Involvement of Bcl-xL in Neuronal Function and Development
Abstract
1. Introduction
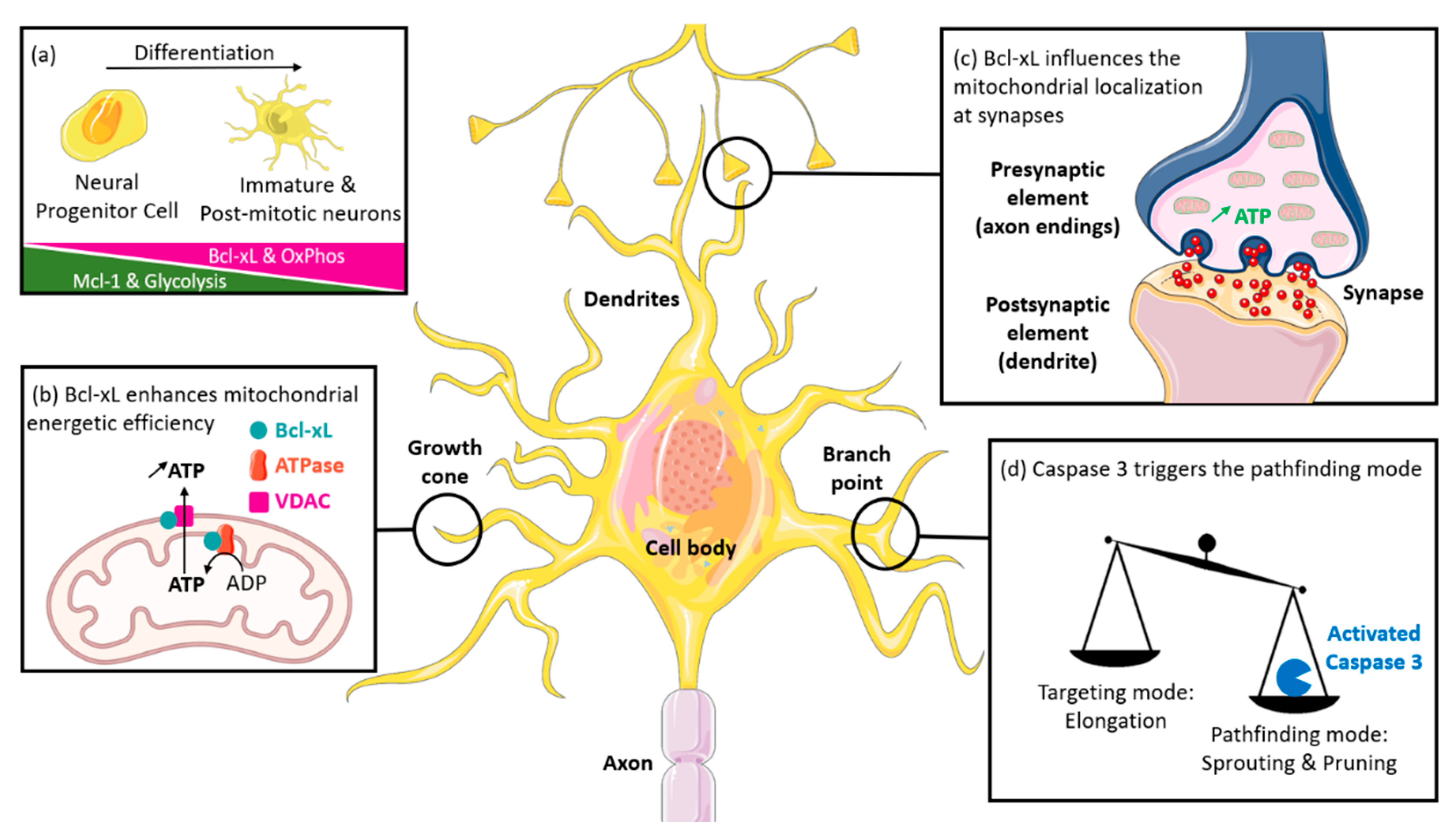
2. Mitochondria Are Crucial for Neuronal Functions and Bcl-xL Can Control Their Metabolic Activity
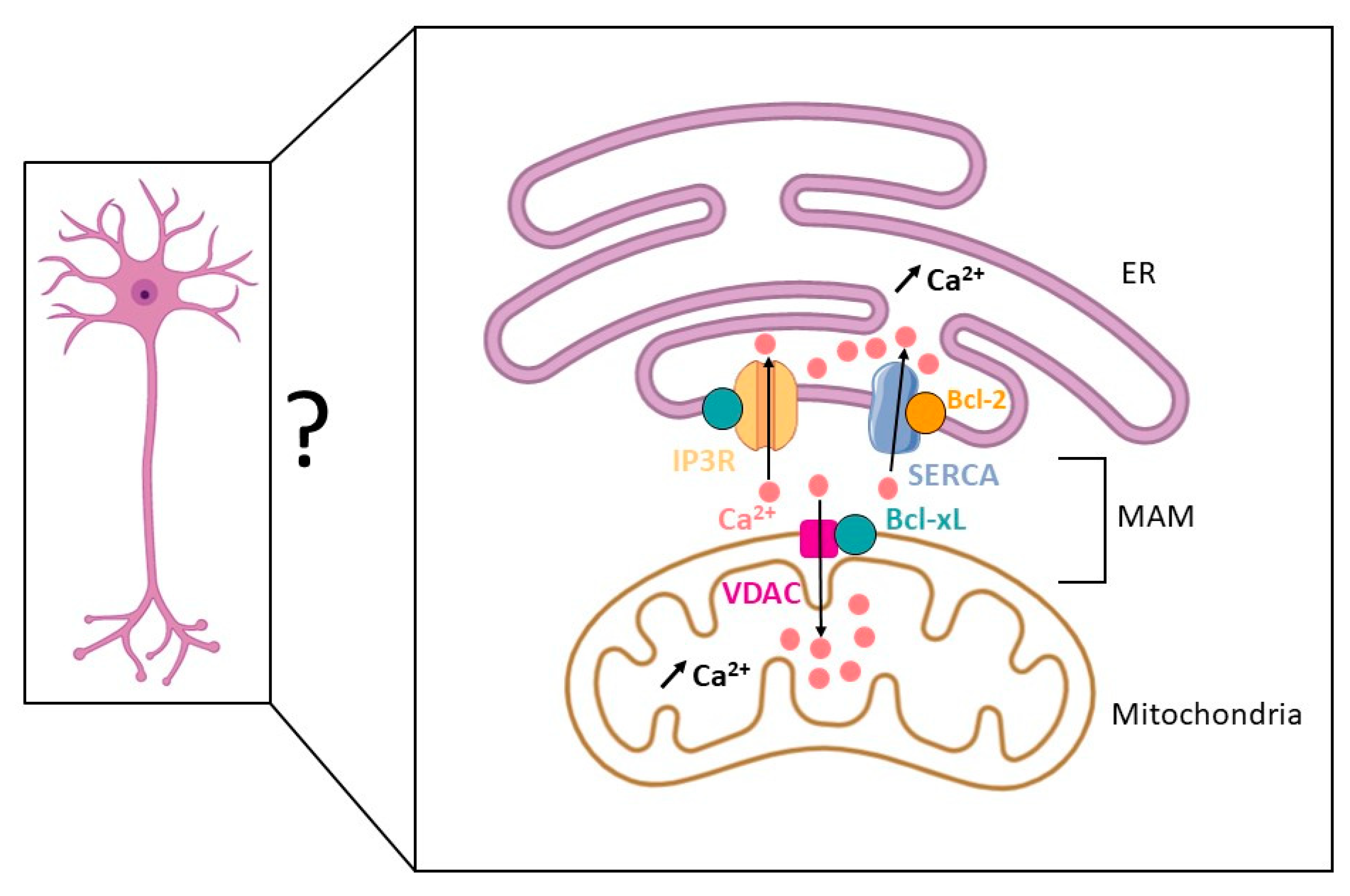
3. Bcl-xL and Calcium Homeostasis
4. Bcl-xL Impacts on Neuron Development and Growth
5. Caspases Regulate Neuronal Plasticity
6. Bcl-x Caspase Crosstalk
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Popgeorgiev, N.; Bonneau, B.; Prudent, J.; Gillet, G. Control of Programmed Cell Death During Zebrafish Embryonic Development. In Recent Advances in Zebrafish Researches; Bozkurt, Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Strasser, A.; Vaux, D.L. Cell Death in the Origin and Treatment of Cancer. Mol. Cell 2020, 78, 1045–1054. [Google Scholar] [CrossRef]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Na Dong, Y.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 3, 108698. [Google Scholar] [CrossRef] [PubMed]
- D’Aguanno, S.; Del Bufalo, D. Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer. Cells 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: Relevance of BH4 domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Gabellini, C.; Buglioni, S.; Pallocca, M.; Alessandrini, G.; D’Aguanno, S.; Del Bufalo, D. BCL-XL overexpression promotes tumor progression-associated properties. Cell Death Dis. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Bessou, M.; Lopez, J.; Gadet, R.; Deygas, M.; Popgeorgiev, N.; Poncet, D.; Nougarède, A.; Billard, P.; Mikaelian, I.; Gonzalo, P.; et al. The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene 2020, 39, 3056–3074. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Vais, H.; Thompson, C.B.; Foskett, J.K.; White, C. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. USA 2007, 104, 12565–12570. [Google Scholar] [CrossRef]
- Park, H.-A.; Licznerski, P.; Alavian, K.N.; Shanabrough, M.; Jonas, E.A. Bcl-xL Is Necessary for Neurite Outgrowth in Hippocampal Neurons. Antioxid. Redox Signal. 2015, 22, 93–108. [Google Scholar] [CrossRef]
- Arena, G.; Gelmetti, V.; Torosantucci, L.; Vignone, D.; Lamorte, G.; De Rosa, P.; Cilia, E.; Jonas, E.A.; Valente, E.M. PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro-apoptotic cleavage. Cell Death Differ. 2013, 20, 920–930. [Google Scholar] [CrossRef]
- Hollville, E.; Carroll, R.G.; Cullen, S.P.; Martin, S.J. Bcl-2 Family Proteins Participate in Mitochondrial Quality Control by Regulating Parkin/PINK1-Dependent Mitophagy. Mol. Cell 2014, 55, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Brambilla, L.; Valori, C.F.; Bergamaschi, C.; Roncoroni, C.; Aronica, E.; Volterra, A.; Bezzi, P.; Rossi, D. The BH4 domain of Bcl-XL rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Hum. Mol. Genet. 2012, 21, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Garcerá, A.; Mincheva, S.; Gou-Fabregas, M.; Caraballo-Miralles, V.; Lladó, J.; Comella, J.X.; Soler, R.M. A new model to study spinal muscular atrophy: Neurite degeneration and cell death is counteracted by BCL-XL Overexpression in motoneurons. Neurobiol. Dis. 2011, 42, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Tasheva, S.; Obis, E.; Tamarit, J.; Ros, J. Apoptotic cell death and altered calcium homeostasis caused by frataxin depletion in dorsal root ganglia neurons can be prevented by BH4 domain of Bcl-xL protein. Hum. Mol. Genet. 2014, 23, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Swahari, V.; Plestant, C.; Smith, I.; McCoy, E.; Smith, S.; Moy, S.S.; Anton, E.S.; Deshmukh, M. Bcl-xL Is Essential for the Survival and Function of Differentiated Neurons in the Cortex That Control Complex Behaviors. J. Neurosci. 2016, 36, 5448–5461. [Google Scholar] [CrossRef]
- Shim, J.-W.; Koh, H.-C.; Chang, M.-Y.; Roh, E.; Choi, C.-Y.; Oh, Y.J.; Son, H.; Lee, Y.-S.; Studer, L.; Lee, S.-H. Enhanced In Vitro Midbrain Dopamine Neuron Differentiation, Dopaminergic Function, Neurite Outgrowth, and 1-Methyl-4-Phenylpyridium Resistance in Mouse Embryonic Stem Cells Overexpressing Bcl-XL. J. Neurosci. 2004, 24, 843–852. [Google Scholar] [CrossRef]
- Kretz, A.; Kügler, S.; Happold, C.; Bähr, M.; Isenmann, S. Excess Bcl-XL increases the intrinsic growth potential of adult CNS neurons in vitro. Mol. Cell. Neurosci. 2004, 26, 63–74. [Google Scholar] [CrossRef]
- Hansen, M.R.; Roehm, P.C.; Xu, N.; Green, S.H. Overexpression of Bcl-2 or Bcl-xL prevents spiral ganglion neuron death and inhibits neurite growth. Dev. Neurobiol. 2007, 67, 316–325. [Google Scholar] [CrossRef]
- Geden, M.J.; Romero, S.E.; Deshmukh, M. Apoptosis versus axon pruning: Molecular intersection of two distinct pathways for axon degeneration. Neurosci. Res. 2019, 139, 3–8. [Google Scholar] [CrossRef]
- Agostini, M.; Romeo, F.; Inoue, S.; Niklison-Chirou, M.V.; Elia, A.J.; Dinsdale, D.; Morone, N.; Knight, R.A.; Mak, T.W.; Melino, G. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016, 23, 1502–1514. [Google Scholar] [CrossRef]
- Zheng, X.; Boyer, L.; Jin, M.; Mertens, J.; Kim, Y.; Mandel, G.; Hamm, M.; Gage, F.H.; Hunter, T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 2016, 5, 13374. [Google Scholar] [CrossRef]
- Arrázola, M.S.; Andraini, T.; Szelechowski, M.; Mouledous, L.; Arnauné-Pelloquin, L.; Davezac, N.; Belenguer, P.; Rampon, C.; Miquel, M.-C. Mitochondria in Developmental and Adult Neurogenesis. Neurotox. Res. 2018, 36, 257–267. [Google Scholar] [CrossRef]
- Youle, R.J.; Van Der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Jones, A.F.; Sanger, R.H.; Collis, L.P.; Flannery, R.; McNay, E.C.; Yu, T.; Schwarzenbacher, R.; Bossy, B.; et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Alavian, K.N.; Lazrove, E.; Mehta, N.; Jones, A.; Zhang, P.; Licznerski, P.; Graham, M.; Uo, T.; Guo, J.; et al. A Bcl-xL–Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat. Cell Biol. 2013, 15, 773–785. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Tan, W.; Colombini, M. On the Role of VDAC in Apoptosis: Fact and Fiction. J. Bioenerg. Biomembr. 2005, 37, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Colombini, M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.A. Contributions of Bcl-xL to acute and long term changes in bioenergetics during neuronal plasticity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.; Kadenbach, B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000, 466, 130–134. [Google Scholar] [CrossRef]
- Hubbard, M.J.; McHugh, N.J. Mitochondrial ATP synthase F1 -β-subunit is a calcium-binding protein. FEBS Lett. 1996, 391, 323–329. [Google Scholar] [CrossRef]
- Beis, I.; Newsholme, E.A. Effects of calcium ions on adenine nucleotide translocase from cardiac muscle. J. Mol. Cell. Cardiol. 1976, 8, 863–876. [Google Scholar] [CrossRef]
- Ikuko, E.Z.A.W.A.; Etsuro, O.G.A.T.A. Ca2+-Induced Activation of Succinate Dehydrogenase and the Regulation of Mito-chondrial Oxidative Reactions. J. Biochem. 1979, 85, 65–74. [Google Scholar]
- Huang, H.; Hu, X.; Eno, C.O.; Zhao, G.; Li, C.; White, C. An Interaction between Bcl-xL and the Voltage-dependent Anion Channel (VDAC) Promotes Mitochondrial Ca2+ Uptake. J. Biol. Chem. 2013, 288, 19870–19881. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Aon, M.A.; Hsu, Y.-T.; Soane, L.; Teng, X.; McCaffery, J.M.; Cheng, W.-C.; Qi, B.; Li, H.; Alavian, K.N.; et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol. 2011, 195, 263–276. [Google Scholar] [CrossRef]
- De Tudela, M.V.-P.; Delgado-Esteban, M.; Maestre, C.; Bobo-Jiménez, V.; Jiménez-Blasco, D.; Vecino, R.; Bolaños, J.P.; Almeida, A. Regulation of Bcl-xL-ATP Synthase Interaction by Mitochondrial Cyclin B1-Cyclin-Dependent Kinase-1 Determines Neuronal Survival. J. Neurosci. 2015, 35, 9287–9301. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, M.; Yin, A.; Mai, Z.; Wang, Y.; Zhao, M.; Wang, X.; Chen, T. Bcl-xL inhibits PINK1/Parkin-dependent mitophagy by preventing mitochondrial Parkin accumulation. Int. J. Biochem. Cell Biol. 2020, 122, 105720. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, L.K. New Connections: Amyloid-β, Calcium, and the Synapse. Sci. Signal. 2017, 10, eaao3024. [Google Scholar] [CrossRef]
- de Juan-Sanz, J.; Holt, G.T.; Schreiter, E.R.; de Juan, F.; Kim, D.S.; Ryan, T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 2017, 93, 867–881.e6. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Z.; O’Kane, C.J.; Pérez-Moreno, J.J. Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration. Front. Neurosci. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.I.E.; James, A.D. Targeting the Calcium Signalling Machinery in Cancer. Cancers 2020, 12, 2351. [Google Scholar] [CrossRef]
- Dremina, E.S.; Sharov, V.S.; Kumar, K.; Zaidi, A.; Michaelis, E.K.; Schöneich, C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). Biochem. J. 2004, 383, 361–370. [Google Scholar] [CrossRef]
- Distelhorst, C.W.; Lam, M.; McCormick, T.S. Bcl-2 inhibits hydrogen peroxide-induced ER Ca2+ pool depletion. Oncogene 1996, 12, 2051–2055. [Google Scholar]
- Kuo, T.H.; Kim, H.-R.C.; Zhu, L.; Yu, Y.; Lin, H.-M.; Tsang, W. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene 1998, 17, 1903–1910. [Google Scholar] [CrossRef]
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161. [Google Scholar] [CrossRef]
- White, C.W.; Li, C.; Yang, J.; Petrenko, N.B.; Madesh, M.; Thompson, C.B.; Foskett, J.K. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat. Cell Biol. 2005, 7, 1021–1028. [Google Scholar] [CrossRef]
- Mendes, C.C.P.; Gomes, D.A.; Thompson, M.; Souto, N.C.; Goes, T.S.; Goes, A.M.; Rodrigues, M.A.; Gomez, M.V.; Nathanson, M.H.; Leite, M.F. The Type III Inositol 1,4,5-Trisphosphate Receptor Preferentially Transmits Apoptotic Ca2+ Signals into Mitochondria. J. Biol. Chem. 2005, 280, 40892–40900. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Hayashi, T.; Wolozny, D.; Yin, B.; Su, T.-C.; Betenbaugh, M.J.; Su, T.-P. The non-apoptotic action of Bcl-xL: Regulating Ca2+ signaling and bioenergetics at the ER-mitochondrion interface. J. Bioenerg. Biomembr. 2016, 48, 211–225. [Google Scholar] [CrossRef]
- Motoyama, N.; Wang, F.; Roth, K.A.; Sawa, H.; Nakayama, K.; Negishi, I.; Senju, S.; Zhang, Q.; Fujii, S.; Et, A. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 1995, 267, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Mai, J.K.; Zapata, J.M.; Ashwell, K.W.S.; Schendel, S.L.; Reed, J.C.; Krajewski, S. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during devel-opment of murine nervous system. Cell Death Differ. 2002, 9, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, L.C.; Song, B.; Suppiah, Y.; Hasan, S.M.; Martin, H.C.; Hogan, S.E.; Xiong, J.; Vanderluit, J.L. Bcl-xL dependency coincides with the onset of neurogenesis in the developing mammalian spinal cord. Mol. Cell. Neurosci. 2016, 77, 34–46. [Google Scholar] [CrossRef]
- Fogarty, L.C.; Flemmer, R.T.; Geizer, B.A.; Licursi, M.; Karunanithy, A.; Opferman, J.T.; Hirasawa, K.; Vanderluit, J.L. Mcl-1 and Bcl-xL are essential for survival of the developing nervous system. Cell Death Differ. 2019, 26, 1501–1515. [Google Scholar] [CrossRef]
- Nikolaev, A.Y.; McLaughlin, T.; O’Leary, D.D.M.; Tessier-Lavigne, M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nat. Cell Biol. 2009, 457, 981–989. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. Caspase-3 Mediated Cell Death in the Normal Development of the Mammalian Cerebellum. Int. J. Mol. Sci. 2018, 19, 3999. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Gamage, K.K.; Heffron, D.S.; Leblanc, A.C.; Deppmann, C.D.; Mandell, J.W. Caspase-mediated cleavage of actin and tubulin is a common feature and sensitive marker of axonal degeneration in neural development and injury. Acta Neuropathol. Commun. 2014, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Gilbert, J.P.; Huo, Y.; Sharaflari, R.; Nee, M.; Qiao, H.; Man, H.-Y. The Autism Protein Ube3A/E6AP Remodels Neuronal Dendritic Arborization via Caspase-Dependent Microtubule Destabilization. J. Neurosci. 2017, 38, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Victor, K.G.; Heffron, D.S.; Sokolowski, J.D.; Majumdar, U.; Leblanc, A.; Mandell, J.W.; Majumder, U. Proteomic identification of synaptic caspase substrates. Synapse 2018, 72, e22014. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-H.; Jiao, S.; Jia, J.-M.; Chen, Y.; Chen, C.Y.; Gucek, M.; Markey, S.P.; Li, Z. The Novel Caspase-3 Substrate Gap43 is Involved in AMPA Receptor Endocytosis and Long-Term Depression. Mol. Cell. Proteom. 2013, 12, 3719–3731. [Google Scholar] [CrossRef]
- Katow, H.; Kanaya, T.; Ogawa, T.; Egawa, R.; Yawo, H. Regulation of axon arborization pattern in the developing chick ciliary ganglion: Possible involvement of caspase 3. Dev. Growth Differ. 2017, 59, 115–128. [Google Scholar] [CrossRef]
- Simon, D.J.; Weimer, R.M.; McLaughlin, T.; Kallop, D.; Stanger, K.; Yang, J.; O’Leary, D.D.M.; Hannoush, R.N.; Tessier-Lavigne, M. A Caspase Cascade Regulating Developmental Axon Degeneration. J. Neurosci. 2012, 32, 17540–17553. [Google Scholar] [CrossRef]
- Cusack, C.L.; Swahari, V.; Henley, W.H.; Ramsey, J.M.; Deshmukh, M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat. Commun. 2013, 4, 1876. [Google Scholar] [CrossRef]
- Ohsawa, S.; Hamada, S.; Kuida, K.; Yoshida, H.; Igaki, T.; Miura, M. Maturation of the olfactory sensory neurons by Apaf-1/caspase-9-mediated caspase activity. Proc. Natl. Acad. Sci. USA 2010, 107, 13366–13371. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, A.; Wang, Y.; Sheng, M. Local Pruning of Dendrites and Spines by Caspase-3-Dependent and Proteasome-Limited Mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef]
- Li, Z.; Jo, J.; Jia, J.-M.; Lo, S.-C.; Whitcomb, D.J.; Jiao, S.; Cho, K.; Sheng, M. Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell 2010, 141, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Imbriani, P.; Tassone, A.; Meringolo, M.; Ponterio, G.; Madeo, G.; Pisani, A.; Bonsi, P.; Martella, G. Loss of Non-Apoptotic Role of Caspase-3 in the PINK1 Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3407. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Oltean, S. Modulation of the Apoptosis Gene Bcl-x Function Through Alternative Splicing. Front. Genet. 2019, 10, 804. [Google Scholar] [CrossRef]
- Clem, R.J.; Cheng, E.H.; Karp, C.L.; Kirsch, D.G.; Ueno, K.; Takahashi, A.; Kastan, M.B.; Griffin, D.E.; Earnshaw, W.C.; Veliuona, M.A.; et al. Modulation of cell death by Bcl-xL through caspase interaction. Proc. Natl. Acad. Sci. USA 1998, 95, 554–559. [Google Scholar] [CrossRef]
- Geden, M.J.; Deshmukh, M. Axon degeneration: Context defines distinct pathways. Curr. Opin. Neurobiol. 2016, 39, 108–115. [Google Scholar] [CrossRef]
- Simon, D.J.; Pitts, J.; Hertz, N.T.; Yang, J.; Yamagishi, Y.; Olsen, O.; Mark, M.T.; Molina, H.; Tessier-Lavigne, M. Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell 2016, 164, 1031–1045. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Linh, N.H.; Shih, Y.H.; Yu, H.-M.; Li, M.S.; Chen, Y.-R. Alzheimer’s Amyloid-β Sequesters Caspase-3 in Vitro via Its C-Terminal Tail. ACS Chem. Neurosci. 2016, 7, 1097–1106. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas, J.; Nguyen, T.; Gillet, G. Involvement of Bcl-xL in Neuronal Function and Development. Int. J. Mol. Sci. 2021, 22, 3202. https://doi.org/10.3390/ijms22063202
Bas J, Nguyen T, Gillet G. Involvement of Bcl-xL in Neuronal Function and Development. International Journal of Molecular Sciences. 2021; 22(6):3202. https://doi.org/10.3390/ijms22063202
Chicago/Turabian StyleBas, Julie, Trang Nguyen, and Germain Gillet. 2021. "Involvement of Bcl-xL in Neuronal Function and Development" International Journal of Molecular Sciences 22, no. 6: 3202. https://doi.org/10.3390/ijms22063202
APA StyleBas, J., Nguyen, T., & Gillet, G. (2021). Involvement of Bcl-xL in Neuronal Function and Development. International Journal of Molecular Sciences, 22(6), 3202. https://doi.org/10.3390/ijms22063202








