Immunoglobulins and Transcription Factors in Otitis Media
Abstract
1. Introduction
2. Types and Functions of B cells
2.1. Types and Distribution of B cells
2.2. Functions of B cells
3. B cells in Otorhinolaryngologic Fields
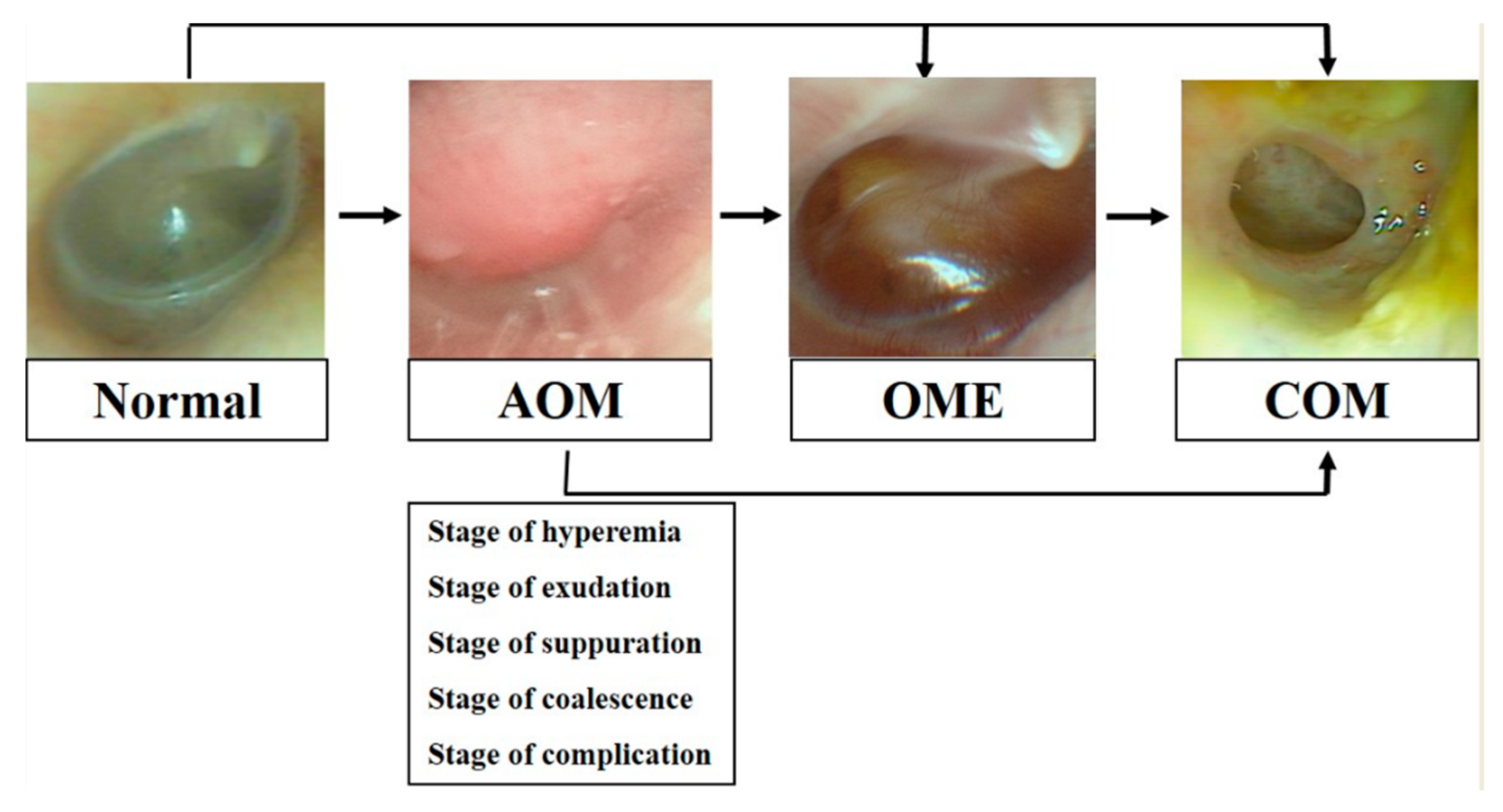
4. Antibody Formation in Otitis Media
5. Antibodies and Related Transcription Factors in Otitis Media
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bluestone, C.D.; Klein, J.O. Otitis media and eustachian tube dysfunction. In Pediatric Otolaryngology, 4th ed.; Bluestone, C.D., Stool, S.E., Alper, C.M., Eds.; Saunders: Philadelphia, PA, USA, 2003; pp. 474–685. [Google Scholar]
- Yeo, S.G. Acute otitis media. In Korean Society of Otorhinolaryngology—Head and Neck Surgery, 3rd ed.; KoonJa: Seoul, Korea, 2018; pp. 363–383. [Google Scholar]
- Christov, F.; Gluth, M.B. Histopathology of the Mucosa of Eustachian Tube Orifice at the Middle Ear in Chronic Otitis Media With Effusion: Possible Insight Into Tuboplasty Failure. Ann. Otol. Rhinol. Laryngol. 2018, 127, 817–822. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Shan, Y.J.; Han, Y.; Zhu, L.W.; Ma, Z.X. Pathological study of otitis media with effusion after treatment with intranasal pulmonary surfactant. Laryngoscope 2013, 123, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.S.; Leach, A.J. Acute and chronic otitis media. Pediatr. Clin. N. Am. 2009, 56, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Budenz, C.L.; El-Kashlan, H.K.; Shelton, C.; Aygun, N.; Niparko, J.K. Complications of temporal bone infections. In Cummings Otolaryngology—Head and Neck Surgery; Flint, P.W., Haughey, B.H., Lund, V.J., Eds.; Saunders-Elsevier: Philadelphia, PA, USA, 2014; pp. 2156–2176. [Google Scholar]
- Forséni, M.; Bagger-Sjöbäck, D.; Hultcrantz, M. A study of inflammatory mediators in the human tympanosclerotic middle ear. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 559–564. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, T.L. Cutting Edge: Commentary: Two B-1 or Not to Be One. J. Immunol. 2002, 168, 4257–4261. [Google Scholar] [CrossRef] [PubMed]
- Ulevitch, R.J. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 2004, 4, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Yeo, S.G.; Kim, S.W.; Cho, J.S.; Cha, C.I. Characteristic features of immune B cells in murine cervical lymph node. Korean J. Otolaryngol. 2005, 48, 241–246. [Google Scholar]
- Morris, D.L.; Rothstein, T.L. Analysis of B-1 cell activation. Methods 1995, 8, 11–25. [Google Scholar] [CrossRef]
- Hamilton, A.M.; Lehuen, A.; Kearney, J.F. Immunofluorescence analysis of B-1 cell ontogeny in the mouse. Int. Immunol. 1994, 6, 355–361. [Google Scholar] [CrossRef]
- Ferlin, W.G.; Severinson, E.; Strom, L.; Heath, A.W.; Coffman, R.L.; Ferrick, D.A.; Howard, M.C. CD40 signaling induces interleukin-4 independent IgE switching in vivo. Eur. J. Immunol. 1996, 26, 2911–2915. [Google Scholar] [CrossRef]
- Davey, E.J.; Thyberg, J.; Conrad, D.; Severinson, E. Regulation of cell morphology in B lymphocytes by interleukin 4: Evidence for induced cytoskeletal changes. J. Immunol. 1998, 160, 5366–5373. [Google Scholar] [PubMed]
- Calmame, K.L.; Lin, K.I.; Tunyaplin, C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003, 21, 205–230. [Google Scholar] [CrossRef]
- Sidman, C.L.; Shultz, L.D.; Hardy, R.R.; Hayakawa, K.; Herzenberg, L.A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science 1986, 232, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Ohdan, H.; Zhao, G.; Yang, Y.G.; Sykes, M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 2003, 171, 5406–5414. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.G.; Cha, C.I.; Park, D.C. Differences in Their Proliferation and Differentiation between B-1 and B-2 Cell. Immune Netw. 2006, 6, 1–5. [Google Scholar] [CrossRef][Green Version]
- Piatelli, M.J.; Tanguay, D.; Rothstein, T.L.; Chiles, T.C. Cell cycle control mechanisms in B-1 and B-2 lymphoid subsets. Immunol. Res. 2003, 27, 31–52. [Google Scholar] [CrossRef]
- Tanguay, D.A.; Colarusso, T.P.; Doughty, C.; Pavlovic-Ewers, S.; Rothstein, T.L.; Chiles, T.C. Cutting edge: Differential signaling requirements for activation of assembled cyclin D3-cdk4 complexes in B-1 and B-2 lymphocyte subsets. J. Immunol. 2001, 166, 4273–4277. [Google Scholar] [CrossRef]
- Tanguay, D.A.; Colarusso, T.P.; Pavlovic, S.; Irigoyen, M.; Howard, R.G.; Bartek, J.; Chiles, T.C.; Rothstein, T.L. Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J. Exp. Med. 1999, 189, 1685–1690. [Google Scholar] [CrossRef]
- Rothstein, T.L.; Kolber, D.L. Anti-Ig Ab inhibits the phorbol ester-induced stimulation of peritoneal B cells. J. Immunol. 1988, 141, 4089–4093. [Google Scholar]
- Segura, A.S.; Brieva, J.A.; Rodriguez, C. Regualtion of immunoglobulin secretion of plasma cells infiltrating nasal polyps. Laryngoscope 2000, 110, 1183–1188. [Google Scholar] [CrossRef]
- Suenaga, S.; Kodama, S.; Ueyama, S.; Suzuki, M.; Mogi, G. Mucosal Immunity of the Middle Ear: Analysis at the single cell level. Laryngoscope 2001, 111, 290–296. [Google Scholar] [CrossRef]
- Arita, M.; Kodama, S.; Suzuki, M.; Mogi, G. Single cell analysis of adenoid CD5+B cells and their protective contributions to nasopharyngeal immunity. Laryngoscope 2003, 113, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.G.; Cho, J.S.; Park, D.C.; Rothstein, T.L. B-1 Cells Differ from Conventional B (B-2) Cells: Difference in Proliferation. Immune Netw. 2004, 4, 155–160. [Google Scholar] [CrossRef]
- Yeo, S.G.; Tumang, J.R.; Rothstein, T.L. Characteristic features of B cells in murine cervical lymph nodes. Acta Otolaryngol. 2006, 126, 56–61. [Google Scholar]
- Roh, J.L.; Seong, W.J.; Sung, M.W.; Lee, D.W.; Park, B.J.; Park, S.W.; Kim, K.H. Frequency and Distribution of Lymphocytes Related to Innate Immunity in Palatine Tonsils and Adenoids. Korean J. Otorhinolaryngol. Head Neck Surg. 2001, 44, 1073–1079. [Google Scholar]
- Vohlonen, I.; Terho, E.O.; Koivikko, A.; Vanato, T.; Holmén, A.; Heinonen, O.P. Reproducibility of the skin prick test. Allergy 1989, 44, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.G.; Yeo, S.G.; Chung, H.; Chung, D.H.; Kim, M.G.; Byun, J.Y.; Park, M.S.; Cha, C.I. Immunoglobulin E and Transcription Factor in Adenoid of Children with Allergy. Korean J. Otorhinolaryngol. Head Neck Surg. 2009, 52, 594–598. [Google Scholar] [CrossRef]
- Bernstein, J.M. Role of allergy in eustachian tube blockage and otitis media with effusion: A review. Otolaryngol. Head Neck Surg. 1996, 114, 562–568. [Google Scholar] [CrossRef]
- Sloyer, J.L.; Howie, V.M.; Ploussard, J.H.; Schiffman, G.; Johnston, R.B. Immune response to acute otitis media: Association between middle ear fluid antibody and the clearing of clinical infection. J. Clin. Microbiol. 1976, 4, 306–308. [Google Scholar] [PubMed]
- Veenhoven, R.; Rijkers, G.; Schilder, A.; Adelmeijer, J.; Uiterwaal, C.; Kuis, W.; Sanders, E. Immunoglobulins in otitis-prone children. Pediatr. Res. 2004, 55, 159–163. [Google Scholar] [CrossRef][Green Version]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Howie, V.M.; Ploussard, J.H.; Sloyer, J.L.; Johnston, R.B., Jr. Immunoglobulins of the Middle Ear Fluid in Acute Otitis Media: Relationship to Serum Immunoglobulin Concentrations and Bacterial Cultures. Infect. Immun. 1973, 7, 589–593. [Google Scholar] [CrossRef]
- Sloyer, J.L., Jr.; Howie, V.M.; Ploussard, J.H.; Ammann, A.J.; Austrian, R.; Johnston, R.B., Jr. Immune response to acute otitis media in children. I. Serotypes isolated and serum and middle ear fluid antibody in pneumococcal otitis media. Infect. Immun. 1974, 9, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Sloyer, J.L., Jr.; Cate, C.C.; Howie, V.M.; Ploussard, J.H.; Johnston, R.B., Jr. The immune response to acute otitis media in children. II. Serum and middle ear fluid antibody in otitis media due to Haemophilus influenza. J. Infect. Dis. 1975, 132, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Sloyer, J.L.; Howie, V.M.; Ploussard, J.H.; Bradac, J.; Habercorn, M.; Ogra, P.L. Immune response to acute otitis media in children III. Implications of viral antibody in middle ear fluid. J. Immunol. 1977, 118, 248–250. [Google Scholar] [PubMed]
- Ren, D.; Almudevar, A.L.; Murphy, T.F.; Lafontaine, E.R.; Campagnari, A.A.; Luke-Marshall, N.; Casey, J.R.; Pichichero, M.E. Serum antibody response to Moraxella catarrhalis proteins OMP CD, OppA, Msp22, Hag, and PilA2 after nasopharyngeal colonization and acute otitis media in children. Vaccine 2015, 33, 5809–5814. [Google Scholar] [CrossRef]
- Kaur, R.; Kim, T.; Casey, J.R.; Pichichero, M.E. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin. Vaccine Immunol. 2012, 19, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Casey, J.R.; Pichichero, M.E. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr. Infect. Dis. J. 2011, 30, 645–650. [Google Scholar] [CrossRef]
- Kaur, R.; Casey, J.R.; Pichichero, M.E. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine 2011, 29, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Corscadden, K.J.; Kirkham, L.-A.S.; Thornton, R.B.; Vijayasekaran, S.; Coates, H.L.; Richmond, P.C.; Wiertsema, S.P. High pneumococcal serotype specific IgG, IgG1 and IgG2 levels in serum and the middle ear of children with recurrent acute otitis media receiving ventilation tubes. Vaccine 2013, 31, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; Corscadden, K.J.; Mowe, E.N.; Zhang, G.; Vijayasekaran, S.; Coates, H.L.; Mitchell, T.J.; Thomas, W.R.; Richmond, P.C.; Kirkham, L.-A.S. IgG responses to Pneumococcal and Haemophilus influenzae protein antigens are not impaired in children with a history of recurrent acute otitis media. PLoS ONE 2012, 7, e49061. [Google Scholar] [CrossRef][Green Version]
- Krakau, M.; Dagöö, B.R.; Hammarström, L.; Granath, A. Normalized immunoglobulin patterns in adults with recurrent acute otitis media and low IgG2 levels during early childhood. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Freijd, A.; Hammarstrom, L.; Persson, M.A.; Smith, C.I. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin. Exp. Immunol. 1984, 56, 233–238. [Google Scholar]
- Verhaegh, S.J.C.; Stol, K.; de Vogel, C.P.; Riesbeck, K.; Lafontaine, E.R.; Murphy, T.F.; van Belkum, A.; Hermans, P.W.M.; Hays, J.P. Comparative analysis of the humoral immune response to Moraxella catarrhalis and Streptococcus pneumoniae surface antigens in children suffering from recurrent acute otitis media and chronic otitis media with effusion. Clin. Vaccine Immunol. 2012, 19, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.H.; Shin, O.Y.; Cha, S.-H.; Kim, Y.I.; Lee, J.W.; Yeo, S.G. IgA and Differentiation-associated Transcription Factors in Chronic Otitis Media with Effusion. Clin. Exp. Otorhinolaryngol. 2009, 2, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Somekawa, Y.; Suzuki, T.; Kataura, A. Immunologic and cytologic studies in otitis media with effusion. Acta Oto-Laryngol. 1987, 104, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.G.; Park, D.C.; Lee, S.K.; Cha, C.I. Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Drake-Lee, A.B.; Hughes, R.G.; Dunn, C. Serum IgA and IgG functional antibodies and their subclasses to Streptococcus pneumoniae capsular antigen found in two aged-matched cohorts of children with and without otitis media with effusion. Clin. Otolaryngol. Allied Sci. 2003, 28, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.H.; Choi, J.Y.; Lee, W.S.; Kim, H.N.; Yoon, J.H. Compositional difference in middle ear effusion: Mucous versus serous. Laryngoscope 2002, 112, 152–155. [Google Scholar] [CrossRef]
- Takada, R.; Harabuchi, Y.; Himi, T.; Kataura, A. Antibodies specific to outer membrane antigens of Moraxella catarrhalis in sera and middle ear effusions from children with otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 1998, 46, 185–195. [Google Scholar] [CrossRef]
- Faden, H.; Hong, J.; Murphy, T. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect. Immun. 1992, 60, 3824–3829. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, A.O.; Arinola, O.G.; Bakare, R.A. Serum and middle ear immunoglobulins in suppurative otitis media. ORL J. Otorhinolaryngol Relat. Spec. 2008, 70, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, A.O.; Arinola, O.G.; Olayemi, O. Role of elevated immunoglobulin E levels in suppurative otitis media. Ann. Trop. Paediatr. 2008, 28, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Tumang, J.R.; Frances, R.; Yeo, S.G.; Rothstein, T.L. Cutting edge: Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 2005, 174, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- Kusam, S.; Dent, A. Common mechanisms for the regulation of B cell differentiation and transformation by the transcriptional repressor protein BCL-6. Immunol. Res. 2007, 37, 177–186. [Google Scholar] [CrossRef]
- Shaffer, A.L.; Yu, X.; He, Y.; Boldrick, J.; Chan, E.P.; Staudt, L.M. Bcl-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000, 13, 199–212. [Google Scholar] [CrossRef]
- Angelin-Duclos, C.; Cattoretti, G.; Lin, K.I.; Calame, K. Commitment of B lymphocytes to a plasma cell fate is associated with blimp-1 expression In vivo. J. Immunol. 2000, 165, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
| Authors and Reference | Study Design | Species/Type of OM | Type of Samples or Specimens | Detection Methods | Targets | Results/Conclusion |
|---|---|---|---|---|---|---|
| Virgil et al. [37] | Prospective study | Human (255 specimens of MEF from 165 episodes of AOM in children) | MEF vs. serum | Radial immunodiffusion | IgG, IgM, IgA | IgA: MEF > serum in almost half the patients - over 9 months of age: culture(−) was dominant in case of MEF > serum IgA IgG and IgM: MEF < serum The MEF of AOM represents primarily a secretory response to inflammation rather than a transudate. |
| Sloyer et al. [38] | Prospective study | Human (61 AOM childrenl) | MEF, serum | IFA, IHA | IgG, IgM, IgA (pneumococcal Ab serotype 1, 3, 6, 14, 18, 19, and 23) | Serum: IgG, IgM: predominantly detected MEF: equally detected all three classes Approximately 25% of the patients (16 of 61) had a positive immune response to their infection as evidenced by increased levels of pneumococcal antibody in the convalescent serum. |
| Sloyer et al. [39] | Prospective study | Human (40 AOM children) | MEF, serum | IFA, IHA | IgG, IgM, IgA (Ab to Hi) | Serum: IgG> IgM = Ig A: acute phase < convalescent phase MEF: IgG = IgA > IgM |
| Sloyer et al. [40] | Prospective study | Human (103 AOM childrenl) | MEF, serum | IFA, radial immunodiffusion | IgG, IgM, IgA (antibody to measles, mumps, rubella, and polio-1) | IgA: MEF > serum IgG: MEF < serum Mean specific MEF IgA titer: immunized > unimmunized |
| Sloyer et al. [33] | Prospective study | Human (80 patients with AOM) | MEF (cleared vs. not cleared) | Indirect fluorescent antibody, radioimmunoassay | IgG, IgM, IgA (Hi, Spn) | Abs concentration: cleared MEF > not cleared MEF Clearing of the MEF in patients with AOM due to Spn or Hi was significantly associated with the presence and concentration of specific Abs in the MEF at the time of diagnosis. |
| Ren et al. [41] | Prospective study | Human: 35 AOM and 149 controls | Serum | Western blot, ELISA | Serum Ab response to Mcat (OMP CD, OppA, Msp22, Hag and PilA2) | Serum IgG in all cases: Msp22 = OppA > OMP CD = Hag = PilA2. Serum antibody to Mcat increased with age in naturally immunized children age 6–30 months following Mcat NP colonization and AOM. In AOM group: IgG against OMP CD: acute phase < convalescent phase High antibody levels against OppA, Msp22, and Hag correlated with reduced carriage. |
| Kaur et al. [42] | Prospective cohort study | Human (137 AOM) | Serum, MEF, NW | ELISA, Western blot | IgG, IgA, sIgA | IgG: NW < MEF ≈ serum IgA: NW > MEF ≈ serum sIgA: MEF (+) IgA in MEF: originated from serum > NW |
| Kaur et al. [43] | Prospective study (3.5 years) | Human: 34 AOM vs. 35 rAOM vs. 25 AOMTF | Serum | ELISA | Serum IgG antibody titers of 5 different Spn proteins (PhtD, LytB, PcpA, PhtE, and Ply) | (1) Acute phase: IgG to PhtD, LytB, PhtE, Ply: rAOM < AOM = AOMTF (2) Convalescent phase: IgG to PhtD, LytB, PhtE, Ply: rAOM = AOMTF < AOM Otitis-prone and AOMTF children mount less of an IgG serum antibody response as compared with non-otitis-prone children to Spn proteins after AOM. |
| Kaur et al. [44] | Prospective study (3.5 years) | Human: 26 AOM vs. 32 rAOM vs. 27 AOMTF | Serum | ELISA | Serum Ab response to outer membrane protein D, P6, OMP26 of NTHi | (1) Acute phase: IgG against PD: rAOM < other two groups IgG against P6, OMP26: rAOM < AOMTF (2) Convalescent phase: rAOM and AOMTF: no change in total IgG against all the three proteins AOM: increased to PD The data on acute sera of otitis prone vs. non-otitis prone children and the acute-to-convalescence response in non-otitis prone children point to a possible link of anti-PD to protection. Further, otitis prone children should be evaluated for their responses to PD, P6, and OMP26 vaccine antigens of NTHi. |
| Veenhoven et al. [34] | Prospective study | Human (365 AOM children: rAOM vs. non-rAOM) | Serum | radial immunodiffusion | IgG (IgG1, IgG2), IgM, IgA | IgG (IgG1, IgG2), IgM, IgA: rAOM < non-rAOM In rAOM groups (compared with normal value): IgG, IgM, IgA, IgG1; increased levels IgG2: decreased levels Lower Ig levels in rAOM children suggest a generalized decreased Abs response in rAOM children. |
| Corscadden et al. [45] | Cross-sectional study | Human (166 rAOM children vs. 61 healthy controls) | Serum, MEF | multiplex bead-based assay, microsphere-based flow cytometric assay | IgG, IgG1, and IgG2 (against 11 pneumococcal polysaccharides: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F 1) | IgG and IgG1 against serotype 5: rAOM > control All pneumococcal serotype specific IgG in rAOM: serum = MEF |
| Wiertsema et al. [46] | Cross-sectional study | Human (172 rAOM vs. 63 controls) | Serum | Multiplex bead assay | IgG: against 4 pneumococcal (PspA1, PspA 2, CbpA, and Ply) and 3 NTHi (P4, P6, and PD) | IgG against NTHi P4, P6, PD: rAOM > control IgG against pneumococcal protein antigens: rAOM ≈ control |
| Krakau et al. [47] | Prospective study | Human: 28 adults with low IgG2 level during childhood (15: a history of rAOM during childhood vs. 13 controls) | Serum | Nephelometry, ELISA | total IgG and IgG subclasses 1–4 | All Igs: rAOM = control Study subjects who had rAOM combined with low IgG2 levels during childhood had a normalized immunoglobulin pattern as adults. |
| Freijd et al. [48] | Prospective study | Human (15 pOME children vs. 15 age matched healthy control children vs. 15 healthy adults) | Plasma samples | ELISA | Spn Abs of different IgG subclasses | Adults: IgG1 < IgG2 Children: IgG1 > IgG2 (pOME < control) |
| Verhaegh et al. [49] | Prospective cohort study | Human (rAOM vs. cOME) | Serum, MEF | Luminex xMAP technology | IgG, IgM, IgA (against Spn and Mcat) | Serum and MEF antigen-specific IgG, IgM, IgA: rAOM ≈ cOME Serum or MEF IgG, IgM, IgA level were not different and not related to bacterial identification in both groups Serum and MEF show strong correlation for only IgG in both groups |
| Shin et al. [50] | Prospective study | Human (29 p-OME vs. 32 OME children) | MEF | ELISA | IgG, IgM, IgA | IgA: pOME < OME. Lower concentrations of IgA in middle ear fluid of patients with OME may be related to OME recurrence and chronicity. |
| Yamanaka et al. [51] | Prospective study | Human (320 OME ears: acute, 10.3%; subacute, 16.6%; chronic, 73.1%) | MEF | ELISA | IgG, IgM, IgA | IgG-ICs: highest positive rate was found in acute cases IgA-ICs: highest positive rate was found in subacute cases neutrophil dominant type in chronic cases: highest IgG-ICs level ICs formed in the MEF might play an important role in the prolonged inflammatory process of OME through the complement activation following chemotaxis of neutrophils. |
| Yeo et al. [52] | Prospective study | Human (58 cOME vs. 64 controls) | MEF, serum | ELISA, nephelometry | IgG, IgM, IgA | Serum IgG, Ig M, IgA: cOME < control MEF Ig concentration–the presence of bacteria: no correlation MEF Ig concentration–serum Ig concentration: no correlation Serum Ig concentration: bacteria (+) > bacteria (−) The presence of effusion bacteria in OME may be related to systemic immunity, but the concentration of Ig in effusion fluid may not be affected by the presence of effusion bacteria. |
| Drake-Lee et al. [53] | Two age-matched cohorts study | Human (50 OME vs. 50 age-matched controls) | Serum | ELISA, radial immunodiffusion | Total IgG, IgG subclass, total IgA, IgA subclass | Total IgA, IgA2, total IgG, IgG2: OME ≈ control normal antibody response between both groups of patients. |
| Chung et al. [54] | Prospective study | Human (27 mucoid OME vs. 18 serous OME) | MEF | Immunoblot assay | sIgA | sIgA: mucoid OME > serous OME |
| Takada et al. [55] | Prospective study | Human (59 OME children) | MEF, serum | ELISA | IgG, IgM, IgA, and sIgA antibodies specific to outer membrane antigens of Mcat | Serum: IgG > IgA > IgM MEF: IgG > IgM > sIgA > IgA All Ig: MEF > serum IgG and IgM in MEF: acute phase > subacute/chronic phase sIgA in MEF: acute phase < subacute/chronic phase IgG in serum or MEF: recurrent/persistent OME group < nonrecurrent/non-persistent OME group Decreased serum and MEF IgG antibody levels specific to outer membrane antigens of Mcat may lead to failure to eliminate this organism, resulting in persistent and/or recurrent appearance of MEF. |
| Faden et al. [56] | Prospective study | Human (14 OME) | Serum, MEF | Ab assay by 96-well microtiter plate | IgG, IgM, IgA | Serum: IgG > IgM > IgA MEF: IgG > IgA > IgM The IgG- and IgA-specific antibody present in middle ear effusions appeared to represent local production rather than passive diffusion from the systemic circulation. |
| Lasisi et al. [57] | Prospective study | Human (20 cSOM vs. 17 aSOM vs. 15 controls) | MEF, serum | ELISA | IgG, IgM | Serum IgG: cSOM > control > aSOM MEF IgG: cSOM > aSOM Serum IgM: aSOM > cSOM> control MEF IgM: aSOM > cSOM |
| Lasisi et al. [58] | Prospective study | Human: 20cSOM vs. 17 aSOM vs. 15 controls | Serum, MEF | Radial immunodiffusion | IgE | Serum IgE: cSOM > aSOM > control MEF IgE: cSOM > aSOM MEF/serum IgE ratio: cSOM > aSOM Serum and MEF showed correlation in cSOM. Allergy appears to play a contributory role in CSOM and elevated IgE in the MES suggests a likely mucosal response. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Kim, D.; Park, D.C.; Lee, E.H.; Choi, Y.-S.; Ryu, J.; Kim, S.H.; Yeo, S.G. Immunoglobulins and Transcription Factors in Otitis Media. Int. J. Mol. Sci. 2021, 22, 3201. https://doi.org/10.3390/ijms22063201
Jung SY, Kim D, Park DC, Lee EH, Choi Y-S, Ryu J, Kim SH, Yeo SG. Immunoglobulins and Transcription Factors in Otitis Media. International Journal of Molecular Sciences. 2021; 22(6):3201. https://doi.org/10.3390/ijms22063201
Chicago/Turabian StyleJung, Su Young, Dokyoung Kim, Dong Choon Park, Eun Hye Lee, Yong-Sung Choi, Jeewon Ryu, Sang Hoon Kim, and Seung Geun Yeo. 2021. "Immunoglobulins and Transcription Factors in Otitis Media" International Journal of Molecular Sciences 22, no. 6: 3201. https://doi.org/10.3390/ijms22063201
APA StyleJung, S. Y., Kim, D., Park, D. C., Lee, E. H., Choi, Y.-S., Ryu, J., Kim, S. H., & Yeo, S. G. (2021). Immunoglobulins and Transcription Factors in Otitis Media. International Journal of Molecular Sciences, 22(6), 3201. https://doi.org/10.3390/ijms22063201







