Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research
Abstract
1. Introduction
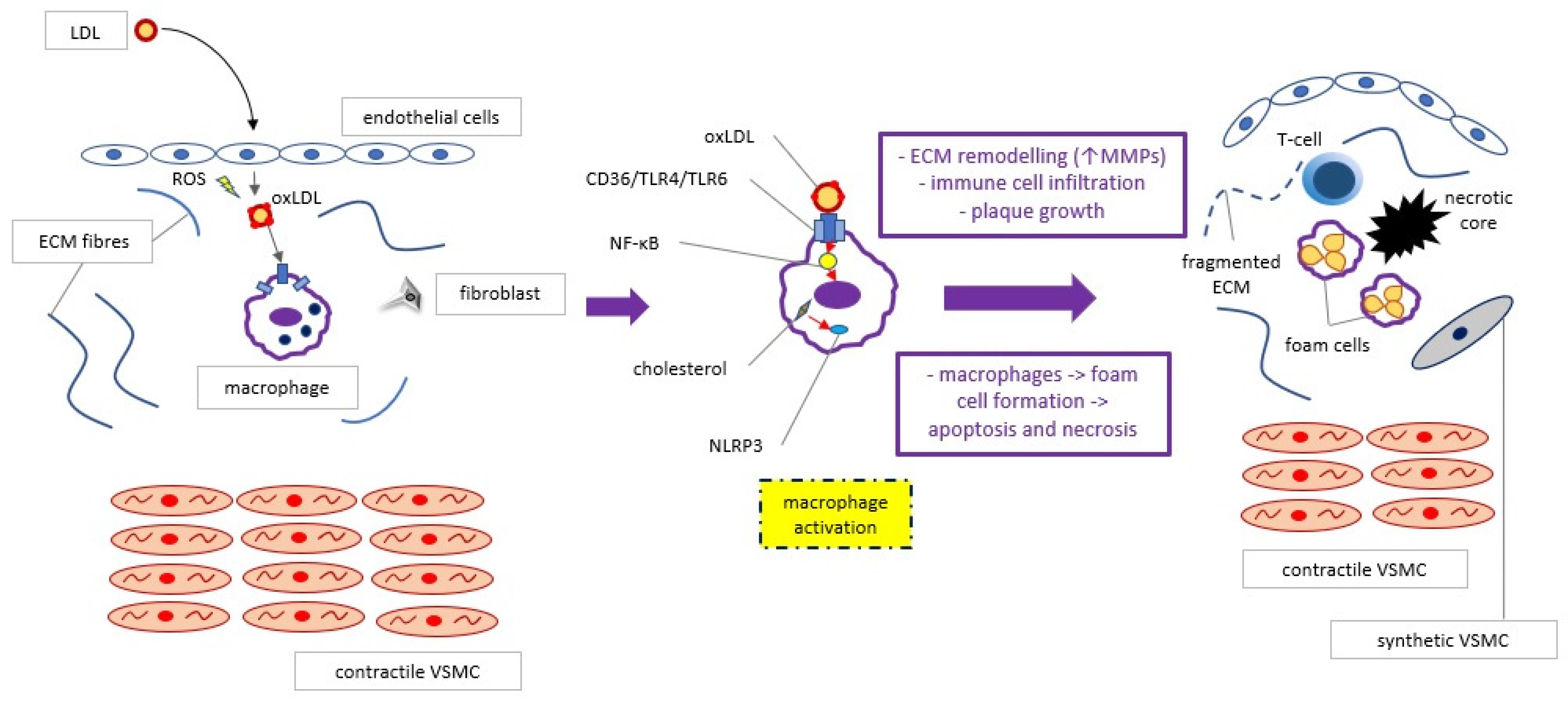
2. Basic Knowledge
3. Exploration of Regulatory Proteins and Pathways
4. Exploration of Epigenetic Regulations and Genetic Patterns
5. Exploration of the Influence of Biophysical Factors upon Atherogenesis
6. Exploration of Tissues and Microorganisms Affecting Atherogenesis
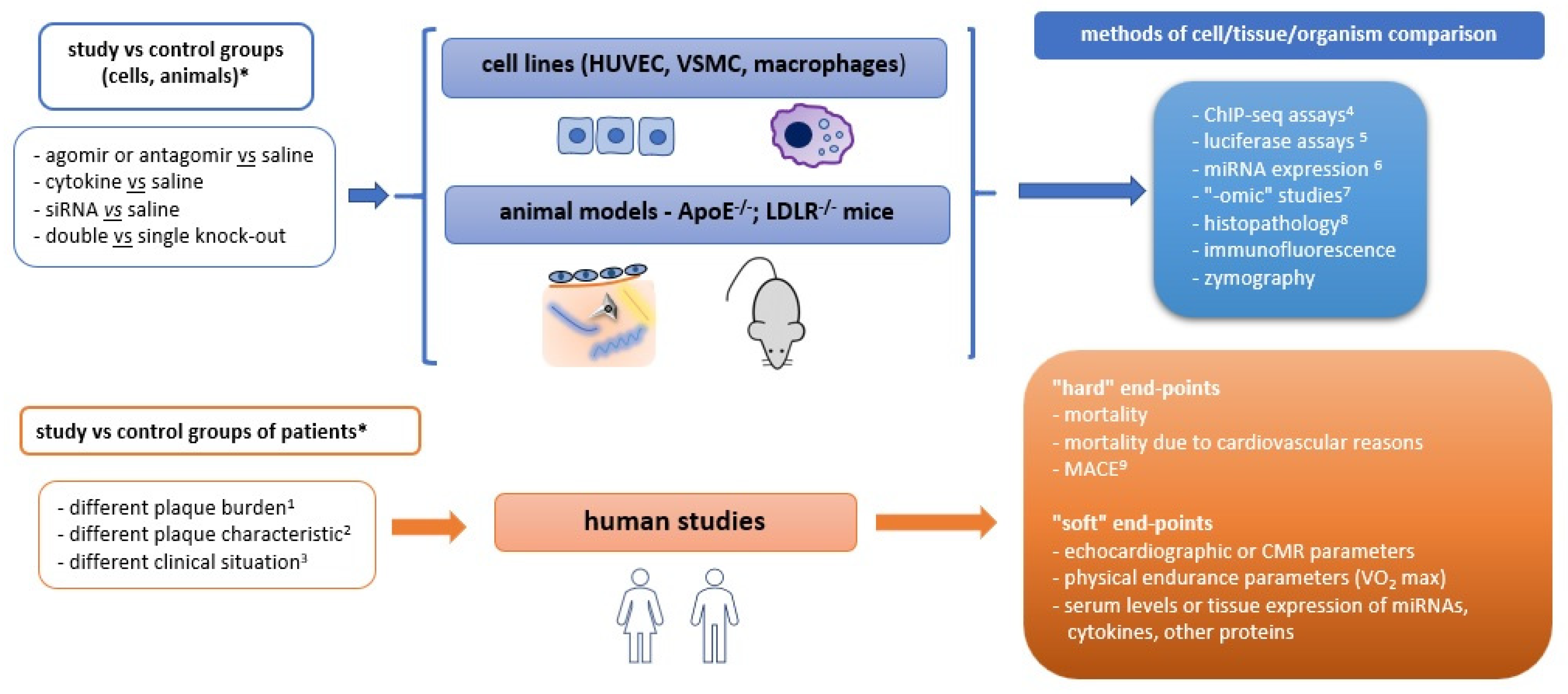
7. New Inspiring Ideas for Further Investigation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Yurdagul, A., Jr.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.; Goncalves, I.; Hultgardh-Nilsson, A. Collagen and related extracellular matrix proteins in atherosclerotic plaque development. Curr. Opin. Lipidol. 2014, 25, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta. 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Dallegri, F.; Montecucco, F.; Carbone, F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb. Haemost. 2017, 117, 7–18. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Mandl, M.; Muller, M.; Neideck, C.; Jansen, Y.; Hristov, M.; Gencer, S.; Peters, L.J.F.; Meiler, S.; Feld, M.; et al. Hematopoietic ChemR23 (Chemerin Receptor 23) Fuels Atherosclerosis by Sustaining an M1 Macrophage-Phenotype and Guidance of Plasmacytoid Dendritic Cells to Murine Lesions-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 685–693. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Nikiforov, N.G.; Elizova, N.V.; Orekhov, A.N. Macrophages and Their Contribution to the Development of Atherosclerosis. Results Probl. Cell Differ. 2017, 62, 273–298. [Google Scholar]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Courties, G.; Heidt, T.; Sebas, M.; Iwamoto, Y.; Jeon, D.; Truelove, J.; Tricot, B.; Wojtkiewicz, G.; Dutta, P.; Sager, H.B.; et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 2014, 63, 1556–1566. [Google Scholar] [CrossRef]
- Seneviratne, A.N.; Edsfeldt, A.; Cole, J.E.; Kassiteridi, C.; Swart, M.; Park, I.; Green, P.; Khoyratty, T.; Saliba, D.; Goddard, M.E.; et al. Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis. Circulation 2017, 136, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Robichaud, S.; Nguyen, M.A.; Geoffrion, M.; Wyatt, H.; Cottee, M.L.; Dennison, T.; Pietrangelo, A.; Lee, R.; Lagace, T.A.; et al. Loss of MLKL (Mixed Lineage Kinase Domain-Like Protein) Decreases Necrotic Core but Increases Macrophage Lipid Accumulation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1155–1167. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhang, X.; Cao, X.; Wu, C.; Guo, P. Cordycepin stimulates autophagy in macrophages and prevents atherosclerotic plaque formation in ApoE(-/-) mice. Oncotarget 2017, 8, 94726–94737. [Google Scholar] [CrossRef]
- Evans, T.D.; Jeong, S.J.; Zhang, X.; Sergin, I.; Razani, B. TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 2018, 14, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Sergin, I.; Evans, T.D.; Zhang, X.; Bhattacharya, S.; Stokes, C.J.; Song, E.; Ali, S.; Dehestani, B.; Holloway, K.B.; Micevych, P.S.; et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 2017, 8, 15750. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, C.V.; Pereira, F.E.; Smith, J.D.; Shapiro, L.H.; Ghosh, M. CD13 deficiency leads to increased oxidative stress and larger atherosclerotic lesions. Atherosclerosis 2019, 287, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.M.; Zhang, X.; Bandyopadhyay, C.; Rotllan, N.; Sugiyama, M.G.; Aryal, B.; Liu, X.; He, S.; Kraehling, J.R.; Ulrich, V.; et al. Caveolin-1 Regulates Atherogenesis by Attenuating Low-Density Lipoprotein Transcytosis and Vascular Inflammation Independently of Endothelial Nitric Oxide Synthase Activation. Circulation 2019, 140, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ramirez, C.M.; Aryal, B.; Madrigal-Matute, J.; Liu, X.; Diaz, A.; Torrecilla-Parra, M.; Suarez, Y.; Cuervo, A.M.; Sessa, W.C.; et al. Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Marzolla, V.; Armani, A.; Mammi, C.; Moss, M.E.; Pagliarini, V.; Pontecorvo, L.; Antelmi, A.; Fabbri, A.; Rosano, G.; Jaffe, I.Z.; et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int. J. Cardiol. 2017, 232, 233–242. [Google Scholar] [CrossRef]
- Zhuang, T.; Liu, J.; Chen, X.; Zhang, L.; Pi, J.; Sun, H.; Li, L.; Bauer, R.; Wang, H.; Yu, Z.; et al. Endothelial Foxp1 Suppresses Atherosclerosis via Modulation of Nlrp3 Inflammasome Activation. Circ. Res. 2019, 125, 590–605. [Google Scholar] [CrossRef]
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.H. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013, 127, 1028–1039. [Google Scholar] [CrossRef]
- Rohm, I.; Atiskova, Y.; Drobnik, S.; Fritzenwanger, M.; Kretzschmar, D.; Pistulli, R.; Zanow, J.; Kronert, T.; Mall, G.; Figulla, H.R.; et al. Decreased regulatory T cells in vulnerable atherosclerotic lesions: Imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediat. Inflamm. 2015, 2015, 364710. [Google Scholar] [CrossRef]
- Cochain, C.; Ait-Oufella, H.; Zernecke, A. Neutrophils promote atherosclerotic plaque destabilization in a mouse model of endotoxinaemia. Cardiovasc. Res. 2018, 114, 1573–1574. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Liu, Y.H.; Kanellakis, P.; Kallies, A.; Li, Y.; Cao, A.; Hosseini, H.; Tipping, P.; Toh, B.H.; Bobik, A.; et al. Follicular B Cells Promote Atherosclerosis via T Cell-Mediated Differentiation Into Plasma Cells and Secreting Pathogenic Immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e71–e84. [Google Scholar] [CrossRef] [PubMed]
- Marvin, J.; Rhoads, J.P.; Major, A.S. FcgammaRIIb on CD11c(+) cells modulates serum cholesterol and triglyceride levels and differentially affects atherosclerosis in male and female Ldlr(-/-) mice. Atherosclerosis 2019, 285, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Luo, S.; Wang, M.; Huang, Q.; Deng, Z.; de Febbo, C.; Daoui, A.; Liew, P.X.; Sukhova, G.K.; et al. IgE Contributes to Atherosclerosis and Obesity by Affecting Macrophage Polarization, Macrophage Protein Network, and Foam Cell Formation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 597–610. [Google Scholar] [CrossRef]
- Ma, T.; Gao, Q.; Zhu, F.; Guo, C.; Wang, Q.; Gao, F.; Zhang, L. Th17 cells and IL-17 are involved in the disruption of vulnerable plaques triggered by short-term combination stimulation in apolipoprotein E-knockout mice. Cell Mol. Immunol. 2013, 10, 338–348. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Barasch, E.; Gottdiener, J.S.; Aurigemma, G.; Kitzman, D.W.; Han, J.; Kop, W.J.; Tracy, R.P. The relationship between serum markers of collagen turnover and cardiovascular outcome in the elderly: The Cardiovascular Health Study. Circ. Heart Fail. 2011, 4, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Bruikman, C.S.; Vreeken, D.; Zhang, H.; van Gils, M.J.; Peter, J.; van Zonneveld, A.J.; Hovingh, G.K.; van Gils, J.M. The identification and function of a Netrin-1 mutation in a pedigree with premature atherosclerosis. Atherosclerosis 2020, 301, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bruikman, C.S.; Vreeken, D.; Hoogeveen, R.M.; Bom, M.J.; Danad, I.; Pinto-Sietsma, S.J.; van Zonneveld, A.J.; Knaapen, P.; Hovingh, G.K.; Stroes, E.S.G.; et al. Netrin-1 and the Grade of Atherosclerosis Are Inversely Correlated in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 462–472. [Google Scholar] [CrossRef]
- Ozaki, K.; Ohnishi, Y.; Iida, A.; Sekine, A.; Yamada, R.; Tsunoda, T.; Sato, H.; Sato, H.; Hori, M.; Nakamura, Y.; et al. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat. Genet. 2002, 32, 650–654. [Google Scholar] [CrossRef]
- Eales, J.M.; Maan, A.A.; Xu, X.; Michoel, T.; Hallast, P.; Batini, C.; Zadik, D.; Prestes, P.R.; Molina, E.; Denniff, M.; et al. Human Y Chromosome Exerts Pleiotropic Effects on Susceptibility to Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2386–2401. [Google Scholar] [CrossRef]
- Roadmap Epigenomics, C.; Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; et al. Integrative analysis of 111 reference human epigenomes. Nature 2015, 518, 317–330. [Google Scholar]
- Dron, J.S.; Hegele, R.A. Genetics of Triglycerides and the Risk of Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Lacey, M.; Baribault, C.; Ehrlich, K.C.; Ehrlich, M. Atherosclerosis-associated differentially methylated regions can reflect the disease phenotype and are often at enhancers. Atherosclerosis 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Harman, J.L.; Dobnikar, L.; Chappell, J.; Stokell, B.G.; Dalby, A.; Foote, K.; Finigan, A.; Freire-Pritchett, P.; Taylor, A.L.; Worssam, M.D.; et al. Epigenetic Regulation of Vascular Smooth Muscle Cells by Histone H3 Lysine 9 Dimethylation Attenuates Target Gene-Induction by Inflammatory Signaling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2289–2302. [Google Scholar] [CrossRef]
- Aravani, D.; Morris, G.E.; Jones, P.D.; Tattersall, H.K.; Karamanavi, E.; Kaiser, M.A.; Kostogrys, R.B.; Ghaderi Najafabadi, M.; Andrews, S.L.; Nath, M.; et al. HHIPL1, a Gene at the 14q32 Coronary Artery Disease Locus, Positively Regulates Hedgehog Signaling and Promotes Atherosclerosis. Circulation 2019, 140, 500–513. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver. Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kowara, M.; Paskal, W.; Gondek, A.; Glowczynska, R.; Opolski, G.; Wlodarski, P.; Cudnoch-Jedrzejewska, A. Increased serum microRNA21 levels reflect cardiac necrosis rather than plaque vulnerability in patients with acute coronary syndrome: A pilot study. Kardiol. Pol. 2019, 77, 1074–1077. [Google Scholar]
- Yang, S.; Ye, Z.M.; Chen, S.; Luo, X.Y.; Chen, S.L.; Mao, L.; Li, Y.; Jin, H.; Yu, C.; Xiang, F.X.; et al. MicroRNA-23a-5p promotes atherosclerotic plaque progression and vulnerability by repressing ATP-binding cassette transporter A1/G1 in macrophages. J. Mol. Cell Cardiol. 2018, 123, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Wei, Z.; Na, M.; Sun, G.; Zheng, S.; Zhu, X.; Xue, Y.; Zhou, Q.; Guo, S.; Xu, J.; et al. The PGC-1alpha/NRF1/miR-378a axis protects vascular smooth muscle cells from FFA-induced proliferation, migration and inflammation in atherosclerosis. Atherosclerosis 2020, 297, 136–145. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Musunuru, K. CRISPR-Cas9 Genome Editing for Treatment of Atherogenic Dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Furgurson, M.; Lagor, W.R. CRISPR: A promising tool for lipid physiology and therapeutics. Curr. Opin. Lipidol. 2019, 30, 172–176. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, T.S.; Li, S.; Hong, H.C.; Chen, Z.; Martin, M.; Zhou, X.; Huang, H.Y.; Su, S.H.; Zhang, J.; et al. Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Kruppel-Like Factor 4)-Mediated Histone Modifications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kostidis, S.; Tiemeier, G.L.; Sol, W.; de Vries, M.R.; Giera, M.; Carmeliet, P.; van den Berg, B.M.; Rabelink, T.J. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 350–364. [Google Scholar] [CrossRef]
- Nakamura, T.; Horikoshi, T.; Kugiyama, K. Relationship of a thinned medial layer to the attenuated contractile response in atherosclerotic coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H135–H142. [Google Scholar] [CrossRef]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Mancio, J.; Oikonomou, E.K.; Antoniades, C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018, 104, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, X.; Villacorta, L.; Rom, O.; Garcia-Barrio, M.T.; Guo, Y.; Fan, Y.; Zhu, T.; Zhang, J.; Zeng, R.; et al. Brown Adipocyte-Specific PPARgamma (Peroxisome Proliferator-Activated Receptor gamma) Deletion Impairs Perivascular Adipose Tissue Development and Enhances Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; van der Velden, S.; Rios-Morales, M.; van Faassen, M.J.R.; Loreti, M.G.; de Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Ascher, S.; Reinhardt, C. The gut microbiota: An emerging risk factor for cardiovascular and cerebrovascular disease. Eur. J. Immunol. 2018, 48, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Dwivedi, G.; O’Gara, F.; Caparros-Martin, J.; Ward, N.C. The gut microbiome and cardiovascular disease: Current knowledge and clinical potential. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H923–H938. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, B.; Kuypers, S.; van den Akker, N.M.S.; Molin, D.G.M.; Michiels, L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front. Immunol. 2018, 9, 1789. [Google Scholar] [CrossRef]
- Gasecka, A.; Pluta, K.; Solarska, K.; Rydz, B.; Eyileten, C.; Postula, M.; van der Pol, E.; Nieuwland, R.; Budnik, M.; Kochanowski, J.; et al. Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling. Biology 2021, 10, 97. [Google Scholar] [CrossRef]
- Williams, J.W.; Winkels, H.; Durant, C.P.; Zaitsev, K.; Ghosheh, Y.; Ley, K. Single Cell RNA Sequencing in Atherosclerosis Research. Circ. Res. 2020, 126, 1112–1126. [Google Scholar] [CrossRef]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Jae, N.; Dimmeler, S. Noncoding RNAs in Vascular Diseases. Circ. Res. 2020, 126, 1127–1145. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.D.; Fang, X.H.; Zhu, J.N.; Yang, J.; Pan, R.; Yuan, S.J.; Zeng, N.; Yang, Z.Z.; Yang, H.; et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovasc. Res. 2020, 116, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Bornfeldt, K.E. Intracellular and Intercellular Aspects of Macrophage Immunometabolism in Atherosclerosis. Circ. Res. 2020, 126, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Borck, P.C.; Guo, L.W.; Plutzky, J. BET Epigenetic Reader Proteins in Cardiovascular Transcriptional Programs. Circ. Res. 2020, 126, 1190–1208. [Google Scholar] [CrossRef]
- Moschonas, I.C.; Tselepis, A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019, 288, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Doring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox. Biol. 2019, 21, 101063. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Wang, M. Hv1 proton channel possibly promotes atherosclerosis by regulating reactive oxygen species production. Med. Hypotheses. 2020, 141, 109724. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowara, M.; Cudnoch-Jedrzejewska, A. Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. Int. J. Mol. Sci. 2021, 22, 3513. https://doi.org/10.3390/ijms22073513
Kowara M, Cudnoch-Jedrzejewska A. Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. International Journal of Molecular Sciences. 2021; 22(7):3513. https://doi.org/10.3390/ijms22073513
Chicago/Turabian StyleKowara, Michal, and Agnieszka Cudnoch-Jedrzejewska. 2021. "Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research" International Journal of Molecular Sciences 22, no. 7: 3513. https://doi.org/10.3390/ijms22073513
APA StyleKowara, M., & Cudnoch-Jedrzejewska, A. (2021). Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. International Journal of Molecular Sciences, 22(7), 3513. https://doi.org/10.3390/ijms22073513




