Pleiotropic Roles of ABC Transporters in Breast Cancer
Abstract
1. Chemoresistance in Breast Cancer
2. The Role of ABCB1 in Breast Cancer Chemoresistance
2.1. Structure and Mechanism of ABCB1
2.2. Expression and Function of ABCB1 in Breast Cancer
2.3. Inhibition of ABCB1 in Breast Cancer
3. The Role of ABC Transporters in Breast Cancer Development and Metastasis
3.1. ABC Transporters and Breast Cancer Development
3.2. ABC Transporter and Breast Cancer Metastasis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ABC subfamily B member 1 |
| ADP | Adenosine diphosphate |
| AKT | Protein kinase B |
| ALDH1 | Aldehyde dehydrogenase 1 |
| ATP | Adenosine Triphosphate |
| BBB | Blood-brain barrier |
| BCRP | Breast cancer resistance protein |
| BCSC | Breast cancer stem cell |
| BPD | Benzoporphyrin derivative |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| CD44-ICD | Intracellular domain fragment of CD44 |
| CS | Collateral sensitivity |
| CSF | Blood-cerebrospinal-fluid barrier |
| DFS | Disease-free survival |
| EMT | Epithelial-to-mesenchymal transition |
| ERK1/2 | Extracellular signal-regulated protein kinase 1/2 |
| FAC | 5-fluorouracil, Adriamycin and cyclophosphamide |
| FEC | 5-fluorouracil, epirubicin and cyclophosphamide |
| HGSC | High-grade serous ovarian cancer |
| HR | Homologous recombination |
| MAPK | Mitogen-activated protein kinases |
| MBC | Metastatic breast cancer |
| MDR | Multidrug resistance |
| MDR1 | Multidrug resistance protein 1 |
| MMP | Metalloproteinase |
| MRP1 | MDR-associated protein 1 |
| NBDs | Nucleotide-binding domains |
| NSP | Non-side population |
| NTRK | Neurotrophic tropomyosin receptor kinase |
| OS | Overall survival |
| P-gp | P-glycoprotein |
| PARP | Poly (ADP-ribose) polymerase |
| PDT | Photodynamic therapy |
| PFS | Progression-free survival |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP | Phosphatidylinositol-3-phosphate |
| PKA | protein kinase A |
| S1P | Sphingosine-1-phosphate |
| SP | Side population |
| SNPs | Single nucleotide polymorphisms |
| SPAG5 | Sperm-associated antigen 5 |
| TAM | Tamoxifen |
| TMDs | Transmembrane domains |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Yu, D.; Hung, M.-C. Breast Cancer Chemosensitivity; Springer Science & Business Media: Basel, Switzerland, 2009; Volume 608. [Google Scholar]
- De Laurentiis, M.; Cancello, G.; D’Agostino, D.; Giuliano, M.; Giordano, A.; Montagna, E.; Lauria, R.; Forestieri, V.; Esposito, A.; Silvestro, L. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J. Clin. Oncol. 2008, 26, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Peto, R. Early Breast Cancer Trialists’ Collaborative Group. The Worldwide Overview: New Results for Systemic Adjuvant Therapies. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 13–16 December 2007; pp. 13–16. [Google Scholar]
- Kataja, V.; Castiglione, M. Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2008, 19, ii11–ii13. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Survival Rates. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 25 November 2020).
- Kessel, D. Circumvention of resistance to anthracyclines by calcium antagonists and other membrane-perturbing agents. Cancer Surv. 1986, 5, 109–127. [Google Scholar] [PubMed]
- Biedler, J.L.; Riehm, H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: Cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970, 30, 1174–1184. [Google Scholar]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.; Armstrong, M.; Oehler, M.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef]
- Hollenstein, K.; Dawson, R.J.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Olsen, S.L.; Qiu, W.; Deeley, R.G.; Cole, S.P. Mutation of a single conserved tryptophan in multidrug resistance protein 1 (MRP1/ABCC1) results in loss of drug resistance and selective loss of organic anion transport. J. Biol. Chem. 2001, 276, 15616–15624. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Delou, J.M.A.; Vignal, G.M.; Indio-do-Brasil, V.; Accioly, M.T.S.; da Silva, T.S.L.; Piranda, D.N.; Sobral-Leite, M.; de Carvalho, M.A.; Capella, M.A.M.; Vianna-Jorge, R. Loss of constitutive ABCB1 expression in breast cancer associated with worse prognosis. Breast Cancer 2017, 9, 415–428. [Google Scholar] [CrossRef]
- Blazquez, A.M.G.; Macias, R.I.R.; Cives-Losada, C.; de la Iglesia, A.; Marin, J.J.G.; Monte, M.J. Lactation during cholestasis: Role of ABC proteins in bile acid traffic across the mammary gland. Sci. Rep. 2017, 7, 7475. [Google Scholar] [CrossRef]
- Schimanski, S.; Wild, P.J.; Treeck, O.; Horn, F.; Sigruener, A.; Rudolph, C.; Blaszyk, H.; Klinkhammer-Schalke, M.; Ortmann, O.; Hartmann, A.; et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm. Metab. Res. 2010, 42, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; van de Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar]
- Gros, P.; CROoP, J.; Roninson, I.; Varshavsky, A.; Housman, D.E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc. Natl. Acad. Sci. USA 1986, 83, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Cornwell, M.M.; Gottesman, M.M.; Pastan, I.; Roninson, I.B.; Ling, V.; Riordan, J.R. The mdrl gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem. Biophys. Res. Commun. 1986, 141, 956–962. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 64, 138–153. [Google Scholar] [CrossRef]
- Sparreboom, A.; Van Asperen, J.; Mayer, U.; Schinkel, A.H.; Smit, J.W.; Meijer, D.K.; Borst, P.; Nooijen, W.J.; Beijnen, J.H.; Van Tellingen, O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 1997, 94, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bruin, M.A.C.; Gan, C.; Lebre, M.C.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of tivozanib is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein) in mice. Int. J. Pharm. 2020, 581, 119277. [Google Scholar] [CrossRef]
- Li, W.; Tibben, M.; Wang, Y.; Lebre, M.C.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (MDR1/ABCB1) controls brain accumulation and intestinal disposition of the novel TGF-beta signaling pathway inhibitor galunisertib. Int. J. Cancer 2020, 146, 1631–1642. [Google Scholar] [CrossRef]
- Li, W.; Sparidans, R.; El-Lari, M.; Wang, Y.; Lebre, M.C.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (ABCB1/MDR1) limits brain accumulation and Cytochrome P450-3A (CYP3A) restricts oral availability of the novel FGFR4 inhibitor fisogatinib (BLU-554). Int. J. Pharm. 2020, 573, 118842. [Google Scholar] [CrossRef]
- van Hoppe, S.; Jamalpoor, A.; Rood, J.J.M.; Wagenaar, E.; Sparidans, R.W.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of osimertinib and its active metabolite AZ5104 is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein). Pharmacol. Res. 2019, 146, 104297. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; Martinez-Chavez, A.; van Dongen, M.G.J.; Beijnen, J.H.; Schinkel, A.H.; Huitema, A.D.R.; Steeghs, N. Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib. Clin. Pharmacokinet. 2020, 59, 1501–1520. [Google Scholar] [CrossRef]
- Slot, A.J.; Molinski, S.V.; Cole, S.P. Mammalian multidrug-resistance proteins (MRPs). Essays Biochem. 2011, 50, 179–207. [Google Scholar] [CrossRef]
- Esser, L.; Zhou, F.; Pluchino, K.M.; Shiloach, J.; Ma, J.; Tang, W.-k.; Gutierrez, C.; Zhang, A.; Shukla, S.; Madigan, J.P. Structures of the multidrug transporter P-glycoprotein reveal asymmetric ATP binding and the mechanism of polyspecificity. J. Biol. Chem. 2017, 292, 446–461. [Google Scholar] [CrossRef]
- Johnson, Z.L.; Chen, J. Structural basis of substrate recognition by the multidrug resistance protein MRP1. Cell 2017, 168, 1075–1085.e1079. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.M.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X.B. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.S.; Chen, L.; Ding, W.X.; Li, K.; Wu, J.J. Elevated expression of both MDR1 and MMP-2 genes in metastasized lymph node of invasive ductal breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2037–2043. [Google Scholar] [PubMed]
- Trock, B.J.; Leonessa, F.; Clarke, R. Multidrug resistance in breast cancer: A meta-analysis of MDR1/gp170 expression and its possible functional significance. J. Natl. Cancer Inst. 1997, 89, 917–931. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Ciarmiello, A.; Potena, M.I.; Carriero, M.V.; Mainolfi, C.; Botti, G.; Thomas, R.; Cerra, M.; D’Aiuto, G.; Tsuruo, T. In vivo detection of multidrug-resistant (MDR1) phenotype by technetium-99m sestamibi scan in untreated breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 1997, 24, 150–159. [Google Scholar] [CrossRef]
- Sun, S.-S.; Hsieh, J.-F.; Tsai, S.-C.; Ho, Y.-J.; Lee, J.-K.; Kao, C.-H. Expression of mediated P-glycoprotein multidrug resistance related to Tc-99m MIBI scintimammography results. Cancer Lett. 2000, 153, 95–100. [Google Scholar] [CrossRef]
- Kao, C.-H.; Tsai, S.-C.; Liu, T.-J.; Ho, Y.-J.; Wang, J.-J.; Ho, S.-T.; ChangLai, S.-P. P-Glycoprotein and multidrug resistance-related protein expressions in relation to technetium-99m methoxyisobutylisonitrile scintimammography findings. Cancer Res. 2001, 61, 1412–1414. [Google Scholar]
- Hlaváč, V.; Brynychová, V.; Václavíková, R.; Ehrlichová, M.; Vrána, D.; Pecha, V.; Koževnikovová, R.; Trnková, M.; Gatěk, J.; Kopperová, D. The expression profile of ATP-binding cassette transporter genes in breast carcinoma. Pharmacogenomics 2013, 14, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Foekens, J.A.; Look, M.P.; Meijer-van Gelder, M.E.; Klijn, J.G.; Wiemer, E.A.; Stoter, G.; Nooter, K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: Correlation with chemotherapeutic response. Clin. Cancer Res. 2003, 9, 827–836. [Google Scholar]
- Vaclavikova, R.; Klajic, J.; Brynychova, V.; Elsnerova, K.; Alnaes, G.I.G.; Tost, J.; Kristensen, V.N.; Rob, L.; Kodet, R.; Skapa, P.; et al. Development of high--resolution melting analysis for ABCB1 promoter methylation: Clinical consequences in breast and ovarian carcinoma. Oncol. Rep. 2019, 42, 763–774. [Google Scholar] [CrossRef]
- Ankathil, R. ABCB1 genetic variants in leukemias: Current insights into treatment outcomes. Pharmgenomics. Pers. Med. 2017, 10, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Wong, Z.W.; Sandanaraj, E.; Xiang, X.; Ang, P.C.; Lee, E.J.; Chowbay, B. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008, 99, 816–823. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Peeters, M.; van Dam, P.; Dieudonne, A.S.; Hatse, S.; et al. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res. Treat. 2014, 147, 557–570. [Google Scholar] [CrossRef]
- Priyadarshini, R.; Raj, G.M.; Kayal, S.; Ramesh, A.; Shewade, D.G. Influence of ABCB1 C3435T and C1236T gene polymorphisms on tumour response to docetaxel-based neo-adjuvant chemotherapy in locally advanced breast cancer patients of South India. J. Clin. Pharm. Ther. 2019, 44, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Caram, M.V.; Kidwell, K.M.; Thibert, J.N.; Gersch, C.; Seewald, N.J.; Smerage, J.; Rubenfire, M.; Henry, N.L.; Cooney, K.A.; et al. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics 2016, 17, 231–240. [Google Scholar] [CrossRef]
- Imperio, G.E.; Javam, M.; Lye, P.; Constantinof, A.; Dunk, C.E.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G.; Ortiga-Carvalho, T.M.; et al. Gestational age-dependent gene expression profiling of ATP-binding cassette transporters in the healthy human placenta. J. Cell. Mol. Med. 2019, 23, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Merino, G.; Musters, S.; van Herwaarden, A.E.; Bolscher, E.; Wagenaar, E.; Mesman, E.; Dale, T.C.; Schinkel, A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005, 11, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aspitia, A.; Perez, E.A. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin. Proc. 2009, 84, 533–545. [Google Scholar] [CrossRef]
- Nemcova-Furstova, V.; Kopperova, D.; Balusikova, K.; Ehrlichova, M.; Brynychova, V.; Vaclavikova, R.; Daniel, P.; Soucek, P.; Kovar, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Izumi, H.; Ito, K.I. ABCB1 and ABCC11 confer resistance to eribulin in breast cancer cell lines. Oncotarget 2016, 7, 70011–70027. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Xu, L.; Wang, R.; Lu, X.; Sang, J. miR-381 overcomes cisplatin resistance in breast cancer by targeting MDR1. Cell Biol. Int. 2019, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mechetner, E.; Kyshtoobayeva, A.; Zonis, S.; Kim, H.; Stroup, R.; Garcia, R.; Parker, R.J.; Fruehauf, J.P. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin. Cancer Res. 1998, 4, 389–398. [Google Scholar]
- Pajic, M.; Iyer, J.K.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Jonkers, J.; Borst, P.; Rottenberg, S. Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res. 2009, 69, 6396–6404. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Sol, W.; Kersbergen, A.; Schlicker, A.; Guyader, C.; Xu, G.; Wessels, L.; Borst, P.; Jonkers, J.; Rottenberg, S. BRCA2-deficient sarcomatoid mammary tumors exhibit multidrug resistance. Cancer Res. 2015, 75, 732–741. [Google Scholar] [CrossRef]
- Rottenberg, S.; Nygren, A.O.; Pajic, M.; van Leeuwen, F.W.; van der Heijden, I.; van de Wetering, K.; Liu, X.; de Visser, K.E.; Gilhuijs, K.G.; van Tellingen, O.; et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 12117–12122. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Vollebergh, M.A.; de Hoon, B.; de Ronde, J.; Schouten, P.C.; Kersbergen, A.; Zander, S.A.; Pajic, M.; Jaspers, J.E.; Jonkers, M.; et al. Impact of intertumoral heterogeneity on predicting chemotherapy response of BRCA1-deficient mammary tumors. Cancer Res. 2012, 72, 2350–2361. [Google Scholar] [CrossRef]
- Shukla, S.; Chen, Z.-S.; Ambudkar, S.V. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist. Updat. 2012, 15, 70–80. [Google Scholar] [CrossRef]
- Lombard, A.P.; Liu, C.; Armstrong, C.M.; D’Abronzo, L.S.; Lou, W.; Chen, H.; Dall’Era, M.; Ghosh, P.M.; Evans, C.P.; Gao, A.C. Overexpressed ABCB1 Induces Olaparib-Taxane Cross-Resistance in Advanced Prostate Cancer. Transl. Oncol. 2019, 12, 871–878. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; Sol, W.; van Deemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013, 3, 68–81. [Google Scholar] [CrossRef]
- He, J.; Green, A.R.; Li, Y.; Chan, S.Y.T.; Liu, D.X. SPAG5: An Emerging Oncogene. Trends Cancer 2020, 6, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.P.; Fan, H.; Fisher, R.A.; Lusvarghi, S.; Pancholi, S.; Ambudkar, S.V.; Martin, L.A.; Charles Coombes, R.; Buluwela, L.; Ali, S. ABC-transporter upregulation mediates resistance to the CDK7 inhibitors THZ1 and ICEC0942. Oncogene 2020, 39, 651–663. [Google Scholar] [CrossRef]
- Baglo, Y.; Liang, B.J.; Robey, R.W.; Ambudkar, S.V.; Gottesman, M.M.; Huang, H.C. Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells. Cancer Lett. 2019, 457, 110–118. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Nies, A.T.; Keppler, D. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 2007, 453, 643–659. [Google Scholar] [CrossRef]
- Borst, P.; de Wolf, C.; van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007, 453, 661–673. [Google Scholar] [CrossRef]
- Russel, F.G.; Koenderink, J.B.; Masereeuw, R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol. Sci. 2008, 29, 200–207. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—an update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef]
- Arumugam, A.; Subramani, R.; Nandy, S.B.; Terreros, D.; Dwivedi, A.K.; Saltzstein, E.; Lakshmanaswamy, R. Silencing growth hormone receptor inhibits estrogen receptor negative breast cancer through ATP-binding cassette sub-family G member 2. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bugde, P.; Biswas, R.; Merien, F.; Lu, J.; Liu, D.-X.; Chen, M.; Zhou, S.; Li, Y. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Bhatia, R.; Morse, C.L.; Woock, A.E.; Zoghbi, S.S.; Shetty, H.U.; Pike, V.W.; Innis, R.B. Increased permeability-glycoprotein inhibition at the human blood-brain barrier can be safely achieved by performing PET during peak plasma concentrations of tariquidar. J. Nucl. Med. 2015, 56, 82–87. [Google Scholar] [CrossRef]
- Bauer, M.; Karch, R.; Zeitlinger, M.; Philippe, C.; Romermann, K.; Stanek, J.; Maier-Salamon, A.; Wadsak, W.; Jager, W.; Hacker, M.; et al. Approaching complete inhibition of P-glycoprotein at the human blood-brain barrier: An (R)-[11C]verapamil PET study. J. Cereb. Blood Flow Metab. 2015, 35, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Wanek, T.; Kuntner, C.; Bankstahl, J.P.; Bankstahl, M.; Stanek, J.; Sauberer, M.; Mairinger, S.; Strommer, S.; Wacheck, V.; Loscher, W.; et al. A comparative small-animal PET evaluation of [11C]tariquidar, [11C]elacridar and (R)-[11C]verapamil for detection of P-glycoprotein-expressing murine breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 149–159. [Google Scholar] [CrossRef][Green Version]
- Koch, K.M.; Smith, D.A.; Botbyl, J.; Arya, N.; Briley, L.P.; Cartee, L.; White, J.H.; Beyer, J.; Dar, M.M.; Chung, H.C.; et al. Effect of lapatinib on oral digoxin absorption in patients. Clin. Pharmacol. Drug Dev. 2015, 4, 449–453. [Google Scholar] [CrossRef]
- Tournier, N.; Goutal, S.; Auvity, S.; Traxl, A.; Mairinger, S.; Wanek, T.; Helal, O.B.; Buvat, I.; Soussan, M.; Caille, F.; et al. Strategies to Inhibit ABCB1- and ABCG2-Mediated Efflux Transport of Erlotinib at the Blood-Brain Barrier: A PET Study on Nonhuman Primates. J. Nucl. Med. 2017, 58, 117–122. [Google Scholar] [CrossRef]
- Verheijen, R.B.; Yaqub, M.; Sawicki, E.; van Tellingen, O.; Lammertsma, A.A.; Nuijen, B.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R.; Hendrikse, N.H.; et al. Molecular Imaging of ABCB1 and ABCG2 Inhibition at the Human Blood-Brain Barrier Using Elacridar and (11)C-Erlotinib PET. J. Nucl. Med. 2018, 59, 973–979. [Google Scholar] [CrossRef]
- Bauer, M.; Karch, R.; Wulkersdorfer, B.; Philippe, C.; Nics, L.; Klebermass, E.M.; Weber, M.; Poschner, S.; Haslacher, H.; Jager, W.; et al. A Proof-of-Concept Study to Inhibit ABCG2- and ABCB1-Mediated Efflux Transport at the Human Blood-Brain Barrier. J. Nucl. Med. 2019, 60, 486–491. [Google Scholar] [CrossRef]
- van de Ven, R.; Scheffer, G.L.; Scheper, R.J.; de Gruijl, T.D. The ABC of dendritic cell development and function. Trends Immunol. 2009, 30, 421–429. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Smits, A.H.; Ziebell, F.; Joberty, G.; Zinn, N.; Mueller, W.F.; Clauder-Munster, S.; Eberhard, D.; Falth Savitski, M.; Grandi, P.; Jakob, P.; et al. Biological plasticity rescues target activity in CRISPR knock outs. Nat. Methods 2019, 16, 1087–1093. [Google Scholar] [CrossRef]
- Borst, P. Looking back at multidrug resistance (MDR) research and ten mistakes to be avoided when writing about ABC transporters in MDR. FEBS Lett. 2020. [Google Scholar] [CrossRef]
- Christie, E.L.; Pattnaik, S.; Beach, J.; Copeland, A.; Rashoo, N.; Fereday, S.; Hendley, J.; Alsop, K.; Brady, S.L.; Lamb, G.; et al. Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat. Commun. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Borst, P.; Jonkers, J.; Rottenberg, S. What makes tumors multidrug resistant? Cell Cycle 2007, 6, 2782–2787. [Google Scholar] [CrossRef]
- Grun, D.; van Oudenaarden, A. Design and analysis of single-cell sequencing experiments. Cell 2015, 163, 799–810. [Google Scholar] [CrossRef]
- Mandric, I.; Schwarz, T.; Majumdar, A.; Hou, K.; Briscoe, L.; Perez, R.; Subramaniam, M.; Hafemeister, C.; Satija, R.; Ye, C.J.; et al. Optimized design of single-cell RNA sequencing experiments for cell-type-specific eQTL analysis. Nat. Commun. 2020, 11, 5504. [Google Scholar] [CrossRef]
- Wind, N.S.; Holen, I. Multidrug resistance in breast cancer: From in vitro models to clinical studies. Int. J. Breast Cancer 2011, 2011, 967419. [Google Scholar] [CrossRef]
- Szybalski, W.; Bryson, V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 1952, 64, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Handley, M.D.; Gottesman, M.M. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009, 30, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhao, X.; Zuo, Z.; Sun, J.; Yao, Y.; Wang, T.; Liu, D.; Zhao, L. Combating P-glycoprotein-mediated multidrug resistance with 10-O-phenyl dihydroartemisinin ethers in MCF-7 cells. Eur. J. Med. Chem. 2016, 108, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, F.; Ren, Q.; Zhao, Q.; Ren, H.; Lu, S.; Zhang, L.; Han, Z. Suppression of ABCG2 inhibits cancer cell proliferation. Int. J. Cancer 2010, 126, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Low, F.G.; Shabir, K.; Brown, J.E.; Bill, R.M.; Rothnie, A.J. Roles of ABCC1 and ABCC4 in Proliferation and Migration of Breast Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7664. [Google Scholar] [CrossRef]
- Yamada, A.; Nagahashi, M.; Aoyagi, T.; Huang, W.C.; Lima, S.; Hait, N.C.; Maiti, A.; Kida, K.; Terracina, K.P.; Miyazaki, H.; et al. ABCC1-Exported Sphingosine-1-phosphate, Produced by Sphingosine Kinase 1, Shortens Survival of Mice and Patients with Breast Cancer. Mol. Cancer Res. 2018, 16, 1059–1070. [Google Scholar] [CrossRef]
- Mochida, Y.; Taguchi, K.-i.; Taniguchi, S.; Tsuneyoshi, M.; Kuwano, H.; Tsuzuki, T.; Kuwano, M.; Wada, M. The role of P-glycoprotein in intestinal tumorigenesis: Disruption of mdr1a suppresses polyp formation in Apc Min/+ mice. Carcinogenesis 2003, 24, 1219–1224. [Google Scholar] [CrossRef]
- Henderson, M.J.; Haber, M.; Porro, A.; Munoz, M.A.; Iraci, N.; Xue, C.; Murray, J.; Flemming, C.L.; Smith, J.; Fletcher, J.I.; et al. ABCC multidrug transporters in childhood neuroblastoma: Clinical and biological effects independent of cytotoxic drug efflux. J. Natl. Cancer Inst. 2011, 103, 1236–1251. [Google Scholar] [CrossRef]
- Zander, S.A.; Kersbergen, A.; Sol, W.; Gonggrijp, M.; van de Wetering, K.; Jonkers, J.; Borst, P.; Rottenberg, S. Lack of ABCG2 shortens latency of BRCA1-deficient mammary tumors and this is not affected by genistein or resveratrol. Cancer Prev. Res. 2012, 5, 1053–1060. [Google Scholar] [CrossRef]
- Balaji, S.A.; Udupa, N.; Chamallamudi, M.R.; Gupta, V.; Rangarajan, A. Role of the Drug Transporter ABCC3 in Breast Cancer Chemoresistance. PLoS ONE 2016, 11, e0155013. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Update 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Yamada, A.; Ishikawa, T.; Ota, I.; Kimura, M.; Shimizu, D.; Tanabe, M.; Chishima, T.; Sasaki, T.; Ichikawa, Y.; Morita, S.; et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res. Treat. 2013, 137, 773–782. [Google Scholar] [CrossRef]
- Ishiguro, J.; Ito, H.; Tsukamoto, M.; Iwata, H.; Nakagawa, H.; Matsuo, K. A functional single nucleotide polymorphism in ABCC11, rs17822931, is associated with the risk of breast cancer in Japanese. Carcinogenesis 2019, 40, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ota, I.; Sakurai, A.; Toyoda, Y.; Morita, S.; Sasaki, T.; Chishima, T.; Yamakado, M.; Kawai, Y.; Ishidao, T.; Lezhava, A.; et al. Association between breast cancer risk and the wild-type allele of human ABC transporter ABCC11. Anticancer Res. 2010, 30, 5189–5194. [Google Scholar]
- Lang, T.; Justenhoven, C.; Winter, S.; Baisch, C.; Hamann, U.; Harth, V.; Ko, Y.D.; Rabstein, S.; Spickenheuer, A.; Pesch, B.; et al. The earwax-associated SNP c.538G>A (G180R) in ABCC11 is not associated with breast cancer risk in Europeans. Breast Cancer Res. Treat. 2011, 129, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Na, A.Y.; Heo, J.C.; Sung, J.Y.; Lee, J.H.; Kim, Y.N.; Kim, D.K. No Association of the rs17822931 Polymorphism in ABCC11 with Breast Cancer Risk in Koreans. Asian Pac. J. Cancer Prev. 2016, 17, 2625–2628. [Google Scholar]
- Miletti-Gonzalez, K.E.; Chen, S.; Muthukumaran, N.; Saglimbeni, G.N.; Wu, X.; Yang, J.; Apolito, K.; Shih, W.J.; Hait, W.N.; Rodriguez-Rodriguez, L. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005, 65, 6660–6667. [Google Scholar] [CrossRef] [PubMed]
- Ouhtit, A.; Rizeq, B.; Saleh, H.A.; Rahman, M.M.; Zayed, H. Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int. J. Biol. Sci. 2018, 14, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, S.; Seyed Forootan, F.; Seyed Forootan, S.; Nasr Esfahani, M.H.; Gure, A.O.; Ghaedi, K. Mechanistic Pathways of Malignancy in Breast Cancer Stem Cells. Front. Oncol. 2020, 10, 452. [Google Scholar] [CrossRef]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitao, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as cancer stem cell markers: An enduring ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, H.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef] [PubMed]
- Idowu, M.O.; Kmieciak, M.; Dumur, C.; Burton, R.S.; Grimes, M.M.; Powers, C.N.; Manjili, M.H. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum. Pathol. 2012, 43, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, I.; Sebastiao, A.P.M.; Lima, R.S.; Urban, C.A.; Junior, E.S.; Anselmi, K.F.; Elifio-Esposito, S.; De Noronha, L.; Moreno-Amaral, A.N. Cancer stem cell markers ALDH1 and CD44+/CD24- phenotype and their prognosis impact in invasive ductal carcinoma. Eur. J. Histochem. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.J.; Ahn, S.H.; Son, B.H.; Kim, S.B.; Ahn, J.H.; Noh, W.C.; Gong, G. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast 2011, 20, 78–85. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Toccacieli, L.; Bozzuto, G.; Stringaro, A.; Gentile, M.; Cianfriglia, M.; Ciervo, A.; Caraglia, M.; Budillon, A.; et al. The multidrug transporter P-glycoprotein: A mediator of melanoma invasion? J. Investig. Dermatol. 2008, 128, 957–971. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef]
- Yang, J.M.; Vassil, A.; Hait, W.N. Involvement of phosphatidylinositol-3-kinase in membrane ruffling induced by P-glycoprotein substrates in multidrug-resistant carcinoma cells. BioChem. Pharmacol. 2002, 63, 959–966. [Google Scholar] [CrossRef]
- Yao, J.; Yao, X.; Tian, T.; Fu, X.; Wang, W.; Li, S.; Shi, T.; Suo, A.; Ruan, Z.; Guo, H.; et al. ABCB5-ZEB1 Axis Promotes Invasion and Metastasis in Breast Cancer Cells. Oncol. Res. 2017, 25, 305–316. [Google Scholar] [CrossRef]
- Kochel, T.J.; Reader, J.C.; Ma, X.; Kundu, N.; Fulton, A.M. Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget 2017, 8, 6540–6554. [Google Scholar] [CrossRef]
- Mourskaia, A.A.; Amir, E.; Dong, Z.; Tiedemann, K.; Cory, S.; Omeroglu, A.; Bertos, N.; Ouellet, V.; Clemons, M.; Scheffer, G.L.; et al. ABCC5 supports osteoclast formation and promotes breast cancer metastasis to bone. Breast Cancer Res. 2012, 14, R149. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Camenisch, T.D.; Stevens, M.V.; Sands, B.J.; McDonald, J.; Schroeder, J.A. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005, 65, 6755–6763. [Google Scholar] [CrossRef]
- Tse, G.M.; Tan, P.H.; Ma, T.K.; Gilks, C.B.; Poon, C.S.; Law, B.K. CD44s is useful in the differentiation of benign and malignant papillary lesions of the breast. J. Clin. Pathol. 2005, 58, 1185–1188. [Google Scholar] [CrossRef]
- Fang, X.J.; Jiang, H.; Zhao, X.P.; Jiang, W.M. The role of a new CD44st in increasing the invasion capability of the human breast cancer cell line MCF-7. BMC Cancer 2011, 11, 290. [Google Scholar] [CrossRef]
- Fang, X.J.; Jiang, H.; Zhu, Y.Q.; Zhang, L.Y.; Fan, Q.H.; Tian, Y. Doxorubicin induces drug resistance and expression of the novel CD44st via NF-kappaB in human breast cancer MCF-7 cells. Oncol. Rep. 2014, 31, 2735–2742. [Google Scholar] [CrossRef]
- Chen, D.D.; Ji, J.A.; Yan, H.C.; Huang, G.H.; Fang, X.J. Effect of CD44st and HER2 expression on the postoperative prognosis of breast cancer patients. Onco Targets Ther. 2019, 12, 577–585. [Google Scholar] [CrossRef]
- Ying Zhi, L.; Xu, Z.; Ning, L.; Jia Jin, L.; Hai Cui, Y.; Hong, H.G.; Fang, X.J. A correlation study of the expression of HA-CD44st and HER-2 in breast cancer. Onco Targets Ther. 2018, 11, 5677–5688. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Tanić, N.; Prvanović, M.; Milovanović, Z.; Tanić, N. Friend or foe: ABCG2, ABCC1 and ABCB1 expression in triple-negative breast cancer. Breast Cancer 2021. [Google Scholar] [CrossRef]
- Sensorn, I.; Sukasem, C.; Sirachainan, E.; Chamnanphon, M.; Pasomsub, E.; Trachu, N.; Supavilai, P.; Pinthong, D.; Wongwaisayawan, S. ABCB1 and ABCC2 and the risk of distant metastasis in Thai breast cancer patients treated with tamoxifen. Onco Targets Ther. 2016, 9, 2121–2129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, N.K.; Brown, T.J. ABC transporters do not contribute to extracellular translocation of hyaluronan in human breast cancer in vitro. Exp. Cell Res. 2010, 316, 1241–1253. [Google Scholar] [CrossRef]
- Xiang, L.; Su, P.; Xia, S.; Liu, Z.; Wang, Y.; Gao, P.; Zhou, G. ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma. Diagn. Pathol. 2011, 6, 90. [Google Scholar] [CrossRef]
- Omran, O.M. The prognostic value of breast cancer resistance protein (BCRB/ABCG2) expression in breast carcinomas. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 367–376. [Google Scholar] [CrossRef]
- Collina, F.; Di Bonito, M.; Li Bergolis, V.; De Laurentiis, M.; Vitagliano, C.; Cerrone, M.; Nuzzo, F.; Cantile, M.; Botti, G. Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer. Biomed. Res. Int. 2015, 2015, 158682. [Google Scholar] [CrossRef]
- Britton, K.M.; Eyre, R.; Harvey, I.J.; Stemke-Hale, K.; Browell, D.; Lennard, T.W.J.; Meeson, A.P. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012, 323, 97–105. [Google Scholar] [CrossRef]
- Nakanishi, T.; Chumsri, S.; Khakpour, N.; Brodie, A.H.; Leyland-Jones, B.; Hamburger, A.W.; Ross, D.D.; Burger, A.M. Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br. J. Cancer 2010, 102, 815–826. [Google Scholar] [CrossRef]
- Zochbauer-Muller, S.; Filipits, M.; Rudas, M.; Brunner, R.; Krajnik, G.; Suchomel, R.; Schmid, K.; Pirker, R. P-glycoprotein and MRP1 expression in axillary lymph node metastases of breast cancer patients. Anticancer Res. 2001, 21, 119–124. [Google Scholar]
- Giordano, A.; Gao, H.; Cohen, E.N.; Anfossi, S.; Khoury, J.; Hess, K.; Krishnamurthy, S.; Tin, S.; Cristofanilli, M.; Hortobagyi, G.N.; et al. Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann. Oncol. 2013, 24, 2515–2521. [Google Scholar] [CrossRef]
- Theodoropoulos, P.A.; Polioudaki, H.; Agelaki, S.; Kallergi, G.; Saridaki, Z.; Mavroudis, D.; Georgoulias, V. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010, 288, 99–106. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]

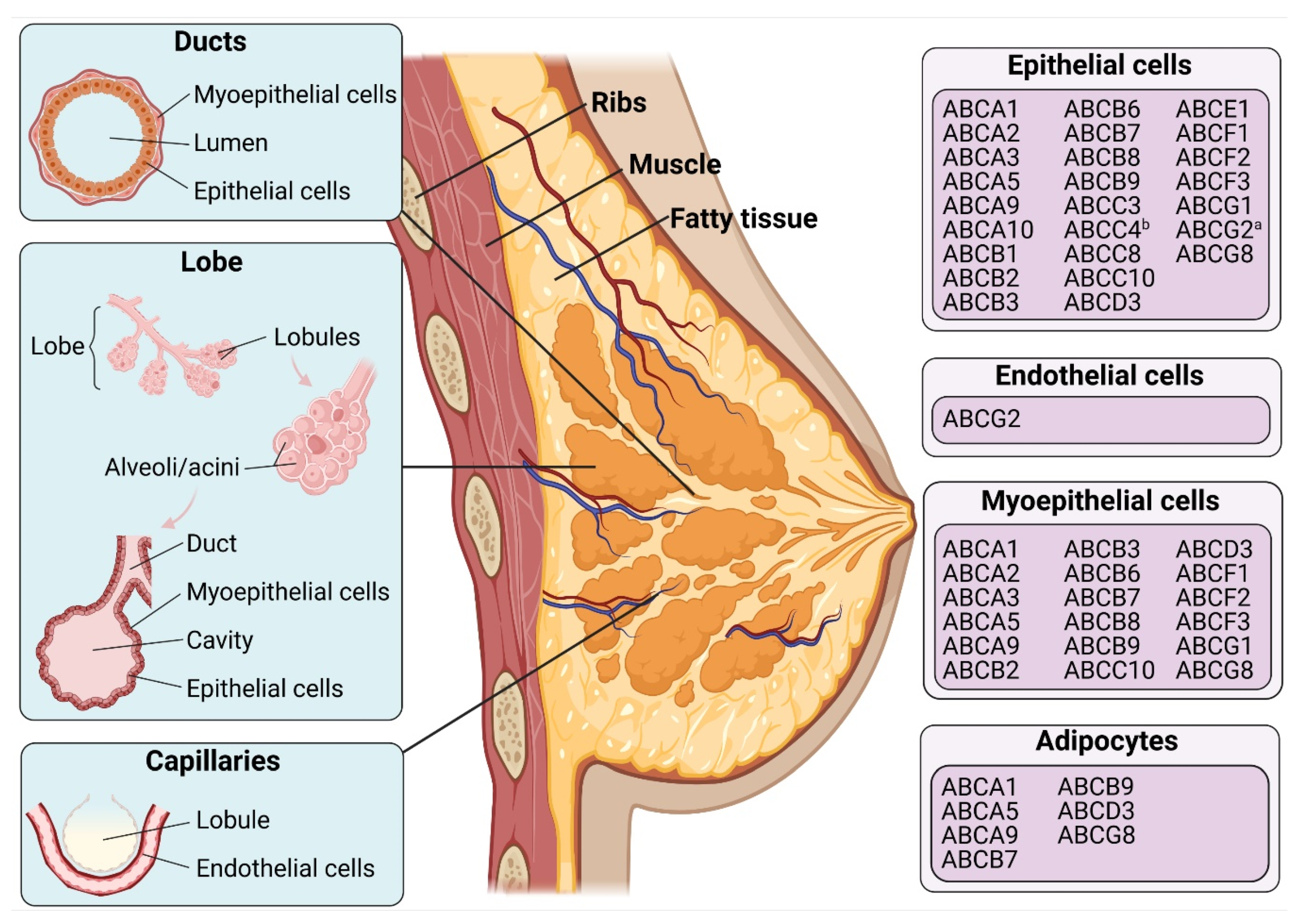
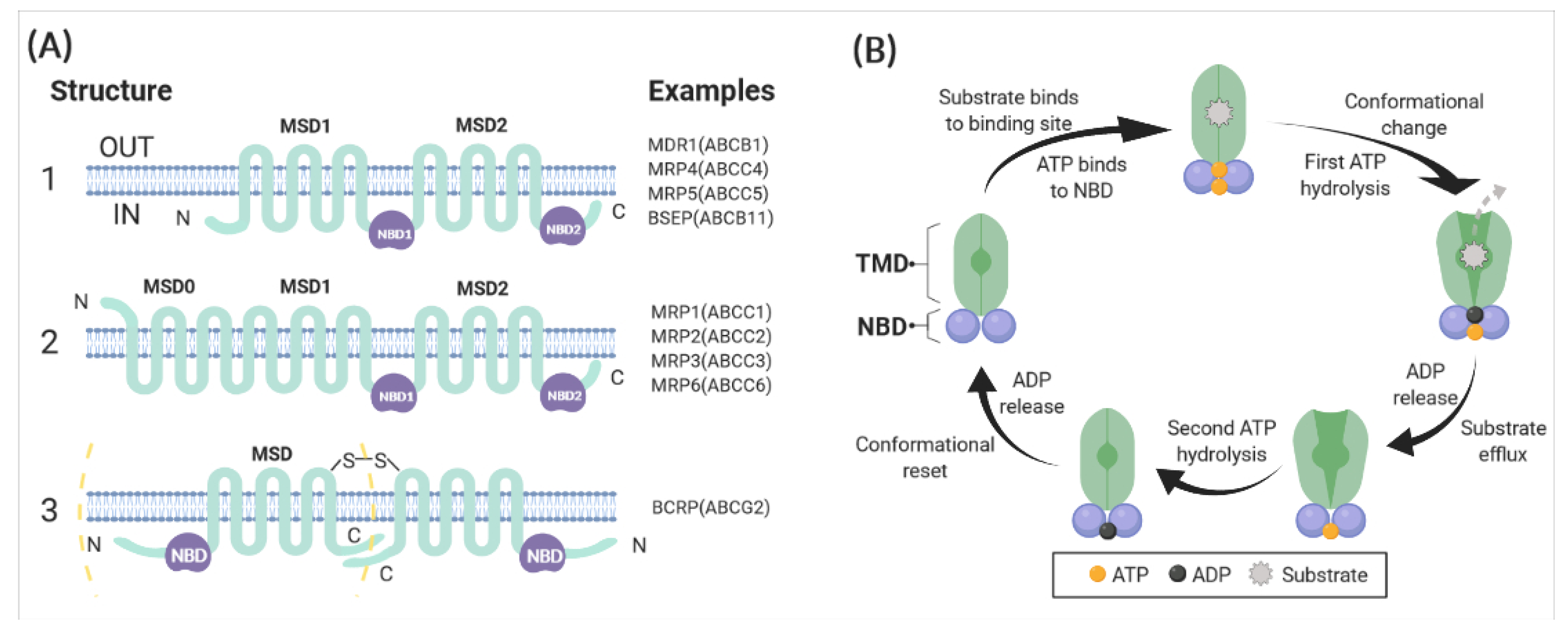
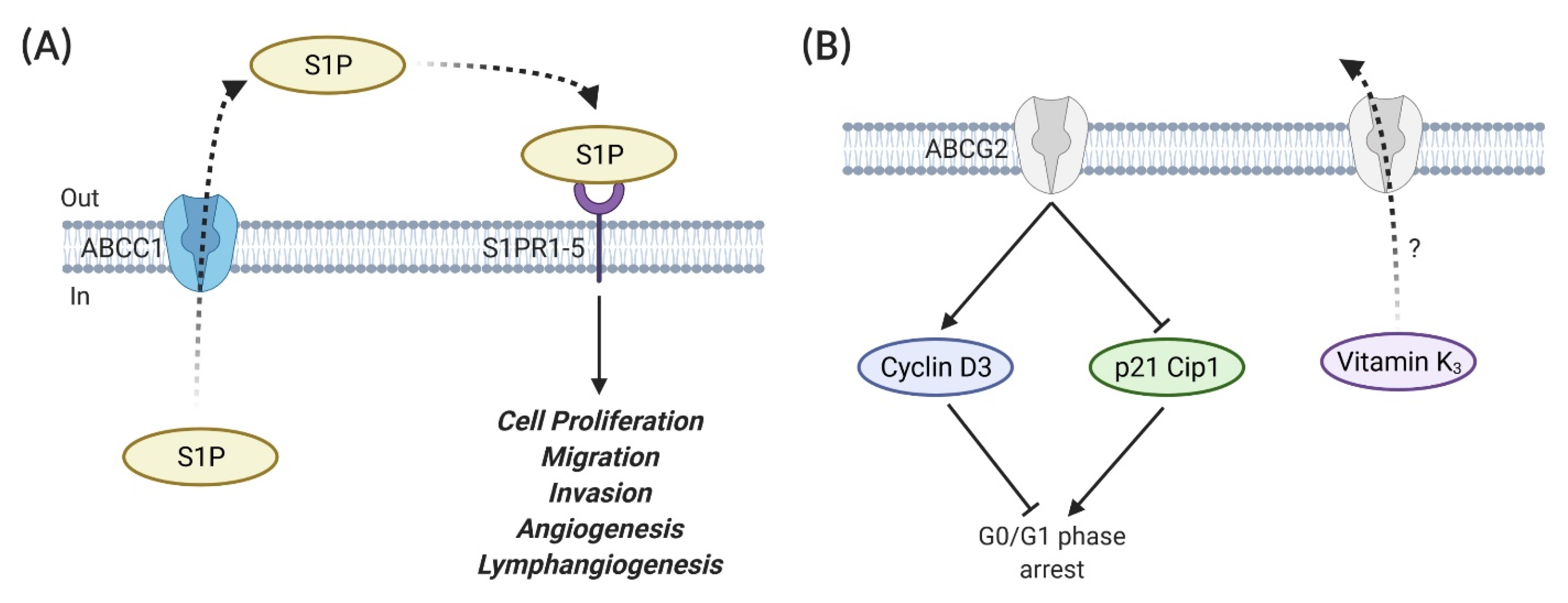
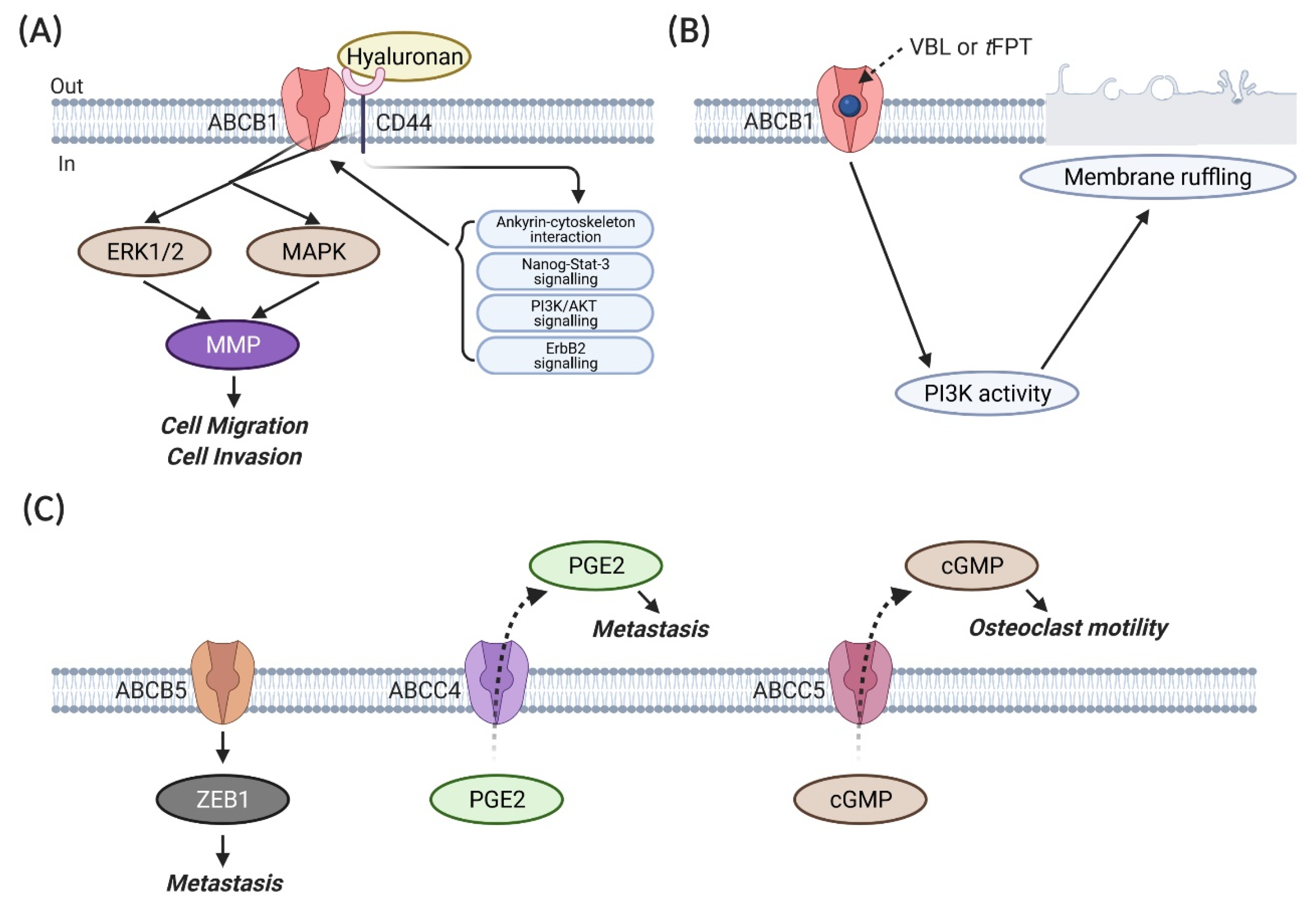
| Names | Substrates | References |
|---|---|---|
| ABCB1 | Olaparib, Doxorubicin, Epirubicin, Docetaxel, Paclitaxel, Cisplatin, 5-Fluorouracil, Palbociclib, Ribociclib, Abemaciclib, ICEC0942, THZ1, Larotrectinib, Tivozanib, Galunisertib, Fisogatinib, Osimertinib, Actinomycin D, Bisantrene, Dasatinib, Daunorubicin, Digoxin, Etoposide/VP-16, Homoharringtonine, Irinotecan, Mitoxantrone, Teniposide, Topotecan, Vinblastine, Vincristine, Vindesine, Vinorelbine, Methotrexate, Mithramycin Mitomycin C | [14,27,29,30,31,32,33,58,65,69] |
| ABCC1 | Doxorubicin, Epirubicin, Daunorubicin, Eribulin, Chlorambucil, Idarubicin, Etoposide/VP-16, Glucuronide, Teniposide, Vincristine, Vinblastine, Vinorelbine, Topotecan, Irinotecan/SN-38, Mitoxantrone, saquinavir, Arsenite, Trivalent antimony, Imatinib, Melphalan, Methotrexate, SN-38 | [14,27] |
| ABCC2 | Doxorubicin, Epirubicin, Paclitaxel, Carboplatin, Cisplatin, Etoposide/VP-16, Irinotecan, Irinotecan/CPT-11, Methotrexate, Mitoxantrone, saquinavir, SN-38, Sulfinpyrazone, Topotecan, Vinblastine, Vincristine | [14,72] |
| ABCC3 | Methotrexate, Etoposide/VP-16, Teniposide, Vincristine | [14,73] |
| ABCC4 | Methotrexate, 5-Fluorouracil, 6-mercaptopurine, 6-thioguanine, Bisantrene, Irinotecan/CPT11, Nucleoside monophosphates, Topotecan, Vinblastine, SN-38 | [14,73,74] |
| ABCC5 | Doxorubicin, Gemcitabine, Methotrexate, 5-Fluorouacil, 6-mercaptopurine, 6-thioguanine, Bisantrene, Mitoxantrone, Nucleoside monophosphates | [14,73] |
| ABCG2 | Doxorubicin, Epirubicin, Methotrexate, Palbociclib, Abemeciclib, THZ1, Larotrectinib, Tivozanib, Osimertinib, Bisantrene, Dasatinib, Docetaxel, Daunorubicin, Flavopiridol, Irinotecan/CPT-11, Mitoxantrone, Nilotinib, SN-38, topotecan, Tyrosine Kinase inhibitors | [14,29,30,32,69,75,76,77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Fortunati, E.; Liu, D.-X.; Li, Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3199. https://doi.org/10.3390/ijms22063199
He J, Fortunati E, Liu D-X, Li Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. International Journal of Molecular Sciences. 2021; 22(6):3199. https://doi.org/10.3390/ijms22063199
Chicago/Turabian StyleHe, Ji, Erika Fortunati, Dong-Xu Liu, and Yan Li. 2021. "Pleiotropic Roles of ABC Transporters in Breast Cancer" International Journal of Molecular Sciences 22, no. 6: 3199. https://doi.org/10.3390/ijms22063199
APA StyleHe, J., Fortunati, E., Liu, D.-X., & Li, Y. (2021). Pleiotropic Roles of ABC Transporters in Breast Cancer. International Journal of Molecular Sciences, 22(6), 3199. https://doi.org/10.3390/ijms22063199







