Abstract
The recent pandemic Sars-CoV2 infection and studies on previous influenza epidemic have drawn attention to the association between the obesity and infectious diseases susceptibility and worse outcome. Metabolic complications, nutritional aspects, physical inactivity, and a chronic unbalance in the hormonal and adipocytokine microenvironment are major determinants in the severity of viral infections in obesity. By these pleiotropic mechanisms obesity impairs immune surveillance and the higher leptin concentrations produced by adipose tissue and that characterize obesity substantially contribute to such immune response dysregulation. Indeed, leptin not only controls energy balance and body weight, but also plays a regulatory role in the interplay between energy metabolism and immune system. Since leptin receptor is expressed throughout the immune system, leptin may exert effects on cells of both innate and adaptive immune system. Chronic inflammatory states due to metabolic (i.e., obesity) as well as infectious diseases increase leptin concentrations and consequently lead to leptin resistance further fueling inflammation. Multiple factors, including inflammation and ER stress, contribute to leptin resistance. Thus, if leptin is recognized as one of the adipokines responsible for the low grade inflammation found in obesity, on the other hand, impairments of leptin signaling due to leptin resistance appear to blunt the immunologic effects of leptin and possibly contribute to impaired vaccine-induced immune responses. However, many aspects concerning leptin interactions with inflammation and immune system as well as the therapeutical approaches to overcome leptin resistance and reduced vaccine effectiveness in obesity remain a challenge for future research.
1. Introduction
Obesity is a worldwide pandemic resulting from a combination of genetic, behavioral, and environmental variables and that dramatically increases morbidity and mortality. Indeed, it has been recognized as a leading cause of major health issues, particularly metabolic, cardiovascular, and oncologic diseases [1,2,3].
The pathophysiological interactions between obesity and viral infectious diseases have recently gained increasing attention after a strong association between obesity and poor outcome in pandemic H1N1 influenza and Sars-CoV2 infections was observed [4,5]. Although the underlying mechanisms are not well established, a number of potential factors may be involved [6].
Obesity complications, respiratory dysfunction, and pharmacological issues have been proposed as possible mechanisms [7]. However, obesity is also associated with impaired immune responses, suggesting a link between metabolic control and immune tolerance [8].
Among the multiple mechanisms by which obesity could impair immunity, the chronic unbalance in the hormonal and adipocytokine microenvironment seems to be a major determinant in the severity of viral infections in obesity.
Herein we review the available evidence on the various aspects of the association between obesity and viral infections and the current understanding of the possible mechanisms underlying this susceptibility to worse outcomes, with particular focus on the role of leptin.
2. Obesity and Risk of Infections
Beyond causing non-communicable conditions, chronically positive energy balance and obesity represent an ideal set-up for getting communicable diseases. Indeed, several epidemiological data suggest that body weight is associated with an increased infection risk [9].
However, with regard to viral infections, data in obese population are so far limited. Two metanalysis showed that patients with obesity had a significantly higher risk for influenza infection (OR: 1.29, 95% CI: 1.11–1.49) [10] with a U-shaped relationship between BMI and the risk of influenza-related pneumonia [11]. In The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Clinical Data Report 20 November 2020, among the reported 95966 clinical COVID-19 cases (93.3% laboratory-confirmed cases) from 42 countries across multiple continents, the prevalence of obesity was 13.4% [12]. A pooled data analysis of 20 studies assessing the association between individuals with obesity and the risk of testing positive for COVID-19 showed that the odds of obese individuals being COVID-19 positive were 46% (OR: 1.46; 95% CI: 1.30–1.65) higher than not obese individuals [13]. Obesity was also more prevalent in patients admitted to intensive care unit (ICU) for COVID-19 compared to a control group of patients admitted to ICU for a non SARS-CoV-2-related acute respiratory disease during the previous year, suggesting a greater susceptibility of obese patients to SARS-CoV-2 infection [14].
The evidence is more substantial for bacterial infections, and among these, in particular for nosocomial rather than community-acquired infections. This discrepancy may be imputable to the fact that the BMI is usually recorded in surgical patients, enabling retrospective studies of this association, but not in hospital admissions for other reasons or in case of community-acquired infections [6]. As a matter of fact, obesity is recognized as a strong independent risk factor of postoperative surgical site infection [15,16,17,18,19]. But, more extensively, obesity has been also identified as an independent risk factor for nosocomial pneumonia, Clostridium difficile colitis, bacteremia, and wound infections [6,20,21]. The effect of obesity on infection risk was especially addressed in a registry-based analysis of 37,808 participants from the Danish Blood Donor Study in which obesity resulted to be associated to a 50% increased rate of nosocomial infections. Respiratory tract infections and those of the skin and subcutaneous tissue were more frequent in obese females and males respectively, whereas abscesses [22], urinary tract infections, and pyelonephritis [23] were more frequent in obese irrespective of gender. Increased rates of bacterial or fungal skin infections in obese have been also reported in other studies [24,25,26].
3. Obesity and Viral Infection Outcomes, from Influenza A Virus to SARS-COV-2
Recent data suggest that obesity increases not only the risk of getting infections but also infection-related rates of hospitalization, morbidity, and mortality.
One hundred years back, the influenza virus triggered the 1918 “Spanish Flu” pandemic, which caused 50–100 million deaths [27]. However, in those days obesity was not widespread in the society, while rather undernutrition was an issue. Since then, other pandemics caused by zoonotic transfer of the virus from animals to humans have taken place, but none created such an adverse impact as the Spanish Flu in 1918.
Only following the H1N1 pandemic in 2009, obesity was recognized as an independent risk factor for hospitalization and increased mortality due to influenza [4,9]. Indeed, in California 51% of 534 hospitalized adult patients with H1N1 infection were obese, and 61% of the mortality occurred in these individuals [28]. Obese individuals infected with H1N1 virus had a two-fold higher risk to be admitted in ICU [29] and, once admitted to ICU, were at greater risk of developing pneumonitis [30] and consumed more ICU resources compared to non-obese [31]. Obesity was also shown to increase both the length of stay in ICU and the need for mechanical ventilation [32] and, among hospitalized patients, obese and morbidly obese required earlier antiviral therapy for severe influenza H1N1 than non-obese patients [33].
Data analysis from cohort studies indicated that obese individuals were at higher risk of hospitalization and had a longer hospital stay also as a result of seasonal (A/H3N2) influenza infection compared with normal weight individuals [34,35].
Prior to the 21st century, coronaviruses (CoVs) were considered pathogens of great relevance in veterinary medicine but with a reduced impact on human health [36]. However, in the past two decades novel CoVs have emerged and caused global concern for human health with the epidemics of severe acute respiratory syndrome (SARS) in 2002–2003 and Middle East respiratory syndrome (MERS) in 2012 [37,38]. Though these viruses did not spread efficiently from human to human, both SARS and MERS had a high fatality rate of 9.5 and 34.4%, respectively [39]. Invariably, chronic conditions resulted in increased MERS severity [40,41,42,43] and, according to a metanalysis, obesity was present in 16% of the hospitalized patients [44].
Currently, we have been facing a novel CoV (SARS-CoV-2) pandemic since the end of 2019 causing a respiratory disease, called COVID-19. According to COVID-19 surveillance reports of European Centre for Disease Prevention and Control (ECDC) and Centers for Disease Control and Prevention (CDC) from US, about 91.5% of adult patients with severe COVID-19 present at least one underlying medical condition [45] including obesity as well as hypertension, diabetes, cardiovascular disease, chronic respiratory disease, chronic kidney disease, immune compromised status, cancer, and smoking [46]. Thus, also in COVID-19 pandemic, patients with obesity appear at greater risk for increased disease severity and hospitalization [47]. In a data analysis from 5700 SARS-CoV-2 patients admitted to hospitals belonging to Northwell Health of New York between 1 March 2020 and 4 April 2020, hypertension (56.6%), obesity (41.7%), and diabetes (33.8%) were the most common comorbidities [48]. Obesity was even the most commonly associated comorbidity accounting for 48% of the ICU admissions due to SARS-CoV-2 in a Spanish report [49]. Similarly, at a single center in France, 47.6% of patients admitted to ICU had BMI > 30 Kg/m2 and 28.2% BMI > 35 Kg/m2 [14]. Positive associations between increasing BMI and the risk of hospitalization and adverse outcomes were highlighted in several reports [50]. Of interest, available data suggest that the relationship between BMI and severity of SARS-CoV-2 infection is more relevant in younger people. Indeed, although older age increased the risk for severe COVID-19, in younger patients the most critical form of the infection was more likely associated to obesity [51]. Accordingly, in retrospective analysis of a cohort of 10,862 individuals hospitalized for COVID-19 the association between increased BMI and disease severity stratified by age was stronger for people <59 years old [52].
4. Obesity and Viral Shedding
Prolonged viral shedding has been described in people with obesity [53]. Indeed, symptomatic obese adults shed influenza A virus 42% longer, with predicted mean shedding times of 5.23 days versus 3.68 days in lean subjects, potentially causing long-term transmission [54]. In a small cohort of 100 consecutive patients with COVID-19, longer time (19 ± 8 days) to SARS-CoV-2 negativity in nasopharyngeal swabs was reported compared to nonobese patients (13 ± 7 days) [55], although in some other studies marginal or no difference in time of viral load clearance was observed [50].
This could be imputable to the fact that several types of viruses, including adenovirus Ad-36, influenza A virus, HIV, and cytomegalovirus, can utilize the adipose tissue as a reservoir [56], thus benefiting from the obesity status. With regard to SARS-CoV-2, its binding to angiotensin-converting enzyme 2 (ACE2) receptor has been recognized as a critical step for virus entry into cells, so that ACE2-expressing tissues become direct viral targets displaying progressive pathological alterations up to organ failure in most severe cases [57]. According to a search of a public gene expression database, in human subcutaneous and visceral adipose depots ACE2 expression seems even higher than in respiratory epithelium, enabling adipose tissue to serve as a functional reservoir also for SARS-CoV-2, especially in obese individuals [58]. Nevertheless, this hypothesis needs more substantive experimental proofs since ACE2 mRNA transcripts in visceral adipose tissue were not different in obese compared with normal and underweight individuals [59].
The prolonged viral shedding in obesity has been also putatively attributed to the higher ventilation volumes of obese individuals. This is suggested by the observation that the positive association between BMI and influenza virus RNA in aerosols from exhaled breath was significant especially in males [60].
6. Mechanisms That Make Obese Patients Vulnerable to Infections
As mentioned, obesity implicates a risk of complication, comorbid secondary infections, prolonged hospitalization [68], and the development of acute respiratory distress syndrome [69]. As potential causes of this worse clinical outcome, the dynamic of pulmonary ventilation with reduced diaphragmatic excursions and a relative increase in anatomical death space in obese individuals should be considered [70]. Other mechanisms include decreased pulmonary perfusion, metabolic and vascular complications of obesity, hormonal axys dysregulation, as well as practical considerations when managing obese patients in critical care settings [71] (Figure 1).
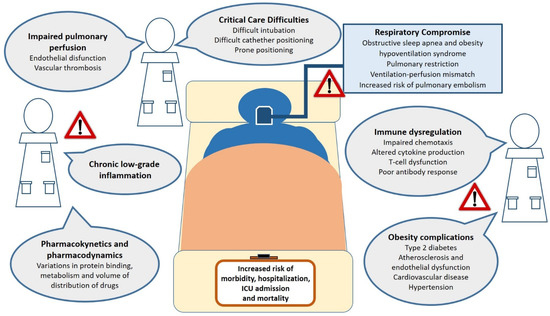
Figure 1.
Obesity-related factors associated to adverse clinical outcomes from viral infections in both specialty and intensive care settings. Potential mechanisms by which obesity may augment the risk of critical illness and death from viral infections. These include chronic inflammation, impairment of respiratory function and pulmonary perfusion, critical care management difficulties, immune dysfunction, metabolic and cardiovascular complications of obesity. Abbreviations: ICU, intensive care unit.
In addition, as known, the adipose tissue is dynamically involved in the pathogenesis of obesity complications through a complex network of endocrine, autocrine, and paracrine signals mediated by various adipokines. The obesity-related chronic low-grade inflammation, characterized by raised levels of pro-inflammatory molecules (such as leptin, resistin, chemokine (C-C motif) ligand 2 (CCL2), interleukin (IL)-6, IL-1β, IL-8, tumor necrosis factor (TNF)-α) and decreased levels of adiponectin, may have negative effects on the lung parenchyma and bronchi [72]. This chronic inflammatory milieu, together with T-helper (Th)1 (antiviral action of interferon gamma –IFN-γ-) to Th2 (anti-inflammatory interleukins such as IL-4, -5, -10, and -13) immune response shift induced by viruses to evade host immunity, can be detrimental to the endothelium leading to vascular complications [73].
Moreover, many reports support the notion that a chronic inflammatory status entails a chronic state of immune deregulation, which may interfere with immune homeostasis and the effectiveness of the innate and adaptive immune response [74,75].
With regard to the innate response, when an antigen is presented, as above-mentioned, the obesity-related chronic inflammation hinders further macrophage production of IFN-γ that is known to have direct antiviral actions [76]. In line with this, monocytes isolated from individuals with obesity and diabetes exhibited greater susceptibility to SARS-CoV-2 infection ex vivo [77]. This is due to an upregulation of suppressor of cytokine signaling (SOCS) proteins which are involved in the inhibition of IFN-α/β JAK/STAT signaling. Indeed, obese subjects exhibit increased basal levels of SOCS3 but a significantly lower expression of SOCS3 and SOCS1 in peripheral blood mononuclear cells (PBMCs) after stimulation compared with non-obese subjects, with consequent decreased ability to produce IFN-α and IFN-β in response to Toll-like receptor ligands [78].
The reduced and delayed capacity to produce IFNs in contrast to viral replication could also sustain viral RNA replication and also favor the appearance of novel, more virulent viral strains [79].
Besides macrophages, various effects of obesity on other leukocyte subpopulations including dendritic cells (DCs), natural killer (NK) cells, and neutrophils have been reported but the findings are often contradictory [80,81]. In particular, in some obese human cohorts, circulating DCs were reduced in number and less responsive to ex vivo stimulation with TLR agonists, NK cells were decreased and neutrophils showed increased production of inflammatory free radicals upon stimulation compared to non-obese controls [75,82] but not in others [83,84]. This could have a negative impact on the bridge between non-specific innate immunity and antigen-specific adaptive immunity which in fact relies on appropriate actions of immune cells from both the lymphoid and myeloid lineages.
As a matter of fact, the adaptive (B- and T-cell) responses show reduced efficacy in obesity and obese mice showed a decrease in key transcripts for early lymphoid commitment likely driven by defects in bone marrow environment [85]. Obesity has been associated with increased activation of pro-inflammatory Th1 and Th17 cells and reduction in anti-inflammatory Th2 and regulatory T (Treg) cells [86]. However, the literature on how human obesity modulates B and T cells’ specific response to viruses is limited. After ex vivo stimulation with H1N1 virus, CD8+ and CD4+ T cells produced significantly more IL-5, whereas IFN-γ production trended lower, compared to normal weight subjects [83]. The T-cell response is increasingly considered pivotal in reducing susceptibility to SARS-CoV-2 infection as well as disease severity [87]. With regard to specific T-cell subsets, while in obese donors no difference in the levels of αβ T cells has been reported, γδ T cells exhibit number reduction, a skewed maturation, and a blunted antiviral IFN-γ response to antigen presentation [88] mirroring the immune deficits seen in elderly and thus supporting the “adipaging” hypothesis [89].
As far as B cells are concerned, few studies in humans have directly investigated whether B cell function is impaired in obesity. In human B cells ex vivo, IgM but not IgG levels positively correlated with increasing BMI upon BCR/TLR9 stimulation [90]. Also diet-induce obesity (DIO) mice studies suggest that obesity impairs antibody production [91] as hemagglutination inhibition titers were reduced and completely blunted by 7 and 35 days post influenza infection, respectively [90].
The mechanisms by which obesity impairs immunity are likely pleiotropic. Besides the contribution of obesity complications such as type 2 diabetes, several other factors exert immunomodulatory effects possibly contributing to the increased infection risk. These factors include physical inactivity, nutritional aspects, and a chronic unbalance in the adipocytokine microenvironment.
Reduced physical activity, which is common among obese patients, per se impairs immune response [92]. In fact, exercise not only has a positive impact on energy balance, but also restores leptin response, type I IFN responsiveness, anti-influenza virus-specific IgG2c antibody production, and CD8+ T-cell percentage in bronchoalveolar lavage in obese mice [93].
Fatty acid status could also play a role, since circulating essential long chain n-3 polyunsaturated fatty acids (PUFA), including docosahexaenoic acid (DHA), that are low in obese individuals compared to lean [94], display immunomodulatory properties, namely impairing many aspects of innate and adaptive immunity [95].
Finally, also the higher leptin concentrations, encompassed in the above-mentioned chronic unfavorable hormone milieu observed in obesity, seem to contribute substantially to such dysregulation of the immune response, as detailed next.
7. Leptin and Immunometabolism
A link between body weight, adipose tissue, and immunity has been hypothesized for a long time, but the precise molecular mediators were unknown until the discovery of leptin in 1994. Leptin is a non-glycosylated hormone of 146 aminoacids with a tertiary structure resembling that of members of the long-chain helical cytokine family (that includes IL-6, IL-11, IL-12, LIF, G-CSF, CNTF, and oncostatin M) [96]. Functionally, leptin is mainly synthesized in adipose cells in response to food intake and energy balance to provide information to the brain to control feeding and metabolism. Indeed, leptin circulating levels are proportional to the body fat mass and dramatically altered in condition of both chronic negative and positive energy balance so that malnutrition leads to hypoleptinemia, while obesity to hyperleptinemia [97]. Besides its central role in appetite and body weight homeostasis by inducing anorexigenic factors (as cocaine-amphetamine-related transcript), suppressing orexigenic neuropeptides (as neuropeptide Y) in hypothalamus and by influencing energy expenditure, leptin has a regulatory role in multiple important physiologic functions within immune, hematopoietic, neuroendocrine, and reproductive systems, as well as in bone metabolism and inflammation [98].
Leptin has an emerging regulatory role in metabolism-immune system interplay [99], being a cornerstone of the new field of research termed immunometabolism. Indeed, the capacity to store energy finely regulated by the crosstalk between the adipose tissue and the brain and mainly orchestrated by leptin, is entangled with the capacity of this adipokine to activate or extinguish certain physiological responses on the basis of energy availability. One of these responses consists in the activation of immune system to control infections. The connection between metabolism and immune system relies on a complex network of cytokines and neuropeptides that act on peripheral nervous system and endocrine axes, and are ultimately regulated in a central manner [100].
In this context, the central effect of leptin in the hypothalamus is mediated by the activation of the sympathetic nervous system [101] and, to a lesser extent, by the inhibition of the hypothalamic-pituitary-adrenal axis [102].
8. Leptin and Immune System
Leptin is also produced by inflammatory regulatory cells and upregulated in response to several inflammatory mediators (i.e., LPS and inflammatory cytokines such as TNF-α, IL-6, and IL-1β), so that serum leptin concentrations highly increase during acute infection, inflammation, and sepsis [103]. Since immune cells express leptin receptor (LepR) on their surface, leptin can perpetuate its effects on these cells through autocrine and/or paracrine signals and contribute to the development of a loop of acute phase reactants [104] and chronic inflammation.
So it is not surprising that leptin, which is almost invariably elevated in obese patients, has been recognized as one of the adipokines mediating the pro-inflammatory state of obesity [105]. In this context, inflammatory cytokines not only stimulate leptin mRNA expression, but also the short-term release of leptin stored in adipose tissue [106].
Not exclusively adipose and immune cells produce leptin in the context of the inflammatory response upon infection. Indeed, human and mice lung epithelial cells in bacterial and viral pneumonia have shown significantly greater leptin staining as compared with uninfected lungs. Concentrations of leptin also increase in bronchoalveolar lavage samples after endotoxin inhalation in both humans and mice [107], in intestinal epithelium upon infection [108] or in animal model of sepsis [109].
Besides its role as a mediator of inflammation, leptin has multiple direct and regulatory immune functions (Figure 2). The first evidence of a possible effect of leptin in immune system regulation arises from the observation that LepR belongs to class I cytokine superfamily. In particular, LepR shares signaling capabilities with IL-6-type cytokine receptors relying on JAK/STAT pathway for signal transduction [110]. Alternatively, LepR could activate ERK 1/2, p38 MAPK, JNK, PKC, and PI3K/Akt pathways [111]. Even the wide distribution of LepR which, as said, is expressed on the membrane of both peripheral and bone marrow-derived immune cells, supports the multiple roles of leptin in the immune system [112].
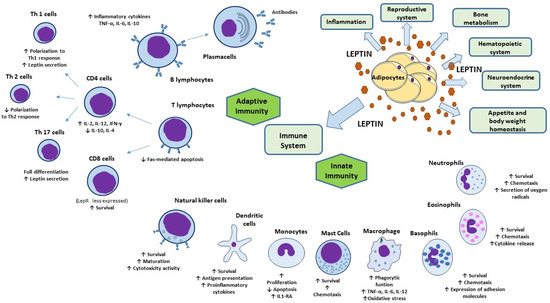
Figure 2.
Physiologic functions of leptin and its effects on innate and adaptive immunity. Besides its key role in appetite and body weight homeostasis, leptin exerts physiological functions within immune, hematopoietic, neuroendocrine, and reproductive systems, as well as in bone metabolism and inflammation. In innate immunity, leptin modulates the activity and function of neutrophils, monocytes/macrophages, eosinophils, mast cells, NK cells, and DCs. In adaptive immunity, leptin affects the maturation and survival of T cells. On memory T cells leptin favors the switch toward Th1 cell responses and facilitate Th17 responses and, contrariwise, negatively affects the expansion of Treg. Leptin activates also B-cell responses. Abbreviations: DC, dendritic cells; IL, interleukin; IL1-RA, interleukin 1 receptor-agonist; IFN-γ, interferon-γ; NK, natural killer; Th, T helper; TNF-α, tumor necrosis factor-α; Treg, regulatory T cells.
Additionally, conditions of hypoleptinemia have proven that leptin is necessary for the full functionality of immune system. In fact, leptin deficiency increases susceptibility to infections [113] and infection-related mortality [114] and is associated to cytokine dysregulation.
In human monocytes, leptin stimulates proliferation [115], prevents apoptosis [116], upregulates the expression of activation markers, such as CD25, CD38, CD69, CD71 [115] and interferon-gamma-inducible protein-10 (IP-10) [117], the secretion of interleukin 1 receptor antagonist and the synthesis of leukotriene [116], cholesterol acyltransferases-1 and cyclooxygenase 2 [118].
Leptin promotes the inflammatory infiltrates by acting as a monocyte/macrophage chemoattractant [119] and by stimulating phagocytic function via phospholipase activation [99], the expression of adhesion molecules and the secretion of proinflammatory cytokine such as TNF-α (in an early phase), IL-6 (in a tardive phase), and IL-12 in monocytes [120]. Finally, leptin has been found to increase the oxidative stress in macrophages [121].
Leptin may also promote the survival and maturation of DCs via the PI3K-Akt signaling pathway, and functionally direct these cells toward Th1 priming increasing their production of proinflammatory cytokines [122].
Leptin seems to behave as a prosurvival cytokine also for neutrophils, delaying the mitochondrial release of cytochrome C and second mitochondria-derived activator of caspase, and the activation of caspase-3 and -8 in these cells [115]. Leptin promotes the expression of CD11b, neutrophils chemotaxis [113], and secretion of oxygen radicals through direct and indirect mechanisms [115].
In addition, leptin enhances survival, chemotaxis, cytokine release and migration in eosinophils and basophils, as well as the expression of adhesion molecules, such as ICAM-1 and CD18 [99]. Leptin and LepR expression has been reported in mast cells suggesting paracrine and/or autocrine pro-inflammatory effects on mast cells [123] as confirmed by the opposite effects of leptin deficiency [124].
Leptin has been demonstrated also to influence the adaptive immune response.
Leptin modulates the activation and proliferation of human T lymphocytes [125], plays a role in maintaining lymphocyte survival by inhibition of Fas-mediated apoptosis. It polarizes T cells toward a Th1 response inducing the synthesis of IL-2, IL-12, and IFN-γ and inhibiting the production of IL-10 and IL-4 [125]. Contrariwise, leptin deficiency in mice and humans leads to a shift from Th1 to Th2 phenotype and to a reduction in total CD4+ T cell [112,126]. Accordingly, LepR is much more expressed in peripheral CD4+ T cells than in CD8+ T cells [99]. Leptin facilitates Th17 cells, which are a CD4+ proinflammatory T-cell subset generated to hinder infections, such that LepR on T-cell membrane is required for full Th17 differentiation [127]. Leptin is also secreted by Th1/Th17 lymphocytes potentiating its own effect by an autocrine loop of secretion [128]. Conversely, leptin inhibits the expansion of human regulatory Foxp3+ CD4+ CD25+ T cells (Treg) [129] which are mediators of immune tolerance and limit inflammation.
Finally, although fewer studies addressed this issue, leptin seems to modulate B-cell compartment, activating B cells to secrete inflammatory cytokines (i.e., TNF-α, IL-6, IL-10) [130].
Immune system impairments attributed to leptin deficiency, as in cases of genetic mutations, are treatable with leptin replacement therapy. Indeed, in several prospective case studies, leptin replacement therapy (with subcutaneous human recombinant methionyl leptin) for 4 to 8 months has been shown to improve immune dysfunction in patients with generalized forms of lipodystrophy, normalizing absolute number and relative percentages of T lymphocyte subsets and increasing TNF-α secretion in PBMC [131]. In addition, long-term (up to four years) leptin replacement therapy in congenital leptin deficiency caused lymphocytes, neutrophils, and monocytes increase and restored Th1/Th2 cytokine balance [132] along with T-cell responsiveness [133].
The immunostimulatory potential of leptin cannot be neglected in vaccines development either [134]. Researchers explored the adjuvant role of leptin in mucosal (either intragastric or intranasal) vaccination against a Gram-positive bacterial pneumonia caused by Rhodococcus equi infection [135]. In this study, only mice vaccinated with LL-VapA (a native Lactococcus lactis vector expressing virulence-associated protein-A of Rhodococcus equi) combined with LL-leptin (a recombinant strain of Lactococcus lactis secreting biologically active leptin) were able to develop protective immunity [135]. In detail, the co-administration of LL-Lep strain, enhanced the Th1 response evoked by intranasally LL-VapA-immunized mice whereas only the co-administration of LL-Lep induced a protective immune response in intragastric vaccinated mice, associated with a mixed Th1/Th2 response [135]. Similarly, LepR knockout mice (db/db) were not protected by prophylactic vaccination against Helicobacter pylori [136], indicating the crucial role of leptin and its signaling in the generation of host protective immune response.
9. Leptin In Viral Infections
The role of leptin in the immune system response to infections has been investigated mostly working on mice models. Many researchers used leptin knockout (ob/ob) and db/db mice. However, in both ob/ob and db/db models the lack of leptin-driven satiety cues results in hyperphagia and obesity, which complicates data interpretation. Thus far, it has been difficult to differentiate the effects of energy imbalances from the effects that leptin directly exerts on immune function, as they are experimentally, as well as physiologically, interconnected.
For instance, thymic atrophy, increased circulating monocytes, and reduced lymphocytes number, NK cell cytotoxicity, and antigen-specific T-cell proliferation have been reported in ob/ob and/or db/db mice [112].
During influenza A pneumonia infection, db/db or malnourished mice showed reduced viral clearance, lung IFNγ level and survival [137]. However, in mice lacking functional LepR uniquely in macrophages and lung epithelial cells, mortality rate was reduced, suggesting that leptin signaling in non-myeloid cells, such as NK and T cells, mostly mediates the immunomodulatory role of leptin upon virus challenge [138]. Both diet- and genetic-induced obese mice exhibited greater lung damage during the pH1N1 infection [67]. Although a number of potential mechanisms may be responsible, Tregs are critical regulators of immunopathology and have been shown to limit the inflammatory response to respiratory syncytial and influenza viruses in mice [139,140]. Tregs isolated from lung airways of DIO mice during pH1N1 challenge are fewer and with impaired suppressive capacity compared to Tregs from lean mice [141]. The key role of leptin in the negative control of Treg has been confirmed by the opposite observation of an increased percentage of peripheral Tregs in ob/ob mice compared to wild-type mice [142]. In human bronchial epithelial cells infected with respiratory syncytial virus, leptin was overexpressed enhancing the differentiation of Th17 subset and ERK1/2 phosphorylation, and suppressing Th2 subset differentiation [143].
In antiretroviral-naïve HIV-1-infected patients, serum leptin levels were inversely associated with viral replication, independent of adipose tissue amount and disease progression (i.e., cellular activation and innate immunity effector levels) [144]. In HIV infection, leptin levels correlates with CD4+ T lymphocytes number and increases along with them during highly active antiretroviral therapy [145]. Interestingly, leptin stimulation of HIV+ monocytes, which are characterized by an increased expression of LepR [145], partially inhibited the production of reactive oxygen species (ROS), an indicator of programmed cell-death in these cells, resulting in an attenuation of HIV-induced oxidative burst [146]. This is in contrast with the expected proinflammatory function of leptin and could be explained either by the induction of a hypo-inflammatory/anergy state in HIV+ monocytes, as observed in other inflammatory conditions such as sepsis under LPS stimulation, or by an anti-apoptotic effect of leptin on these cells [116].
Encephalomyocarditis virus infection in ob/ob mice caused a more severe inflammatory myocardial damage through enhanced expression of TNF-α compared to that of wild-type mice [147]. Similarly, db/db mice exhibited higher susceptibility to Coxsackie virus B4 infection than heterozygous normal (db/+) and normal (+/+) genotypic mice [148].
Many viruses (i.e., influenza, hepatitis B, HIV, Epstein Barr, Sars-Cov2) are capable of inducing SOCS3 to enhance self-replication and evade host immunity. Indeed, SOCS3 negatively regulates signaling leptin as well as of various cytokines and growth factors (i.e., IFN, IL-6, and G-CSF) by inhibiting IFN-α/β JAK/STAT pathways [149]. Therefore, an upregulation of SOCS3 during viral infections may, both directly and by decreasing leptin expression, dampen innate and adaptive immunity.
Notably, although lymphopenia and the predominance of innate immune macrophages which are features of the SARS-CoV2 infection could reflect a strategy adopted by the CoV to suppress host antiviral response, the finding that reduced lymphocyte counts correlated with increased leptin levels may suggest that leptin-induced monocyte changes may also result in insufficient virus-specific T-cell priming [150].
Furthermore, influenza A virus as well as other viral infections produce endoplasmic reticulum (ER) stress which in turn activates unfolded protein response (UPR) pathways (i.e., inositol-requiring protein-1). The activation of UPR has been recognized as critical for influenza A viral replication because it impairs T- and B-cell development, plasma cells differentiation and leptin signaling [151].
10. Leptin in Obesity: What Role during Viral Infections?
Because leptin reduces food intake and body weight, the coexistence of high serum leptin concentrations and obesity is widely interpreted as evidence of an attenuation of leptin signaling termed “leptin resistance” [152]. However, there are currently no methods for assessing leptin sensitivity in a clinical setting. Although hyperleptinemia is often considered as a key marker of leptin resistance, it should be noted that serum leptin concentrations depend not only on its transcription levels but also on its clearance rate and the efficiency of its signal transduction [153]. Moreover, the observation that obese individuals exhibit changes in appetite and energy expenditure after moderate weight loss and that these changes are blunted by the administration of leptin, suggests that the high leptin concentrations in obesity may be biologically relevant [154]. Accordingly, in DIO mouse model challenged with H1N1 influenza infection, hyperleptinemia was associated with increased mortality, viral spread, and lung inflammation, which were all significantly improved by the administration of anti-leptin antibody [155].
In this complex contest in which it is not feasible to define leptin resistance in a universal and quantifiable manner, several mechanisms that may underlie the attenuated responsiveness to leptin in vivo have been described. First, leptin signaling is potently antagonized by an upregulation of SOCS3 which specifically binds to LepR via phosphorylated tyrosine-985, resulting in JAK2 dephosphorylation, so that the key pathway affected is JAK/STAT [149]. Leptin itself, when chronically elevated as in the obese state, can induce SOCS3 expression as a mechanism of negative feedback. There are also multiple protein tyrosine phosphatases (i.e., RPTPe, PTP1B and TCPTP) that are capable of dephosphorylating JAK2 and that are upregulated in a high-fat diet and obesity [156]. Second, ER stress triggered by chronic positive energy balance activates the UPR that, if ER stress persists, switches from a pro-survival to a pro-apoptotic response and induces leptin resistance by inhibiting leptin-induced STAT3 phosphorylation [157]. An additional factor implicated in leptin resistance pathogenesis is the downregulation of the short- and long-form isotypes of LepR [152]. Leptin transport across the blood-brain barrier occurs via short-form leptin receptors, whose downregulation in the hypothalamus, in combination with high serum leptin concentrations, leads to a saturable, unidirectional transport system [152]. Finally, circulating factors such as C-reactive protein and clusterin may bind leptin altering its bioavailability and activity [158].
Leptin resistance has been demonstrated in immune cells such as NK cells [159], T cells [160], and monocytes [161], and this might contribute to suboptimal immune responses to viral infection in obese individuals. LepR desensitization could be perceived by immune cells as a condition of leptin deficiency, leading to immune dysfunction similarly to malnutrition and genetic leptin deficiency (Figure 3).
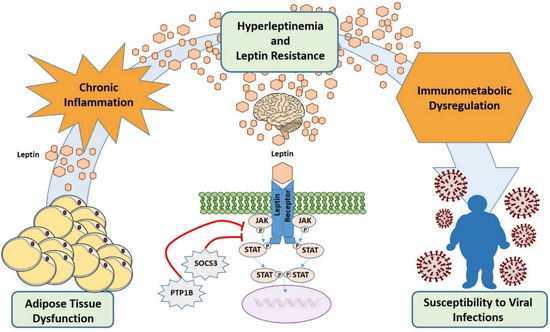
Figure 3.
Hyperleptinemia and leptin resistance contribute to immune dysregulation and increased susceptibility to viral infections in obesity. In obesity, adipose tissue produces high levels of leptin (hyperleptinemia) that causes desensitization of target cells for leptin signaling (leptin resistance) by several mechanisms including overexpression of SOCS3. This results in diminished immune cells response and, finally, in an increased susceptibility to infections. In addition, the hyperleptinemia contributes to the obesity low-grade inflammatory background.
Nevertheless, both direct and indirect effects of leptin on the immune system are likely to account for the immune defects observed in leptin-resistance conditions. In fact, when db/db bone marrow cells were transplanted into wild-type mice to generate bone marrow chimeras, thymus cellularity and cellular and humoral immune responses resulted normal, suggesting that direct effects of leptin on bone marrow-derived cells and thymic stromal cells are not necessary for T-lymphocyte maturation and immune response. Rather, the major effects of leptin on immune system are indirect via changes in the systemic environment [162].
After all, ob/ob and db/db mice display multiple neuroendocrine and metabolic alterations. These include the overactivation of the hypothalamic-pituitary-adrenal axis with subsequent hypercortisolism, decreased activity of the sympathetic nervous system, and altered production of various neuropeptides, which are all known to have immunomodulatory effects. In line with this, the negative effects of leptin deficiency on the hepatic innate immune system appear mostly related to the decreased sympathetic tone and β-adrenergic signaling [163].
Instead, with regard to hypercortisolism, even though it is known to induce immune suppression and favor thymic atrophy, in db/db mice the relative proportions of CD4+, CD8+ and CD4 and CD8 double positive thymocyte populations are not altered, in contrast with the preferential depletion of CD4 and CD8 double positive thymocytes induced by corticosteroid excess [164], suggesting for leptin a noncorticosteroid-related mechanism of action.
In obesity, adipose tissue dysfunction leads to chronic inflammation not only locally [165] but also in the brain. In particular, hypothalamic inflammation observed in chronic inflammatory conditions such as obesity or upon fat rich diet [166] is considered crucial in leptin resistance development.
In the hypothalamus, leptin activates the sympathetic nervous system [101] and, to a lesser extent, inhibits the hypothalamic-pituitary-adrenal axys [102]. In obesity, as result of leptin resistance at central level, the sympathetic nervous system, which has a major anti-inflammatory role in the brain-immune cross-talk, results less activated [101], thus fostering obesity low-grade inflammatory background [99].
Therefore, if excess leptin secretion from adipocytes can be envisaged as an inflammatory signal that induces proinflammatory environmental and cellular changes, on the other direction chronic inflammation due to metabolic, infectious, or autoimmune diseases impairs leptin signaling as a part of a maladaptive response.
Said that cytokine storms play an important role in the process of COVID-19 aggravation and are considered one of the major determinants of acute respiratory distress syndrome and multiple-organ failure, leptin levels have been found to be not only increased in COVID-19 patients compared with healthy controls, but also significantly higher in severe COVID-19 patients than in mild patients [150]. Notably, in this cohort leptin correlated with BMI, decreased lymphocyte counts and disease progression, showing high consistency with TNF-α and CXCL-10 in predicting disease severity. Leptin correlated with monocyte M1 (inflammatory) polarization in COVID-19 patients and mechanistic experiments revealed that leptin activated STAT3/NF-κB signaling and promoted M1-polarization marker gene and TLR gene expression in immortalized monocyte THP-1 cells [150]. Therefore, it seems that, upon infection, subjects with fat mass excess are prone to produce more leptin which in turn activates monocytes promoting a positive feedback loop and severe cytokine storms.
Leptin can also be inhibited by ACE2 via alamandine production and activation of MrgD-receptor/c/Src/p38MAPK pathway [167]. Therefore, it has been hypothesized that in obese patients infected by SARS-CoV-2, the impairment of ACE2 function due to viral binding may further increase the leptin levels. Consequently, hyperleptinemia combined with local ACE2-angiotensin II dysbalance could contribute to the hyperinflammatory pulmonary response found in obese patients [168].
While impairments attributed to leptin genetic mutations are treatable with recombinant methionyl human leptin administration, as result of leptin resistance replacement therapy in individuals with obesity does not provide therapeutic benefit.
However, in leptin resistance conditions in which vaccine-induced immune responses are dampened, the use of SOCS3 and protein tyrosine phosphatase-1B (PTP1B) inhibitors as viral vaccine adjuvants has been proposed since it may help restore immune homeostasis [134].
Another potential therapy candidate to prevent obesity-related vaccine failure is represented by chemical chaperones that, by decreasing ER stress induced by obesity, can improve leptin sensitivity [169]. Also saponins derived from fungal endophytes could be potential inhibitors of leptin, reverse its resistance in obesity, and potentiate host immune response against multiple diseases [170].
11. Conclusions
Metabolic complications, nutritional aspects, physical inactivity, and the chronic imbalance in the hormonal and adipocytokine microenvironment are major determinants in the severity of viral infections in obesity.
Besides the pleiotropic mechanisms by which obesity impairs immunity, the higher leptin concentrations which characterize obesity substantially contribute to such dysregulation of the immune response.
Indeed, chronic inflammatory states due to metabolic (i.e., obesity) or infectious diseases may increase leptin concentrations and lead to leptin resistance further fueling inflammation.
On the other hand, leptin plays an important role in numerous metabolic and immunologic functions, and impairments of leptin signaling due to leptin resistance appear to hamper these processes and possibly contribute to impaired vaccine-induced immune responses.
However, many aspects concerning leptin interactions with inflammation and immune system, as well as the therapeutical approaches to overcome leptin resistance and reduced vaccine effectiveness in obesity remain a challenge for future research.
Author Contributions
Conceptualization, V.G.; writing—review and editing, V.G. and L.C.; supervision, V.G., M.D., and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministero dell’Istruzione, dell’Università e della Ricerca, prot. 2017L8Z2EM (PRIN to Valeria Guglielmi).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E.R. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. J. Lab. Clin. Med. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37–43. [Google Scholar] [CrossRef]
- Huttunen, R.; Syrjanen, J. Obesity and the outcome of infection. Lancet Infect. Dis. 2010, 10, 442–443. [Google Scholar] [CrossRef]
- Hussain, A.; Mahawar, K.; Xia, Z.; Yang, W.; El-Hasani, S. Obesity and mortality of COVID-19. Meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Falagas, M.E.; Athanasoulia, A.P.; Peppas, G.; Karageorgopoulos, D.E. Effect of body mass index on the outcome of infections: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 280–289. [Google Scholar] [CrossRef]
- Carbone, F.; La Rocca, C.; De Candia, P.; Procaccini, C.; Colamatteo, A.; Micillo, T.; De Rosa, V.; Matarese, G. Metabolic control of immune tolerance in health and autoimmunity. Semin. Immunol. 2016, 28, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Beck, M.A. The impact of obesity on the immune response to infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gang, X.; He, G.; Li, Z.; Lv, Y.; Han, Q.; Wang, G. Obesity Increases the Severity and Mortality of Influenza and COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 595109. [Google Scholar] [CrossRef]
- Phung, D.T.; Wang, Z.; Rutherford, S.; Huang, C.; Chu, C. Body mass index and risk of pneumonia: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 839–857. [Google Scholar] [CrossRef]
- Hall, M.; Pritchard, M.; Dankwa, E.A.; Baillie, J.K.; Carson, G.; Citarella, B.W.; Docherty, A.; Donnelly, C.A.; Dunning, J.; Fraser, C.; et al. ISARIC Clinical Data Report 20 November 2020. medRxiv. 2020. Available online: https://isaric.org/wp-content/uploads/2021/01/ISARIC-Clinical-Data-Report-20.11.202 (accessed on 4 February 2021).
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Winfield, R.D.; Reese, S.; Bochicchio, K.; Mazuski, J.E.; Bochicchio, G.V. Obesity and the Risk for Surgical Site Infection in Abdominal Surgery. Am. Surg. 2016, 82, 331–336. [Google Scholar] [CrossRef]
- Zahr, F.; Genovese, E.; Mathier, M.; Shullo, M.; Lockard, K.; Zomak, R.; McNamara, D.; Toyoda, Y.; Kormos, R.L.; Teuteberg, J.J. Obese patients and mechanical circulatory support: Weight loss, adverse events, and outcomes. Ann. Thorac. Surg. 2011, 92, 1420–1426. [Google Scholar] [CrossRef]
- Crabtree, T.D.; Codd, J.E.; Fraser, V.J.; Bailey, M.S.; Olsen, M.A.; Damiano, R.J., Jr. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 53–61. [Google Scholar] [CrossRef]
- Tjeertes, E.K.; Hoeks, S.E.; Beks, S.B.; Valentijn, T.M.; Hoofwijk, A.G.; Stolker, R.J. Obesity--a risk factor for postoperative complications in general surgery? Bmc Anesthesiol. 2015, 15, 112. [Google Scholar] [CrossRef]
- Vilar-Compte, D.; Mohar, A.; Sandoval, S.; de la Rosa, M.; Gordillo, P.; Volkow, P. Surgical site infections at the National Cancer Institute in Mexico: A case-control study. Am. J. Infect. Control. 2000, 28, 14–20. [Google Scholar] [CrossRef]
- Huttunen, R.; Karppelin, M.; Syrjanen, J. Obesity and nosocomial infections. J. Hosp. Infect. 2013, 85, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, E.; Rosen, H.; Bader, A.M.; Lipsitz, S.R.; Rogers, S.O., Jr.; Eriksson, E. Percent body fat and prediction of surgical site infection. J. Am. Coll. Surg. 2010, 210, 381–389. [Google Scholar] [CrossRef]
- Kaspersen, K.A.; Pedersen, O.B.; Petersen, M.S.; Hjalgrim, H.; Rostgaard, K.; Moller, B.K.; Juul-Sorensen, C.; Kotze, S.; Dinh, K.M.; Erikstrup, L.T.; et al. Obesity and risk of infection: Results from the Danish Blood Donor Study. Epidemiology 2015, 26, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Semins, M.J.; Shore, A.D.; Makary, M.A.; Weiner, J.; Matlaga, B.R. The impact of obesity on urinary tract infection risk. Urology 2012, 79, 266–269. [Google Scholar] [CrossRef]
- Thorsteinsdottir, B.; Tleyjeh, I.M.; Baddour, L.M. Abdominal wall cellulitis in the morbidly obese. Scand. J. Infect. Dis. 2005, 37, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Sigurgeirsson, B.; Korting, H.C.; Kerl, H.; Paul, C. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: A case-control study. Dermatology 2004, 209, 301–307. [Google Scholar] [CrossRef]
- Garcia Hidalgo, L. Dermatological complications of obesity. Am. J. Clin. Dermatol. 2002, 3, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K. Influenza Cataclysm, 1918. N. Engl. J. Med. 2018, 379, 2285–2287. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T.; California Pandemic Working Group. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza A (H1N1). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 301–312. [Google Scholar] [CrossRef]
- Fezeu, L.; Julia, C.; Henegar, A.; Bitu, J.; Hu, F.B.; Grobbee, D.E.; Kengne, A.P.; Hercberg, S.; Czernichow, S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 653–659. [Google Scholar] [CrossRef]
- Kok, J.; Blyth, C.C.; Foo, H.; Bailey, M.J.; Pilcher, D.V.; Webb, S.A.; Seppelt, I.M.; Dwyer, D.E.; Iredell, J.R. Viral pneumonitis is increased in obese patients during the first wave of pandemic A(H1N1) 2009 virus. PLoS ONE 2013, 8, e55631. [Google Scholar] [CrossRef]
- Diaz, E.; Rodriguez, A.; Martin-Loeches, I.; Lorente, L.; Del Mar Martin, M.; Pozo, J.C.; Montejo, J.C.; Estella, A.; Arenzana, A.; Rello, J.; et al. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest 2011, 139, 382–386. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, H.E.; Evans, R.; Arshad, S.; Zervos, M.J.; Archer, C.; Kaye, K.S.; Martin, E.T. The impact of obesity and timely antiviral administration on severe influenza outcomes among hospitalized adults. J. Med. Virol. 2018, 90, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Campitelli, M.A.; Rosella, L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: A cohort study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 53, 413–421. [Google Scholar] [CrossRef]
- Martin, V.; Castilla, J.; Godoy, P.; Delgado-Rodriguez, M.; Soldevila, N.; Fernandez-Villa, T.; Molina, A.J.; Astray, J.; Castro, A.; Gonzalez-Candelas, F.; et al. High Body Mass Index as a Risk Factor for Hospitalization Due to Influenza: A Case-Control Study. Arch. Bronconeumol. 2016, 52, 299–307. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.G.; Moreli, M.L.; Saivish, M.V. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch. Virol. 2020, 165, 1517–1526. [Google Scholar] [CrossRef]
- Drosten, C.; Gunther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Assiri, A.; McGeer, A.; Perl, T.M.; Price, C.S.; Al Rabeeah, A.A.; Cummings, D.A.; Alabdullatif, Z.N.; Assad, M.; Almulhim, A.; Makhdoom, H.; et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013, 369, 407–416. [Google Scholar] [CrossRef]
- Memish, Z.A.; Assiri, A.M.; Al-Tawfiq, J.A. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: An observational analysis with infection control implications. Int. J. Infect. Dis. Ijid Off. Publ. Int. Soc. Infect. Dis. 2014, 29, 307–308. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Arifi, A.A.; Balkhy, H.H.; Najm, H.; Aldawood, A.S.; Ghabashi, A.; Hawa, H.; Alothman, A.; Khaldi, A.; Al Raiy, B. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014, 160, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet. Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef]
- Badawi, A.; Ryoo, S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. Ijid Off. Publ. Int. Soc. Infect. Dis. 2016, 49, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVIDView. A Wkly Surveill Summ US COVID-19 Act. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 28 January 2021).
- European Centre for Disease Prevention and Control. ECDC’s Weekly COVID-19 Surveillance Report. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/epidemiology (accessed on 28 January 2021).
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Barrasa, H.; Rello, J.; Tejada, S.; Martin, A.; Balziskueta, G.; Vinuesa, C.; Fernandez-Miret, B.; Villagra, A.; Vallejo, A.; San Sebastian, A.; et al. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth. Crit. Care Pain Med. 2020, 39, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021. [Google Scholar] [CrossRef]
- Kass, D.A.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Recalde, M.; Pistillo, A.; Fernandez-Bertolin, S.; Roel, E.; Aragon, M.; Freisling, H.; Prieto-Alhambra, D.; Burn, E.; Duarte-Salles, T. Body mass index and risk of COVID-19 diagnosis, hospitalisation, and death: A population-based multi-state cohort analysis including 2,524,926 people in Catalonia, Spain. medRxiv 2020. [Google Scholar] [CrossRef]
- Maier, H.E.; Lopez, R.; Sanchez, N.; Ng, S.; Gresh, L.; Ojeda, S.; Burger-Calderon, R.; Kuan, G.; Harris, E.; Balmaseda, A.; et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. J. Infect. Dis. 2018, 218, 1378–1382. [Google Scholar] [CrossRef]
- Milner, J.J.; Rebeles, J.; Dhungana, S.; Stewart, D.A.; Sumner, S.C.; Meyers, M.H.; Mancuso, P.; Beck, M.A. Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice. J. Immunol. 2015, 194, 4846–4859. [Google Scholar] [CrossRef]
- Moriconi, D.; Masi, S.; Rebelos, E.; Virdis, A.; Manca, M.L.; De Marco, S.; Taddei, S.; Nannipieri, M. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes. Res. Clin. Pract. 2020, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Gorwood, J.; Barrail-Tran, A.; Lagathu, C.; Capeau, J.; Desjardins, D.; Le Grand, R.; Damouche, A.; Bereziat, V.; Lambotte, O. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front. Microbiol. 2019, 10, 2837. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef]
- Al-Benna, S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef]
- Pinheiro, T.A.; Barcala-Jorge, A.S.; Andrade, J.M.O.; Pinheiro, T.A.; Ferreira, E.C.N.; Crespo, T.S.; Batista-Jorge, G.C.; Vieira, C.A.; Lelis, D.F.; Paraiso, A.F.; et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017, 48, 74–82. [Google Scholar] [CrossRef]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; Consortium, E. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Bailey, D.; Thomas, D. Interaction of obesity and infections. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 1017–1029. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Frasca, D.; Ferracci, F.; Diaz, A.; Romero, M.; Lechner, S.; Blomberg, B.B. Obesity decreases B cell responses in young and elderly individuals. Obesity 2016, 24, 615–625. [Google Scholar] [CrossRef]
- Joshi, S.S.; Davis, R.P.; Ma, M.M.; Tam, E.; Cooper, C.L.; Ramji, A.; Kelly, E.M.; Jayakumar, S.; Swain, M.G.; Jenne, C.N.; et al. Reduced immune responses to hepatitis B primary vaccination in obese individuals with nonalcoholic fatty liver disease (NAFLD). Npj Vaccines 2021, 6, 9. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- O’Brien, K.B.; Vogel, P.; Duan, S.; Govorkova, E.A.; Webby, R.J.; McCullers, J.A.; Schultz-Cherry, S. Impaired wound healing predisposes obese mice to severe influenza virus infection. J. Infect. Dis. 2012, 205, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W., Jr.; Stearns, J.D.; Colantuoni, E.; Robinson, K.A.; Stierer, T.; Mitter, N.; Pronovost, P.J.; Needham, D.M. The impact of obesity on outcomes after critical illness: A meta-analysis. Intensive Care Med. 2009, 35, 1152–1170. [Google Scholar] [CrossRef]
- Anzueto, A.; Frutos-Vivar, F.; Esteban, A.; Bensalami, N.; Marks, D.; Raymondos, K.; Apezteguia, C.; Arabi, Y.; Hurtado, J.; Gonzalez, M.; et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax 2011, 66, 66–73. [Google Scholar] [CrossRef]
- Jones, R.L.; Nzekwu, M.M. The effects of body mass index on lung volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Ravussin, E.; Heymsfield, S. COVID 19 and the Patient with Obesity—The Editors Speak Out. Obesity 2020, 28, 847. [Google Scholar] [CrossRef]
- Petrovic, V.; Radenkovic, D.; Radenkovic, G.; Djordjevic, V.; Banach, M. Pathophysiology of Cardiovascular Complications in COVID-19. Front. Physiol. 2020, 11, 575600. [Google Scholar] [CrossRef]
- Huttunen, R.; Syrjanen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Sohn, S.H.; Lee, S.Y.; Park, H.L.; Park, Y.W.; Kim, H.; Nam, J.H. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ. Toxicol. Pharmacol. 2015, 40, 924–930. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.B.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1alpha/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446 e435. [Google Scholar] [CrossRef]
- Teran-Cabanillas, E.; Montalvo-Corral, M.; Caire-Juvera, G.; Moya-Camarena, S.Y.; Hernandez, J. Decreased interferon-alpha and interferon-beta production in obesity and expression of suppressor of cytokine signaling. Nutrition 2013, 29, 207–212. [Google Scholar] [CrossRef]
- Honce, R.; Karlsson, E.A.; Wohlgemuth, N.; Estrada, L.D.; Meliopoulos, V.A.; Yao, J.; Schultz-Cherry, S. Obesity-Related Microenvironment Promotes Emergence of Virulent Influenza Virus Strains. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Kern, P.A.; Nikolajczyk, B.S. The Immune System in Obesity: Developing Paradigms Amidst Inconvenient Truths. Curr. Diabetes Rep. 2017, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Green, W.D.; Beck, M.A. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann. Am. Thorac. Soc. 2017, 14, S406–S409. [Google Scholar] [CrossRef]
- O’Shea, D.; Corrigan, M.; Dunne, M.R.; Jackson, R.; Woods, C.; Gaoatswe, G.; Moynagh, P.N.; O’Connell, J.; Hogan, A.E. Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int. J. Obes. 2013, 37, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Paich, H.A.; Sheridan, P.A.; Handy, J.; Karlsson, E.A.; Schultz-Cherry, S.; Hudgens, M.G.; Noah, T.L.; Weir, S.S.; Beck, M.A. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity 2013, 21, 2377–2386. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.I.; Henson, D.A.; Butterworth, D.E.; Fagoaga, O.R.; Warren, B.J.; Rainwater, M.K. Immune response to obesity and moderate weight loss. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1996, 20, 353–360. [Google Scholar]
- Chan, M.E.; Adler, B.J.; Green, D.E.; Rubin, C.T. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4855–4863. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.E.; Taylor, K.R.; Dutt, S.; Han, P.P.; Fujioka, K.; Jameson, J.M. Obesity impairs gammadelta T cell homeostasis and antiviral function in humans. PLoS ONE 2015, 10, e0120918. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. ‘Adipaging’: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, R.; Guesdon, W.; Crouch, M.J.; Teague, H.L.; Sullivan, E.M.; Karlsson, E.A.; Schultz-Cherry, S.; Gowdy, K.; Bridges, L.C.; Reese, L.R.; et al. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J. Immunol. 2017, 198, 4738–4752. [Google Scholar] [CrossRef]
- Arai, S.; Maehara, N.; Iwamura, Y.; Honda, S.; Nakashima, K.; Kai, T.; Ogishi, M.; Morita, K.; Kurokawa, J.; Mori, M.; et al. Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Rep. 2013, 3, 1187–1198. [Google Scholar] [CrossRef]
- Zheng, Q.; Cui, G.; Chen, J.; Gao, H.; Wei, Y.; Uede, T.; Chen, Z.; Diao, H. Regular Exercise Enhances the Immune Response Against Microbial Antigens Through Up-Regulation of Toll-like Receptor Signaling Pathways. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 37, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.J.; Olson, M.M.; Thompson, N.J.; Cahill, M.L.; Wyatt, T.A.; Yoon, K.J.; Loiacono, C.M.; Kohut, M.L. Exercise Improves Host Response to Influenza Viral Infection in Obese and Non-Obese Mice through Different Mechanisms. PLoS ONE 2015, 10, e0129713. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Munro, I.; Phang, M.; Garg, M. Plasma n-3 Polyunsaturated Fatty Acids are negatively associated with obesity. Br. J. Nutr. 2009, 102, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. The adipocyte: Storage depot or node on the energy information superhighway? Cell 1995, 80, 15–18. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Fernandez-Riejos, P.; Martin-Gonzalez, J.; Segura-Egea, J.J.; Sanchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef]
- Satoh, N.; Ogawa, Y.; Katsuura, G.; Numata, Y.; Masuzaki, H.; Yoshimasa, Y.; Nakao, K. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci. Lett. 1998, 249, 107–110. [Google Scholar] [CrossRef]
- Gaillard, R.C.; Spinedi, E.; Chautard, T.; Pralong, F.P. Cytokines, leptin, and the hypothalamo-pituitary-adrenal axis. Ann. N. Y. Acad. Sci. USA 2000, 917, 647–657. [Google Scholar] [CrossRef]
- Zakrzewska, K.E.; Cusin, I.; Sainsbury, A.; Rohner-Jeanrenaud, F.; Jeanrenaud, B. Glucocorticoids as counterregulatory hormones of leptin: Toward an understanding of leptin resistance. Diabetes 1997, 46, 717–719. [Google Scholar] [CrossRef]
- Gualillo, O.; Eiras, S.; Lago, F.; Dieguez, C.; Casanueva, F.F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000, 67, 2433–2441. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Reviews. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ubags, N.D.; Vernooy, J.H.; Burg, E.; Hayes, C.; Bement, J.; Dilli, E.; Zabeau, L.; Abraham, E.; Poch, K.R.; Nick, J.A.; et al. The role of leptin in the development of pulmonary neutrophilia in infection and acute lung injury. Crit. Care Med. 2014, 42, e143–e151. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Roberts, M.R.; Becker, S.M.; Podd, B.; Zhang, Y.; Chua, S.C., Jr.; Myers, M.G., Jr.; Duggal, P.; Houpt, E.R.; Petri, W.A., Jr. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011, 4, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Khankin, E.V.; Van Meurs, M.; Shih, S.C.; Lu, S.; Yano, M.; Castro, P.R.; Maratos-Flier, E.; Parikh, S.M.; Karumanchi, S.A.; et al. Leptin exacerbates sepsis-mediated morbidity and mortality. J. Immunol. 2010, 185, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393, 7–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martin-Romero, C.; Perez-Perez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Matarese, G.; Moschos, S.; Mantzoros, C.S. Leptin in immunology. J. Immunol. 2005, 174, 3137–3142. [Google Scholar] [CrossRef]
- Ozata, M.; Ozdemir, I.C.; Licinio, J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999, 84, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh-Esfahani, H.; Pockley, G.; Metcalfe, R.A.; Bidlingmaier, M.; Wu, Z.; Ajami, A.; Weetman, A.P.; Strasburger, C.J.; Ross, R.J. High-dose leptin activates human leukocytes via receptor expression on monocytes. J. Immunol. 2001, 167, 4593–4599. [Google Scholar] [CrossRef]
- Najib, S.; Sanchez-Margalet, V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell. Immunol. 2002, 220, 143–149. [Google Scholar] [CrossRef]
- Balogh, Z.; Foris, G.; Kosztaczky, B.; Paragh, G., Jr.; Seres, I.; Zsiros, E.; Konya, G.; Paragh, G. The concentration dependent biphasic effect of leptin on endogenous cholesterol synthesis in human monocytes. Peptides 2007, 28, 2081–2083. [Google Scholar] [CrossRef]
- Meier, C.A.; Chicheportiche, R.; Dreyer, M.; Dayer, J.M. IP-10, but not RANTES, is upregulated by leptin in monocytic cells. Cytokine 2003, 21, 43–47. [Google Scholar] [CrossRef]
- Curat, C.A.; Miranville, A.; Sengenes, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumie, A. From blood monocytes to adipose tissue-resident macrophages: Induction of diapedesis by human mature adipocytes. Diabetes 2004, 53, 1285–1292. [Google Scholar] [CrossRef]
- Loffreda, S.; Yang, S.Q.; Lin, H.Z.; Karp, C.L.; Brengman, M.L.; Wang, D.J.; Klein, A.S.; Bulkley, G.B.; Bao, C.; Noble, P.W.; et al. Leptin regulates proinflammatory immune responses. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 57–65. [Google Scholar]
- Gruen, M.L.; Hao, M.; Piston, D.W.; Hasty, A.H. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell Physiol. 2007, 293, C1481–C1488. [Google Scholar] [CrossRef] [PubMed]
- Lam, Q.L.; Liu, S.; Cao, X.; Lu, L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur. J. Immunol. 2006, 36, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- Caldefie-Chezet, F.; Poulin, A.; Tridon, A.; Sion, B.; Vasson, M.P. Leptin: A potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 2001, 69, 414–418. [Google Scholar]
- Zhou, Y.; Yu, X.; Chen, H.; Sjoberg, S.; Roux, J.; Zhang, L.; Ivoulsou, A.H.; Bensaid, F.; Liu, C.L.; Liu, J.; et al. Leptin Deficiency Shifts Mast Cells toward Anti-Inflammatory Actions and Protects Mice from Obesity and Diabetes by Polarizing M2 Macrophages. Cell Metab. 2015, 22, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sanchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Asp. Med. 2012, 33, 35–45. [Google Scholar] [CrossRef]
- Reis, B.S.; Lee, K.; Fanok, M.H.; Mascaraque, C.; Amoury, M.; Cohn, L.B.; Rogoz, A.; Dallner, O.S.; Moraes-Vieira, P.M.; Domingos, A.I.; et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol. 2015, 194, 5253–5260. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Cali, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.A.; Javor, E.D.; Ding, L.; Uzel, G.; Cochran, E.K.; Young, J.R.; DePaoli, A.M.; Holland, S.M.; Gorden, P. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J. Clin. Endocrinol. Metab. 2006, 91, 621–628. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Paz-Filho, G.; Wong, M.L.; Licinio, J. Ten years of leptin replacement therapy. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, e315–e323. [Google Scholar] [CrossRef]
- White, S.J.; Taylor, M.J.; Hurt, R.T.; Jensen, M.D.; Poland, G.A. Leptin-based adjuvants: An innovative approach to improve vaccine response. Vaccine 2013, 31, 1666–1672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cauchard, S.; Bermudez-Humaran, L.G.; Blugeon, S.; Laugier, C.; Langella, P.; Cauchard, J. Mucosal co-immunization of mice with recombinant lactococci secreting VapA antigen and leptin elicits a protective immune response against Rhodococcus equi infection. Vaccine 2011, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, A.; Aebischer, T.; Meyer, T.F.; Walduck, A.K. Leptin receptor signaling is required for vaccine-induced protection against Helicobacter pylori. Helicobacter 2008, 13, 94–102. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Radigan, K.A.; Morales-Nebreda, L.; Soberanes, S.; Nicholson, T.; Nigdelioglu, R.; Cho, T.; Chi, M.; Hamanaka, R.B.; Misharin, A.V.; Perlman, H.; et al. Impaired clearance of influenza A virus in obese, leptin receptor deficient mice is independent of leptin signaling in the lung epithelium and macrophages. PLoS ONE 2014, 9, e108138. [Google Scholar] [CrossRef]
- Bedoya, F.; Cheng, G.S.; Leibow, A.; Zakhary, N.; Weissler, K.; Garcia, V.; Aitken, M.; Kropf, E.; Garlick, D.S.; Wherry, E.J.; et al. Viral antigen induces differentiation of Foxp3+ natural regulatory T cells in influenza virus-infected mice. J. Immunol. 2013, 190, 6115–6125. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.B.; Meyerholz, D.K.; Varga, S.M. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 2010, 185, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Sheridan, P.A.; Karlsson, E.A.; Schultz-Cherry, S.; Shi, Q.; Beck, M.A. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J. Immunol. 2013, 191, 2474–2485. [Google Scholar] [CrossRef]
- De Rosa, V.; Procaccini, C.; Cali, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- Qin, L.; Tan, Y.R.; Hu, C.P.; Liu, X.A.; He, R.X. Leptin Is Oversecreted by Respiratory Syncytial Virus-Infected Bronchial Epithelial Cells and Regulates Th2 and Th17 Cell Differentiation. Int. Arch. Allergy Immunol. 2015, 167, 65–71. [Google Scholar] [CrossRef]
- Sinha, U.; Sinharay, K.; Sengupta, N.; Mukhopadhyay, P. Benefits of leptin therapy in HIV patients. Indian J. Endocrinol. Metab. 2012, 16, S637–S643. [Google Scholar] [CrossRef]
- Matarese, G.; Castelli-Gattinara, G.; Cancrini, C.; Bernardi, S.; Romiti, M.L.; Savarese, C.; Di Giacomo, A.; Rossi, P.; Racioppi, L. Serum leptin and CD4+ T lymphocytes in HIV+ children during highly active antiretroviral therapy. Clin. Endocrinol. 2002, 57, 643–646. [Google Scholar] [CrossRef]
- Sanchez-Pozo, C.; Rodriguez-Bano, J.; Dominguez-Castellano, A.; Muniain, M.A.; Goberna, R.; Sanchez-Margalet, V. Leptin stimulates the oxidative burst in control monocytes but attenuates the oxidative burst in monocytes from HIV-infected patients. Clin. Exp. Immunol. 2003, 134, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yu, F.; Saegusa, S.; Sumino, H.; Nakahashi, T.; Iwai, K.; Morimoto, S.; Kurabayashi, M.; Kanda, T. Impaired expression of cardiac adiponectin in leptin-deficient mice with viral myocarditis. Int. Heart J. 2006, 47, 107–123. [Google Scholar] [CrossRef]
- Webb, S.R.; Loria, R.M.; Madge, G.E.; Kibrick, S. Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J. Exp. Med. 1976, 143, 1239–1248. [Google Scholar] [CrossRef]
- Krebs, D.L.; Hilton, D.J. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhang, X.; Wang, S.; Peng, Z.; Guo, J.; Jiang, H.; Liu, J.; Xie, Y.; Wang, J.; et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J. Leukoc. Biol. 2021. [Google Scholar] [CrossRef]
- Hassan, I.H.; Zhang, M.S.; Powers, L.S.; Shao, J.Q.; Baltrusaitis, J.; Rutkowski, D.T.; Legge, K.; Monick, M.M. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 2012, 287, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; LeDuc, C.A.; Watanabe, K.; Mueller, B.R.; Skowronski, A.; Rosenbaum, M.; Leibel, R.L. Effects of chronic leptin infusion on subsequent body weight and composition in mice: Can body weight set point be reset? Mol. Metab. 2014, 3, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Murphy, E.M.; Heymsfield, S.B.; Matthews, D.E.; Leibel, R.L. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 2002, 87, 2391–2394. [Google Scholar] [CrossRef]
- Zhang, A.J.; To, K.K.; Li, C.; Lau, C.C.; Poon, V.K.; Chan, C.C.; Zheng, B.J.; Hung, I.F.; Lam, K.S.; Xu, A.; et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J. Infect. Dis. 2013, 207, 1270–1280. [Google Scholar] [CrossRef]
- St-Pierre, J.; Tremblay, M.L. Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 2012, 15, 292–297. [Google Scholar] [CrossRef]
- Ozcan, L.; Ergin, A.S.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.G., Jr.; Ozcan, U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef]
- Martin, S.S.; Sperling, L.S.; Blaha, M.J.; Wilson, P.W.F.; Gluckman, T.J.; Blumenthal, R.S.; Stone, N.J. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: Importance to implementation of the 2013 ACC/AHA Guidelines. J. Am. Coll. Cardiol. 2015, 65, 1361–1368. [Google Scholar] [CrossRef]
- Nave, H.; Mueller, G.; Siegmund, B.; Jacobs, R.; Stroh, T.; Schueler, U.; Hopfe, M.; Behrendt, P.; Buchenauer, T.; Pabst, R.; et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: A novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology 2008, 149, 3370–3378. [Google Scholar] [CrossRef]
- Papathanassoglou, E.; El-Haschimi, K.; Li, X.C.; Matarese, G.; Strom, T.; Mantzoros, C. Leptin receptor expression and signaling in lymphocytes: Kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J. Immunol. 2006, 176, 7745–7752. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Pappa, V.; Raptis, S.A.; Tsigos, C. Expression of the long and short leptin receptor isoforms in peripheral blood mononuclear cells: Implications for leptin’s actions. Metab. Clin. Exp. 2000, 49, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Aurrand-Lions, M.; Contassot, E.; Talabot-Ayer, D.; Ducrest-Gay, D.; Vesin, C.; Chobaz-Peclat, V.; Busso, N.; Gabay, C. Indirect effects of leptin receptor deficiency on lymphocyte populations and immune response in db/db mice. J. Immunol. 2006, 177, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Oben, J.A.; Yang, S.; Lin, H.; Stafford, E.A.; Soloski, M.J.; Thomas, S.A.; Diehl, A.M. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology 2004, 40, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Odaka, C.; Mizuochi, T. Macrophages are involved in DNA degradation of apoptotic cells in murine thymus after administration of hydrocortisone. Cell Death Differ. 2002, 9, 104–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guglielmi, V.; Sbraccia, P. Obesity phenotypes: Depot-differences in adipose tissue and their clinical implications. Eat. Weight Disord. Ewd 2018, 23, 3–14. [Google Scholar] [CrossRef]
- de Git, K.C.; Adan, R.A. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Okajima, F.; Mogi, C.; Tobo, A.; Tomono, S.; Sato, K. Alamandine reduces leptin expression through the c-Src/p38 MAP kinase pathway in adipose tissue. PLoS ONE 2017, 12, e0178769. [Google Scholar] [CrossRef]
- van der Voort, P.H.J.; Moser, J.; Zandstra, D.F.; Muller Kobold, A.C.; Knoester, M.; Calkhoven, C.F.; Hamming, I.; van Meurs, M. Leptin levels in SARS-CoV-2 infection related respiratory failure: A cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon 2020, 6, e04696. [Google Scholar] [CrossRef]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef]
- Chandra Mouli, K.; Pragathi, D.; Naga Jyothi, U.; Shanmuga Kumar, V.; Himalaya Naik, M.; Balananda, P.; Suman, B.; Seshadri Reddy, V.; Vijaya, T. Leptin inhibitors from fungal endophytes (LIFEs): Will be novel therapeutic drugs for obesity and its associated immune mediated diseases. Med. Hypotheses 2016, 92, 48–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).