Immunological Prognostic Factors in Multiple Myeloma
Abstract
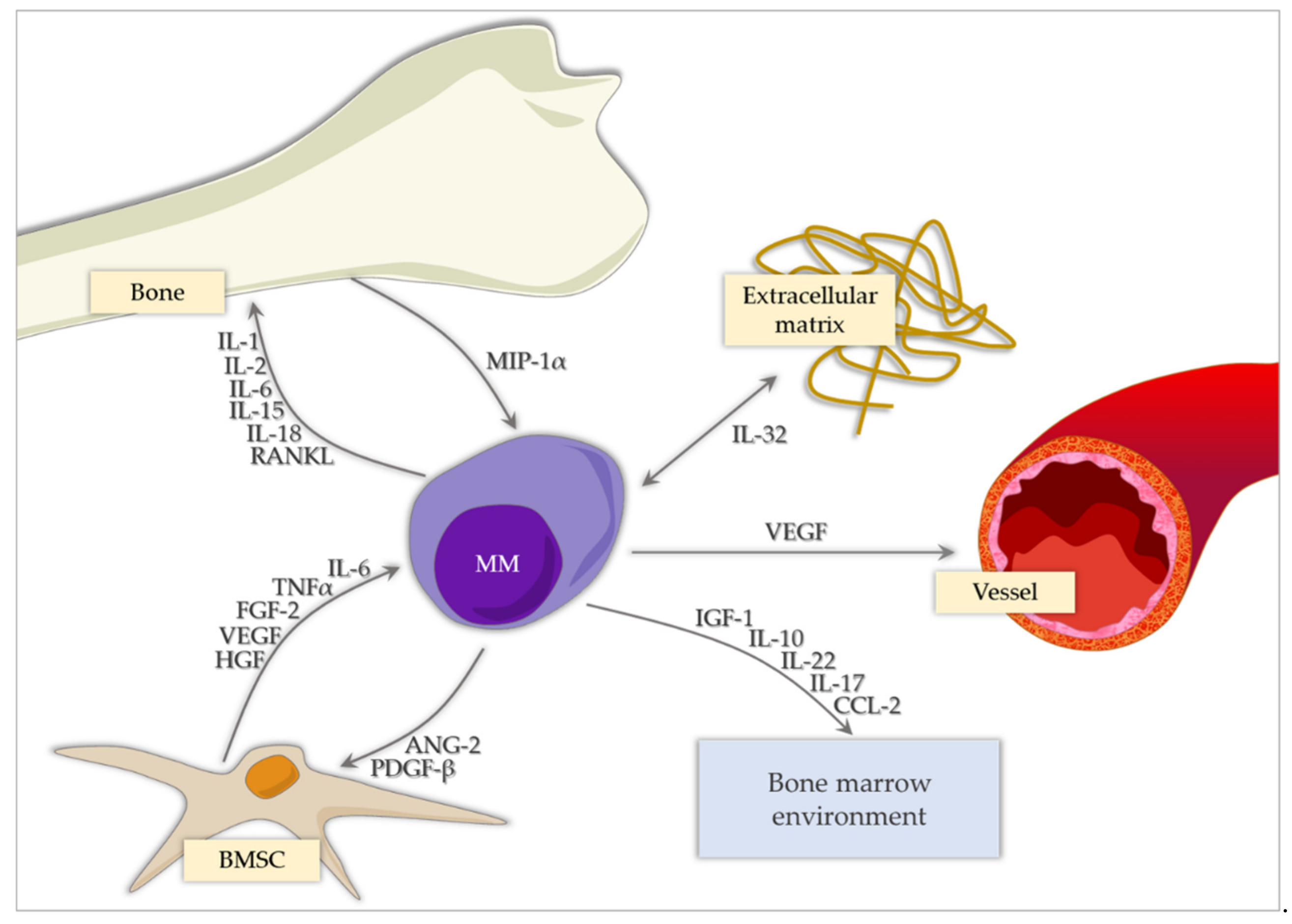
1. Introduction
2. Cytokines and Chemokines
2.1. Pro-Inflammatory Cytokines
2.1.1. IL-1
2.1.2. IL-2
2.1.3. IL-6
2.1.4. IL-8
2.1.5. IL-12
2.1.6. IL-15
2.1.7. IL-17
2.1.8. IL-18
2.1.9. IL-32
2.1.10. Tumour Necrosis Factor Family
2.1.11. Interferon-𝛾 (IFN-𝛾)
2.2. Anti-Inflammatory Cytokines
2.2.1. IL-1Ra
2.2.2. IL-4
2.2.3. IL-10
2.2.4. IL-22
2.3. Growth Factors
2.4. Chemokines
2.4.1. MIP-1 (Macrophage Inflammatory Protein-1, CCL3) and CCL2 (MCP1, Monocyte Chemoattractant Protein 1)
2.4.2. CCL2 (MCP1, Monocyte Chemoattractant Protein 1) and CCL3
3. Immune System Cells
3.1. Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR), and Platelet to Lymphocyte Ratio (PLR)
3.2. T-cell Subpopulations
3.3. Circulating Plasma Cells
4. Other Markers
4.1. Junctional Adhesion Molecule-A (JAM-A)
4.2. Autophagic Markers: Beclin-1, LC3
4.3. Immune Checkpoint Sygnalling: PD-L1
5. Further Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kehrer, M.; Koob, S.; Strauss, A.; Wirtz, D.C.; Schmolders, J. Multiples Myelom—aktuelle Standards in Diagnostik und Therapie. Z. Orthop. Unfallchir. 2017, 155, 575–586. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; Van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [CrossRef]
- Legarda, M.A.; Cejalvo, M.J.; De La Rubia, J. Recent Advances in the Treatment of Patients with Multiple Myeloma. Cancers 2020, 12, 3576. [Google Scholar] [CrossRef]
- Qian, J.; Jin, J.; Luo, H.; Jin, C.; Wang, L.; Qian, W.; Meng, H. Analysis of clinical characteristics and prognostic factors of multiple myeloma: A retrospective single-center study of 787 cases. Hematology 2017, 22, 1–5. [Google Scholar] [CrossRef][Green Version]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Ludwig, H.; Dimopoulos, M.A.; Bladé, J.; Mateos, M.V.; Rosiñol, L.; Boccadoro, M.; Cavo, M.; Lokhorst, H.; et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood 2011, 118, 4519–4529. [Google Scholar] [CrossRef]
- Hanbali, A.; Hassanein, M.; Rasheed, W.; Aljurf, M.; Alsharif, F. The Evolution of Prognostic Factors in Multiple Myeloma. Adv. Hematol. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Węgłowska, E.; Dróżdż, I.; Mikulski, D.; Jarych, D.; Ferlińska, M.; Wawrzyniak, E.; Misiewicz, M.; Smolewski, P.; Fendler, W.; et al. Cytokine and Chemokine Profile in Patients with Multiple Myeloma Treated with Bortezomib. Mediat. Inflamm. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ali, T.A.; Faiyaz, A.; Khan, O.S.; Raza, S.S.; Kulinski, M.; El Omri, H.; Bhat, A.A.; Uddin, S. Cytokine-Mediated Dysregulation of Signaling Pathways in the Pathogenesis of Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5002. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1–24. [Google Scholar] [CrossRef]
- Costes, V.; Portier, M.; Lu, Z.Y.; Rossi, J.F.; Bataille, R.; Klein, B. Interleukin-1 in multiple myeloma: Producer cells and their role in the control of IL-6 production. Br. J. Haematol. 1998, 103, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Lust, J.A.; Lacy, M.Q.; Zeldenrust, S.R.; Witzig, T.E.; Moon-Tasson, L.L.; Dinarello, C.A.; Donovan, K.A. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am. J. Hematol. 2016, 91, 571–574. [Google Scholar] [CrossRef]
- Goh, A.X.H.; Bertin-Maghit, S.; Yeo, S.P.; Ho, A.W.S.; Derks, H.; Mortellaro, A.; Wang, C.I. A novel human anti-interleukin-1β neutralizing monoclonal antibody showing in vivo efficacy. MAbs 2014, 6, 765–773. [Google Scholar] [CrossRef]
- Menssen, H.D.; Harnack, U.; Erben, U.; Neri, D.; Hirsch, B.; Dürkop, H. Antibody-based delivery of tumor necrosis factor (L19-TNFα) and interleukin-2 (L19-IL2) to tumor-associated blood vessels has potent immunological and anticancer activity in the syngeneic J558L BALB/c myeloma model. J. Cancer Res. Clin. Oncol. 2018, 144, 499–507. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T Cell Receptor γδ Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef]
- Fazzi, R.; Petrini, I.; Giuliani, N.; Morganti, R.; Carulli, G.; Palma, B.D.; Notarfranchi, L.; Galimberti, S.; Buda, G. Phase II Trial of Maintenance Treatment With IL2 and Zoledronate in Multiple Myeloma After Bone Marrow Transplantation: Biological and Clinical Results. Front. Immunol. 2021, 11, 573156. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Di Stefano, R.; Frassanito, A.; Iodice, G.; Dammacco, F. A disturbance of the IL-2/IL-2 receptor system parallels the activity of multiple myeloma. Clin. Exp. Immunol. 1991, 84, 429–434. [Google Scholar]
- Lauta, V.M. Interleukin-6 and the Network of Several Cytokines in Multiple Myeloma: An Overview of Clinical and Experimental Data. Cytokine 2001, 16, 79–86. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.H.; Liu, W.J.; Wang, W.D.; Wang, H.; Chen, X.Q.; Geng, Q.R.; Lu, Y.; Xia, Z.J. High level of soluble interleukin-2 receptor in serum predicts treatment resistance and poor progression-free survival in multiple myeloma. Ann. Hematol. 2017, 96, 2079–2088. [Google Scholar] [CrossRef]
- Lauta, V.M. A Review of the Cytokine Network in Multiple Myeloma: Diagnostic, Prognostic, and Therapeutic Implications. Cancer 2003, 97, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, X.; Zhang, Y.; Bao, C.; Zhou, Z.; Jin, J. Cytokine profiles in patients with newly diagnosed multiple myeloma: Survival is associated with IL-6 and IL-17A levels. Cytokine 2021, 138, 155358. [Google Scholar] [CrossRef] [PubMed]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine profile in multiple myeloma. Cytokine 2020, 136, 155271. [Google Scholar] [CrossRef]
- Mondello, P.; Cuzzocrea, S.; Navarra, M.; Mian, M. Bone marrow micro-environment is a crucial player for myelomagenesis and disease progression. Oncotarget 2017, 8, 20394–20409. [Google Scholar] [CrossRef]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of Interleukin-6 in the Pathogenesis of Multiple Myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Špička, I.; et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015, 90, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Brighton, T.A.; Khot, A.; Harrison, S.J.; Ghez, D.; Weiss, B.M.; Kirsch, A.; Magen, H.; Gironella, M.; Oriol, A.; Streetly, M.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3772–3775. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef]
- Stasi, R.; Brunetti, M.; Parma, A.; Di Giulio, C.; Terzoli, E.; Pagano, A. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer 1998, 82, 1860–1866. [Google Scholar] [CrossRef]
- Ohtani, K.; Ninomiya, H.; Hasegawa, Y.; Kobayashi, T.; Kojima, H.; Nagasawa, T.; Abe, T. Clinical significance of elevated soluble interleukin-6 receptor levels in the sera of patients with plasma cell dyscrasias. Br. J. Haematol. 1995, 91, 116–210. [Google Scholar] [CrossRef] [PubMed]
- Kyrtsonis, M.C.; Dedoussis, G.; Zervas, C.; Perifanis, V.; Baxevanis, C.; Stamatelou, M.; Maniatis, A.; Perifanis, V. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br. J. Haematol. 1996, 93, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Gernone, A.; Dammacco, F. Molecular alterations of IL-6R, lck and c-myc genes in transforming monoclonal gammopathies of undetermined significance. Br. J. Haematol. 1996, 93, 623–631. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Schorr, W. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 2000, 7, 178–182. [Google Scholar] [CrossRef]
- Shahzad, A.; Knapp, M.; Lang, I.; Koehler, G. Interleukin 8 (IL-8): A universal biomarker? Int. Arch. Med. 2010, 3, 11. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ghobrial, I.M.; Roodman, G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006, 34, 1289–1295. [Google Scholar] [CrossRef]
- Ang, Y. The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. 2005, 10, 853–865. [Google Scholar] [CrossRef]
- Shapiro, V.S.; Mollenauer, M.N.; Weiss, A. Endogenous CD28 expressed on myeloma cells up-regulates interleukin-8 production: Implications for multiple myeloma progression. Blood 2001, 98, 187–193. [Google Scholar] [CrossRef]
- Pellegrino, A.; Ria, R.; Di Pietro, G.; Cirulli, T.; Surico, G.; Pennisi, A.; Morabito, F.; Ribatti, D.; Vacca, A. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br. J. Haematol. 2005, 129, 248–256. [Google Scholar] [CrossRef]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Hsieh, C.; Macatonia, S.; Tripp, C.; Wolf, S.; O’Garra, A.; Murphy, K. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–549. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Stern, A.S.; Magram, J.; Presky, D.H. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996, 58, 639–654. [Google Scholar] [CrossRef]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological Properties and Clinical Application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef]
- Hjorth-Hansen, H.; Waage, A.; Börset, M. Interleukin-15 blocks apoptosis and induces proliferation of the human myeloma cell line OH-2 and freshly isolated myeloma cells. Br. J. Haematol. 1999, 106, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pappa, C.; Miyakis, S.; Tsirakis, G.; Sfiridaki, A.; Alegakis, A.; Kafousi, M.; Stathopoulos, E.; Alexandrakis, M. Serum levels of Interleukin-15 and Interleukin-10 and their correlation with proliferating cell nuclear antigen in multiple myeloma. Cytokine 2007, 37, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Prabhala, R.H.; Pelluru, D.; Fulciniti, M.; Prabhala, H.K.; Nanjappa, P.; Song, W.; Pai, C.; Amin, S.; Tai, Y.T.; Richardson, P.G.; et al. Elevated IL-17 produced by Th17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 2010, 115, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Y.; Yang, R.Y.; Li, C.; Duan, L.J. Correlation of Th17 Cells and IL-17 Level in Multiple Myeloma Patients with Pathogenesis of Multiple Myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 147–150. [Google Scholar] [PubMed]
- El-Gamal, R.; Al-Feky, M.; El Gohary, G.; Kamal, G. Interleukin-17 and interleukin-27 in newly diagnosed multiple myeloma patients: Interrelationship and correlation with leukocyte differential counts. Egypt. J. Haematol. 2016, 41, 132. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Pappa, C.A.; Miyakis, S.; Sfiridaki, A.; Kafousi, M.; Alegakis, A.; Stathopoulos, E.N. Serum interleukin-17 and its relationship to angiogenic factors in multiple myeloma. Eur. J. Intern. Med. 2006, 17, 412–416. [Google Scholar] [CrossRef]
- Song, X.N.; Yang, J.Z.; Sun, L.X.; Meng, J.B.; Zhang, J.Q.; Lv, H.Y.; Kong, L.J. Expression levels of IL-27 and IL-17 in multiple myeloma patients: A higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leuk. Res. 2013, 37, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Vacca, A.; Ribatti, D. A Comprehensive Biological and Clinical Perspective Can Drive a Patient-Tailored Approach to Multiple Myeloma: Bridging the Gaps between the Plasma Cell and the Neoplastic Niche. J. Oncol. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kassem, S.; Cleynen, A.; Chrétien, M.L.; Guillerey, C.; Putz, E.M.; Bald, T.; Förster, I.; Vuckovic, S.; Hill, G.R.; et al. Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer Cell 2018, 33, 634–648.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Yan, Z.; Li, H.; Chen, L.; Zhang, Z.; Fan, G.; Xu, K.; Li, Z. Dysregulation of the NLRP3 inflammasome complex and related cytokines in patients with multiple myeloma. Hematology 2015, 21, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.; Passam, F.; Sfiridaki, K.; Moschandrea, J.; Pappa, C.; Liapi, D.; Petreli, E.; Roussou, P.; Kyriakou, D. Interleukin-18 in multiple myeloma patients: Serum levels in relation to response to treatment and survival. Leuk. Res. 2004, 28, 259–266. [Google Scholar] [CrossRef]
- Lin, X.; Yang, L.; Wang, G.; Zi, F.; Yan, H.; Guo, X.; Chen, J.; Chen, Q.; Huang, X.; Li, Y.; et al. Interleukin-32α promotes the proliferation of multiple myeloma cells by inducing production of IL-6 in bone marrow stromal cells. Oncotarget 2017, 8, 92841–92854. [Google Scholar] [CrossRef]
- Yan, H.; Dong, M.; Liu, X.; Shen, Q.; He, D.; Huang, X.; Zhang, E.; Lin, X.; Chen, Q.; Guo, X.; et al. Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. 2019, 446, 38–48. [Google Scholar] [CrossRef]
- Zahoor, M.; Westhrin, M.; Aass, K.R.; Moen, S.H.; Misund, K.; Psonka-Antonczyk, K.M.; Giliberto, M.; Buene, G.; Sundan, A.; Waage, A.; et al. Hypoxia promotes IL-32 expression in myeloma cells, and high expression is associated with poor survival and bone loss. Blood Adv. 2017, 1, 2656–2666. [Google Scholar] [CrossRef]
- Wang, G.; Ning, F.Y.; Wang, J.H.; Yan, H.M.; Kong, H.W.; Zhang, Y.T.; Shen, Q. Expression of interleukin-32 in bone marrow of patients with myeloma and its prognostic significance. World J. Clin. Cases 2019, 7, 4234–4244. [Google Scholar] [CrossRef]
- Jourdan, M.; Tarte, K.; Legouffe, E.; Brochier, J.; Rossi, J.F.; Klein, B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur. Cytokine Netw. 1999, 10, 65–70. [Google Scholar]
- Neben, K.; Mytilineos, J.; Moehler, T.M.; Preiss, A.; Kraemer, A.; Ho, A.D.; Opelz, G.; Goldschmidt, H. Polymorphisms of the tumor necrosis factor-α gene promoter predict for outcome after thalidomide therapy in relapsed and refractory multiple myeloma. Blood 2002, 100, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Hou, J.; Zhao, Y.; Ke, X.; Wang, Z.; Qiu, L.; Xi, H.; Wang, F.; Wei, N.; Liu, Y.; et al. A multicenter, open-label phase II study of recombinant CPT (Circularly Permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am. J. Hematol. 2014, 89, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Hideshima, T.; Chauhan, D.; Schlossman, R.; Richardson, P.; Anderson, K.C. The role of tumor necrosis factor α in the pathophysiology of human multiple myeloma: Therapeutic applications. Oncogene 2001, 20, 4519–4527. [Google Scholar] [CrossRef]
- Dmoszynska, A.; Bojarska-Junak, A.; Domanski, D.; Roliński, J.; Hus, M.; Soroka-Wojtaszko, M. Production of Proangiogenic Cytokines During Thalidomide Treatment of Multiple Myeloma. Leuk. Lymphoma 2002, 43, 401–406. [Google Scholar] [CrossRef]
- Kawano, Y.; Moschetta, M.; Manier, S.; Glavey, S.; Görgün, G.T.; Roccaro, A.M.; Anderson, K.C.; Ghobrial, I.M. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 2015, 263, 160–172. [Google Scholar] [CrossRef]
- Jurišić, V.; Colovic, M. Correlation of Sera TNF-α with Percentage of Bone Marrow Plasma Cells, LDH, β2-Microglobulin, and Clinical Stage in Multiple Myeloma. Med Oncol. 2002, 19, 133–140. [Google Scholar] [CrossRef]
- Wang, X.S.; Shi, Q.; Williams, L.A.; Shah, N.D.; Mendoza, T.R.; Cohen, E.N.; Reuben, J.M.; Cleeland, C.S.; Orlowski, R.Z. Longitudinal analysis of patient-reported symptoms post-autologous stem cell transplant and their relationship to inflammation in patients with multiple myeloma. Leuk. Lymphoma 2014, 56, 1335–1341. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Rème, T.; Lugagne, C.; Moine, P.; Rossi, J.F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef]
- Fragioudaki, M.; Tsirakis, G.; Pappa, C.; Aristeidou, I.; Tsioutis, C.; Alegakis, A.; Kyriakou, D.; Stathopoulos, E.; Alexandrakis, M. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk. Res. 2012, 36, 1004–1008. [Google Scholar] [CrossRef]
- Pan, J.; Sun, Y.; Zhang, N.; Li, J.; Ta, F.; Wei, W.; Yu, S.; Ai, L. Characteristics of BAFF and APRIL factor expression in multiple myeloma and clinical significance. Oncol. Lett. 2017, 14, 2657–2662. [Google Scholar] [CrossRef]
- Neri, P.; Kumar, S.; Fulciniti, M.T.; Vallet, S.; Chhetri, S.; Mukherjee, S.; Tai, Y.; Chauhan, D.; Tassone, P.; Venuta, S.; et al. Neutralizing B-Cell–Activating Factor Antibody Improves Survival and Inhibits Osteoclastogenesis in a Severe Combined Immunodeficient Human Multiple Myeloma Model. Clin. Cancer Res. 2007, 13, 5903–5909. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Roussou, P.; Pappa, C.A.; Messaritakis, I.; Xekalou, A.; Goulidaki, N.; Boula, A.; Tsirakis, G. Relationship between Circulating BAFF Serum Levels with Proliferating Markers in Patients with Multiple Myeloma. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- González-Suárez, E.; Sanz-Moreno, A. RANK as a therapeutic target in cancer. FEBS J. 2016, 283, 2018–2033. [Google Scholar] [CrossRef]
- Renema, N.; Navet, B.; Heymann, M.F.; Lezot, F.; Heymann, D. RANK-RANKL Signalling in Cancer. Biosci. Rep. 2016, 36, e00366. [Google Scholar] [CrossRef]
- Sordillo, E.M.; Pearse, R.N. RANK-Fc: A therapeutic antagonist for RANK-L in myeloma. Cancer 2003, 97, 802–812. [Google Scholar] [CrossRef]
- Jakob, C.; Goerke, A.; Terpos, E.; Sterz, J.; Heider, U.; Kühnhardt, D.; Ziefle, S.; Kleeberg, L.; Mieth, M.; Von Metzler, I.; et al. Serum Levels of Total-RANKL in Multiple Myeloma. Clin. Lymphoma Myeloma 2009, 9, 430–435. [Google Scholar] [CrossRef]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor κB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef]
- Spanoudakis, E.; Papoutselis, M.; Terpos, E.; Dimopoulos, M.A.; Tsatalas, C.; Margaritis, D.; Rahemtulla, A.; Kotsianidis, I.; Karadimitris, A. Overexpression of RANKL by invariant NKT cells enriched in the bone marrow of patients with multiple myeloma. Blood Cancer J. 2016, 6, e500. [Google Scholar] [CrossRef]
- Palumbo, A.; Bruno, B.; Boccadoro, M.; Pileri, A. Interferon-γ in Multiple Myeloma. Leuk. Lymphoma 1995, 18, 215–219. [Google Scholar] [CrossRef]
- Bladé, J.; Esteve, J. Viewpoint on the Impact of Interferon in the Treatment of Multiple Myeloma: Benefit for a Small Proportion of Patients? Med. Oncol. 2000, 17, 77–84. [Google Scholar] [CrossRef]
- Kamińska, T.; Dmoszyńska, A.; Cioch, M.; Hus, I.; Jawniak, D.; Szuster-Ciesielska, A.; Kandefer-Szerszeń, M. Interferon gamma as immunomodulator in a patient with multiple myeloma. Arch. Immunol. Ther. Exp. 1999, 47, 107–112. [Google Scholar]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Zhao, M.; Flynt, F.L.; Hong, M.; Chen, H.; Gilbert, C.A.; Briley, N.T.; Bolick, S.C.; Wright, K.L.; Piskurich, J.F. MHC class II transactivator (CIITA) expression is upregulated in multiple myeloma cells by IFN-γ. Mol. Immunol. 2007, 44, 2923–2932. [Google Scholar] [CrossRef]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef]
- Treon, S.P.; Pilarski, L.M.; Belch, A.R.; Kelliher, A.; Preffer, F.I.; Shima, Y.; Mitsiades, C.S.; Mitsiades, N.S.; Szczepek, A.J.; Ellman, L.; et al. CD20-Directed Serotherapy in Patients With Multiple Myeloma: Biologic Considerations and Therapeutic Applications. J. Immunother. 2002, 25, 72–81. [Google Scholar] [CrossRef]
- Yigit, M.; Değirmencioğlu, S.; Ugurlu, E.; Yaren, A. Effect of serum interleukin-1 receptor antagonist level on survival of patients with non-small cell lung cancer. Mol. Clin. Oncol. 2017, 6, 708–712. [Google Scholar] [CrossRef]
- Lust, J.A.; Lacy, M.Q.; Zeldenrust, S.R.; Dispenzieri, A.; Gertz, M.A.; Witzig, T.E.; Kumar, S.; Hayman, S.R.; Russell, S.J.; Buadi, F.K.; et al. Induction of a Chronic Disease State in Patients With Smoldering or Indolent Multiple Myeloma by Targeting Interleukin 1β-Induced Interleukin 6 Production and the Myeloma Proliferative Component. Mayo Clin. Proc. 2009, 84, 114–122. [Google Scholar] [CrossRef]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef]
- Cao, Y.; Luetkens, T.; Kobold, S.; Hildebrandt, Y.; Gordic, M.; Lajmi, N.; Meyer, S.; Bartels, K.; Zander, A.R.; Bokemeyer, C.; et al. The cytokine/chemokine pattern in the bone marrow environment of multiple myeloma patients. Exp. Hematol. 2010, 38, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Jarych, D.; Mikulski, D.; Dróżdż, I.; Węgłowska, E.; Kotkowska, A.; Misiewicz, M.; Smolewski, P.; Stawiski, K.; Fendler, W.; et al. The Prognostic Value of Whole-Blood PSMB5, CXCR4, POMP, and RPL5 mRNA Expression in Patients with Multiple Myeloma Treated with Bortezomib. Cancers 2021, 13, 951. [Google Scholar] [CrossRef]
- Donovan, K.A.; Moon-Tasson, L.L.; Lust, J.A. Interplay Between IL-1, IL-6 and IL-17 in IL-1 Receptor Antagonist (IL-1Ra) Treated Multiple Myeloma Patients. Blood 2012, 120, 1874. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Naz, S.; Mesaik, M.A. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev. 2016, 32, 3–15. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Qin, Z. Paradoxical Roles of IL-4 in Tumor Immunity. Cell. Mol. Immunol. 2009, 6, 415–422. [Google Scholar] [CrossRef]
- Howard, M.; O’Garra, A.; Ishida, H.; Malefyt, R.D.W.; De Vries, J. Biological properties of interleukin 10. J. Clin. Immunol. 1992, 12, 239–247. [Google Scholar] [CrossRef]
- Shekarriz, R.; Janbabaei, G.; Kenari, S.A. Prognostic Value of IL-10 and Its Relationship with Disease Stage in Iranian Patients with Multiple Myeloma. Asian Pac. J. Cancer Prev. 2018, 19, 27–32. [Google Scholar]
- Raja, K.R.M.; Kubiczkova, L.; Říhová, L.; Piskacek, M.; Všianská, P.; Hézová, R.; Pour, L.; Hajek, R. Functionally Suppressive CD8 T Regulatory Cells Are Increased in Patients with Multiple Myeloma: A Cause for Immune Impairment. PLoS ONE 2012, 7, e49446. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Goulidaki, N.; Pappa, C.A.; Boula, A.; Psarakis, F.; Neonakis, I.; Tsirakis, G. Interleukin-10 Induces Both Plasma Cell Proliferation and Angiogenesis in Multiple Myeloma. Pathol. Oncol. Res. 2015, 21, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Chi, P.D.; Wang, W.D.; Chen, X.Q.; Geng, Q.R.; Xia, Z.J.; Lu, Y. High level of interleukin-10 in serum predicts poor prognosis in multiple myeloma. Br. J. Cancer 2016, 114, 463–468. [Google Scholar] [CrossRef]
- Tsirakis, G.; Pappa, C.A.; Kolovou, A.; Kokonozaki, M.; Neonakis, I.; Alexandrakis, M.G. Clinical significance of interleukin-22 in multiple myeloma. Hematology 2014, 20, 143–147. [Google Scholar] [CrossRef]
- De La Puente, P.; Muz, B.; Azab, F.; Azab, A.K. Cell Trafficking of Endothelial Progenitor Cells in Tumor Progression. Clin. Cancer Res. 2013, 19, 3360–3368. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D. Bone marrow angiogenesis in multiple myeloma. Leukemia 2005, 20, 193–199. [Google Scholar] [CrossRef]
- De La Puente, P.; Muz, B.; Azab, F.; Luderer, M.; Azab, A.K. Molecularly Targeted Therapies in Multiple Myeloma. Leuk. Res. Treat. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Späth, F.; Wibom, C.; Krop, E.J.M.; Santamaria, A.I.; Johansson, A.S.; Bergdahl, I.A.; Hultdin, J.; Vermeulen, R.; Melin, B. Immune marker changes and risk of multiple myeloma: A nested case-control study using repeated pre-diagnostic blood samples. Haematology 2019, 104, 2456–2464. [Google Scholar] [CrossRef]
- Vermeulen, R.; Hosnijeh, F.S.; Bodinier, B.; Portengen, L.; Liquet, B.; Garrido-Manriquez, J.; Lokhorst, H.; Bergdahl, I.A.; Kyrtopoulos, S.A.; Johansson, A.; et al. Pre-diagnostic blood immune markers, incidence and progression of B-cell lymphoma and multiple myeloma: Univariate and functionally informed multivariate analyses. Int. J. Cancer 2018, 143, 1335–1347. [Google Scholar] [CrossRef]
- Korc, M.; Friesel, R.E. The Role of Fibroblast Growth Factors in Tumor Growth. Curr. Cancer Drug Targets 2009, 9, 639–651. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Pappa, C.A.; Kokonozaki, M.; Boula, A.; Vyzoukaki, R.; Staphylaki, D.; Papadopoulou, A.; Androulakis, N.; Tsirakis, G.; Sfiridaki, A. Circulating serum levels of IL-20 in multiple myeloma patients: Its significance in angiogenesis and disease activity. Med Oncol. 2015, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, T.; Butrym, A.; Łacina, P.; Rybka, J.; Gębura, K.; Mazur, G.; Bogunia-Kubik, K. BFGF Polymorphism Is Associated with Disease Progression and Response to Chemotherapy in Multiple Myeloma Patients. Anticancer Res. 2017, 37, 1799–1804. [Google Scholar] [CrossRef]
- Clinical Trials.gov. A Phase 2 Trial of MP0250 Plus Bortezomib + Dexamethasone in Patients With Multiple Myeloma. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03136653?term=VEGF&recrs=e&cond=Multiple+Myeloma&draw=2&rank=1 (accessed on 6 March 2021).
- Clinical Trials.gov. Efficacy Study of ZD6474 to Treat Multiple Myeloma Cancer. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT00047788?term=VEGF&recrs=e&cond=Multiple+Myeloma&draw=2&rank=2 (accessed on 6 March 2021).
- Clinical Trials.gov. Bevacizumab, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Stage II or III Multiple Myeloma. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT00410605?term=VEGF&recrs=e&cond=Multiple+Myeloma&draw=2&rank=3 (accessed on 6 March 2021).
- Clinical Trials.gov. Lenalidomide in Combination With Bevacizumab, Sorafenib, Temsirolimus, or 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX). 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT01183663?term=VEGF&recrs=e&cond=Multiple+Myeloma&draw=2&rank=10 (accessed on 6 March 2021).
- Ganesan, P.; Piha-Paul, S.; Naing, A.; Falchook, G.; Wheler, J.; Fu, S.; Hong, D.S.; Kurzrock, R.; Janku, F.; Laday, S.; et al. Phase I clinical trial of lenalidomide in combination with sorafenib in patients with advanced cancer. Investig. New Drugs 2013, 32, 279–286. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Imaging in MGUS, SMM and MM. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01237054?term=VEGF&recrs=e&cond=Multiple+Myeloma&draw=2&rank=7 (accessed on 6 March 2021).
- Bhutani, M.; Turkbey, B.; Tan, E.; Korde, N.; Kwok, M.; Manasanch, E.E.; Tageja, N.; Mailankody, S.; Roschewski, M.; Mulquin, M.; et al. Bone marrow abnormalities and early bone lesions in multiple myeloma and its precursor disease: A prospective study using functional and morphologic imaging. Leuk. Lymphoma 2016, 57, 1114–1121. [Google Scholar] [CrossRef][Green Version]
- Bhutani, M.; Turkbey, B.; Tan, E.; Kemp, T.J.; Pinto, L.A.; Berg, A.R.; Korde, N.; Minter, A.R.; Weiss, B.M.; Mena, E.; et al. Bone marrow angiogenesis in myeloma and its precursor disease: A prospective clinical trial. Leukemia 2013, 28, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Derksen, P.W.B.; De Gorter, D.J.J.; Meijer, H.P.; Bende, R.J.; Van Dijk, M.; Lokhorst, H.M.; Bloem, A.C.; Spaargaren, M.; Pals, S.T. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 2003, 17, 764–774. [Google Scholar] [CrossRef]
- Borset, M.; Hjorth, H.H.; Seidel, C.; Sundan, A.; Waage, A. Hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group. Blood 1988, 91, 806–812. [Google Scholar]
- Børset, M.; Seidel, C.; Hjorth-Hansen, H.; Waage, A.; Sundan, A. The Role of Hepatocyte Growth Factor and its Receptor C-Met in Multiple Myeloma and Other Blood Malignancies. Leuk. Lymphoma 1999, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.O.; Sahin, B.; Günesacar, R.; Unsal, C. Clinical significance of hepatocyte growth factor, platelet-derived growth factor-AB, and transforming growth factor-α in bone marrow and peripheral blood of patients with multiple myeloma. Adv. Ther. 2006, 23, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Tsirakis, G.; Pappa, C.A.; Kanellou, P.; Stratinaki, M.A.; Xekalou, A.; Psarakis, F.E.; Sakellaris, G.; Alegakis, A.; Stathopoulos, E.N.; Alexandrakis, M.G. Role of platelet-derived growth factor-AB in tumour growth and angiogenesis in relation with other angiogenic cytokines in multiple myeloma. Hematol. Oncol. 2011, 30, 131–136. [Google Scholar] [CrossRef]
- Belloni, D.; Marcatti, M.; Ponzoni, M.; Ciceri, F.; Veschini, L.; Corti, A.; Cappio, F.C.; Ferrarini, M.; Ferrero, E. Angiopoietin-2 in Bone Marrow milieu promotes Multiple Myeloma-associated angiogenesis. Exp. Cell Res. 2015, 330, 1–12. [Google Scholar] [CrossRef]
- Giuliani, N.; Colla, S.; Lazzaretti, M.; Sala, R.; Roti, G.; Mancini, C.; Bonomini, S.; Lunghi, P.; Hojden, M.; Genestreti, G.; et al. Proangiogenic properties of human myeloma cells: Production of angiopoietin-1 and its potential relationship to myeloma-induced angiogenesis. Blood 2003, 102, 638–645. [Google Scholar] [CrossRef]
- Terpos, E.; Matsaridis, D.; Koutoulidis, V.; Zagouri, F.; Christoulas, D.; Fontara, S.; Panourgias, E.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.A.; et al. Dynamic contract -enhanced magnetic resonance imaging parameters correlate with advanced revised-ISS and angiopoietin-1/angiopoietin-2 ratio in patients with multiple myeloma. Ann. Hematol. 2017, 96, 1707–1714. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Mitsiades, N.; Poulaki, V.; Schlossman, R.; Akiyama, M.; Chauhan, D.; Hideshima, T.; Treon, S.P.; Munshi, N.C.; Richardson, P.G.; et al. Activation of NF-κB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: Therapeutic implications. Oncogene 2002, 21, 5673–5683. [Google Scholar] [CrossRef]
- Xu, F.; Gardner, A.; Tu, Y.; Michl, P.; Prager, D.; Lichtenstein, A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. Br. J. Haematol. 1997, 97, 429–440. [Google Scholar] [CrossRef]
- Menu, E.; Kooijman, R.; Van Valckenborgh, E.; Asosingh, K.; Bakkus, M.; Van Camp, B.; Vanderkerken, K. Specific roles for the PI3K and the MEK–ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: Study in the 5T33MM model. Br. J. Cancer 2004, 90, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Vanderkerken, K.; Asosingh, K.; Braet, F.; Van Riet, I.; Van Camp, B. Insulin-Like Growth Factor-1 Acts as a Chemoattractant Factor for 5T2 Multiple Myeloma Cells. Blood 1999, 93, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Podar, K.; Catley, L.; Tseng, Y.H.; Akiyama, M.; Shringarpure, R.; Burger, R.; Hideshima, T.; Chauhan, D.; Mitsiades, N.; et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidyli-nositol 3′-kinase/AKT signaling. Cancer Res. 2003, 63, 5850–5858. [Google Scholar] [PubMed]
- Qiang, Y.W.; Yao, L.; Tosato, G.; Rudikoff, S. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood 2004, 103, 301–308. [Google Scholar] [CrossRef]
- Peng, Y.; Li, F.; Zhang, P.; Wang, X.; Shen, Y.; Feng, Y.; Jia, Y.; Zhang, R.; Hu, J.; He, A. IGF-1 promotes multiple myeloma progression through PI3K/Akt-mediated epithelial-mesenchymal transition. Life Sci. 2020, 249, 117503. [Google Scholar] [CrossRef]
- Terpos, E.; Politou, M.; Viniou, N.; Rahemtulla, A. Significance of macrophage inflammatory protein-1 alpha (MIP-1α) in multiple myeloma. Leuk. Lymphoma 2005, 46, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Politou, M.; Szydlo, R.; Goldman, J.M.; Apperley, J.F.; Rahemtulla, A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1α) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br. J. Haematol. 2003, 123, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Hiura, K.; Wilde, J.; Moriyama, K.; Hashimoto, T.; Ozaki, S.; Wakatsuki, S.; Kosaka, M.; Kido, S.; Inoue, D.; et al. Role for macrophage inflammatory protein (MIP)-1aplha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood 2002, 100, 2195–2202. [Google Scholar] [CrossRef]
- Oyajobi, B.O.; Mundy, G.R. Receptor activator of NF-?B ligand, macrophage inflammatory protein-1α, and the proteasome. Cancer 2003, 97, 813–817. [Google Scholar] [CrossRef]
- Arendt, B.K.; Miller, A.L.; Arora, T.; Tschumper, R.C.; Jelinek, D.F. Evidence for functional chemokine receptors on myeloma cells. Blood 1998, 92, 100a. [Google Scholar]
- Roodman, G.D.; Choi, S.J. MIP-1 Alpha and Myeloma Bone Disease. Cancer Treat. Res. 2004, 118, 83–100. [Google Scholar] [CrossRef]
- Wang, X.T.; He, Y.C.; Zhou, S.Y.; Jiang, J.Z.; Huang, Y.M.; Liang, Y.Z.; Lai, Y.R. Bone marrow plasma macrophage inflammatory protein protein-1 alpha(MIP-1 alpha) and sclerostin in multiple myeloma: Relationship with bone disease and clinical characteristics. Leuk. Res. 2014, 38, 525–531. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Li, T.; Wang, Q.; Qian, J.; Lü, Y.; Zhang, M.; Bi, E.; Yang, M.; Reu, F.; et al. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget 2015, 6, 24218–24229. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Yan, H.; Zhang, E.; Huang, X.; Chen, Q.; Chen, J.; Qu, J.; Liu, Y.; He, J.; et al. CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell Death Dis. 2019, 10, 781–817. [Google Scholar] [CrossRef]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef]
- Kumar, S.; E Witzig, T.; Timm, M.; Haug, J.S.; E Wellik, L.; Fonseca, R.; Greipp, P.R.; Rajkumar, S.V. Expression of VEGF and its receptors by myeloma cells. Leukemia 2003, 17, 2025–2031. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Faria, S.S.; Fernandes, P.C.; Barbosa Silva, M.J.; Lima, V.C.; Fontes, W.; Freitas, R., Jr.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, S.; Yuan, Q.; Zhu, P.; Li, B.; Lin, Q.; Shi, W.; Min, Y.; Ge, Q.; Shao, Y. The Prognostic Significance of Combined Pretreatment Fibrinogen and Neutrophil-Lymphocyte Ratio in Various Cancers: A Systematic Review and Meta-Analysis. Dis. Markers 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Yin, X.; Xiao, Y.; Li, F.; Qi, S.; Yin, Z.; Gao, J. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Prostate Cancer. Medicine 2016, 95, e2544. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ding, P.R.; Li, Y.H.; Wang, F.H.; Shi, Y.X.; Wang, Z.Q.; He, Y.J.; Xu, R.H.; Jiang, W.Q. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010, 15, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Nakashima, J.; Ohori, M.; Tanaka, A.; Hashimoto, T.; Gondo, T.; Hatano, T.; Tachibana, M. Clinical variables for predicting metastatic renal cell carcinoma patients who might not benefit from cytoreductive nephrectomy: Neutrophil-to-lymphocyte ratio and performance status. Int. J. Clin. Oncol. 2013, 19, 139–145. [Google Scholar] [CrossRef]
- Gondo, T.; Nakashima, J.; Ohno, Y.; Choichiro, O.; Horiguchi, Y.; Namiki, K.; Yoshioka, K.; Ohori, M.; Hatano, T.; Tachibana, M. Prognostic Value of Neutrophil-to-lymphocyte Ratio and Establishment of Novel Preoperative Risk Stratification Model in Bladder Cancer Patients Treated With Radical Cystectomy. Urology 2012, 79, 1085–1091. [Google Scholar] [CrossRef]
- Mallappa, S.; Sinha, A.K.; Gupta, S.; Chadwick, S.J.D. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Color. Dis. 2013, 15, 323–328. [Google Scholar] [CrossRef]
- Cho, H.; Hur, H.W.; Kim, S.W.; Kim, S.H.; Kim, J.H.; Kim, Y.T.; Lee, K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. 2009, 58, 15–23. [Google Scholar] [CrossRef]
- Pirozzolo, G.; Gisbertz, S.S.; Castoro, C.; Henegouwen, M.I.V.B.; Scarpa, M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, 3136–3145. [Google Scholar] [CrossRef]
- Shi, L.; Qin, X.; Wang, H.; Xia, Y.; Li, Y.; Chen, X.; Shang, L.; Tai, Y.T.; Feng, X.; Acharya, P.; et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 2016, 8, 18792–18801. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a Prognostic Factor for Overall Survival in Advanced Carcinomas, Sarcomas, and Lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Porrata, L.F.; Rsitow, K.; Inwards, D.J.; Ansell, S.M.; Micallef, I.N.; Johnston, P.B.; Habermann, T.M.; E Witzig, T.; Colgan, J.P.; Nowakowski, G.S.; et al. Lymphopenia assessed during routine follow-up after immunochemotherapy (R-CHOP) is a risk factor for predicting relapse in patients with diffuse large B-cell lymphoma. Leukemia 2010, 24, 1343–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Y.; Zhan, X.; Wang, N.; Peng, F.; Feng, X.; Wu, X. Monocyte/Lymphocyte Ratio and Cardiovascular Disease Mortality in Peritoneal Dialysis Patients. Mediat. Inflamm. 2020, 2020, 9852507–9852509. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Y.; Fan, Z.; Zuo, B.; Jian, X.; Li, L.; Liu, T. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc. Disord. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Neofytou, K.; Smyth, E.C.; Giakoustidis, A.; Khan, A.Z.; Williams, R.; Cunningham, D.; Mudan, S. The Preoperative Lymphocyte-to-Monocyte Ratio is Prognostic of Clinical Outcomes for Patients with Liver-Only Colorectal Metastases in the Neoadjuvant Setting. Ann. Surg. Oncol. 2015, 22, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef]
- Gui, L.; Wang, F.; Shi, J.; Chen, B. The Significance of Inflammatory Markers in the Prognosis of Newly Diagnosed Multiple Myeloma Patients. Blood 2020, 136, 15. [Google Scholar] [CrossRef]
- Ye, G.L.; Chen, Q.; Chen, X.; Liu, Y.Y.; Yin, T.T.; Meng, Q.H.; Wei, H.Q.; Zhou, Q.H. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Solmaz, S.; Uzun, O.; Acar, C.; Sevindik, O.G.; Piskin, O.; Ozsan, H.G.; Demirkan, F.; Undar, B.; Alacacioglu, A.; Ozcan, M.A.; et al. Is the platelet-to-lymphocyte ratio a new prognostic marker in multiple myeloma? J. Lab. Physicians 2018, 10, 363–369. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; George, G.; Chaiwatcharayut, W.; Biso, S.; Candelario, N.; Mittal, V.; Pomerantz, S.; Varadi, G. The Prognostic Significance of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Patients with Multiple Myeloma. J. Clin. Lab. Anal. 2016, 30, 1208–1213. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Huang, Y. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. OncoTargets Ther. 2013, 6, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Salman, T.; Kazaz, S.N.; Varol, U.; Oflazoglu, U.; Unek, I.T.; Kucukzeybek, Y.; Alacacioglu, A.; Atag, E.; Semiz, H.S.; Cengiz, H.; et al. Prognostic Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Patients with Neuroendocrine Tumors: An Izmir Oncology Group Study. Chemotherapy 2016, 61, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Krzystanek, M.; Jósa, V.; Dede, K.; Agoston, E.; Szász, A.M.; Sinko, D.; Szarvas, V.; Salamon, F.; Eklund, A.C.; et al. The comparison of thrombocytosis and plate-let-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb. Haemost. 2014, 111, 483–490. [Google Scholar] [PubMed]
- Jung, S.H.; Kim, J.S.; Lee, W.S.; Oh, S.J.; Ahn, J.S.; Yang, D.H.; Kim, Y.K.; Kim, H.J.; Lee, J.J. Prognostic value of the inverse platelet to lymphocyte ratio (iPLR) in patients with multiple myeloma who were treated up front with a novel agent-containing regimen. Ann. Hematol. 2015, 95, 55–61. [Google Scholar] [CrossRef]
- O’Garra, A.; Vieira, P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004, 10, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Kochanek, M.; Darabi, K.; Popov, A.; Jensen, M.; Endl, E.; Knolle, P.A.; Thomas, R.K.; Von Bergwelt-Baildon, M.; Debey, S.; et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005, 106, 2018–2025. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef]
- Tang, Q.; A Bluestone, J. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Disis, M.L.; Salazar, L.G. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol. Immunother. 2006, 56, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Curotto de Lafaille, M.A.; Lafaille, J.J. Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity 2009, 30, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.R.M.; Rihova, L.; Zahradova, L.; Klincova, M.; Penka, M.; Hajek, R. Increased T Regulatory Cells Are Associated with Adverse Clinical Features and Predict Progression in Multiple Myeloma. PLoS ONE 2012, 7, e47077. [Google Scholar] [CrossRef]
- Braga, W.M.T.; Da Silva, B.R.; De Carvalho, A.C.; Maekawa, Y.H.; Bortoluzzo, A.B.; Gil Rizzatti, E.; Atanackovic, D.; Colleoni, G.W.B. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4+ T regulatory cells. Cancer Immunol. Immunother. 2014, 63, 1189–1197. [Google Scholar] [CrossRef]
- Nowakowski, G.S.; Witzig, T.E.; Dingli, D.; Tracz, M.J.; Gertz, M.A.; Lacy, M.Q.; Lust, J.A.; Dispenzieri, A.; Greipp, P.R.; Kyle, R.A.; et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005, 106, 2276–2279. [Google Scholar] [CrossRef]
- Chng, W.J.; Dispenzieri, A.; Chim, C.S.; Fonseca, R.; Goldschmidt, H.; Lentzsch, S.; Munshi, N.C.; Palumbo, A.; Miguel, J.S.; Sonneveld, P. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014, 28, 269–277. [Google Scholar] [CrossRef]
- Ferrucci, A.; Moschetta, M.; Frassanito, M.A.; Berardi, S.; Catacchio, I.; Ria, R.; Racanelli, V.; Caivano, A.; Solimando, A.G.; Vergara, D.; et al. A HGF/cMET Autocrine Loop Is Operative in Multiple Myeloma Bone Marrow Endothelial Cells and May Represent a Novel Therapeutic Target. Clin. Cancer Res. 2014, 20, 5796–5807. [Google Scholar] [CrossRef]
- Malergue, F.; Galland, F.; Martin, F.; Mansuelle, P.; Aurrand-Lions, M.; Naquet, P. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol. Immunol. 1998, 35, 1111–1119. [Google Scholar] [CrossRef]
- Severson, E.A.; Lee, W.Y.; Capaldo, C.T.; Nusrat, A.; Parkos, C.A. Junctional Adhesion Molecule A Interacts with Afadin and PDZ-GEF2 to Activate Rap1A, Regulate β1 Integrin Levels, and Enhance Cell Migration. Mol. Biol. Cell 2009, 20, 1916–1925. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Vià, M.C.; Leone, P.; Borrelli, P.; Croci, G.A.; Tabares, P.; Brandl, A.; Di Lernia, G.; Bianchi, F.P.; Tafuri, S.; et al. Halting the vicious cycle within the multiple myeloma ecosystem: Blocking JAM-A on bone marrow endothelial cells restores the angiogenic homeostasis and suppresses tumor progression. Haematology 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8+ T cell subset with suppressive function in myeloma patients. OncoImmunology 2019, 8, e1486949. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Croner, R.S.; Weinländer, K.; Hohenberger, W.; Sturzl, M.; Naschberger, E. Endothelial cells of human colorectal cancer and healthy colon reveal phenotypic differences in culture. Lab. Investig. 2007, 87, 1159–1170. [Google Scholar] [CrossRef][Green Version]
- Severson, E.A.; Parkos, C.A. Mechanisms of Outside-in Signaling at the Tight Junction by Junctional Adhesion Molecule A. Ann. N. Y. Acad. Sci. 2009, 1165, 10–18. [Google Scholar] [CrossRef]
- Solimando, A.G.; Brandl, A.; Mattenheimer, K.; Graf, C.; Ritz, M.; Ruckdeschel, A.; Stühmer, T.; Mokhtari, Z.; Rudelius, M.; Dotterweich, J.; et al. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia 2018, 32, 736–743. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, V.; Saltarella, I.; Lamanuzzi, A.; Mariggiò, M.; Racanelli, V.; Vacca, A.; Frassanito, M. Autophagy: A New Mechanism of Prosurvival and Drug Resistance in Multiple Myeloma. Transl. Oncol. 2018, 11, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Xia, H.G.; Yuan, J. Pharmacologic agents targeting autophagy. J. Clin. Investig. 2015, 125, 5–13. [Google Scholar] [CrossRef]
- Kenific, C.M.; Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015, 25, 37–45. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Jung, G.; Roh, J.; Lee, H.; Gil, M.; Yoon, H.; Suh, C.; Jang, S.; Park, C.J.; Huh, J.; Park, C.S. Autophagic Markers BECLIN 1 and LC3 Are Associated with Prognosis of Multiple Myeloma. Acta Haematol. 2015, 134, 17–24. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Zhang, X.; Schwartz, J.C.D.; Guo, X.; Bhatia, S.; Cao, E.; Chen, L.; Zhang, Z.Y.; Edidin, M.A.; Nathenson, S.G.; Almo, S.C. Structural and Functional Analysis of the Costimulatory Receptor Programmed Death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signaling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, Y.; Kim, J.H.; Kang, K.W.; Lee, S.J.; Kim, S.J.; Kim, B.S. PD-L1 expression in bone marrow plasma cells as a biomarker to predict multiple myeloma prognosis: Developing a nomogram-based prognostic model. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007, 110, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Ishibashi, M.; Sunakawa-Kii, M.; Inokuchi, K. PD-L1–PD-1 Pathway in the Pathophysiology. Cancers 2020, 12, 924. [Google Scholar] [CrossRef]
- Guan, J.; Wang, R.; Hasan, S.; Tao, L.; Wazir, M.; Jain, A.G.; Zhu, X.; Perkins, S.; Mohamed, S.; Chang, C.C.; et al. Prognostic Significance of the Dynamic Change of Programmed Death-ligand 1 Expression in Patients with Multiple Myeloma. Cureus 2019, 11, e4401. [Google Scholar] [CrossRef]
- D’Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia 2019, 34, 21–34. [Google Scholar] [CrossRef]
- Amodio, N.; D’Aquila, P.; Passarino, G.; Tassone, P.; Bellizzi, D. Epigenetic modifications in multiple myeloma: Recent advances on the role of DNA and histone methylation. Expert Opin. Ther. Targets 2016, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Gulla’, A.; Anderson, K.C. Multiple myeloma: The (r)evolution of current therapy and a glance into future. Haematology 2020, 105, 2358–2367. [Google Scholar] [CrossRef]
| Factor | Function in MM | Prognostic Value | Reference | |
|---|---|---|---|---|
| Pro-inflammatory interleukins | IL-1 | Promoting the invasiveness and progression of the tumour; Stimulation of IL-6 production; IL-1 plays a role in the conversion of latent myeloma to active MM. | High levels of IL-1 correlate with poor prognosis. | [11,12,14] |
| IL-2 | The IL-2–IL-2R system plays a key role in maintaining the proper functioning of T-cells. | Disturbance of the IL-2–IL-2R system may be a prognostic marker in the diagnosis of MM in comparison with MGUS; malignancy marker; high levels of sIL-2R correlate with poor PFS. | [19,20,21] | |
| IL-6 | Promotion of tumour growth and reduction in apoptosis in myeloma cells by the JAK/STAT and RAS/MAPKs pathways. | High levels of sIL-6R are associated with shorter survival; elevated levels of IL-6 and sIL-6R reflect the level of disease activity and indicate poor prognosis; high cellular expression of IL-6 mRNA in MGUS patients may predict the development of MM. | [12,22,26,33] | |
| IL-15 | The production of IL-15 in the stroma influences the growth of myeloma cells independent of IL-6; overexpression of IL-15 in MM plasma cells protect them against apoptosis. | The levels of IL-15 in stage III MM patients are increased, compared to stage I and II patients. | [12,48] | |
| IL-17 | IL-17A promotes the growth of MM cells and inhibit immune functions in the tumour environment. | High levels of IL-17 indicate poor prognosis, negative response to therapy, and correlate with disease severity; IL-27: IL-17 ratio in newly diagnosed MM correlates with disease progression. | [23,49,50,51,52,53] | |
| IL-18 | IL-18 influences the formation of MDSC cells and increased levels of angiogenic cytokines. | High levels of IL-18 correlate with poorer patient survival and the severity of the disease. | [55,56,57] | |
| IL-32 | IL-32α induces IL-6 production in BMSC and creates a feedback loop that promotes MM cells growth; IL-32 promotes macrophage immunosuppression. | High expression of the IL-32 gene in plasma cells correlates with worse survival and more advanced clinical stage of MM; lower IL-32 levels result in more positive PFS and OS; high levels of IL-32 likely enable the early identification of resistant MM cells in patients with complete disease remission. | [59,60,61] | |
| Anti-inflammatory interleukins | IL-1Ra | Regulation of IL-1 activity. | Serum IL-1Ra levels were higher in stage III patients than in stage I/II patients after bortezomib therapy; high levels of IL-1Ra are associated with bone involvement in MM. | [1,2,3] |
| IL-10 | Abnormal levels of IL-10 released by CD8+ T-cells and MM cells may support the MM immunosuppressive environment by abolishing DC function; IL-10 acts as a proliferative factor for plasma cells, but also promotes angiogenesis in MM. | Elevated serum IL-10 levels in patients with initial MM negatively affected PFS and OS, response to treatment, and prognosis, but is also associated with disease severity in patients with MM. | [48,99,100,101,102] | |
| IL-22 | IL-22 can stimulate the growth of MM and influence the development of immunosuppression in the tumour environment. | High levels of IL-22 correlate with the severity of the disease. | [103] | |
| Tumour necrosis factor family | TNF-α | TNF-α stimulates the production of autocrine IL-6. | High levels of TNF-α in patients with MM correlate with the severity of the disease and indicated the occurrence of severe symptoms during maintenance treatment. | [67,68] |
| BAFF | BAFF promotes the growth of tumour MM by an autocrine loop. | Increased BAFF levels in patients with MM correlate with decreased survival. | [24,71,72,73] | |
| RANK | RANK signalling and its RANKL ligand are involved in tumour formation and growth. | Level of RANK reflecting disease severity, lytic bone damage and poor prognosis for MM patients; high RANKL: OPG ratio characterize MM patients with shorter survival, and high levels of soluble RANKL correlated with the degree of bone damage. | [77,78,79,80,81] | |
| Growth factors | FGF-2 | FGF-2 promotes cancer progression and angiogenic potential; FGF-2, along with IL-6, can increase the proliferation of myeloma cells. | Low FGF-2 levels are associated with a shorter MM progression time; bFGF G allele is associated with worse response to therapy. | [108,109,111,144] |
| VEGF | VEGF promotes cancer progression and angiogenic potential. | VEGF is positively correlated with IL-20 levels and the link between those two parameters may be used as an indicator of the disease progression and angiogenesis process. | [110,145] | |
| HGF | HGF is involved in the regulation of cell proliferation and survival, but also has an anti-apoptotic effect on MM cells. | High level of HGF is associated with an unfavorable prognosis. | [120,121] | |
| PDGF- | PDGF-β promotes cancer angiogenic potential. | PDGF-β may be an important indicator of the immune status of an advanced MM patient. | [123,124] | |
| Ang-2 | Ang-2 promotes cancer angiogenic potential. | Ang-2 is a biomarker of angiogenesis; Ang-1/Ang-2 ratio may be useful in MM. | [125,126] | |
| Interferon | IFN- 𝛾 | IFN-𝛾 inhibits cell proliferation. | IFN-𝛾 has a positive effect on rituximab treatment. | [82,88] |
| Chemokines | CCL2 and CCL3 | CCL2 and CCL3 affect the infiltration of macrophages into the bone marrow, as well as elevated polarization into TAM; CCL3 influences the development of bone disease in MM. | CCL2 and CCL3 are involved in the development of chemoresistance; CCL3 levels correlate with the activity and stage of the disease; high levels of CCL3 are associated with unfavorable diagnosis. | [40,136,141,142,143] |
| Parameter | Level | Ref |
|---|---|---|
| Neutrophil-to-lymphocyte ratio (NLR) | MM > control group | [156] |
| Monocyte-to-lymphocyte ratio (MLR) | MM > control group | [156,164] |
| Platels-to-lymphocyte ratio (PLR) | MM < control group | [156,166] |
| Regulatory T lymphocytes (Tregs) | MM > control group | [179,180] |
| Circulating Plasma Cells | MM > control group | [181] |
| Junctional adhesion molecule-A (JAM-A) | MM > control group | [186,190] |
| Autophagic markers – Beclin-1, LC3 | MM > control group | [196] |
| Programmed death ligand 1 (PD-L1) | MM > control group | [204,205,206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Pasiarski, M.; Sosnowska-Pasiarska, B.; Smarz-Widelska, I.; Góźdź, S.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunological Prognostic Factors in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 3587. https://doi.org/10.3390/ijms22073587
Bębnowska D, Hrynkiewicz R, Grywalska E, Pasiarski M, Sosnowska-Pasiarska B, Smarz-Widelska I, Góźdź S, Roliński J, Niedźwiedzka-Rystwej P. Immunological Prognostic Factors in Multiple Myeloma. International Journal of Molecular Sciences. 2021; 22(7):3587. https://doi.org/10.3390/ijms22073587
Chicago/Turabian StyleBębnowska, Dominika, Rafał Hrynkiewicz, Ewelina Grywalska, Marcin Pasiarski, Barbara Sosnowska-Pasiarska, Iwona Smarz-Widelska, Stanisław Góźdź, Jacek Roliński, and Paulina Niedźwiedzka-Rystwej. 2021. "Immunological Prognostic Factors in Multiple Myeloma" International Journal of Molecular Sciences 22, no. 7: 3587. https://doi.org/10.3390/ijms22073587
APA StyleBębnowska, D., Hrynkiewicz, R., Grywalska, E., Pasiarski, M., Sosnowska-Pasiarska, B., Smarz-Widelska, I., Góźdź, S., Roliński, J., & Niedźwiedzka-Rystwej, P. (2021). Immunological Prognostic Factors in Multiple Myeloma. International Journal of Molecular Sciences, 22(7), 3587. https://doi.org/10.3390/ijms22073587








