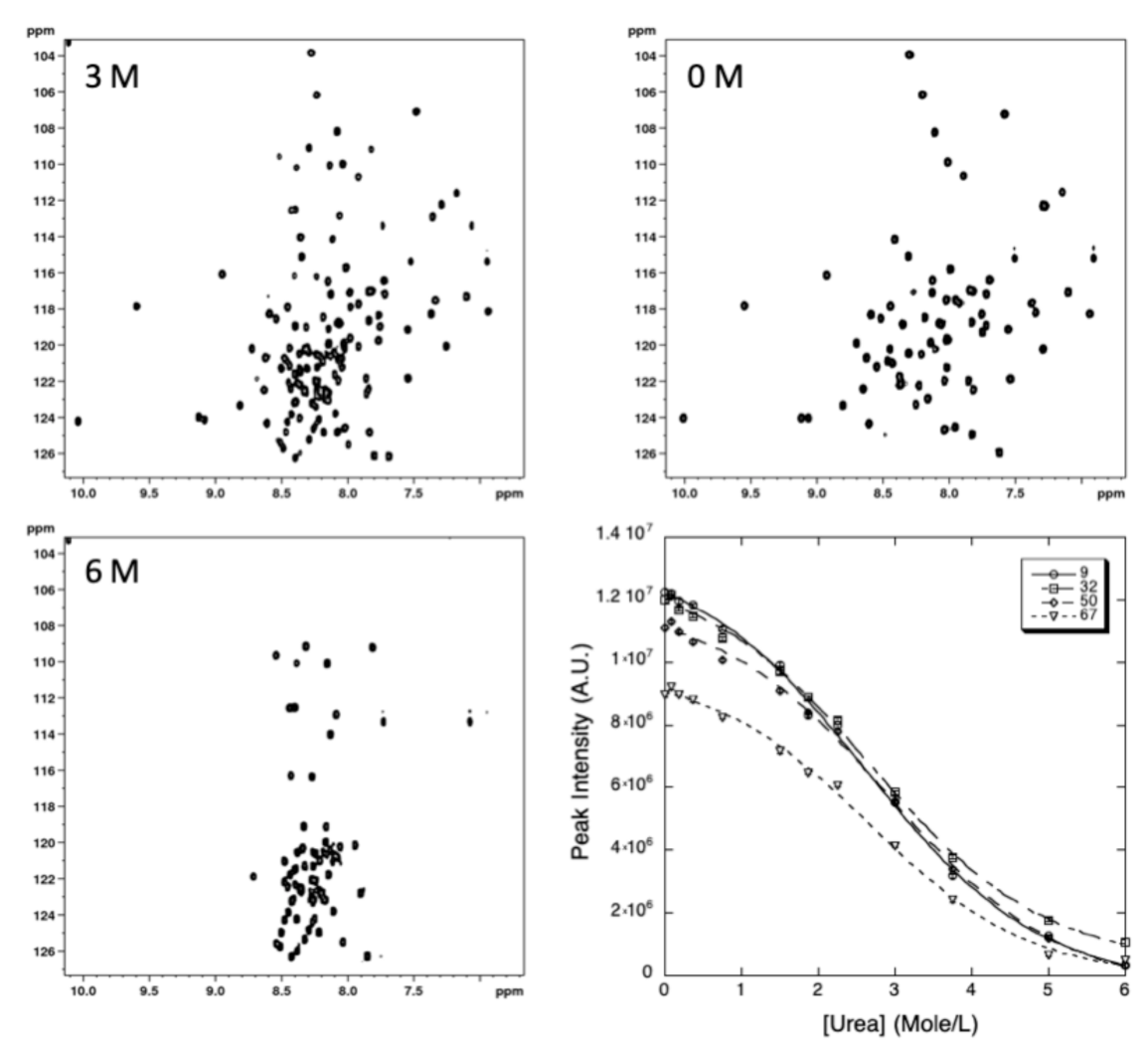
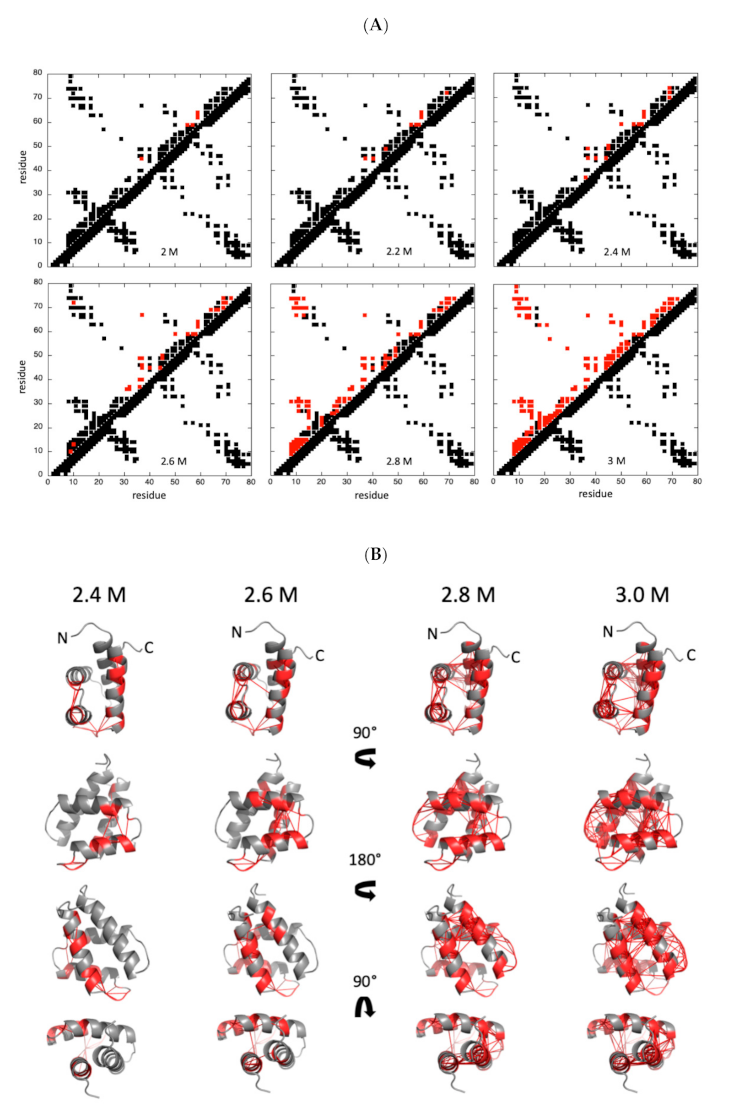
2.2.2. Chemical Denaturation
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. NMR Assignments and Solution Structure
4.3. Relaxation Studies
4.4. Protein Unfolding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclature
| GIPC1 | GAIP Interacting Protein, C-terminus 1 |
| GH2 | C-terminal GIPC-homology 2 |
| H(H)P-NMR | High (Hydrostatic) Pressure Nuclear Magnetic Resonance |
| nOe | nuclear Overhauser enhancement |
References
- Malhotra, P.; Udgaonkar, J.B. How cooperative are protein folding and unfolding transitions? Protein Sci. 2016, 25, 1924–1941. [Google Scholar] [CrossRef]
- Sosnick, T.R.; Barrick, D. The folding of single domain proteins—Have we reached a consensus? Curr. Opin. Struct. Biol. 2011, 21, 12–24. [Google Scholar] [CrossRef]
- Roche, J.; Royer, C.A.; Roumestand, C. Exploring the Protein Folding Pathway with High-Pressure NMR: Steady-State and Kinetics Studies. High Press. Biosci. 2015, 72, 261–278. [Google Scholar]
- Roche, J.; Royer, C.A.; Roumestand, C. Monitoring protein folding through high pressure NMR spectroscopy. Prog. Nucl. Magn. Reason. Spectrosc. 2017, 102, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A.; Roumestand, C. Exploring Protein Conformational Landscapes Using High-Pressure NMR. Methods Enzymol. 2019, 614, 293–320. [Google Scholar]
- Dubois, C.; Herrada, I.; Barthe, P.; Roumestand, C. Combining High-Pressure Perturbation with NMR Spectroscopy for a Structural and Dynamical Characterization of Protein Folding Pathways. Molecules 2020, 25, 5551. [Google Scholar] [CrossRef] [PubMed]
- Rouget, J.; Aksel, T.; Roche, J.; Saldana, J.; Garcia, A.E.; Barrick, D.; Royer, C.A. Size and sequence and the volume change of protein folding. J. Am. Chem. Soc. 2011, 133, 6020–6027. [Google Scholar] [CrossRef]
- Roche, J.; Caro, J.A.; Norberto, D.R.; Barthe, P.; Roumestand, C.; Schlessman, J.L.; Garcia, A.E.; García-Moreno, B.E.; Royer, C.A. Cavities determine the pressure unfolding of proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 6945–6950. [Google Scholar] [CrossRef]
- Roche, J.; Dellarole, M.J.; Caro, A.; Guca, E.; Norberto, D.R.; Yang, Y.-S.; Garcia, A.E.; Roumestand, C.; García-Moreno, B.; Royer, C.A. Remodeling of the folding free-energy landscape of staphylococcal nuclease by cavity-creating mutations. Biochemistry 2012, 51, 9535–9546. [Google Scholar] [CrossRef] [PubMed]
- Herrada, I.; Barthe, P.; Vanheusden, M.; DeGuillen, K.; Mammri, L.; Delbecq, S.; Rico, F.; Roumestand, C. Monitoring Unfolding of Titin I27 Single and Bi Domain with High-Pressure NMR Spectroscopy. Biophys. J. 2018, 115, 341–352. [Google Scholar] [CrossRef]
- Saotome, T.; Doret, M.; Kulkarni, M.; Yang, Y.-S.; Barthe, P.; Kuroda, Y.; Roumestand, C. Folding of the Ig-Like Domain of the Dengue Virus Envelope Protein Analyzed by High-Hydrostatic-Pressure NMR at a Residue-Level Resolution. Biomolecules 2019, 9, 309. [Google Scholar] [CrossRef]
- Fossat, M.J.; Dao, T.P.; Jenkins, K.; Dellarole, M.; Yang, Y.-S.; McCallum, S.A.; Garcia, A.E.; Barrick, D.; Roumestand, C.; Royer, C.A. High-Resolution Mapping of a Repeat Protein Folding Free Energy Landscape. Biophys. J. 2016, 111, 2368–2376. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Stenzoski, N.E.; Zou, J.; Peran, I.; McCallum, S.A.; Raleigh, D.P.; Royer, C.A. Pressure-Temperature Analysis of the Stability of the CTL9 Domain Reveals Hidden Intermediates. Biophys. J. 2019, 116, 445–453. [Google Scholar] [CrossRef]
- Kohn, W.D.; Mant, C.T.; Hodges, R.S. α-Helical protein assembly motifs. J. Biol. Chem. 1997, 272, 2583–2586. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Functional proteomics, human genetics and cancer biology of GIPC family members. Exp. Mol. Med. 2013, 45, e26. [Google Scholar] [CrossRef]
- Shang, G.; Brautigam, C.A.; Chen, R.; Lu, D.; Torres-Vázquez, J.; Zhang, X. Structure analyses reveal a regulated oligomerization mechanism of the PlexinD1/GIPC/myosin VI complex. eLife 2017, 6, e27322. [Google Scholar] [CrossRef]
- Shen, Y.; Bax, A. Protein backbone ans side chain torsion angles predicted from NMR chemical shifts using artificial neural networks. J. Biomol. NMR 2013, 56, 227–241. [Google Scholar] [CrossRef]
- Güntert, P. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 2004, 278, 353–378. [Google Scholar] [PubMed]
- Hwang, T.L.; Mori, S.; van Zijl, P.C. Application of phase-modulated CLEAN chemical EXchange spectroscopy(CLEANEX-PM) to detect water-protein proton exchange and intermolecular NOEs. J. Am. Chem. Soc. 1997, 119, 6203–6204. [Google Scholar] [CrossRef]
- Hwang, T.L.; van Zijl, P.C.; Mori, S. Accurate quantitation of water-amide proton exchange rates using the phase-modulated CLEAN chemical EXchange (CLEANEX-PM) approach with a Fast-HSQC (FHSQC) detection scheme. J. Biomol. NMR 1998, 11, 221–226. [Google Scholar] [CrossRef]
- Abragam, A. Principles of Nuclear Magnetism; Oxford, Science Publication, Clarendon Press: Oxford, UK, 1961. [Google Scholar]
- Lipari, G.; Szabo, A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. J. Am. Chem. Soc. 1982, 104, 4546–4559. [Google Scholar] [CrossRef]
- Clore, G.M.; Driscoll, P.C.; Wingfield, P.T.; Gronenborn, A.M. Analysis of the backbone dynamics of interleukin-1 beta using two-dimensional inverse detected heteronuclear 15N-1H NMR spectroscopy. Biochemistry 1990, 29, 7387–7401. [Google Scholar] [CrossRef] [PubMed]
- Seemann, H.; Winter, R.; Royer, C.A. Volume, expansivity and isothermal compressibility changes associated with temperature and pressure unfolding of Staphylococcal nuclease. J. Mol. Biol. 2001, 307, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, R.; Royer, C.A.; Yamada, H.; Boyer, M.; Saldana, J.L.; Akasaka, K.; Roumestand, C. Equilibrium and pressure-jump relaxation studies of the conformational transitions of P13MTCP1. J. Mol. Biol. 2002, 12, 609–628. [Google Scholar] [CrossRef]
- Baase, W.A.; Liu, L.; Tronrud, D.E.; Matthews, B.W. Lessons from the lysozyme of phage T4. Protein Sci. 2010, 19, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Shirley, B.A.; Hendricks, M.M.; Iimura, S.; Gajiwala, K.; Scholtz, J.M.; et al. Contribution of hydrophobic interactions to protein stability. J. Mol. Biol. 2011, 408, 514–528. [Google Scholar] [CrossRef]
- Shortle, D. Staphylococcal nuclease: A showcase of m-value effects. Adv. Protein Chem. 1995, 46, 217–247. [Google Scholar]
- Pons, J.L.; Malliavin, T.E.; Delsuc, M.A. Gifa V.4: A complete package for NMR data set processing. J. Biomol. NMR 1996, 8, 445–452. [Google Scholar] [CrossRef]
- Sattler, M.; Schleucher, J.; Griesinger, C. Heteronuclear multi-dimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 93–158. [Google Scholar] [CrossRef]
- Nederveen, A.J.; Doreleijers, J.F.; Vranken, W.; Miller, Z.; Spronk, C.A.; Nabuurs, S.B.; Güntert, P.; Livny, M.; Markley, J.L.; Nilges, M.; et al. RECOORD: A recalculated coordinate database of 500+ proteins from the PDB using restraints from the BioMagResBank. Proteins 2005, 59, 662–672. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Moss, D.S.; Thornton, J.M. Main-chain bond lengths and bond angles in protein structures. J. Mol. Biol. 1993, 231, 1049–1067. [Google Scholar] [CrossRef]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Delano, W.L. PyMOL: An open-source molecular graphics tool. CCP4 Newslett. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Peng, J.W.; Wagner, G. Mapping of the spectral densities of N-H bond motion in eglin c using heteronuclear relaxation experiments. Biochemistry 1992, 31, 8571–8586. [Google Scholar] [CrossRef]
- Peng, J.W.; Wagner, G. Mapping of the spectral density functions using heteronuclear NMR relaxation experiments. J. Magn. Reson. 1992, 98, 308–332. [Google Scholar]
- Kay, L.E.; Nicholson, L.K.; Delaglio, F.; Bax, A.; Torchia, D.A. Pulse sequences for removal of the effects of cross correlation between dipolar and chemical-shift anisotropy relaxation mechanisms on the measurement of heteronuclear T1 and T2 values in proteins. J. Magn. Reson. 1992, 97, 359–375. [Google Scholar] [CrossRef]
- Barthe, P.; Chiche, L.; Declerck, N.; Delsuc, M.A.; Lefèvre, J.F.; Malliavin, T.; Mispelter, J.; Stern, M.-H.; Lhoste, J.M.; Roumestand, C. Refined Solution Structure and Backbone Dynamics of 15N-Labeled C12A-p8MTCP1 Studied by NMR Relaxation. J. Biomol. NMR 1999, 15, 271–288. [Google Scholar] [CrossRef]
- Guignard, L.; Padilla, A.; Mispelter, J.; Yang, Y.-S.; Stern, M.-H.; Lhoste, J.M.; Roumestand, C. Backbone dynamics and solution structure refinement of the 15N-labeled human oncogenic protein p13MTCP1: Comparison with X-ray data. J. Biomol. NMR 2000, 17, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Auguin, D.; Barthe, P.; Augé-Sénégas, M.T.; Stern, M.H.; Noguchi, M.; Roumestand, C. Solution structure and backbone dynamics of the pleckstrin homology domain of the human protein kinase B (PKB/Akt). Interaction with inositol phosphates. J. Biomol. NMR 2004, 28, 137–155. [Google Scholar] [CrossRef]
- Farrow, N.A.; Zhang, O.; Szabo, A.; Torchia, D.A.; Kay, L.E. Spectral density function mapping using 15N relaxation data exclusively. J. Biomol. NMR 1995, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ishima, R.; Nagayama, K. Protein backbone dynamics revealed by quasi spectral density function analysis of amide N-15 nuclei. Biochemistry 1995, 34, 3162–3171. [Google Scholar] [CrossRef] [PubMed]
- Ishima, R.; Nagayama, K. Quasi-spectral-density function analysis for nitrogen-15 nuclei in proteins. J. Magn. Reson. B 1995, 108, 73–76. [Google Scholar] [CrossRef]
- Lefèvre, J.-F.; Dayie, K.T.; Peng, J.W.; Wagner, G. Internal mobility in the partially folded DNA binding and dimerization domains of GAL4: NMR analysis of the N-H spectral density functions. Biochemistry 1996, 35, 2674–2686. [Google Scholar] [CrossRef] [PubMed]
- Barthe, P.; Ropars, V.; Roumestand, C. DYNAMOF: A program for the dynamics analysis of relaxation data obtained at multiple magnetic fields. C. R. Chimie 2006, 9, 503–513. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubois, C.; Planelles-Herrero, V.J.; Tillatte-Tripodi, C.; Delbecq, S.; Mammri, L.; Sirkia, E.M.; Ropars, V.; Roumestand, C.; Barthe, P. Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1. Int. J. Mol. Sci. 2021, 22, 3597. https://doi.org/10.3390/ijms22073597
Dubois C, Planelles-Herrero VJ, Tillatte-Tripodi C, Delbecq S, Mammri L, Sirkia EM, Ropars V, Roumestand C, Barthe P. Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1. International Journal of Molecular Sciences. 2021; 22(7):3597. https://doi.org/10.3390/ijms22073597
Chicago/Turabian StyleDubois, Cécile, Vicente J. Planelles-Herrero, Camille Tillatte-Tripodi, Stéphane Delbecq, Léa Mammri, Elena M. Sirkia, Virginie Ropars, Christian Roumestand, and Philippe Barthe. 2021. "Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1" International Journal of Molecular Sciences 22, no. 7: 3597. https://doi.org/10.3390/ijms22073597
APA StyleDubois, C., Planelles-Herrero, V. J., Tillatte-Tripodi, C., Delbecq, S., Mammri, L., Sirkia, E. M., Ropars, V., Roumestand, C., & Barthe, P. (2021). Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1. International Journal of Molecular Sciences, 22(7), 3597. https://doi.org/10.3390/ijms22073597






