Pseudo-Starvation Driven Energy Expenditure Negatively Affects Ovarian Follicle Development
Abstract
1. Introduction
2. Results
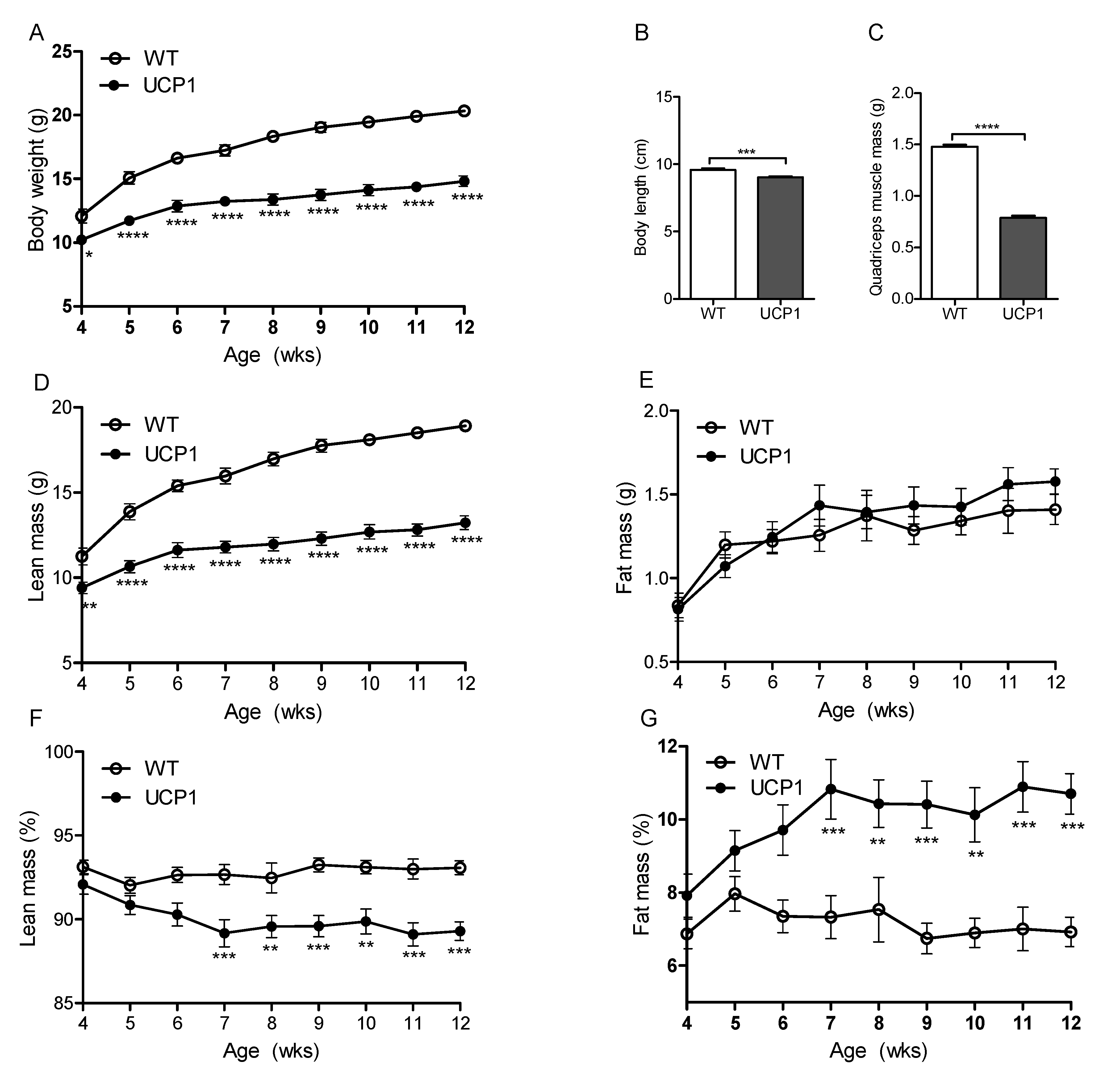
2.1. Morphometric Measurements
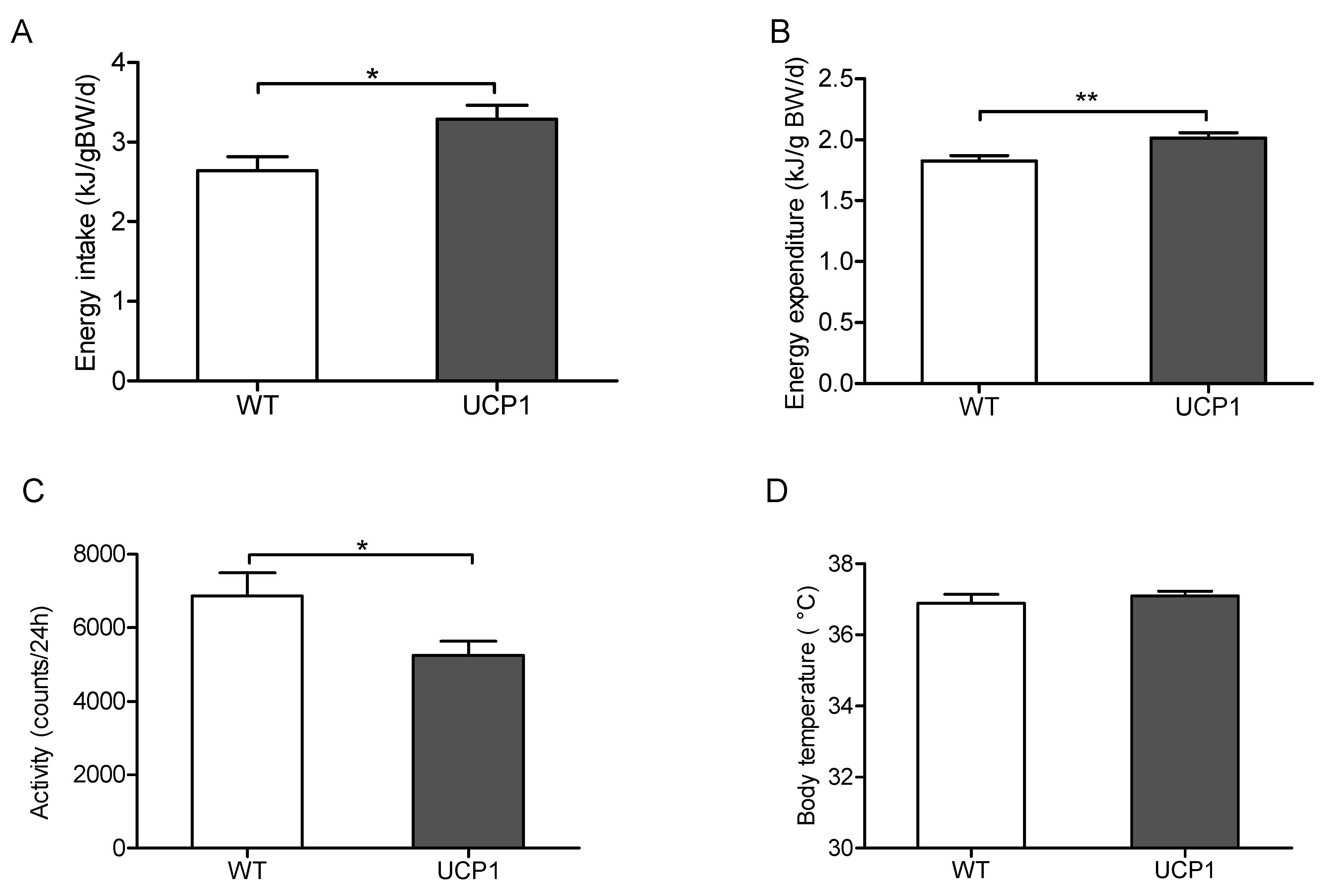
2.2. Energy Metabolism
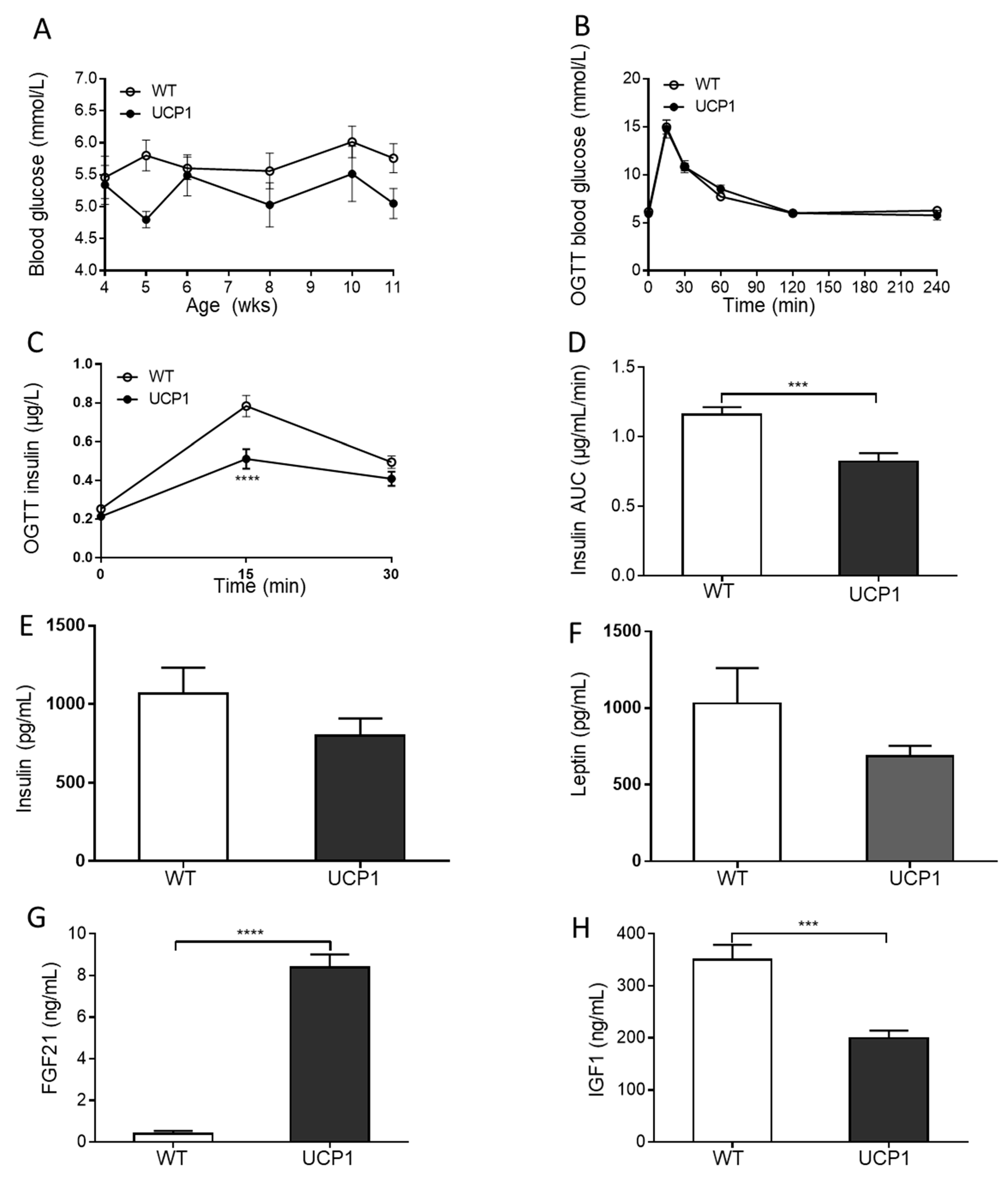
2.3. Plasma Glucose and Hormone Concentrations
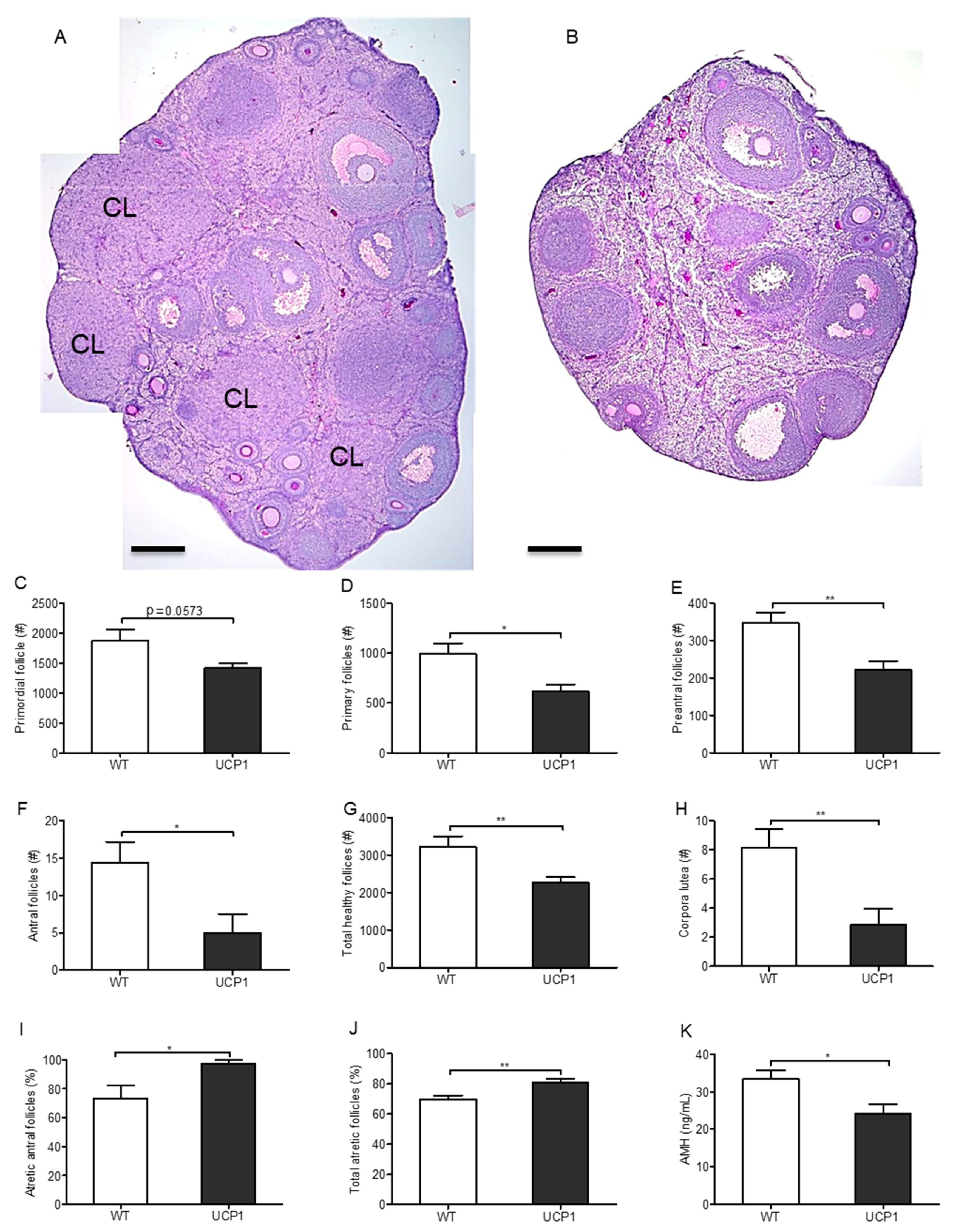
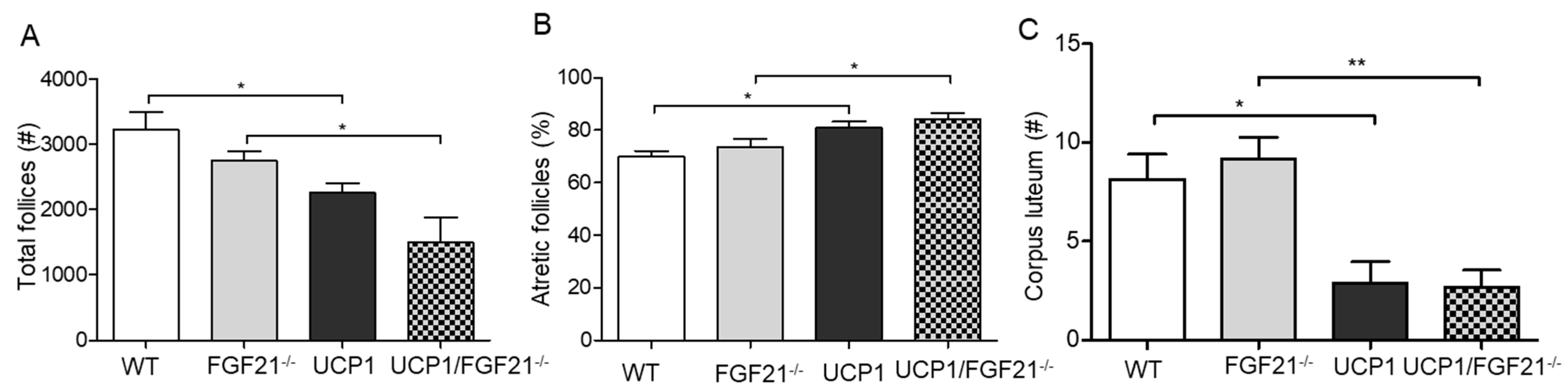
2.4. Follicular Development in UCP1-TG Mice
2.5. Follicular Development in FGF21–/–Mice
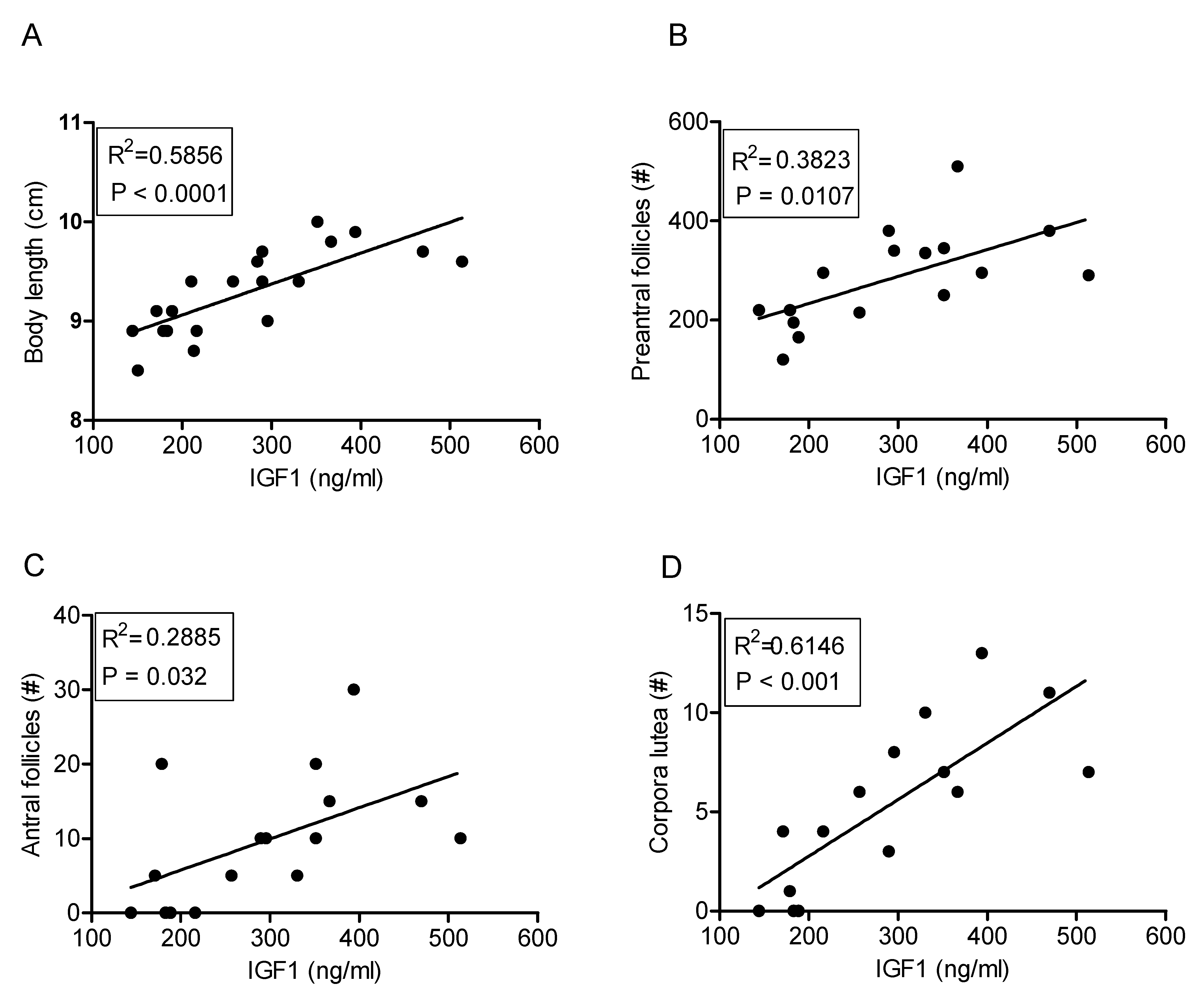
2.6. IGF1 Concentrations and Ovarian Follicle Numbers
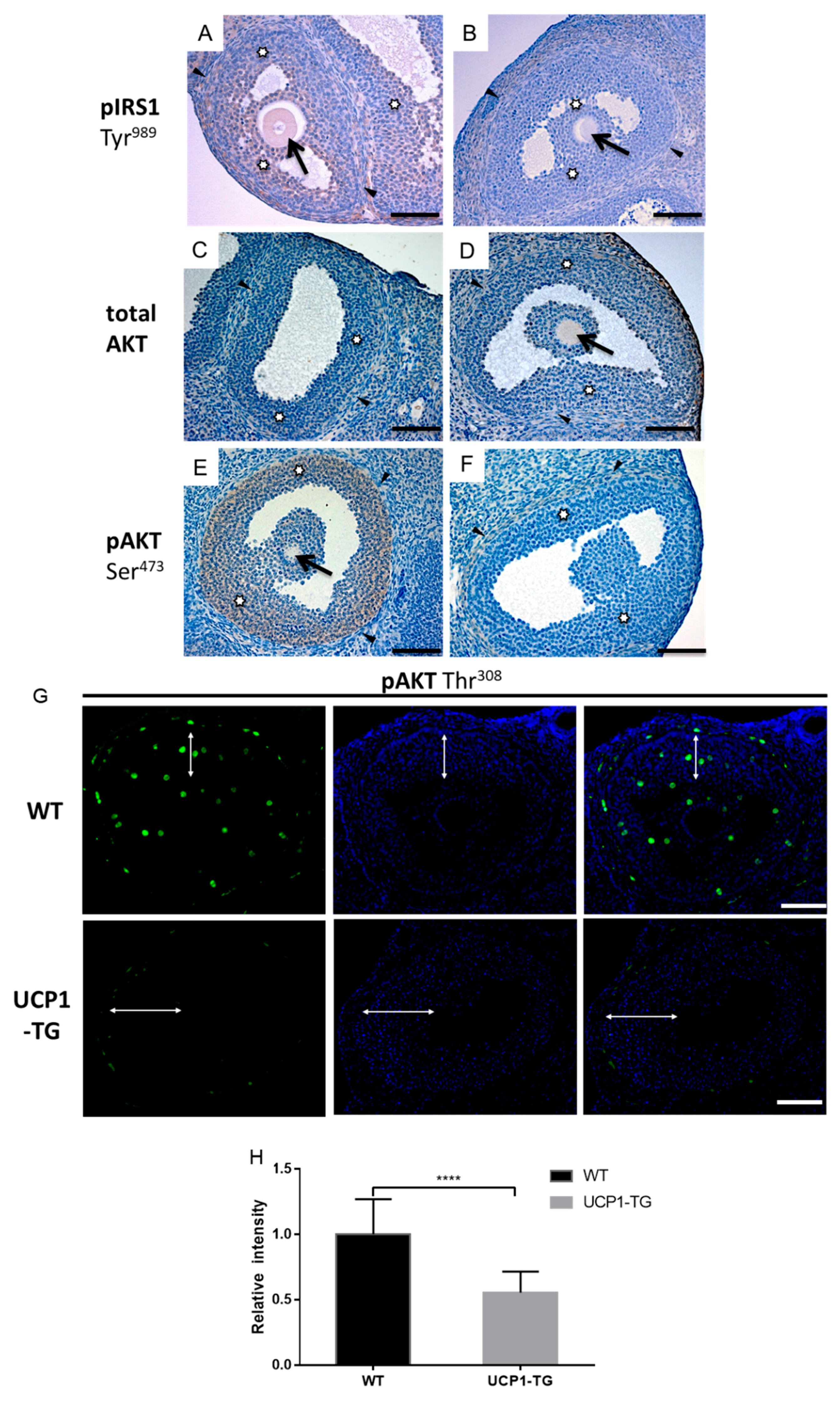
2.7. Effects of UCP1-TG on Phosphorylated IRS1, AKT and IRS2 Expression in Granulosa Cells of Antral Follicles
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Animals
4.3. Anthropometric and Metabolic Assessments
4.4. Plasma IGF1, AMH and Leptin Measurements
4.5. Histological Evaluation of Ovarian Tissues
4.6. Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef]
- Speakman, J.R.; Krol, E. The heat dissipation limit theory and evolution of life histories in endotherms—Time to dispose of the disposable soma theory? Integr. Comp. Biol. 2010, 50, 793–807. [Google Scholar] [CrossRef]
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development and longevity. Physiol. Rev. 2013, 93, 571–598. [Google Scholar] [CrossRef]
- Evans, J.J.; Anderson, G.M. Balancing ovulation and anovulation: Integration of the reproductive and energy balance axes by neuropeptides. Hum. Reprod. Update 2012, 18, 313–332. [Google Scholar] [CrossRef]
- Visscher, M.B.; King, J.T.; Lee, Y.C. Further studies on influence of age and diet upon reproductive senescence in strain A female mice. Am. J. Physiol. 1952, 170, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, E.M.; Cowen, J. The effect of protein deficiency and calorie deficiency on the reproduction of rats. Br. J. Nutr. 1972, 27, 85–95. [Google Scholar] [CrossRef][Green Version]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Schneider, J.E.; Li, H.Y. Control of fertility by metabolic cues. Am. J. Physiol. 1996, 270, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Ball, Z.B.; Barnes, R.H.; Visscher, M.B. The effects of dietary caloric restriction on maturity and senescence, with particular reference to fertility and longevity. Am. J. Physiol. 1947, 150, 511–519. [Google Scholar] [CrossRef]
- Selesniemi, K.; Lee, H.J.; Tilly, J.L. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008, 7, 622–629. [Google Scholar] [CrossRef]
- Couplan, E.; Gelly, C.; Goubern, M.; Fleury, C.; Quesson, B.; Silberberg, M.; Thiaudiere, E.; Mateo, P.; Lonchampt, M.; Levens, N.; et al. High level of uncoupling protein 1 expression in muscle of transgenic mice selectively affects muscles at rest and decreases their IIb fiber content. J. Biol. Chem. 2002, 277, 43079–43088. [Google Scholar] [CrossRef]
- Klaus, S.; Rudolph, B.; Dohrmann, C.; Wehr, R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol. Genom. 2005, 21, 193–200. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Gurmaches, J.; Hung, C.M.; Guertin, D.A. Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 2016, 26, 313–326. [Google Scholar] [CrossRef]
- Klaus, S.; Casteilla, L.; Bouillaud, F.; Ricquier, D. The uncoupling protein UCP: A membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int. J. Biochem. 1991, 23, 791–801. [Google Scholar] [CrossRef]
- Ost, M.; Keipert, S.; van Schothorst, E.M.; Donner, V.; van der Stelt, I.; Kipp, A.P.; Petzke, K.J.; Jove, M.; Pamplona, R.; Portero-Otin, M.; et al. Muscle mitohormesis promotes cellular survival via serine/glycine pathway flux. FASEB J. 2015, 29, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Weinstein, D.; Bentov, I. Similarities and differences between insulin and IGF-I: Structures, receptors and signalling pathways. Arch. Physiol. Biochem. 2008, 114, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Adashi, E.Y. The IGF family and folliculogenesis. J. Reprod. Immunol. 1998, 39, 13–19. [Google Scholar] [CrossRef]
- Slot, K.A.; Kastelijn, J.; Bachelot, A.; Kelly, P.A.; Binart, N.; Teerds, K.J. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction 2006, 131, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Gutierrez, C.G.; Baxter, G.; Glazyrin, A.L.; Mann, G.E.; Woad, K.J.; Hogg, C.O.; Webb, R. Expression of mRNA encoding IGF-I, IGF-II and type 1 IGF receptor in bovine ovarian follicles. J. Endocrinol. 2000, 165, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hastie, P.M.; Haresign, W. Expression of mRNAs encoding insulin-like growth factor (IGF) ligands, IGF receptors and IGF binding proteins during follicular growth and atresia in the ovine ovary throughout the oestrous cycle. Anim. Reprod. Sci. 2006, 92, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chin, E.; Bondy, C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinology 1991, 129, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Wandji, S.A.; Wood, T.L.; Crawford, J.; Levison, S.W.; Hammond, J.M. Expression of mouse ovarian insulin growth factor system components during follicular development and atresia. Endocrinology 1998, 139, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellve, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [CrossRef]
- Zhou, J.; Kumar, T.R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef]
- Glister, C.; Tannetta, D.S.; Groome, N.P.; Knight, P.G. Interactions between follicle-stimulating hormone and growth factors in modulating secretion of steroids and inhibin-related peptides by nonluteinized bovine granulosa cells. Biol. Reprod. 2001, 65, 1020–1028. [Google Scholar] [CrossRef]
- Zhou, P.; Baumgarten, S.C.; Wu, Y.; Bennett, J.; Winston, N.; Hirshfeld-Cytron, J.; Stocco, C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013, 27, 511–523. [Google Scholar] [CrossRef]
- Hu, C.L.; Cowan, R.G.; Harman, R.M.; Quirk, S.M. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol. Endocrinol. 2004, 18, 326–338. [Google Scholar] [CrossRef]
- Sun, G.W.; Kobayashi, H.; Suzuki, M.; Kanayama, N.; Terao, T. Follicle-stimulating hormone and insulin-like growth factor I synergistically induce up-regulation of cartilage link protein (Crtl1) via activation of phosphatidylinositol-dependent kinase/Akt in rat granulosa cells. Endocrinology 2003, 144, 793–801. [Google Scholar] [CrossRef][Green Version]
- Burks, D.J.; Font de Mora, J.; Schubert, M.; Withers, D.J.; Myers, M.G.; Towery, H.H.; Altamuro, S.L.; Flint, C.L.; White, M.F. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000, 407, 377–382. [Google Scholar] [CrossRef]
- Law, N.C.; Hunzicker-Dunn, M.E. Insulin Receptor Substrate 1, the Hub Linking Follicle-stimulating Hormone to Phosphatidylinositol 3-Kinase Activation. J. Biol. Chem. 2016, 291, 4547–4560. [Google Scholar] [CrossRef]
- Gates, A.C.; Bernal-Mizrachi, C.; Chinault, S.L.; Feng, C.; Schneider, J.G.; Coleman, T.; Malone, J.P.; Townsend, R.R.; Chakravarthy, M.V.; Semenkovich, C.F. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007, 6, 497–505. [Google Scholar] [CrossRef]
- Katterle, Y.; Keipert, S.; Hof, J.; Klaus, S. Dissociation of obesity and insulin resistance in transgenic mice with skeletal muscle expression of uncoupling protein 1. Physiol. Genom. 2008, 32, 352–359. [Google Scholar] [CrossRef]
- Keipert, S.; Voigt, A.; Klaus, S. Dietary effects on body composition, glucose metabolism, and longevity are modulated by skeletal muscle mitochondrial uncoupling in mice. Aging Cell 2011, 10, 122–136. [Google Scholar] [CrossRef]
- Neschen, S.; Katterle, Y.; Richter, J.; Augustin, R.; Scherneck, S.; Mirhashemi, F.; Schurmann, A.; Joost, H.G.; Klaus, S. Uncoupling protein 1 expression in murine skeletal muscle increases AMPK activation, glucose turnover and insulin sensitivity in vivo. Physiol. Genom. 2008, 33, 333–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ost, M.; Igual Gil, C.; Coleman, V.; Keipert, S.; Efstathiou, S.; Vidic, V.; Weyers, M.; Klaus, S. Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress. EMBO Rep. 2020, 21, e48804. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Ost, M.; Johann, K.; Imber, F.; Jastroch, M.; van Schothorst, E.M.; Keijer, J.; Klaus, S. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E469–E482. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. What is the “ovarian reserve”? Fertil. Steril. 2015, 103, 628–630. [Google Scholar] [CrossRef]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef]
- Holehan, A.M.; Merry, B.J. The control of puberty in the dietary restricted female rat. Mech. Ageing Dev. 1985, 32, 179–191. [Google Scholar] [CrossRef]
- Warren, M.P. The effects of exercise on pubertal progression and reproductive function in girls. J. Clin. Endocrinol. Metab. 1980, 51, 1150–1157. [Google Scholar] [CrossRef]
- Gudmundsdottir, S.L.; Flanders, W.D.; Augestad, L.B. Physical activity and fertility in women: The North-Trondelag Health Study. Hum. Reprod. 2009, 24, 3196–3204. [Google Scholar] [CrossRef]
- Selesniemi, K.; Lee, H.J.; Muhlhauser, A.; Tilly, J.L. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 2011, 108, 12319–12324. [Google Scholar] [CrossRef] [PubMed]
- Ost, M.; Coleman, V.; Voigt, A.; van Schothorst, E.M.; Keipert, S.; van der Stelt, I.; Ringel, S.; Graja, A.; Ambrosi, T.; Kipp, A.P.; et al. Muscle mitochondrial stress adaptation operates independently of endogenous FGF21 action. Mol. Metab. 2016, 5, 79–90. [Google Scholar] [CrossRef]
- Neganova, I.; Al-Qassab, H.; Heffron, H.; Selman, C.; Choudhury, A.I.; Lingard, S.J.; Diakonov, I.; Patterson, M.; Ghatei, M.; Bloom, S.R.; et al. Role of central nervous system and ovarian insulin receptor substrate 2 signaling in female reproductive function in the mouse. Biol. Reprod. 2007, 76, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Law, N.C.; White, M.F.; Hunzicker-Dunn, M.E. G protein-coupled receptors (GPCRs) that signal via protein kinase A (PKA) cross-talk at insulin receptor substrate 1 (IRS1) to activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. J. Biol. Chem. 2016, 291, 27160–27169. [Google Scholar] [CrossRef]
- Law, N.C.; Donaubauer, E.M.; Zeleznik, A.J.; Hunzicker-Dunn, M.J.E. How protein kinase a activates canonical tyrosine kinase signaling pathways to promote granulosa cell differentiation. Endocrinology 2017, 158, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Owen, B.M.; Bookout, A.L.; Ding, X.; Lin, V.Y.; Atkin, S.D.; Gautron, L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 2013, 19, 1153–1156. [Google Scholar] [CrossRef]
- Singhal, G.; Douris, N.; Fish, A.J.; Zhang, X.; Adams, A.C.; Flier, J.S.; Pissios, P.; Maratos-Flier, E. Fibroblast growth factor 21 has no direct role in regulating fertility in female mice. Mol. Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Nakamura, H.; Konishi, M.; Murata, Y.; Takagi, H.; Matsumura, S.; Inoue, K.; Fushiki, T.; Itoh, N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 2009, 150, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.J.; Hardeman, E.C. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J. Biol. Chem. 1993, 268, 719–725. [Google Scholar] [CrossRef]
- Tidball, J.G.; Spencer, M.J. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J. Physiol. 2002, 545, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorde, L.P.; van Schothorst, E.M.; Swarts, H.M.; Kuda, O.; Steenbergh, E.; Termeulen, S.; Kopecky, J.; Keijer, J. A Difference in Fatty Acid Composition of Isocaloric High-Fat Diets Alters Metabolic Flexibility in Male C57BL/6JOlaHsd Mice. PLoS ONE 2015, 10, e0128515. [Google Scholar] [CrossRef] [PubMed]
- Flaws, J.A.; Abbud, R.; Mann, R.J.; Nilson, J.H.; Hirshfield, A.N. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol. Reprod. 1997, 57, 1233–1237. [Google Scholar] [CrossRef]
- Meng, L.; Rijntjes, E.; Swarts, H.; Bunschoten, A.; van der Stelt, I.; Keijer, J.; Teerds, K. Dietary-Induced Chronic Hypothyroidism Negatively Affects Rat Follicular Development and Ovulation Rate and Is Associated with Oxidative Stress. Biol. Reprod. 2016, 94, 90. [Google Scholar] [CrossRef]
- Tilly, J.L. Ovarian follicle counts—Not as simple as 1, 2, 3. Reprod. Biol. Endocrinol. 2003, 1, 11. [Google Scholar] [CrossRef]
- Meng, L.; Jan, S.Z.; Hamer, G.; van Pelt, A.M.; van der Stelt, I.; Keijer, J.; Teerds, K.J. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol. Reprod. 2018, 99, 853–863. [Google Scholar] [CrossRef]
- Logothetopoulos, J.; Dorrington, J.; Bailey, D.; Stratis, M. Dynamics of follicular growth and atresia of large follicles during the ovarian cycle of the guinea pig: Fate of the degenerating follicles, a quantitative study. Anat. Rec. 1995, 243, 37–48. [Google Scholar] [CrossRef]
- Teerds, K.J.; Dorrington, J.H. Immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase in the rat ovary during follicular development and atresia. Biol. Reprod. 1993, 49, 989–996. [Google Scholar] [CrossRef][Green Version]
- Dijkstra, G.; de Rooij, D.G.; de Jong, F.H.; van den Hurk, R. Effect of hypothyroidism on ovarian follicular development, granulosa cell proliferation and peripheral hormone levels in the prepubertal rat. Eur. J. Endocrinol. 1996, 134, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Teerds, K.; Tao, J.; Wei, H.; Jaklofsky, M.; Zhao, Z.; Liang, Y.; Li, L.; Wang, C.C.; Zhang, S. Characteristics of Circular RNA Expression Profiles of Porcine Granulosa Cells in Healthy and Atretic Antral Follicles. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, Z.; Zhao, K.; Tao, J.; Chit, T.; Zhang, S.; Wang, C.C.; Teerds, K. Transcriptome Analysis of Porcine Granulosa Cells in Healthy and Atretic Follicles: Role of Steroidogenesis and Oxidative Stress. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Coleman, V.; Zhao, Y.; Ost, M.; Voigt, A.; Bunschoten, A.; Keijer, J.; Teerds, K.; Klaus, S. Pseudo-Starvation Driven Energy Expenditure Negatively Affects Ovarian Follicle Development. Int. J. Mol. Sci. 2021, 22, 3557. https://doi.org/10.3390/ijms22073557
Meng L, Coleman V, Zhao Y, Ost M, Voigt A, Bunschoten A, Keijer J, Teerds K, Klaus S. Pseudo-Starvation Driven Energy Expenditure Negatively Affects Ovarian Follicle Development. International Journal of Molecular Sciences. 2021; 22(7):3557. https://doi.org/10.3390/ijms22073557
Chicago/Turabian StyleMeng, Li, Verena Coleman, Yu Zhao, Mario Ost, Anja Voigt, Annelies Bunschoten, Jaap Keijer, Katja Teerds, and Susanne Klaus. 2021. "Pseudo-Starvation Driven Energy Expenditure Negatively Affects Ovarian Follicle Development" International Journal of Molecular Sciences 22, no. 7: 3557. https://doi.org/10.3390/ijms22073557
APA StyleMeng, L., Coleman, V., Zhao, Y., Ost, M., Voigt, A., Bunschoten, A., Keijer, J., Teerds, K., & Klaus, S. (2021). Pseudo-Starvation Driven Energy Expenditure Negatively Affects Ovarian Follicle Development. International Journal of Molecular Sciences, 22(7), 3557. https://doi.org/10.3390/ijms22073557







