Hyaluronidases in Human Diseases
Abstract
1. Introduction to Hyaluronidases
2. Background and Mechanism of Action in Humans
3. Hyaluronidases in Human Organs and Cancer
3.1. In Skin
3.2. In the Cardiovascular System
3.3. In the Lungs
3.4. In the Kidneys
3.5. In the Liver
3.6. In the Gastrointestinal Tract
3.7. In Cancer
4. Hyaluronidase-Based Therapeutics
5. Concluding Thoughts
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DAMP | damage-associated molecular pattern |
| ECM | extracellular matrix |
| GAG | glycosaminoglycan |
| GPI | glycosylphosphatidylinositol |
| HA | hyaluronic acid/hyaluronan |
| HARE | HA-receptor for endocytosis |
| HMW-HA | high-molecular-weight hyaluronic acid |
| HYAL | hyaluronidase |
| IBD | inflammatory bowel disease |
| KO | knockout |
| LMW-HA | low-molecular-weight hyaluronic acid |
| LYVE-1 | lymphatic vessel endothelial HA-receptor-1 |
| MW | molecular weight |
| PH | pulmonary hypertension |
References
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Nagy, N.; Sunkari, V.G.; Kaber, G.; Hasbun, S.; Lam, D.N.; Speake, C.; Sanda, S.; McLaughlin, T.L.; Wight, T.N.; Long, S.R.; et al. Hyaluronan levels are increased systemically in human type 2 but not type 1 diabetes independently of glycemic control. Matrix Biol. 2019, 80, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Lin, Y.T.; Chiang, B.L.; Lin, Y.H.; Hou, S.M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr. Cartil. 2006, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Inokoshi, Y.; Tanino, Y.; Wang, X.; Sato, S.; Fukuhara, N.; Nikaido, T.; Fukuhara, A.; Saito, J.; Frevert, C.W.; Munakata, M. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology 2013, 18, 1236–1243. [Google Scholar] [CrossRef]
- Duran-Reynals, F. The effect of extracts of certain organs from normal and immunized animals on the infecting power of vaccine virus. J. Exp. Med. 1929, 50, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Meyer, K.; Rapport, M.M. Hyaluronidases. Adv. Enzym. Relat. Subj. Biochem. 1952, 13, 199–236. [Google Scholar] [CrossRef]
- Jedrzejas, M.J.; Stern, R. Structures of vertebrate hyaluronidases and their unique enzymatic mechanism of hydrolysis. Proteins 2005, 61, 227–238. [Google Scholar] [CrossRef]
- Jedrzejas, M.J. Structural and functional comparison of polysaccharide-degrading enzymes. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 221–251. [Google Scholar] [CrossRef]
- Yuki, H.; Fishman, W.H. Purification and characterization of leech hyaluronic acid-endo-beta-glucuronidase. J. Biol. Chem. 1963, 238, 1877–1879. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R. The properties and turnover of hyaluronan. Ciba Found. Symp. 1986, 124, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. Serum hyaluronan as a disease marker. Ann. Med. 1996, 28, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Stern, R. Devising a pathway for hyaluronan catabolism: Are we there yet? Glycobiology 2003, 13, 105R–115R. [Google Scholar] [CrossRef]
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Weigel, J.A.; Raymond, R.C.; McGary, C.; Singh, A.; Weigel, P.H. A blocking antibody to the hyaluronan receptor for endocytosis (HARE) inhibits hyaluronan clearance by perfused liver. J. Biol. Chem. 2003, 278, 9808–9812. [Google Scholar] [CrossRef]
- Jackson, D.G. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol. Rev. 2009, 230, 216–231. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamamoto, H.; Tobisawa, Y.; Irie, F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol. 2019, 78, 139–146. [Google Scholar] [CrossRef]
- Yoshida, H.; Aoki, M.; Komiya, A.; Endo, Y.; Kawabata, K.; Nakamura, T.; Sakai, S.; Sayo, T.; Okada, Y.; Takahashi, Y. HYBID (alias KIAA1199/CEMIP) and hyaluronan synthase coordinately regulate hyaluronan metabolism in histamine-stimulated skin fibroblasts. J. Biol. Chem. 2020, 295, 2483–2494. [Google Scholar] [CrossRef]
- Yoshino, Y.; Goto, M.; Hara, H.; Inoue, S. The role and regulation of TMEM2 (transmembrane protein 2) in HYBID (hyaluronan (HA)-binding protein involved in HA depolymerization/ KIAA1199/CEMIP)-mediated HA depolymerization in human skin fibroblasts. Biochem. Biophys. Res. Commun. 2018, 505, 74–80. [Google Scholar] [CrossRef]
- Sato, S.; Mizutani, Y.; Yoshino, Y.; Masuda, M.; Miyazaki, M.; Hara, H.; Inoue, S. Pro-inflammatory cytokines suppress HYBID (hyaluronan (HA) -binding protein involved in HA depolymerization/KIAA1199/CEMIP) -mediated HA metabolism in human skin fibroblasts. Biochem. Biophys. Res. Commun. 2021, 539, 77–82. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamazaki, K.; Komiya, A.; Aoki, M.; Kasamatsu, S.; Murata, T.; Sayo, T.; Cilek, M.Z.; Okada, Y.; Takahashi, Y. Inhibitory effects of Sanguisorba officinalis root extract on HYBID (KIAA1199)-mediated hyaluronan degradation and skin wrinkling. Int. J. Cosmet. Sci. 2019, 41, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yamazaki, K.; Komiya, A.; Aoki, M.; Nakamura, T.; Kasamatsu, S.; Murata, T.; Sayo, T.; Okada, Y.; Takahashi, Y. Inhibition of HYBID (KIAA1199)-mediated hyaluronan degradation and anti-wrinkle effect of Geranium thunbergii extract. J. Cosmet. Derm. 2019, 18, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.C.; Atmuri, V.; Hemming, R.J.; Farley, J.; Mort, J.S.; Byers, S.; Hombach-Klonisch, S.; Csoka, A.B.; Stern, R.; Triggs-Raine, B.L. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum. Mol. Genet. 2008, 17, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Jadin, L.; Wu, X.; Ding, H.; Frost, G.I.; Onclinx, C.; Triggs-Raine, B.; Flamion, B. Skeletal and hematological anomalies in HYAL2-deficient mice: A second type of mucopolysaccharidosis IX? FASEB J. 2008, 22, 4316–4326. [Google Scholar] [CrossRef]
- Triggs-Raine, B.; Natowicz, M.R. Biology of hyaluronan: Insights from genetic disorders of hyaluronan metabolism. World J. Biol. Chem. 2015, 6, 110–120. [Google Scholar] [CrossRef]
- Kiykim, E.; Barut, K.; Cansever, M.S.; Zeybek, C.A.; Zubarioglu, T.; Aydin, A.; Kasapcopur, O. Screening Mucopolysaccharidosis Type IX in Patients with Juvenile Idiopathic Arthritis. JIMD Rep. 2016, 25, 21–24. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; Iqbal, S.A.; Bayat, A. Altered expression of hyaluronan synthase and hyaluronidase mRNA may affect hyaluronic acid distribution in keloid disease compared with normal skin. Exp. Derm. 2013, 22, 377–379. [Google Scholar] [CrossRef]
- Neudecker, B.A.; Stern, R.; Connolly, M.K. Aberrant serum hyaluronan and hyaluronidase levels in scleroderma. Br. J. Derm. 2004, 150, 469–476. [Google Scholar] [CrossRef]
- Fiszer-Szafarz, B.; Czartoryska, B.; Tylki-Szymanska, A. Serum hyaluronidase aberrations in metabolic and morphogenetic disorders. Glycoconj. J. 2005, 22, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Derm. 2007, 127, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Wang, X.; King, A.; Le, L.D.; Bhattacharya, S.S.; Moles, C.M.; Butte, M.J.; de Jesus Perez, V.A.; Liechty, K.W.; Wight, T.N.; et al. Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J. 2017, 31, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Damodarasamy, M.; Chan, C.K.; Johnson, M.N.; Wight, T.N.; Vernon, R.B. Cleavage of hyaluronan is impaired in aged dermal wounds. Matrix Biol. 2013, 32, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Balaji, S.; Steen, E.H.; Blum, A.J.; Li, H.; Chan, C.K.; Manson, S.R.; Lu, T.C.; Rae, M.M.; Austin, P.F.; et al. High molecular weight hyaluronan attenuates tubulointerstitial scarring in kidney injury. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Muggenthaler, M.M.; Chowdhury, B.; Hasan, S.N.; Cross, H.E.; Mark, B.; Harlalka, G.V.; Patton, M.A.; Ishida, M.; Behr, E.R.; Sharma, S.; et al. Mutations in HYAL2, Encoding Hyaluronidase 2, Cause a Syndrome of Orofacial Clefting and Cor Triatriatum Sinister in Humans and Mice. PLoS Genet. 2017, 13, e1006470. [Google Scholar] [CrossRef]
- Nassar, P.N.; Hamdan, R.H. Cor Triatriatum Sinistrum: Classification and Imaging Modalities. Eur. J. Cardiovasc. Med. 2011, 1, 84–87. [Google Scholar] [CrossRef]
- Chowdhury, B.; Xiang, B.; Liu, M.; Hemming, R.; Dolinsky, V.W.; Triggs-Raine, B. Hyaluronidase 2 Deficiency Causes Increased Mesenchymal Cells, Congenital Heart Defects, and Heart Failure. Circ. Cardiovasc. Genet. 2017, 10, e001598. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef]
- Reeves, S.R.; Barrow, K.A.; Rich, L.M.; White, M.P.; Shubin, N.J.; Chan, C.K.; Kang, I.; Ziegler, S.F.; Piliponsky, A.M.; Wight, T.N.; et al. Respiratory Syncytial Virus Infection of Human Lung Fibroblasts Induces a Hyaluronan-Enriched Extracellular Matrix That Binds Mast Cells and Enhances Expression of Mast Cell Proteases. Front. Immunol. 2019, 10, 3159. [Google Scholar] [CrossRef] [PubMed]
- Tseng, V.; Ni, K.; Allawzi, A.; Prohaska, C.; Hernandez-Lagunas, L.; Elajaili, H.; Cali, V.; Midura, R.; Hascall, V.; Triggs-Raine, B.; et al. Extracellular Superoxide Dismutase Regulates Early Vascular Hyaluronan Remodeling in Hypoxic Pulmonary Hypertension. Sci. Rep. 2020, 10, 280. [Google Scholar] [CrossRef]
- Ormiston, M.L.; Slaughter, G.R.; Deng, Y.; Stewart, D.J.; Courtman, D.W. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L148–L157. [Google Scholar] [CrossRef]
- Vlahu, C.A.; Lemkes, B.A.; Struijk, D.G.; Koopman, M.G.; Krediet, R.T.; Vink, H. Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol. 2012, 23, 1900–1908. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.C.; Woods, E.L.; Jenkins, R.H.; Brown, C.; Khalid, U.; Chavez, R.; Hascall, V.; Steadman, R.; Phillips, A.O.; Meran, S. Hyaluronidase-2 Regulates RhoA Signaling, Myofibroblast Contractility, and Other Key Profibrotic Myofibroblast Functions. Am. J. Pathol. 2020, 190, 1236–1255. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C. Turnover and metabolism of hyaluronan. Ciba Found. Symp. 1989, 143, 41–53. [Google Scholar] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Gudowska, M.; Cylwik, B.; Chrostek, L. The role of serum hyaluronic acid determination in the diagnosis of liver fibrosis. Acta Biochim. Pol. 2017, 64, 451–457. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Stern, R. Serum hyaluronan and hyaluronidase: Very early markers of toxic liver injury. Clin. Chim. Acta 2004, 348, 189–197. [Google Scholar] [CrossRef]
- Isman, F.K.; Kucur, M.; Baysal, B.; Ozkan, F. Evaluation of serum hyaluronic acid level and hyaluronidase activity in acute and chronic hepatitis C. J. Int. Med. Res. 2007, 35, 346–352. [Google Scholar] [CrossRef]
- Orăşan, O.H.; Sava, M.; Iancu, M.; Cozma, A.; Saplonţai-Pop, A.; Sarlea Ţărmure, S.; Lungoci, C.; Orăşan, R.A.; Patiu, I.M.; Dumitraşcu, D.L. Serum hyaluronic acid in chronic viral hepatitis B and C: A biomarker for assessing liver fibrosis in chronic hemodialysis patients. Int. Urol. Nephrol. 2015, 47, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- El-mezayen, H.A.; Toson el, S.A.; Shiha, G.E. Role of hyaluronic acid, its degrading enzymes, degradation products, and ferritin in the assessment of fibrosis stage in Egyptian patients with chronic hepatitis C. Eur. J. Gastroenterol. Hepatol. 2013, 25, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.M.; Salvén, A.; Kärjä, V.; Rilla, K.; Matilainen, J.; Nieminen, P. Hyaluronan histochemistry-a potential new tool to assess the progress of liver disease from simple steatosis to hepatocellular carcinoma. Glycobiology 2019, 29, 298–306. [Google Scholar] [CrossRef]
- Zhao, Q.; Peng, L.; Huang, W.; Li, Q.; Pei, Y.; Yuan, P.; Zheng, L.; Zhang, Y.; Deng, J.; Zhong, C.; et al. Rare inborn errors associated with chronic hepatitis B virus infection. Hepatology 2012, 56, 1661–1670. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, C.; Li, Y.M.; Huang, Z.L.; Zhao, Q.Y.; Hu, Z.X.; Wang, P.P.; Gu, Y.R.; Gao, Z.L.; Peng, L. TMEM2 inhibits hepatitis B virus infection in HepG2 and HepG2.2.15 cells by activating the JAK-STAT signaling pathway. Cell Death Dis. 2016, 7, e2239. [Google Scholar] [CrossRef]
- Eriksson, S.; Fraser, J.R.; Laurent, T.C.; Pertoft, H.; Smedsrød, B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 1983, 144, 223–228. [Google Scholar] [CrossRef]
- Bourguignon, V.; Flamion, B. Respective roles of hyaluronidases 1 and 2 in endogenous hyaluronan turnover. FASEB J. 2016, 30, 2108–2114. [Google Scholar] [CrossRef]
- Li, L.; Tian, F.Y.; Yuan, Y.; Zhang, T.; Yang, W.B.; Kong, R.; Wang, G.; Chen, H.; Chen, H.Z.; Hu, J.S.; et al. HYAL-1-induced autophagy facilitates pancreatic fistula for patients who underwent pancreaticoduodenectomy. FASEB J. 2020, 34, 2524–2540. [Google Scholar] [CrossRef]
- Petrey, A.C.; Obery, D.R.; Kessler, S.P.; Zawerton, A.; Flamion, B.; de la Motte, C.A. Platelet hyaluronidase-2 regulates the early stages of inflammatory disease in colitis. Blood 2019, 134, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, A.; Albeiroti, S.; West, G.A.; Willard, B.; Fiocchi, C.; de la Motte, C.A. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; de Souza, H.S.P. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 2018, 24, 4622–4634. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.; Rho, H.; West, G.; Fiocchi, C.; Drazba, J.; de la Motte, C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin. Transl. Sci. 2008, 1, 57–61. [Google Scholar] [CrossRef]
- Riehl, T.E.; Ee, X.; Stenson, W.F. Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G377–G388. [Google Scholar] [CrossRef]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; et al. Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, J.; Wang, G.; Yang, Z.; Zhao, C.; Zhang, X.; Wang, J. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J. Exp. Clin. Cancer Res. 2018, 37, 106. [Google Scholar] [CrossRef]
- Wang, D.; Lu, S.; Zhang, X.; Huang, L.; Zhao, H. Co-expression of KIAA1199 and hypoxia-inducible factor 1α is a biomarker for an unfavorable prognosis in hepatocellular carcinoma. Medicine 2020, 99, e23369. [Google Scholar] [CrossRef]
- Tammi, M.I.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K.; Auvinen, P.; Tammi, R.H. Activated hyaluronan metabolism in the tumor matrix—Causes and consequences. Matrix Biol. 2019, 78, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Vitrase full prescribing information. 2004. Available online: https://www.bausch.com/ecp/our-products/rx-pharmaceuticals/rx-pharmaceuticals/vitrase (accessed on 19 March 2021).
- Hylenex recombinant full prescribing information. 2005. Available online: https://hylenex.com/ (accessed on 19 March 2021).
- Amphadase professional prescribing information. 2005. Available online: http://www.amphastar.com/our-products.html (accessed on 19 March 2021).
- Darzalex Faspro full prescribing information. 2020. Available online: https://www.darzalexhcp.com/ (accessed on 19 March 2021).
- Herceptin Hylecta full prescribing information. 2019. Available online: https://www.herceptinhylecta.com/# (accessed on 19 March 2021).
- HyQvia full prescribing information. 2014. Available online: https://www.hyqvia.com/ (accessed on 19 March 2021).
- Rituxan Hycela full prescribing information. 2017. Available online: https://www.rituxanhycela.com/ (accessed on 19 March 2021).
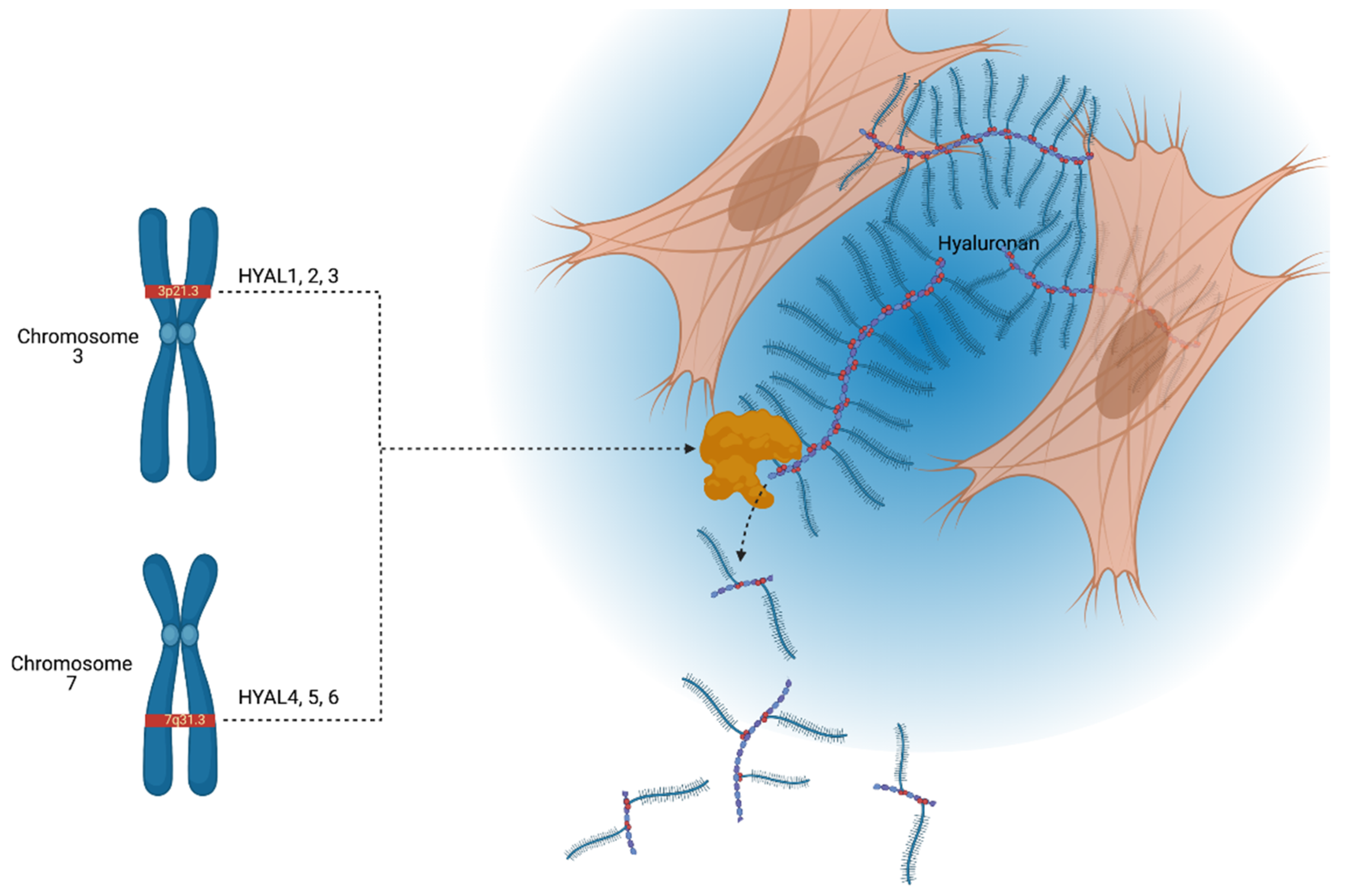
| Chromosomal Location | Gene | Protein | Active pH | Somatically Active |
|---|---|---|---|---|
| 3p21.3 | HYAL1 | HYAL1 | 3–4 | Yes |
| HYAL2 | HYAL2 | 4, 7.5 | Yes | |
| HYAL3 | HYAL3 | / | No | |
| 7q31.3 | HYAL4 | HYAL4 | / | No |
| HYAL5/SPAM1/PH20 | HPH-20 | 4, 7.5 | Yes | |
| HYAL6/HYALP1 | Noncoding | / | No | |
| 15q25.1 | CEMIP | KIAA1199 | / | Yes |
| 9q21.13 | CEMIP2 | TMEM2 | / | Yes |
| Organ System | Disease/Condition | Hyaluronidase(s) Involved | Hyaluronidase Involvement |
|---|---|---|---|
| Skin and connective tissue | Mucopolysaccharidosis type IX (MPS IX) | HYAL1 | Deficiency leads to joint pathology resembling juvenile idiopathic arthritis |
| Keloid scarring | HYALs 1 & 2 | Reduced expression compared to normal skin | |
| Scleroderma Facial wrinkles | HYAL1 KIAA1199 | Reduced in serum Exacerbates facial wrinkles | |
| Cardiovascular | Cor triatriatum sinistrum | HYAL2 | Deficiency |
| Post-myocardial infarction fibrosis | HYAL3 | Increases collagen deposition | |
| Pulmonary | Pulmonary hypertension | HYAL2 | Promotes remodeling of vasculature and pulmonary hypertension |
| Renal | Chronic kidney disease | HYAL1 | Elevated in serum of patients with end-stage renal disease |
| Hepatic | Steatosis, steatohepatitis, cirrhosis | HYALs 1 & 2 | Elevated in diseased liver parenchyma |
| Hepatitis B | TMEM2 | Decreased in chronic infection; may be protective against infection | |
| Hepatitis C | HYALs | Elevated in serum | |
| Gastrointestinal | Pancreaticocutaneous fistula | HYAL1 | Elevated in serum |
| Inflammatory bowel disease | HYAL2 | Reduced in platelets | |
| Crohn’s disease | KIAA1199 | Elevated in colonic fibroblasts | |
| Colorectal cancer | KIAA1199 | Overexpression promotes cancer cell invasion |
| Brand Name | Composition | Mechanism | Use | Species Origin |
|---|---|---|---|---|
| Amphadase | HYAL | Dispersion agent to hydrolyze HA | Adjuvant for hydration | Bovine testicular |
| Darzalex Faspro | Daratumumab, HYAL | HYAL depolymerizes HA to increase absorption of daratumumab | Multiple myeloma | Recombinant human |
| Herceptin Hylecta | Trastuzumab, HYAL | HYAL depolymerizes HA to increase absorption of trastuzumab | Adjuvant for breast cancer | Recombinant human |
| Hylenex | HYAL | Dispersion agent to hydrolyze HA | Adjuvant for hydration | Recombinant human |
| Hyqvia | IgG, HYAL | HYAL depolymerizes HA to increase absorption of immunoglobulin G | Replacement therapy for immunodeficiency | Recombinant human |
| Rituxan Hycela | Rituximab, HYAL | HYAL depolymerizes HA to increase absorption of rituximab | Various lymphomas | Recombinant human |
| Vitrase | HYAL | Dispersion agent to hydrolyze HA | Adjuvant for hydration | Ovine testicular |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaul, A.; Short, W.D.; Wang, X.; Keswani, S.G. Hyaluronidases in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3204. https://doi.org/10.3390/ijms22063204
Kaul A, Short WD, Wang X, Keswani SG. Hyaluronidases in Human Diseases. International Journal of Molecular Sciences. 2021; 22(6):3204. https://doi.org/10.3390/ijms22063204
Chicago/Turabian StyleKaul, Aditya, Walker D. Short, Xinyi Wang, and Sundeep G. Keswani. 2021. "Hyaluronidases in Human Diseases" International Journal of Molecular Sciences 22, no. 6: 3204. https://doi.org/10.3390/ijms22063204
APA StyleKaul, A., Short, W. D., Wang, X., & Keswani, S. G. (2021). Hyaluronidases in Human Diseases. International Journal of Molecular Sciences, 22(6), 3204. https://doi.org/10.3390/ijms22063204








