Mitochondria and Pharmacologic Cardiac Conditioning—At the Heart of Ischemic Injury
Abstract
1. Introduction
2. Mechanisms of Myocardial Damage and Cell Death after Ischemia/Reperfusion
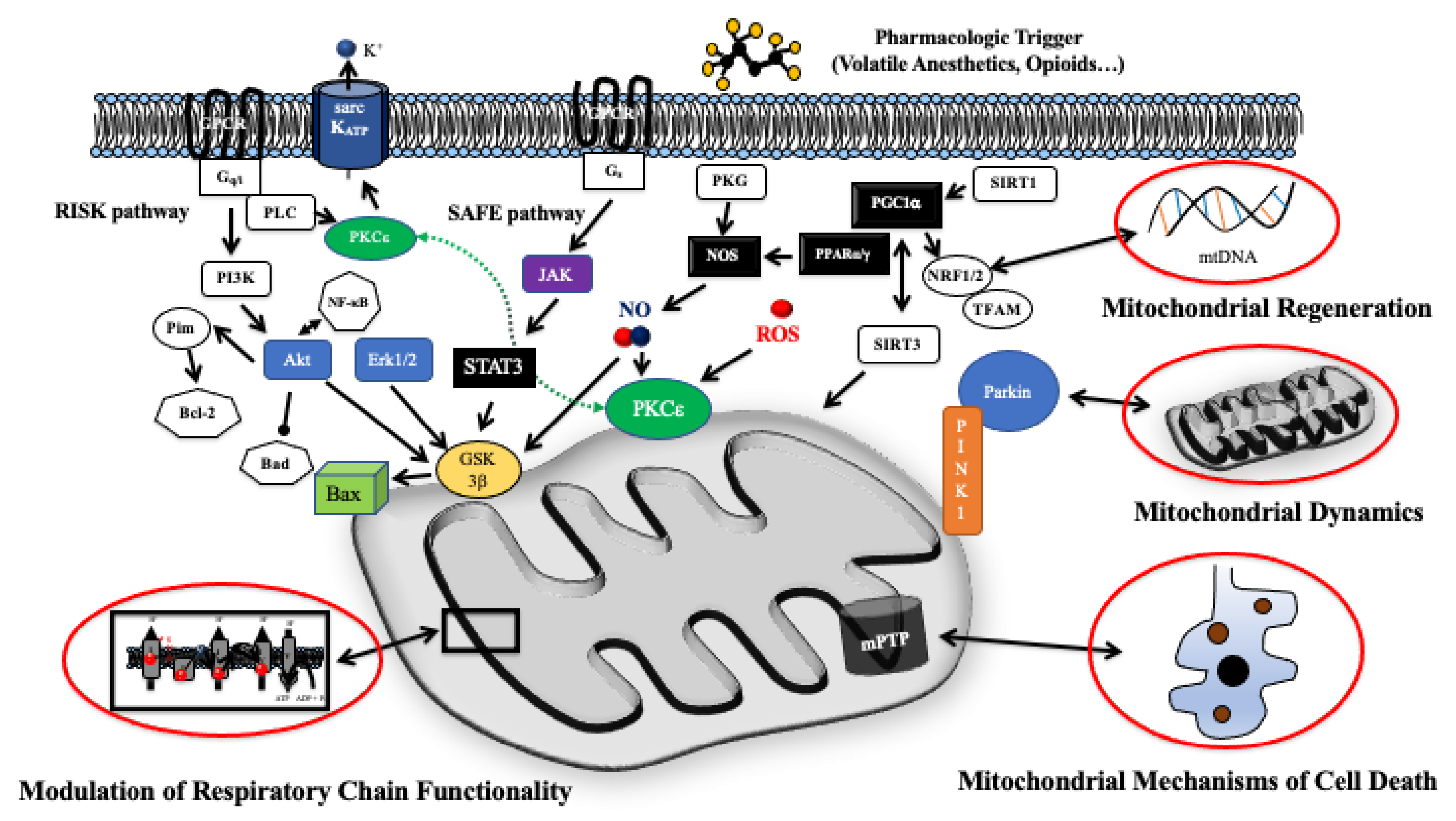
3. Intracellular Pathways of Pharmacologic Cardiac Conditioning
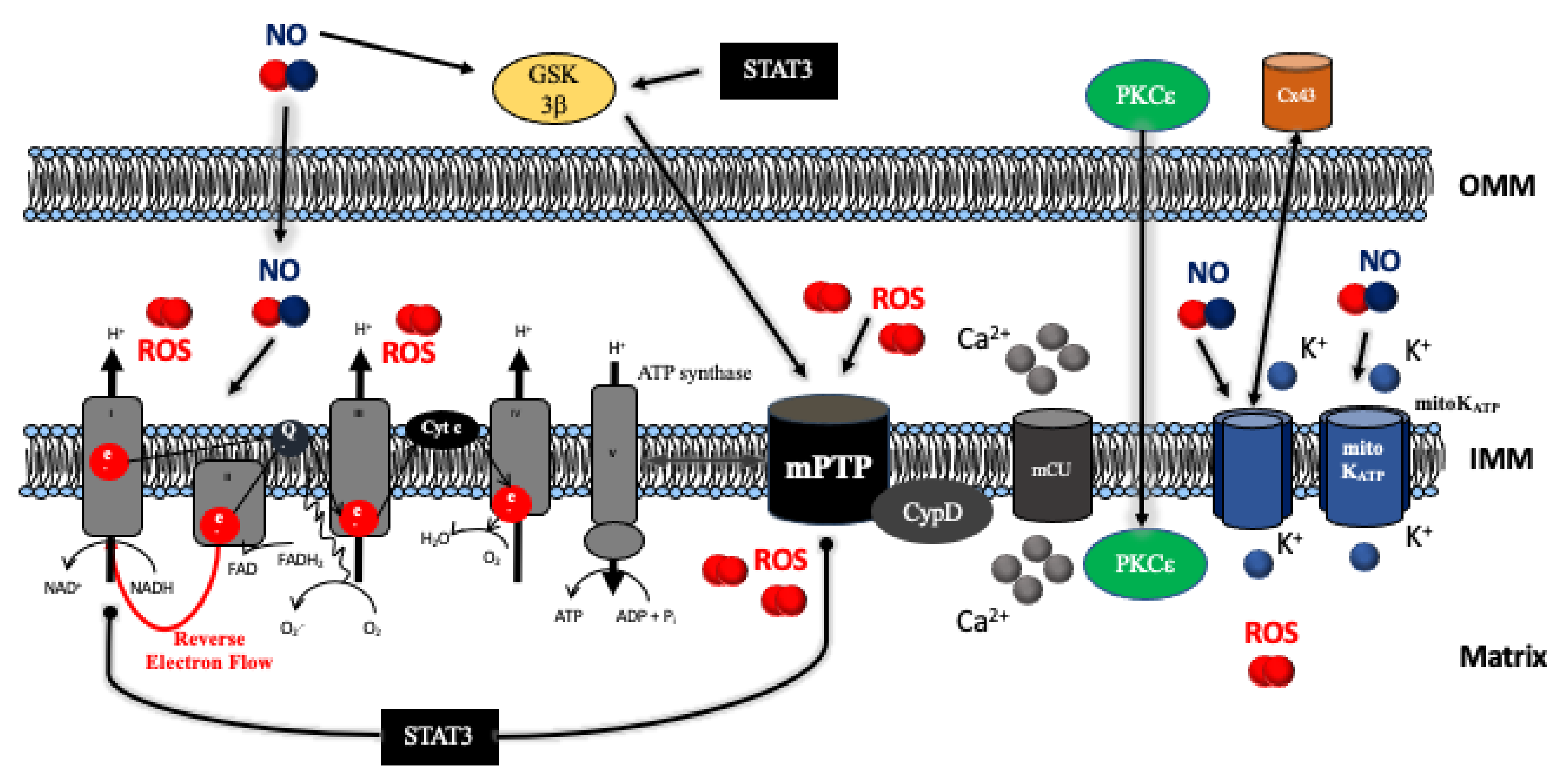
4. Mitochondrial Respiratory Chain and ATP Production
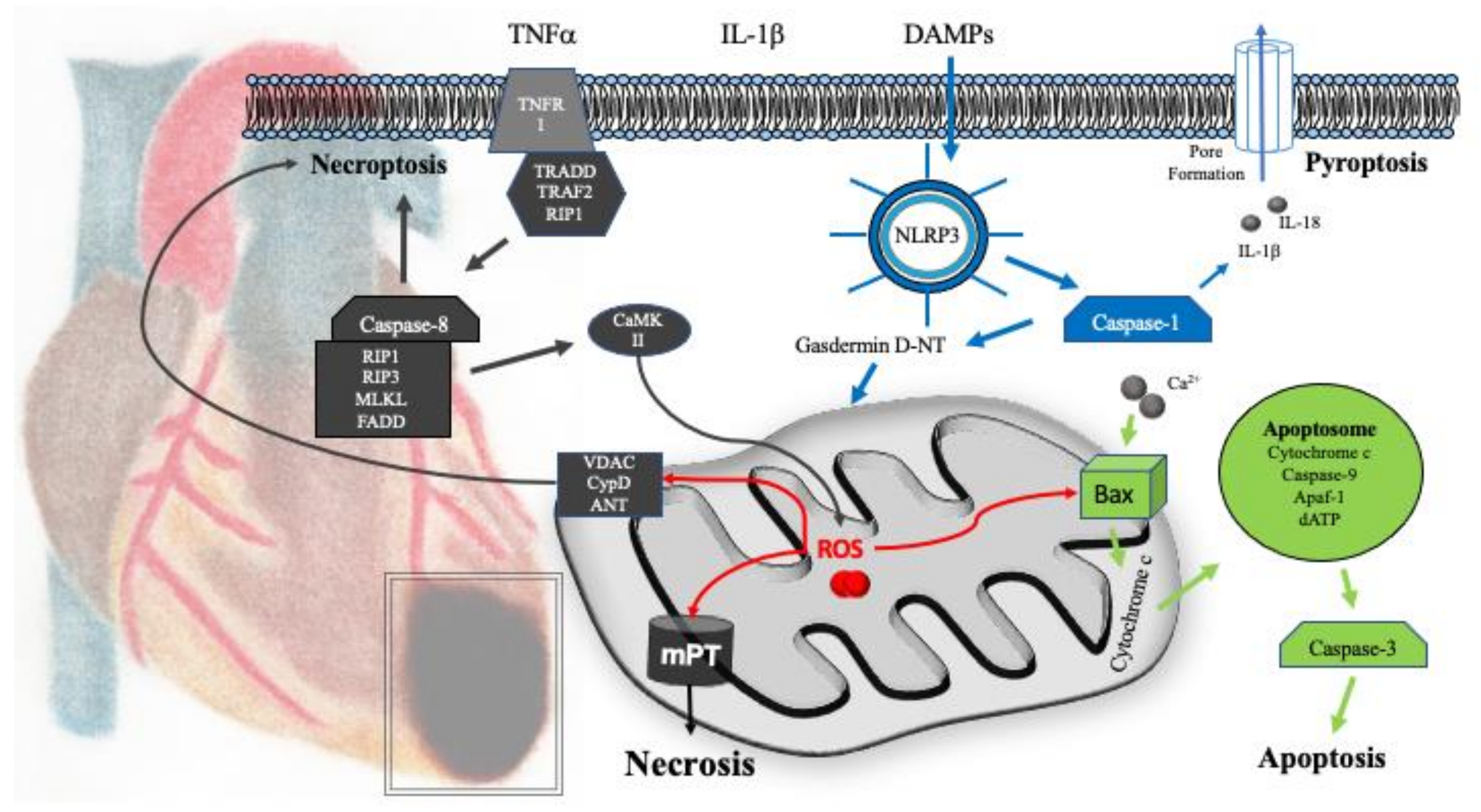
5. Mitochondrial Mechanisms of Cell Death
5.1. Necrosis
5.2. Apoptosis
5.3. Pyroptosis
6. Mitochondrial Dynamics
7. Mitochondrial Mechanisms of Regeneration
8. Conclusions, Clinical Translation, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kersten, J.R.; Riess, M.L. Opioid-induced cardioprotection. Curr. Pharm. Des. 2014, 20, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Kersten, J.R. Anesthetic preconditioning: An anesthesiologist’s tale. 1997. Anesthesiology 2011, 114, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Zitta, K.; Bein, B.; Wennemuth, G.; Broch, O.; Renner, J.; Schuett, T.; Lauer, F.; Maahs, D.; Hummitzsch, L.; et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: A pilot experimental study. Basic Res. Cardiol. 2013, 108, 314. [Google Scholar] [CrossRef]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Boning, A.; Niemann, B.; et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef]
- Schultz, J.E.; Hsu, A.K.; Gross, G.J. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ. Res. 1996, 78, 1100–1104. [Google Scholar] [CrossRef]
- Schultz, J.E.; Rose, E.; Yao, Z.; Gross, G.J. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am. J. Physiol. 1995, 268 Pt 2, H2157–H2161. [Google Scholar] [CrossRef]
- Hall, C.J.; Sanderson, L.E.; Crosier, K.E.; Crosier, P.S. Mitochondrial metabolism, reactive oxygen species, and macrophage function-fishing for insights. J. Mol. Med. (Berl.) 2014, 92, 1119–1128. [Google Scholar] [CrossRef]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Lotz, C.; Lin, A.J.; Black, C.M.; Zhang, J.; Lau, E.; Deng, N.; Wang, Y.; Zong, N.C.; Choi, J.H.; Xu, T.; et al. Characterization, design, and function of the mitochondrial proteome: From organs to organisms. J. Proteome Res. 2014, 13, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.A.; Valverde, C.A.; Sanchez, G.; Said, M.; Rodriguez, J.S.; Portiansky, E.L.; Kaetzel, M.A.; Dedman, J.R.; Donoso, P.; Kranias, E.G.; et al. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2010, 48, 1298–1306. [Google Scholar] [CrossRef]
- Weber, C.R.; Piacentino, V., III; Houser, S.R.; Bers, D.M. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation 2003, 108, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Garbincius, J.F.; Luongo, T.S.; Elrod, J.W. The debate continues—What is the role of MCU and mitochondrial calcium uptake in the heart? J. Mol. Cell. Cardiol. 2020, 143, 163–174. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Luongo, T.S.; Lambert, J.P.; Yuan, A.; Zhang, X.; Gross, P.; Song, J.; Shanmughapriya, S.; Gao, E.; Jain, M.; Houser, S.R.; et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep. 2015, 12, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.P.; Luongo, T.S.; Tomar, D.; Jadiya, P.; Gao, E.; Zhang, X.; Lucchese, A.M.; Kolmetzky, D.W.; Shah, N.S.; Elrod, J.W. MCUB Regulates the Molecular Composition of the Mitochondrial Calcium Uniporter Channel to Limit Mitochondrial Calcium Overload During Stress. Circulation 2019, 140, 1720–1733. [Google Scholar] [CrossRef]
- Lange, M.; Redel, A.; Smul, T.M.; Lotz, C.; Nefzger, T.; Stumpner, J.; Blomeyer, C.; Gao, F.; Roewer, N.; Kehl, F. Desflurane-induced preconditioning has a threshold that is lowered by repetitive application and is mediated by beta 2-adrenergic receptors. J. Cardiothorac. Vasc. Anesth. 2009, 23, 607–613. [Google Scholar] [CrossRef]
- Lange, M.; Smul, T.M.; Redel, A.; Lotz, C.; Jazbutyte, V.; Schnupp, V.; Roewer, N.; Kehl, F. Differential role of calcium/calmodulin-dependent protein kinase II in desflurane-induced preconditioning and cardioprotection by metoprolol: Metoprolol blocks desflurane-induced preconditioning. Anesthesiology 2008, 109, 72–80. [Google Scholar] [CrossRef]
- Redel, A.; Stumpner, J.; Smul, T.M.; Lange, M.; Jazbutyte, V.; Ridyard, D.G.; Roewer, N.; Kehl, F. Endothelial nitric oxide synthase mediates the first and inducible nitric oxide synthase mediates the second window of desflurane-induced preconditioning. J. Cardiothorac. Vasc. Anesth. 2013, 27, 494–501. [Google Scholar] [CrossRef]
- Smul, T.M.; Lange, M.; Redel, A.; Burkhard, N.; Roewer, N.; Kehl, F. Desflurane-induced preconditioning against myocardial infarction is mediated by nitric oxide. Anesthesiology 2006, 105, 719–725. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Song, C.X.; Zheng, Y.T.; Wang, G.W.; Zhang, J.; Wang, O.L.; Guo, Y.; Bolli, R.; Cardwell, E.M.; Ping, P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003, 92, 873–880. [Google Scholar] [CrossRef]
- Qiu, Y.; Ping, P.; Tang, X.L.; Manchikalapudi, S.; Rizvi, A.; Zhang, J.; Takano, H.; Wu, W.J.; Teschner, S.; Bolli, R. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J. Clin. Investig. 1998, 101, 2182–2198. [Google Scholar] [CrossRef] [PubMed]
- Le Good, J.A.; Ziegler, W.H.; Parekh, D.B.; Alessi, D.R.; Cohen, P.; Parker, P.J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 1998, 281, 2042–2045. [Google Scholar] [CrossRef]
- Rossello, X.; Yellon, D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2018, 113, 2. [Google Scholar] [CrossRef]
- Chen, E.; Chen, C.; Niu, Z.; Gan, L.; Wang, Q.; Li, M.; Cai, X.; Gao, R.; Katakam, S.; Chen, H.; et al. Poly(I:C) preconditioning protects the heart against myocardial ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent pathway. Signal Transduct. Target. Ther. 2020, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, F.; Yazdi, M.B.; Zarch, M.B.; Zarch, M.B.; Hekmatimoghaddam, S. Cross talk of the first-line defense TLRs with PI3K/Akt pathway, in preconditioning therapeutic approach. Mol. Cell. Ther. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Kim, S.H.; Pepe, S.; Fu, Q.; Fishbein, K.W.; Ziman, B.D.; Wang, S.; Ytrehus, K.; Antos, C.L.; et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004, 113, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Role of glycogen synthase kinase-3beta in cardioprotection. Circ. Res. 2009, 104, 1240–1252. [Google Scholar] [CrossRef]
- Xuan, Y.T.; Guo, Y.; Han, H.; Zhu, Y.; Bolli, R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc. Natl. Acad. Sci. USA 2001, 98, 9050–9055. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Potla, R.; Chwae, Y.J.; Sepuri, N.B.; Zhang, Q.; Koeck, T.; Derecka, M.; Szczepanek, K.; Szelag, M.; Gornicka, A.; et al. Function of mitochondrial Stat3 in cellular respiration. Science 2009, 323, 793–797. [Google Scholar] [CrossRef]
- Boengler, K.; Ungefug, E.; Heusch, G.; Schulz, R. The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr. Pharm. Des. 2013, 19, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Harhous, Z.; Booz, G.W.; Ovize, M.; Bidaux, G.; Kurdi, M. An Update on the Multifaceted Roles of STAT3 in the Heart. Front. Cardiovasc. Med. 2019, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.A.; Hyun, M.; Cantwell, M.; Raza, A.; Mertens, C.; Raje, V.; Sisler, J.; Tracy, E.; Torres-Odio, S.; Gispert, S.; et al. Stress-induced dynamic regulation of mitochondrial STAT3 and its association with cyclophilin D reduce mitochondrial ROS production. Sci. Signal 2017, 10, eaag2588. [Google Scholar] [CrossRef]
- Pavo, N.; Lukovic, D.; Zlabinger, K.; Zimba, A.; Lorant, D.; Goliasch, G.; Winkler, J.; Pils, D.; Auer, K.; Jan Ankersmit, H.; et al. Sequential activation of different pathway networks in ischemia-affected and non-affected myocardium, inducing intrinsic remote conditioning to prevent left ventricular remodeling. Sci. Rep. 2017, 7, 43958. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977, 252, 8731–8739. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am. J. Physiol. 1986, 250 Pt 2, H741–H748. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Tandler, B.; Ye, J.; Slabe, T.J.; Turkaly, J.; Hoppel, C.L. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. 1997, 273 Pt 2, H1544–H1554. [Google Scholar] [CrossRef]
- Komlodi, T.; Geibl, F.F.; Sassani, M.; Ambrus, A.; Tretter, L. Membrane potential and delta pH dependency of reverse electron transport-associated hydrogen peroxide production in brain and heart mitochondria. J. Bioenerg. Biomembr. 2018, 50, 355–365. [Google Scholar] [CrossRef]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005, 280, 29403–29408. [Google Scholar] [CrossRef]
- Bienengraeber, M.; Pellitteri-Hahn, M.; Hirata, N.; Baye, T.M.; Bosnjak, Z.J.; Olivier, M. Quantitative characterization of changes in the cardiac mitochondrial proteome during anesthetic preconditioning and ischemia. Physiol. Genom. 2013, 45, 163–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, J.; Zhu, M.; Schaub, M.C.; Gehrig, P.; Roschitzki, B.; Lucchinetti, E.; Zaugg, M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: Phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc. Res. 2008, 80, 20–29. [Google Scholar] [CrossRef]
- Hanley, P.J.; Ray, J.; Brandt, U.; Daut, J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J. Physiol. 2002, 544 Pt 3, 687–693. [Google Scholar] [CrossRef]
- Ljubkovic, M.; Mio, Y.; Marinovic, J.; Stadnicka, A.; Warltier, D.C.; Bosnjak, Z.J.; Bienengraeber, M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am. J. Physiol. Cell. Physiol. 2007, 292, C1583–C1590. [Google Scholar] [CrossRef]
- Sedlic, F.; Sepac, A.; Pravdic, D.; Camara, A.K.; Bienengraeber, M.; Brzezinska, A.K.; Wakatsuki, T.; Bosnjak, Z.J. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: Roles of ROS and Ca2+. Am. J. Physiol. Cell. Physiol. 2010, 299, C506–C515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J. Pharmacol. Exp. Ther. 2006, 319, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Novalija, E.; Kevin, L.G.; Eells, J.T.; Henry, M.M.; Stowe, D.F. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology 2003, 98, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Zhang, J.; Fang, C.; Liem, D.; Ping, P. Isoflurane protects the myocardium against ischemic injury via the preservation of mitochondrial respiration and its supramolecular organization. Anesth. Analg. 2015, 120, 265–274. [Google Scholar] [CrossRef]
- Lotz, C.; Stumpner, J.; Smul, T.M. Sevoflurane as opposed to propofol anesthesia preserves mitochondrial function and alleviates myocardial ischemia/reperfusion injury. Biomed. Pharmacother. 2020, 129, 110417. [Google Scholar] [CrossRef]
- Galkin, A.; Abramov, A.Y.; Frakich, N.; Duchen, M.R.; Moncada, S. Lack of oxygen deactivates mitochondrial complex I: Implications for ischemic injury? J. Biol. Chem. 2009, 284, 36055–36061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ross, T.; Hu, Y.; Lesnefsky, E.J. Blockade of electron transport at the onset of reperfusion decreases cardiac injury in aged hearts by protecting the inner mitochondrial membrane. J. Aging Res. 2012, 2012, 753949. [Google Scholar] [CrossRef]
- Stewart, S.; Lesnefsky, E.J.; Chen, Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl. Res. 2009, 153, 224–231. [Google Scholar] [CrossRef]
- Ludwig, L.M.; Tanaka, K.; Eells, J.T.; Weihrauch, D.; Pagel, P.S.; Kersten, J.R.; Warltier, D.C. Preconditioning by isoflurane is mediated by reactive oxygen species generated from mitochondrial electron transport chain complex III. Anesth. Analg. 2004, 99, 1308–1315. [Google Scholar] [CrossRef]
- Kornfeld, O.S.; Hwang, S.; Disatnik, M.H.; Chen, C.H.; Qvit, N.; Mochly-Rosen, D. Mitochondrial reactive oxygen species at the heart of the matter: New therapeutic approaches for cardiovascular diseases. Circ. Res. 2015, 116, 1783–1799. [Google Scholar] [CrossRef]
- Zhang, G.; Sheng, M.; Wang, J.; Teng, T.; Sun, Y.; Yang, Q.; Xu, Z. Zinc improves mitochondrial respiratory function and prevents mitochondrial ROS generation at reperfusion by phosphorylating STAT3 at Ser(727). J. Mol. Cell. Cardiol. 2018, 118, 169–182. [Google Scholar] [CrossRef]
- Szczepanek, K.; Xu, A.; Hu, Y.; Thompson, J.; He, J.; Larner, A.C.; Salloum, F.N.; Chen, Q.; Lesnefsky, E.J. Cardioprotective function of mitochondrial-targeted and transcriptionally inactive STAT3 against ischemia and reperfusion injury. Basic Res. Cardiol. 2015, 110, 53. [Google Scholar] [CrossRef]
- Harhous, Z.; Badawi, S.; Bona, N.G.; Pillot, B.; Augeul, L.; Paillard, M.; Booz, G.W.; Canet-Soulas, E.; Ovize, M.; Kurdi, M.; et al. Critical appraisal of STAT3 pattern in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2019, 131, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Menazza, S.; Holmstrom, K.M.; Parks, R.J.; Liu, J.; Sun, J.; Liu, J.; Pan, X.; Murphy, E. The ins and outs of mitochondrial calcium. Circ. Res. 2015, 116, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Demaurex, N.; Poburko, D.; Frieden, M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim. Biophys. Acta 2009, 1787, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Giorgio, V.; Carrer, A.; Franchin, C.; Arrigoni, G.; Jiko, C.; Abe, K.; Maeda, S.; Shinzawa-Itoh, K.; Bogers, J.F.M.; et al. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 2019, 10, 4341. [Google Scholar] [CrossRef] [PubMed]
- Gerle, C. Mitochondrial F-ATP synthase as the permeability transition pore. Pharmacol. Res. 2020, 160, 105081. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P. The cardiac mitochondrion: Nexus of stress. Annu. Rev. Physiol. 2010, 72, 61–80. [Google Scholar] [CrossRef]
- He, J.; Ford, H.C.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 3409–3414. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Maddock, H.L.; Baxter, G.F.; Yellon, D.M. Inhibiting mitochondrial permeability transition pore opening: A new paradigm for myocardial preconditioning? Cardiovasc. Res. 2002, 55, 534–543. [Google Scholar] [CrossRef]
- Krolikowski, J.G.; Bienengraeber, M.; Weihrauch, D.; Warltier, D.C.; Kersten, J.R.; Pagel, P.S. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: The role of mitochondrial KATP channels. Anesth. Analg. 2005, 101, 1590–1596. [Google Scholar] [CrossRef]
- Pagel, P.S.; Krolikowski, J.G.; Shim, Y.H.; Venkatapuram, S.; Kersten, J.R.; Weihrauch, D.; Warltier, D.C.; Pratt, P.F., Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 2007, 105, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, M.; Behmenburg, F.; Raible, M.; Blase, D.; Grievink, H.; Hollmann, M.W.; Heinen, A.; Huhn, R. Morphine-Induced Preconditioning: Involvement of Protein Kinase A and Mitochondrial Permeability Transition Pore. PLoS ONE 2016, 11, e0151025. [Google Scholar] [CrossRef]
- Obame, F.N.; Plin-Mercier, C.; Assaly, R.; Zini, R.; Dubois-Rande, J.L.; Berdeaux, A.; Morin, D. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J. Pharmacol. Exp. Ther. 2008, 326, 252–258. [Google Scholar] [CrossRef]
- Ong, S.B.; Dongworth, R.K.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Role of the MPTP in conditioning the heart-translatability and mechanism. Br. J. Pharmacol. 2015, 172, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Piriou, V.; Chiari, P.; Gateau-Roesch, O.; Argaud, L.; Muntean, D.; Salles, D.; Loufouat, J.; Gueugniaud, P.Y.; Lehot, J.J.; Ovize, M. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology 2004, 100, 581–588. [Google Scholar] [CrossRef]
- Pravdic, D.; Sedlic, F.; Mio, Y.; Vladic, N.; Bienengraeber, M.; Bosnjak, Z.J. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology 2009, 111, 267–274. [Google Scholar] [CrossRef]
- Boengler, K.; Hilfiker-Kleiner, D.; Heusch, G.; Schulz, R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 2010, 105, 771–785. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Boston-Griffiths, E.A.; Yellon, D.M. Cyclosporin A and cardioprotection: From investigative tool to therapeutic agent. Br. J. Pharmacol. 2012, 165, 1235–1245. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zhe-Wei, S.; Li-Sha, G.; Yue-Chun, L. The Role of Necroptosis in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Koshinuma, S.; Miyamae, M.; Kaneda, K.; Kotani, J.; Figueredo, V.M. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia-reperfusion injury. J. Anesth. 2014, 28, 235–241. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, Y.; Yang, J.; Yin, Y.; Liu, M.; Shi, Z.; Mu, N.; Yu, L.; Ma, H. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J. Mol. Cell. Cardiol. 2018, 125, 185–194. [Google Scholar] [CrossRef]
- Szobi, A.; Farkasova-Ledvenyiova, V.; Lichy, M.; Murarikova, M.; Carnicka, S.; Ravingerova, T.; Adameova, A. Cardioprotection of ischaemic preconditioning is associated with inhibition of translocation of MLKL within the plasma membrane. J. Cell. Mol. Med. 2018, 22, 4183–4196. [Google Scholar] [CrossRef]
- Oerlemans, M.I.; Liu, J.; Arslan, F.; den Ouden, K.; van Middelaar, B.J.; Doevendans, P.A.; Sluijter, J.P. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res. Cardiol. 2012, 107, 270. [Google Scholar] [CrossRef]
- Koudstaal, S.; Oerlemans, M.I.; Van der Spoel, T.I.; Janssen, A.W.; Hoefer, I.E.; Doevendans, P.A.; Sluijter, J.P.; Chamuleau, S.A. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur. J. Clin. Investig. 2015, 45, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Lutz, M.; Carter, N.; Sosna, J.; Jacoby, C.; Vucur, M.; Gautheron, J.; Roderburg, C.; Borg, N.; Reisinger, F.; et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc. Res. 2014, 103, 206–216. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Zhou, X.; Sun, X.; Cao, J.; Liu, Y.; Wang, X. Dexmedetomidine Preconditioning Protects Cardiomyocytes Against Hypoxia/Reoxygenation-Induced Necroptosis by Inhibiting HMGB1-Mediated Inflammation. Cardiovasc. Drugs Ther. 2019, 33, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Tian, S.; Wang, H.; Wu, H.; Zhang, A.; Gao, C. Anti-inflammatory Effects of Perioperative Dexmedetomidine Administered as an Adjunct to General Anesthesia: A Meta-analysis. Sci. Rep. 2015, 5, 12342. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Quaini, F.; Sala, R.; Lagrasta, C.; Corradi, D.; Bonacina, E.; Gambert, S.R.; Cigola, E.; Anversa, P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J. Mol. Cell. Cardiol. 1996, 28, 2005–2016. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef]
- Bialik, S.; Geenen, D.L.; Sasson, I.E.; Cheng, R.; Horner, J.W.; Evans, S.M.; Lord, E.M.; Koch, C.J.; Kitsis, R.N. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Investig. 1997, 100, 1363–1372. [Google Scholar] [CrossRef]
- Abbate, A.; Biondi-Zoccai, G.G.; Bussani, R.; Dobrina, A.; Camilot, D.; Feroce, F.; Rossiello, R.; Baldi, F.; Silvestri, F.; Biasucci, L.M.; et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 753–760. [Google Scholar] [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Budde, J.M.; Morris, C.; Wang, N.P.; Velez, D.A.; Muraki, S.; Guyton, R.A.; Vinten-Johansen, J. Adenosine attenuates reperfusion-induced apoptotic cell death by modulating expression of Bcl-2 and Bax proteins. J. Mol. Cell. Cardiol. 2001, 33, 57–68. [Google Scholar] [CrossRef]
- Oikawa, M.; Wu, M.; Lim, S.; Knight, W.E.; Miller, C.L.; Cai, Y.; Lu, Y.; Blaxall, B.C.; Takeishi, Y.; Abe, J.; et al. Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2013, 64, 11–19. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Zhou, Y.; Su, Q.; Liu, T.; Li, L. Levosimendan Pretreatment Inhibits Myocardial Apoptosis in Swine after Coronary Microembolization. Cell. Physiol. Biochem. 2017, 41, 67–78. [Google Scholar] [CrossRef]
- Okubo, S.; Tanabe, Y.; Takeda, K.; Kitayama, M.; Kanemitsu, S.; Kukreja, R.C.; Takekoshi, N. Ischemic preconditioning and morphine attenuate myocardial apoptosis and infarction after ischemia-reperfusion in rabbits: Role of delta-opioid receptor. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1786–H1791. [Google Scholar] [CrossRef][Green Version]
- Tian, X.; Zhou, Y.; Wang, Y.; Zhang, S.; Feng, J.; Wang, X.; Guo, H.; Fan, R.; Feng, N.; Jia, M.; et al. Mitochondrial Dysfunction and Apoptosis Are Attenuated on kappa-Opioid Receptor Activation through AMPK/GSK-3beta Pathway after Myocardial Ischemia and Reperfusion. J. Cardiovasc. Pharmacol. 2019, 73, 70–81. [Google Scholar] [CrossRef]
- Rong, F.; Peng, Z.; Ye, M.X.; Zhang, Q.Y.; Zhao, Y.; Zhang, S.M.; Guo, H.T.; Hui, B.; Wang, Y.M.; Liang, C.; et al. Myocardial apoptosis and infarction after ischemia/reperfusion are attenuated by kappa-opioid receptor agonist. Arch. Med. Res. 2009, 40, 227–234. [Google Scholar] [CrossRef]
- Raphael, J.; Abedat, S.; Rivo, J.; Meir, K.; Beeri, R.; Pugatsch, T.; Zuo, Z.; Gozal, Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J. Pharmacol. Exp. Ther. 2006, 318, 186–194. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Qiu, H.; Chen, J.; Liu, H. Effects of volatile anesthetic preconditioning on expression of NFkB-regulated genes in aged rat myocardium. J. Biomed. Res. 2017, 33, 264–270. [Google Scholar]
- Sopka, S.; Mertens, C.; Roehl, A.B.; Schiffl, K.; Rossaint, R.; Classen-Linke, I. Effects of xenon and isoflurane on apoptosis and inflammation in a porcine myocardial infarction model. Ann. Anat. 2013, 195, 166–174. [Google Scholar] [CrossRef]
- Roehl, A.B.; Funcke, S.; Becker, M.M.; Goetzenich, A.; Bleilevens, C.; Rossaint, R.; Steendijk, P.; Hein, M. Xenon and isoflurane reduce left ventricular remodeling after myocardial infarction in the rat. Anesthesiology 2013, 118, 1385–1394. [Google Scholar] [CrossRef]
- Jin, Y.C.; Kim, W.; Ha, Y.M.; Shin, I.W.; Sohn, J.T.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Propofol limits rat myocardial ischemia and reperfusion injury with an associated reduction in apoptotic cell death in vivo. Vascul. Pharmacol. 2009, 50, 71–77. [Google Scholar] [CrossRef]
- Su, Z.; Hou, X.K.; Wen, Q.P. Propofol induces apoptosis of epithelial ovarian cancer cells by upregulation of microRNA let-7i expression. Eur. J. Gynaecol. Oncol. 2014, 35, 688–691. [Google Scholar]
- Zhang, J.; Shan, W.F.; Jin, T.T.; Wu, G.Q.; Xiong, X.X.; Jin, H.Y.; Zhu, S.M. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J. Transl. Med. 2014, 12, 279. [Google Scholar] [CrossRef]
- Ibacache, M.; Sanchez, G.; Pedrozo, Z.; Galvez, F.; Humeres, C.; Echevarria, G.; Duaso, J.; Hassi, M.; Garcia, L.; Diaz-Araya, G.; et al. Dexmedetomidine preconditioning activates pro-survival kinases and attenuates regional ischemia/reperfusion injury in rat heart. Biochim. Biophys. Acta 2012, 1822, 537–545. [Google Scholar] [CrossRef]
- Chang, J.H.; Jin, M.M.; Liu, J.T. Dexmedetomidine pretreatment protects the heart against apoptosis in ischemia/reperfusion injury in diabetic rats by activating PI3K/Akt signaling in vivo and in vitro. Biomed. Pharmacother. 2020, 127, 110188. [Google Scholar] [CrossRef]
- Peng, K.; Chen, W.R.; Xia, F.; Liu, H.; Meng, X.W.; Zhang, J.; Liu, H.Y.; Xia, Z.Y.; Ji, F.H. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1alpha signalling. J. Cell. Mol. Med. 2020, 24, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Li, T.; Cao, L.; Yu, Y.Y.; Chen, L.L.; Fan, X.H.; Yang, B.B.; Tan, X.Q. Dexmedetomidine attenuates H2O2-induced neonatal rat cardiomyocytes apoptosis through mitochondria- and ER-medicated oxidative stress pathways. Mol. Med. Rep. 2018, 17, 7258–7264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, F.; Zhao, H.; Peng, K.; Liu, H.; Meng, X.; Chen, C.; Ji, F. Dexmedetomidine-induced cardioprotection is mediated by inhibition of high mobility group box-1 and the cholinergic anti-inflammatory pathway in myocardial ischemia-reperfusion injury. PLoS ONE 2019, 14, e0218726. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, C.J.; Abbate, A.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Collino, M.; De Kleijn, D.P.V.; Downey, J.M.; Pagliaro, P.; Preissner, K.T.; Takahashi, M.; et al. Innate immunity as a target for acute cardioprotection. Cardiovasc. Res. 2019, 115, 1131–1142. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef]
- Mezzaroma, E.; Toldo, S.; Farkas, D.; Seropian, I.M.; Van Tassell, B.W.; Salloum, F.N.; Kannan, H.R.; Menna, A.C.; Voelkel, N.F.; Abbate, A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 19725–19730. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Chojnacki, J.; Toldo, S.; Mezzaroma, E.; Tranchida, N.; Rose, S.W.; Federici, M.; Van Tassell, B.W.; Zhang, S.; Abbate, A. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J. Cardiovasc. Pharmacol. 2014, 63, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, G.P.; Bosch, L.; Ellenbroek, G.H.; de Haan, J.J.; van Solinge, W.W.; Cooper, M.A.; Arslan, F.; de Jager, S.C.; Robertson, A.A.; Pasterkamp, G.; et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 2017, 38, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Sandanger, O.; Gao, E.; Ranheim, T.; Bliksoen, M.; Kaasboll, O.J.; Alfsnes, K.; Nymo, S.H.; Rashidi, A.; Ohm, I.K.; Attramadal, H.; et al. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem. Biophys. Res. Commun. 2016, 469, 1012–1020. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Jong, W.M.; Eerbeek, O.; Koeman, A.; Pulskens, W.P.; Butter, L.M.; Leemans, J.C.; Hollmann, M.W. Deletion of the innate immune NLRP3 receptor abolishes cardiac ischemic preconditioning and is associated with decreased Il-6/STAT3 signaling. PLoS ONE 2012, 7, e40643. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Quirin, C.; Glytsou, C.; Corrado, M.; Urbani, A.; Pellattiero, A.; Calvo, E.; Vazquez, J.; Enriquez, J.A.; Gerle, C.; et al. The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function. Nat. Commun. 2018, 9, 3399. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Prunier, F.; Girao, H.; Dorn, G.; Hausenloy, D.J.; EU-CARDIOPROTECTION COST Action (CA16225). Targeting mitochondrial fusion and fission proteins for cardioprotection. J. Cell. Mol. Med. 2020, 24, 6571–6585. [Google Scholar] [CrossRef]
- Ong, S.B.; Subrayan, S.; Lim, S.Y.; Yellon, D.M.; Davidson, S.M.; Hausenloy, D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.W.; Fang, Y.H.; Han, M.; Zhang, H.J.; Hong, Z.; Banathy, A.; Morrow, E.; Ryan, J.J.; Archer, S.L. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014, 28, 316–326. [Google Scholar] [CrossRef]
- Yu, J.; Maimaitili, Y.; Xie, P.; Wu, J.J.; Wang, J.; Yang, Y.N.; Ma, H.P.; Zheng, H. High glucose concentration abrogates sevoflurane post-conditioning cardioprotection by advancing mitochondrial fission but dynamin-related protein 1 inhibitor restores these effects. Acta Physiol. 2017, 220, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Kwek, X.Y.; Katwadi, K.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Ismail, N.I.; Lin, Y.H.; Yap, E.P.; Lim, S.Y.; Ja, K.; et al. Targeting Mitochondrial Fission Using Mdivi-1 in A Clinically Relevant Large Animal Model of Acute Myocardial Infarction: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 3972. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015, 116, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Youle, R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012, 125 Pt 4, 795–799. [Google Scholar] [CrossRef]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.M.; Hernandez, G.; Lee, P.; Huang, C.; Ratliff, E.P.; Sin, J.; Thornton, C.A.; Damasco, M.V.; Gottlieb, R.A. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal 2014, 21, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Zhang, X.; Lee, Y.; Hanna, R.A.; Quinsay, M.N.; Nguyen, C.K.; Jimenez, R.; Petrosyan, S.; Murphy, A.N.; Gustafsson, A.B. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 2013, 288, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Siddall, H.K.; Yellon, D.M.; Ong, S.B.; Mukherjee, U.A.; Burke, N.; Hall, A.R.; Angelova, P.R.; Ludtmann, M.H.; Deas, E.; Davidson, S.M.; et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS ONE 2013, 8, e62400. [Google Scholar] [CrossRef]
- Saito, T.; Nah, J.; Oka, S.I.; Mukai, R.; Monden, Y.; Maejima, Y.; Ikeda, Y.; Sciarretta, S.; Liu, T.; Li, H.; et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin. Investig. 2019, 129, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Redel, A.; Lotz, C.; Smul, T.M.; Blomeyer, C.; Frank, A.; Stumpner, J.; Roewer, N.; Kehl, F. Desflurane-induced postconditioning is mediated by beta-adrenergic signaling: Role of beta 1- and beta 2-adrenergic receptors, protein kinase A, and calcium/calmodulin-dependent protein kinase II. Anesthesiology 2009, 110, 516–528. [Google Scholar] [CrossRef]
- Tong, H.; Bernstein, D.; Murphy, E.; Steenbergen, C. The role of beta-adrenergic receptor signaling in cardioprotection. FASEB J. 2005, 19, 983–985. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Yu, X.J.; Wang, H.; He, X.; Liu, J.K.; Zang, W.J. Cardioprotection by acetylcholine: A novel mechanism via mitochondrial biogenesis and function involving the PGC-1alpha pathway. J. Cell Physiol. 2013, 228, 1238–1248. [Google Scholar] [CrossRef]
- Yu, L.; Gong, B.; Duan, W.; Fan, C.; Zhang, J.; Li, Z.; Xue, X.; Xu, Y.; Meng, D.; Li, B.; et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: Role of AMPK-PGC-1alpha-SIRT3 signaling. Sci. Rep. 2017, 7, 41337. [Google Scholar] [CrossRef]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef]
- Tao, L.; Bei, Y.; Lin, S.; Zhang, H.; Zhou, Y.; Jiang, J.; Chen, P.; Shen, S.; Xiao, J.; Li, X. Exercise Training Protects Against Acute Myocardial Infarction via Improving Myocardial Energy Metabolism and Mitochondrial Biogenesis. Cell Physiol. Biochem. 2015, 37, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Belhomme, D.; Peynet, J.; Louzy, M.; Launay, J.M.; Kitakaze, M.; Menasche, P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation 1999, 100 (Suppl. 19), II-340–II-344. [Google Scholar] [CrossRef]
- Tritapepe, L.; Landoni, G.; Guarracino, F.; Pompei, F.; Crivellari, M.; Maselli, D.; De Luca, M.; Fochi, O.; D’Avolio, S.; Bignami, E.; et al. Cardiac protection by volatile anaesthetics: A multicentre randomized controlled study in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Eur. J. Anaesthesiol. 2007, 24, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Julier, K.; da Silva, R.; Garcia, C.; Bestmann, L.; Frascarolo, P.; Zollinger, A.; Chassot, P.G.; Schmid, E.R.; Turina, M.I.; von Segesser, L.K.; et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. Anesthesiology 2003, 98, 1315–1327. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Liu, N.; Dong, T.; Li, R. Sevoflurane preconditioning in on-pump coronary artery bypass grafting: A meta-analysis of randomized controlled trials. J. Anesth. 2016, 30, 977–986. [Google Scholar] [CrossRef]
- De Hert, S.; Vlasselaers, D.; Barbe, R.; Ory, J.P.; Dekegel, D.; Donnadonni, R.; Demeere, J.L.; Mulier, J.; Wouters, P. A comparison of volatile and non volatile agents for cardioprotection during on-pump coronary surgery. Anaesthesia 2009, 64, 953–960. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef]
- Ebrahim, Z.; Yellon, D.M.; Baxter, G.F. Ischemic preconditioning is lost in aging hypertensive rat heart: Independent effects of aging and longstanding hypertension. Exp. Gerontol. 2007, 42, 807–814. [Google Scholar] [CrossRef]
- Tanaka, K.; Kehl, F.; Gu, W.; Krolikowski, J.G.; Pagel, P.S.; Warltier, D.C.; Kersten, J.R. Isoflurane-induced preconditioning is attenuated by diabetes. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2018–H2023. [Google Scholar] [CrossRef]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef]
- Heusch, G.; Rassaf, T. Time to Give Up on Cardioprotection? A Critical Appraisal of Clinical Studies on Ischemic Pre-, Post-, and Remote Conditioning. Circ. Res. 2016, 119, 676–695. [Google Scholar] [CrossRef]
| Authors | Model | Protocol | Identified Mechanism |
|---|---|---|---|
| Hanley, P.J. et al. 2002 [44] | Intact ventricular myocytes of guineapigs | Analyses of electron transport chain activity. | Halothane, isoflurane, and sevoflurane inhibit complex I of the electron transport chain. |
| Novalija, E. et al. 2003 [48] | Isolated guinea pig hearts | Sevoflurane preconditioning prior to ischemia reperfusion. | Anesthetic preconditioning preserved mitochondrial ATP production and attenuated mitochondrial ROS overload. |
| Baines, C.P. et al. 2005 [76] | Ppif null mice and cyclophilin D transgenic mice | Ischemia and reperfusion (24 h). | Cyclophilin D is required for Ca2+- and oxidative damage-induced cell death. |
| Krolikowski, J.G. et al. 2005 [67] | Male New Zealand white rabbits | Isoflurane pre- and postconditioning, left coronary artery occlusion and reperfusion. | Isoflurane conditioning inhibits mitochondrial permeability transition. |
| Chen, Q. et al. 2006 [47] | Isolated Fischer-344 rat hearts | Amobarbital preconditioning prior to global ischemia and reperfusion. | Mitochondrial damage occurs mainly during ischemia. Preserved mitochondrial respiration during reperfusion attenuates ROS release and decreases myocardial infarct size. |
| Ljubkovic, M. et al. 2007 [45] | Isolated rat ventricular myocytes | Analysis of mitochondrial membrane potential, redox state and oxygen consumption after isoflurane preconditioning. | Isoflurane preconditioning elicits partial mitochondrial uncoupling and reduces mitochondrial Ca2+ uptake. |
| Feng, J. et al. 2008 [43] | Isolated male adult Wistar rat hearts | Isoflurane pre- and postconditioning with global no-flow ischemia followed by reperfusion. | Identification of 26 potential phosphorylation sites in 19 mitochondrial proteins. Detection of a novel phosphorylation site in adenine nucleotide translocator-1 (ANT1). |
| Pravdic, D. et al. 2009 [73] | Isolated rat ventricular myocytes | In-vivo isoflurane preconditioning in the absence or presence of chelerythrine. | Isoflurane conditioning delays mPTP opening dependent on PKCε activation. |
| Stewart, S.; Lesnefsky, E.; Chen, Q. 2009 [53] | Isolated Fischer-344 rat hearts | Amobarbital postconditioning within global ischemia and reperfusion. | Blockade of the proximal electron transport chain at respiratory complex I attenuated maximal mitochondrial ROS generation during reperfusion. |
| Boengler, K. et al. 2010 [74] | Female STAT3-KO mice | Left coronary artery occlusion and reperfusion, administration of cyclosporine A prior to reperfusion. | STAT3-KO mice exhibited decreased ADP-stimulated mitochondrial respiration accompanied by increased susceptibility to mPTP opening. |
| Sedlic, F. et al. 2010 [46] | Isolated rat ventricular myocytes | Cardiomyocytes exposed to H2O2- after isoflurane preconditioning. | Isoflurane partially decreases mitochondrial membrane potential (ΔΨm), attenuating ROS production, decreasing Ca2+ uptake, and preventing mPTP opening. |
| Bienengraeber, M. et al. 2013 [42] | Male adult Wistar rats | Isoflurane preconditioning with left coronary artery (LCA) ligation and reperfusion. | 14 mitochondrial proteins were up- or downregulated in the conditioning response, the majority belonging to complexes of the electron transport chain. |
| Lotz, C. et al. 2015 [49] | Male adult mice | Isoflurane preconditioning prior to ischemia and reperfusion (second window). | Isoflurane conditioning preserved the activity of respiratory complex III, stabilized mitochondrial supercomplexes III/IV, decreased malondialdehyde formation, and diminished susceptibility to Ca2+-induced swelling. |
| Szczepanek, K. et al. 2015 [57] | Transgenic mice overexpressing mitochondria-targeted, transcriptionally inactive STAT3 (MLS-STAT3E mice) | Global ischemia and reperfusion in isolated hearts. Survival analysis after left coronary artery occlusion followed reperfusion (second window). | Partial and persistent blockade of complex I in MLS-STAT3E mice decreases cardiac injury during reperfusion with concomitantly increased survival. |
| Zhang, G. et al. 2018 [56] | Isolated rat hearts | ZnCl2 postconditioning within regional ischemia and reperfusion. | ZnCl2 prevented ΔΨm dissipation and mitochondrial ROS generation at reperfusion by increasing mitochondrial STAT3 phosphorylation at Ser727 via ERK. |
| Lambert, J.P. et al. 2019 [17] | Tamoxifen-inducible MCUB mutant mice; MCUB knockout cell line (MCUB−/−) | Left coronary artery (LCA) ligation with and without reperfusion. | MCUB is incorporated into the mtCU following ischemic injury limiting mitochondrial Ca2+ overload and cell loss during chronic stress. |
| Urbani, A. et al. 2019 [62] | F-ATP synthase purified from bovine heart mitochondria | Characterization of F-ATP synthase channel activity after reconstitution and patch clamp experiments. | Ca2+ can transform the energy-conserving F-ATP synthase into an energy-dissipating device, i.e., the mPTP. |
| Lotz, C.; Stumpner, J.; Smul, T.M. 2020 [50] | Male New Zealand white rabbits | Sevoflurane compared to propofol anesthesia. Left coronary artery occlusion and reperfusion. | Sevoflurane anesthesia preserved the activities of respiratory complexes I and IV, whereas a higher portion of complex I was in its inactive (dormant) form. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotz, C.; Herrmann, J.; Notz, Q.; Meybohm, P.; Kehl, F. Mitochondria and Pharmacologic Cardiac Conditioning—At the Heart of Ischemic Injury. Int. J. Mol. Sci. 2021, 22, 3224. https://doi.org/10.3390/ijms22063224
Lotz C, Herrmann J, Notz Q, Meybohm P, Kehl F. Mitochondria and Pharmacologic Cardiac Conditioning—At the Heart of Ischemic Injury. International Journal of Molecular Sciences. 2021; 22(6):3224. https://doi.org/10.3390/ijms22063224
Chicago/Turabian StyleLotz, Christopher, Johannes Herrmann, Quirin Notz, Patrick Meybohm, and Franz Kehl. 2021. "Mitochondria and Pharmacologic Cardiac Conditioning—At the Heart of Ischemic Injury" International Journal of Molecular Sciences 22, no. 6: 3224. https://doi.org/10.3390/ijms22063224
APA StyleLotz, C., Herrmann, J., Notz, Q., Meybohm, P., & Kehl, F. (2021). Mitochondria and Pharmacologic Cardiac Conditioning—At the Heart of Ischemic Injury. International Journal of Molecular Sciences, 22(6), 3224. https://doi.org/10.3390/ijms22063224






