Non-Coding RNAs as Prognostic Markers for Endometrial Cancer
Abstract
1. Introduction
2. The Genetics of EC
2.1. TCGA Classification
- (1)
- POLE ultramutated
- (2)
- Microsatellite instability hypermutated
- (3)
- Copy-number low
- (4)
- Copy-number high
2.2. PORTEC-4a Classification
- (1)
- Favorable:
- (2)
- Intermediate:
- (3)
- Unfavorable:
3. The Epigenetics of EC
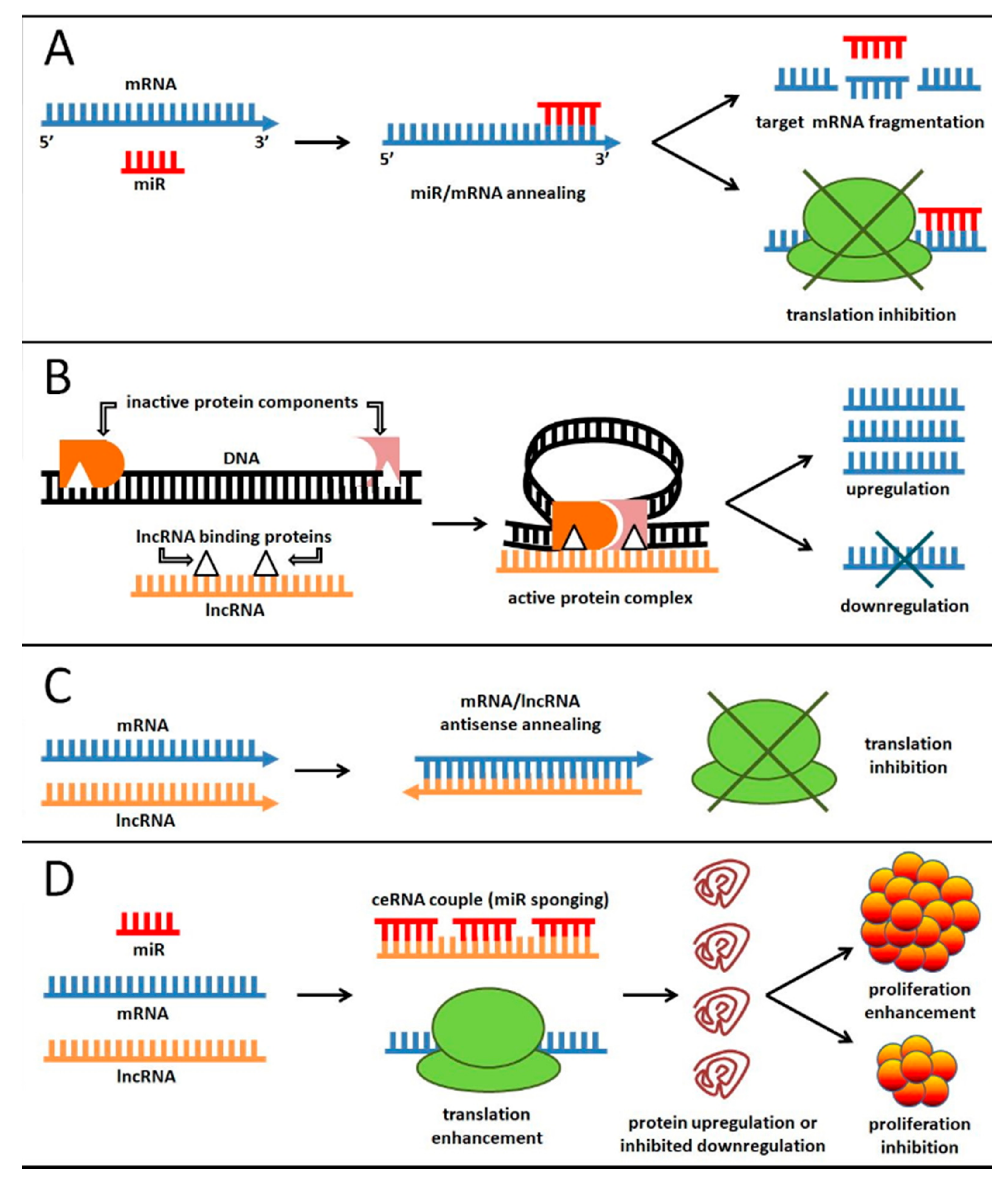
3.1. ncRNA Role in EC Etiology
3.2. ceRNA: At the Crossroad between Small and Long ncRNA Function in EC
3.3. Structural DNA Modifications in EC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 10 January 2021).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Phys. 2016, 93, 468–474. [Google Scholar]
- Lu, K.H.; Schorge, J.O.; Rodabaugh, K.J.; Daniels, M.S.; Sun, C.C.; Soliman, P.T.; White, K.G.; Luthra, R.; Gershenson, D.M.; Broaddus, R.R. Prospective Determination of Prevalence of Lynch Syndrome in Young Women with Endometrial Cancer. J. Clin. Oncol. 2007, 25, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T. Follow-up after Primary Therapy for Endometrial Cancer: A Systematic Review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Juergenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; Van Der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer—Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- PORTEC-4a, Version 2.5.1, 26 June 2020. NCT03469674. Available online: https://clinicaltrials.gov/ct2/show/NCT03469674 (accessed on 10 January 2021).
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-Coding RNAs and Endometrial Cancer. Genes 2018, 9, 187. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, C.; Yao, Y.; Wang, Q.; Li, F.; Li, Y.; Jiang, A.; Wang, G. Identifying Prognostic Biomarkers in Endometrial Carcinoma Based on Cerna Network. J. Cell. Biochem. 2019, 121, 2437–2446. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Howitt, B.E. Molecular Pathology. Surg. Pathol. Clin. 2016, 9, 405–426. [Google Scholar] [CrossRef]
- HGNC. HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:9177 (accessed on 10 January 2021).
- Yu, S.; Shao, H.; Ban, X.; Zhang, H.; You, Y.; Zhou, N.; Mao, X.; Zhao, H.; Chen, J.; Lu, Z. Detection of POLE Subtypes in High-Grade Endometrioid Carcinoma by BaseScope-ISH Assay. Front. Oncol. 2019, 9, 831. [Google Scholar] [CrossRef]
- Oldfield, L.E.; Li, T.; Tone, A.; Aronson, M.; Edwards, M.; Holter, S.; Quevedo, R.; Van De Laar, E.; Lerner-Ellis, J.; Pollett, A.; et al. An Integrative DNA Sequencing and Methylation Panel to Assess Mismatch Repair Deficiency. J. Mol. Diagn. 2021, 23, 242–252. [Google Scholar] [CrossRef]
- Klaus, A.; Birchmeier, W. Wnt Signalling and Its Impact on Development and Cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 Million Years of Evolution and 40 Years of Discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Rathjen, F.; Schachner, M. Immunocytological and Biochemical Characterization of a New Neuronal Cell Surface Component (L1 Antigen) Which Is Involved in Cell Adhesion. EMBO J. 1984, 3, 1–10. [Google Scholar] [CrossRef]
- Zeimet, A.G.; Reimer, D.; Huszar, M.; Winterhoff, B.; Puistola, U.; Azim, S.A.; Müller-Holzner, E.; Ben-Arie, A.; Van Kempen, L.C.; Petru, E.; et al. L1CAM in Early-Stage Type I Endometrial Cancer: Results of a Large Multicenter Evaluation. J. Natl. Cancer Inst. 2013, 105, 1142–1150. [Google Scholar] [CrossRef]
- Ponting, C.P.; Hardison, R.C. What Fraction of the Human Genome Is Functional? Genome Res. 2011, 21, 1769–1776. [Google Scholar] [CrossRef]
- Ling, H.; Vincent, K.; Pichler, M.; Fodde, R.; Berindan-Neagoe, I.; Slack, F.; Calin, G. Junk DNA and the Long Non-Coding RNA Twist in Cancer Genetics. Oncogene 2015, 34, 5003–5011. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Nebbioso, A.; Tambaro, F.P.; Dell’Aversana, C.; Altucci, L. Cancer Epigenetics: Moving Forward. PLoS Genet. 2018, 14, e1007362. [Google Scholar] [CrossRef]
- Kanwal, R.; Gupta, K.; Gupta, S. Cancer Epigenetics: An Introduction. Methods Mol. Biol. 2014, 3–25. [Google Scholar] [CrossRef]
- Jurcevic, S.; Olsson, B.; Klinga-Levan, K. MicroRNA Expression in Human Endometrial Adenocarcinoma. Cancer Cell Int. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Li, R.; Li, Y.; Zhu, X. Construction of a Competitive Endogenous RNA Network in Uterine Corpus Endometrial Carcinoma. Med. Sci. Monit. 2019, 25, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Razavi, Z.S.; Tajiknia, V.; Majidi, S.; Ghandali, M.; Mirzaei, H.R.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Gynecologic Cancers and Non-coding RNAs: Epigenetic Regulators with Emerging Roles. Crit. Rev. Oncol. 2021, 157, 103192. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Lopes, J.M.; Jerónimo, C. Epigenetics in Endometrial Carcinogenesis—Part 1: DNA Methylation. Epigenomics 2017, 9, 737–755. [Google Scholar] [CrossRef]
- Bartosch, C.; Lopes, J.M.; Jerónimo, C. Epigenetics in Endometrial Carcinogenesis—Part 2: Histone Modifications, Chromatin Remodeling and Noncoding RNAs. Epigenomics 2017, 9, 873–892. [Google Scholar] [CrossRef]
- Xia, L.; Wang, Y.; Meng, Q.; Su, X.; Shen, J.; Wang, J.; He, H.; Wen, B.; Zhang, C.; Xu, M. Integrated Bioinformatic Analysis of a Competing Endogenous RNA Network Reveals a Prognostic Signature in Endometrial Cancer. Front. Oncol. 2019, 9, 448. [Google Scholar] [CrossRef]
- Gulìa, C.; Baldassarra, S.; Signore, F.; Rigon, G.; Pizzuti, V.; Gaffi, M.; Briganti, V.; Porrello, A.; Piergentili, R. Role of Non-Coding RNAs in the Etiology of Bladder Cancer. Genes 2017, 8, 339. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.-L.; Chen, S.; Zong, Z.-H.; Guan, X.; Zhao, Y. LncRNA ABHD11-AS1 Promotes the Development of Endometrial Carcinoma by Targeting Cyclin D1. J. Cell. Mol. Med. 2018, 22, 3955–3964. [Google Scholar] [CrossRef]
- Gu, Z.-R.; Liu, Q. The LncRNA AL161431.1 Targets miR-1252-5p and Facilitates Cellular Proliferation and Migration via MAPK Signaling in Endometrial Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2294–2302. [Google Scholar]
- Zhai, W.; Li, X.; Wu, S.; Zhang, Y.; Pang, H.; Chen, W. Microarray Expression Profile of lncRNAs and the Upregulated ASLNC04080 lncRNA in Human Endometrial Carcinoma. Int. J. Oncol. 2015, 46, 2125–2137. [Google Scholar] [CrossRef]
- Wang, D.; Wang, D.; Wang, N.; Long, Z.; Ren, X. Long Non-Coding RNA BANCR Promotes Endometrial Cancer Cell Proliferation and Invasion by Regulating MMP2 and MMP1 via ERK/MAPK Signaling Pathway. Cell. Physiol. Biochem. 2016, 40, 644–656. [Google Scholar] [CrossRef]
- Liu, J.; Nie, S.; Liang, J.; Jiang, Y.; Wan, Y.; Zhou, S.; Cheng, W. Competing Endogenous RNA Network of Endometrial Carcinoma: A Comprehensive Analysis. J. Cell. Biochem. 2019, 120, 15648–15660. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, X.; Zhao, L.; Shi, L.; Cheng, J.; Kang, S.; Zhang, H.; Zhang, J.; Li, L.; Zhang, H.; et al. The rs6983267 SNP and Long Non-coding RNACARLo-5are Associated with Endometrial Carcinoma. Environ. Mol. Mutagen. 2016, 57, 508–515. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, L.; Gao, Y.; Sun, Q.; Liu, B.; Hu, Y.; Han, X. LncRNA CCAT1 Negatively Regulates miR 181a 5p to Promote Endometrial Carcinoma Cell Proliferation and Migration. Exp. Ther. Med. 2019, 17, 4259–4266. [Google Scholar] [CrossRef]
- Treeck, O.; Skrzypczak, M.; Schüler-Toprak, S.; Weber, F.; Ortmann, O. Long Non-coding RNA CCAT1 Is Overexpressed in Endometrial Cancer and Regulates Growth and Transcriptome of Endometrial Adenocarcinoma Cells. Int. J. Biochem. Cell Biol. 2020, 122, 105740. [Google Scholar] [CrossRef]
- Xie, P.; Cao, H.; Li, Y.; Wang, J.; Cui, Z. Knockdown of lncRNA CCAT2 Inhibits Endometrial Cancer Cells Growth and Metastasis via Sponging miR-216b. Cancer Biomarkers 2017, 21, 123–133. [Google Scholar] [CrossRef]
- Shang, C.; Ao, C.N.; Cheong, C.C.; Meng, L. Long Non-coding RNA CDKN2B Antisense RNA 1 Gene Contributes to Paclitaxel Resistance in Endometrial Carcinoma. Front. Oncol. 2019, 9, 27. [Google Scholar] [CrossRef]
- Shi, Y.; Zha, J.; Zuo, M.; Yan, Q.; Song, H. Long Noncoding RNA CHL1-AS1 Promotes Cell Proliferation and Migration by Sponging miR-6076 to Regulate CHL1 Expression in Endometrial Cancer. J. Cell. Biochem. 2019, 121, 2655–2663. [Google Scholar] [CrossRef]
- Shen, Q.; He, T.; Yuan, H. Hsa_circ_0002577 Promotes Endometrial Carcinoma Progression via Regulating miR-197/CTNND1 Axis and Activating Wnt/β-Catenin Pathway. Cell Cycle 2019, 18, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gong, Z.; Shen, Y.; Fang, Y.; Zhong, S. Circular RNA Expression in Extracellular Vesicles Isolated from Serum of Patients with Endometrial Cancer. Epigenomics 2018, 10, 187–197. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y. Hsa_circ_0061140 Promotes Endometrial Carcinoma Progression via Regulating miR-149-5p/STAT3. Gene 2020, 745, 144625. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, S.; Lu, C. Knockdown of Differentiation Antagonizing Non-protein Coding RNA Exerts Anti-tumor Effect by up-Regulating miR-214 in Endometrial Carcinoma. Mol. Cell. Biochem. 2019, 460, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Shen, Z.; Hu, W.; Li, M.; Shi, Y.; Xie, Z.; Wu, D. LncRNA DCST1-AS1 Was Upregulated in Endometrial Carcinoma and May Sponge miR-92a-3p to Upregulate Notch1. Cancer Manag. Res. 2020, ume 12, 1221–1227. [Google Scholar] [CrossRef]
- Shao, W.; Li, Y.; Chen, F.; Jia, H.; Jia, J.; Fu, Y. Long Non-Coding RNA DLEU1 Contributes to the Development of Endometrial Cancer by Sponging miR-490 to Regulate SP1 Expression. Die Pharm. 2018, 73, 379–385. [Google Scholar]
- Du, Y.; Wang, L.; Chen, S.; Liu, Y.; Zhao, Y. lncRNA DLEU1 Contributes to Tumorigenesis and Development of Endometrial Carcinoma by Targeting mTOR. Mol. Carcinog. 2018, 57, 1191–1200. [Google Scholar] [CrossRef]
- Kong, Y.; Ren, Z. Overexpression of LncRNA FER1L4 in Endometrial Carcinoma Is Associated with Favorable Survival Out-come. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8113–8118. [Google Scholar] [CrossRef]
- Qiao, Q.; Li, H. LncRNA FER1L4 Suppresses Cancer Cell Proliferation and Cycle by Regulating PTEN Expression in Endometrial Carcinoma. Biochem. Biophys. Res. Commun. 2016, 478, 507–512. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wang, X.; Ding, Y.; Li, N. The Tumor Suppressive Effect of Long Non-coding RNA FRMD6-AS2 in Uteri Corpus Endometrial Carcinoma. Life Sci. 2020, 243, 117254. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Meng, X.; Zhou, S.; Xiao, S.; Li, X.; Liu, S.; Yu, P. Long Noncoding RNA GAS5 Impairs the Proliferation and In-vasion of Endometrial Carcinoma Induced by High Glucose via Targeting MiR-222-3p/P27. Am. J. Transl. Res. 2019, 11, 2413–2421. [Google Scholar]
- Guo, C.; Song, W.-Q.; Sun, P.; Jin, L.; Dai, H.-Y. LncRNA-GAS5 Induces PTEN Expression through Inhibiting miR-103 in Endometrial Cancer Cells. J. BioMed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef]
- Tanos, V.; Ariel, I.; Prus, D.; De-Groot, N.; Hochberg, A. H19 and IGF2 Gene Expression in Human Normal, Hyperplastic, and Malignant Endometrium. Int. J. Gynecol. Cancer 2004, 14, 521–525. [Google Scholar] [CrossRef]
- Lottin, S.; Adriaenssens, E.; Berteaux, N.; Lepretre, A.; Vilain, M.-O.; Denhez, E.; Coll, J.; Dugimont, T.; Curgy, J.-J. The Human H19 Gene Is Frequently Overexpressed in Myometrium and Stroma during Pathological Endometrial Proliferative Events. Eur. J. Cancer 2005, 41, 168–177. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Chen, W.; Zhai, W.; Pan, J.; Pang, H.; Li, X. H19 Promotes Endometrial Cancer Progression by Modulating Epithelial-Mesenchymal Transition. Oncol. Lett. 2017, 13, 363–369. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.-L.; Yu, P. LncRNA H19 Regulates the Expression of Its Target Gene HOXA10 in Endometrial Carcinoma through Competing With miR-612. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4820–4827. [Google Scholar]
- Zhu, H.; Jin, Y.-M.; Lyu, X.-M.; Fan, L.-M.; Wu, F. Long Noncoding RNA H19 Regulates HIF-1α/AXL Signaling through Inhibiting miR-20b-5p in Endometrial Cancer. Cell Cycle 2019, 18, 2454–2464. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, J.; Tang, X.; Cui, H.; Zhang, Q.; Yang, Q. LncRNA-H19 Regulates Cell Proliferation and Invasion of Ectopic Endometrium by Targeting ITGB3 via Modulating miR-124-3p. Exp. Cell Res. 2019, 381, 215–222. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.C.; Lee, W.Y.W.; Trovik, J.; Chung, T.K.H.; Kwong, J. Long Non-coding RNA HAND2-as1 Inhibits Invasion and Metastasis in Endometrioid Endometrial Carcinoma through Inac-Tivating Neuromedin U. Cancer Lett. 2018, 413, 23–34. [Google Scholar] [CrossRef]
- He, X.; Bao, W.; Li, X.; Chen, Z.; Che, Q.; Wang, H.; Wan, X.-P. The Long Non-coding RNA HOTAIR Is Upregulated in Endometrial Carcinoma and Correlates with Poor Prognosis. Int. J. Mol. Med. 2013, 33, 325–332. [Google Scholar] [CrossRef]
- Huang, J.; Ke, P.; Guo, L.; Wang, W.; Tan, H.; Liang, Y.; Yao, S. Lentivirus-Mediated RNA Interference Targeting the Long Noncoding RNA HOTAIR Inhibits Proliferation and Invasion of Endometrial Carcinoma Cells In Vitro and In Vivo. Int. J. Gynecol. Cancer 2014, 24, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Y.; Zhu, J.-Y.; Zhang, C.-Y.; Zhang, M.; Song, Y.-N.; Rahman, K.; Zhang, L.-J.; Zhang, H. Autophagy Regulated by lncRNA HOTAIR Contributes to the Cisplatin-Induced Resistance in Endometrial Cancer Cells. Biotechnol. Lett. 2017, 39, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Wang, C.; Mao, L.-W.; Wang, Y.-L.; Xia, L.-Q.; Zhao, W.; Shen, J.; Chen, J. Long Noncoding RNA HOTAIR Mediates the Estrogen-Induced Metastasis of Endometrial Cancer Cells via the miR-646/NPM1 Axis. Am. J. Physiol. Physiol. 2018, 314, C690–C701. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Liu, Y.; Zhou, X.; Feng, D.; Xiao, X.; Li, W.; Zhao, Y.; Wang, H. Knockdown of Long Non-coding HOTAIR Enhances the Sensitivity to Progesterone in Endometrial Cancer by Epigenetic Regu-Lation of Progesterone Receptor Isoform B. Cancer Chemother. Pharmacol. 2019, 83, 277–287. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Hu, P.; Xie, Y.-Q.; Kang, Y.-J.; Li, M. Long Noncoding RNA HOTAIR Promotes Endometrial Carcinoma Cell Proliferation by Binding to PTEN via the Activating Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. Mol. Cell. Biol. 2019, 39, e00251-19. [Google Scholar] [CrossRef]
- Li, X.; Pang, L.; Yang, Z.; Liu, J.; Li, W.; Wang, D. LncRNA HOTAIRM1/HOXA1 Axis Promotes Cell Proliferation, Migration and Invasion in Endometrial Cancer. OncoTargets Ther. 2019, ume 12, 10997–11015. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, M.; Jiang, L.; Liu, K. Long Noncoding RNA HOXB-AS1 Is Upregulated in Endometrial Carcinoma and Sponged miR-149-3p to Upregulate Wnt10b. Technol. Cancer Res. Treat. 2020, 19, 153303382096746. [Google Scholar] [CrossRef]
- Xin, W.; Zhao, S.; Han, X.; Zhao, P.; Yu, H.; Gao, X.; Li, P.; Wu, Q.; Ding, J.; Hua, K. lncRNA LA16c-313D11.11 Modulates the Development of Endometrial Cancer by Binding to and Inhibiting microRNA 205 5p Function and Indirectly in-Creasing PTEN activity. Int. J. Oncol. 2020, 57, 355–363. [Google Scholar] [CrossRef]
- Fang, Q.; Sang, L.; Du, S. Long Noncoding RNA LINC00261 Regulates Endometrial Carcinoma Progression by Modulating miRNA/FOXO1 Expression. Cell Biochem. Funct. 2018, 36, 323–330. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, L.; Hu, M.; Li, F.; Deng, J.; An, M.; Wu, S.; Ma, R.; Lu, J.; et al. Long non-coding RNA LINC00672 contributes to p53 protein-mediated gene suppression and promotes endometrial cancer chemosensitivity. J. Biol. Chem. 2017, 292, 5801–5813. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, T.; Sun, X.; Wang, Y. Identification of a Potential Prognostic lncRNA-miRNA-mRNA Signature in Endometrial Cancer Based on the Competing Endogenous RNA Network. J. Cell. Biochem. 2019, 120, 18845–18853. [Google Scholar] [CrossRef]
- Pan, X.; Li, D.; Huo, J.; Kong, F.; Yang, H.; Ma, X. LINC01016 Promotes the Malignant Phenotype of Endometrial Cancer Cells by Regulating the miR-302a-3p/miR-3130-3p/NFYA/SATB1 axis. Cell Death Dis. 2018, 9, 303. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Xu, H.; Li, P.; Zeng, T.; Du, W.; Lu, X. LINC01170 Promotes the Progression of Endometrial Carcinoma by Activating the Akt Pathway. J. BUON Off. J. Balk. Union Oncol. 2019, 23, 1745–1752. [Google Scholar]
- Li, Y.; Kong, C.; Wu, C.; Wang, Y.; Xu, B.; Liang, S.; Ying, X.; Wu, C. Knocking Down of LINC01220 Inhibits Proliferation and Induces Apoptosis of Endometrial Carcinoma through Silencing MAPK11. Biosci. Rep. 2019, 39, 20181794. [Google Scholar] [CrossRef]
- Lu, M.; Ding, N.; Zhuang, S.; Li, Y. LINC01410/miR-23c/CHD7 Functions as a ceRNA Network to Affect the Prognosis of Patients with Endometrial Cancer and Strengthen the Malignant Properties of Endometrial Cancer Cells. Mol. Cell. Biochem. 2020, 469, 9–19. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Wang, J.; Zhang, X.; Liu, K.; Duan, Z. Linc-RNA-RoR Acts as a “Sponge” against Mediation of the Differentiation of Endometrial Cancer Stem Cells by microRNA-145. Gynecol. Oncol. 2014, 133, 333–339. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Li, M.; Kang, Y.-J.; Xie, Y.-Q.; Cao, Y.-X. Long Non-Coding RNA LINP1 Functions as an Oncogene in Endometrial Cancer Progression by Regulating the PI3K/AKT Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci 2019, 23, 6830–6838. [Google Scholar]
- Sun, L.; Zhou, R.; Dong, J.; Liu, S.; Jiao, Y.; Wang, L.; Hu, S.; He, P.; Liu, X.; Zhao, X.; et al. Lnc-NA Inhibits Proliferation and Metastasis in Endometrioid Endometrial Carcinoma through Regulation of NR4A1. J. Cell. Mol. Med. 2019, 23, 4699–4710. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Cai, Y.-X. Inhibition of Lnc-OC1 Induced Cell Apoptosis and Decreased Cell Viability by Releasing miR-34a and Inhibiting PD-L1 in Endometrial Carcinoma. Reprod. Sci. 2020, 27, 1848–1856. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Song, Y.; Feng, C. Long Noncoding RNA-ATB Impairs the Function of Tumor Suppressor miR-126-Mediated Signals in Endometrial Cancer for Tumor Growth and Metastasis. Cancer Biother. Radiopharm. 2019, 34, 47–55. [Google Scholar] [CrossRef]
- Guo, J.-L.; Tang, T.; Li, J.-H.; Yang, Y.-H.; Zhang, L.; Quan, Y. LncRNA HEIH Enhances Paclitaxel-Tolerance of Endometrial Cancer Cells via Activation of MAPK Signaling Pathway. Pathol. Oncol. Res. 2019, 26, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, H.; Zhang, L.; Li, W.; Deng, J.; An, M.; Wu, S.; Lu, X.; Ma, R.; Wang, Y.; et al. X Chromosome-Linked Long Noncoding RNA lnc-XLEC1 regulates c-Myc-Dependent Cell Growth by Collaborating with MBP-1 in Endometrial Cancer. Int. J. Cancer 2019, 145, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, Y.; Ma, Z.; Xu, X.; Qin, L.; Luo, B. LOC134466 Methylation Promotes Oncogenesis of Endometrial Carcinoma Through LOC134466/hsa-miR-196a-5p/TAC1 axis. Aging 2018, 10, 3353–3370. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xing, G.; Liu, S.; Li, B.; He, Y.; Wang, F. LncRNA LOXL1-AS1 Promotes Endometrial Cancer Progression by Sponging miR-28-5p to Upregulate RAP1B Expression. BioMed. Pharmacother. 2020, 125, 109839. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Chen, R.; Xiong, H.; Qiu, F.; Liu, S.; Zhang, M.; Wang, F.; Wang, Y.; Zhou, X.; et al. Disrupting MALAT1/miR-200c Sponge Decreases Invasion and Migration in Endometrioid Endometrial Carcinoma. Cancer Lett. 2016, 383, 28–40. [Google Scholar] [CrossRef]
- Guo, Q.; Qian, Z.; Yan, D.; Li, L.; Huang, L. LncRNA-MEG3 Inhibits Cell Proliferation of Endometrial Carcinoma by Repressing Notch Signaling. BioMed. Pharmacother. 2016, 82, 589–594. [Google Scholar] [CrossRef]
- Sun, K.-X.; Wu, D.-D.; Chen, S.; Zhao, Y.; Zong, Z.-H. LncRNA MEG3 Inhibit Endometrial Carcinoma Tumorigenesis and Progression through PI3K Pathway. Apoptosis 2017, 22, 1543–1552. [Google Scholar] [CrossRef]
- Shi, F.; Wang, T.; Liu, Z.; Zhang, Y.; Wang, J.; Zhang, K.; Su, J. LncRNA miR143HG Up-Regulates p53 In Endometrial Carcinoma by Sponging miR-125a. Cancer Manag. Res. 2019, ume 11, 10117–10123. [Google Scholar] [CrossRef]
- Cui, Z.; An, X.; Li, J.; Liu, Q.; Liu, W. LncRNA MIR22HG Negatively Regulates miR-141-3p to Enhance DAPK1 Expression and Inhibits Endometrial Carcinoma Cells Proliferation. BioMed. Pharmacother. 2018, 104, 223–228. [Google Scholar] [CrossRef]
- Wang, W.; Ge, L.; Xu, X.-J.; Yang, T.; Yuan, Y.; Ma, X.-L.; Zhang, X.-H. LncRNA NEAT1 Promotes Endometrial Cancer Cell Proliferation, Migration and Invasion by Regulating the miR-144-3p/EZH2 Axis. Radiol. Oncol. 2019, 53, 434–442. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Xu, D.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. Long Noncoding RNA NEAT1 Drives Aggressive Endometrial Cancer Progression via Mir-361-Regulated Networks Involving STAT3 and Tumor Microenvi-Ronment-Related Genes. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, R.; He, X.; Deng, Q.; Peng, X.; Li, J.; Luo, X. Investigations on the Mechanism of Progesterone in Inhibiting Endometrial Cancer Cell Cycle and Viability via Regulation of Long Noncoding RNA NEAT1/microRNA-146b-5p Mediated Wnt/β-catenin signaling. IUBMB Life 2019, 71, 223–234. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhao, W.; Mao, L.-W.; Wang, Y.-L.; Xia, L.-Q.; Cao, M.; Shen, J.; Chen, J. Long Non-Coding RNA NIFK-AS1 Inhibits M2 Polarization of Macrophages in Endometrial Cancer through Targeting miR-146a. Int. J. Biochem. Cell Biol. 2018, 104, 25–33. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Mingxin, Y.U. LncRNA NR2F1-AS1 Is Involved in the Progression of Endometrial Cancer by Sponging miR-363 to target SOX4. Die Pharm. 2019, 74, 295–300. [Google Scholar]
- Zhao, X.; Fan, Y.; Lu, C.; Li, H.; Zhou, N.; Sun, G.; Fan, H. PCAT1 Is a Poor Prognostic Factor in Endometrial Carcinoma and Associated with Cancer Cell Proliferation, Migration and Invasion. Bosn. J. Basic Med. Sci. 2019, 19, 274–281. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, S.; Zhang, Y.; Wang, L.; Liu, J.; Fang, F.; Li, P.; Wang, B. LncRNA PCAT1 Promotes Metastasis of Endometrial Carcinoma through Epigenetical Downregulation of E-cadherin Associated with Methyl-Transferase EZH2. Life Sci. 2020, 243, 117295. [Google Scholar] [CrossRef]
- Li, Q.; Shen, F.; Zhao, L. The Relationship between lncRNA PCGEM1 and STAT3 During the Occurrence and Development of Endometrial Carcinoma. BioMed. Pharmacother. 2018, 107, 918–928. [Google Scholar] [CrossRef]
- Kong, F.; Ma, J.; Yang, H.; Yang, D.; Wang, C.; Ma, X. Long Non-Coding RNA PVT1 Promotes Malignancy in Human Endometrial Carcinoma Cells through Negative Regulation of miR-195-5p. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1865, 1479–1490. [Google Scholar] [CrossRef]
- Xing, T.-R.; Chen, P.; Wu, J.-M.; Gao, L.-L.; Yang, W.; Cheng, Y.; Tong, L.-B. UPF1 Participates in the Progression of Endometrial Cancer by Inhibiting the Expression of lncRNA PVT1. OncoTargets Ther. 2020, ume 13, 2103–2114. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Chen, L.; Zhao, L.; Zhu, J.; Zhu, T. The Long Non-Coding RNA-14327.1 Promotes Migration and Invasion Potential of Endometrial Carcinoma Cells by Stabilizing the Potassium Channel Kca3.1. OncoTargets Ther. 2019, ume 12, 10287–10297. [Google Scholar] [CrossRef]
- Gao, L.; Nie, X.; Zhang, W.; Gou, R.; Hu, Y.; Qi, Y.; Li, X.; Liu, Q.; Liu, J.; Lin, B. Identification of Long Noncoding RNA RP11-89K21.1 and RP11-357H14.17 as Prognostic Signature of Endometrial Carcinoma via Integrated Bioinformatics Analysis. Cancer Cell Int. 2020, 20, 1–18. [Google Scholar] [CrossRef]
- Xin, W.; Gao, X.; Zhao, S.; Zhao, P.; Yu, H.; Wu, Q.; Hua, K. LncRNA RP11-395G23.3 Suppresses the Endometrial Cancer Progression via Regulating microRNA-205-5p/PTEN axis. Am. J. Transl. Res 2020, 12, 4422–4433. [Google Scholar]
- Zhang, G.; Ma, A.; Jin, Y.; Pan, G.; Wang, C. LncRNA SNHG16 Induced by TFAP2A Modulates Glycolysis and Proliferation of Endometrial Carcinoma Through miR-490-3p/HK2 axis. Am. J. Transl. Res 2019, 11, 7137–7145. [Google Scholar]
- Li, S.; Shan, Y.; Li, X.; Zhang, C.; Wei, S.; Dai, S.; Zhao, R.; Zhao, X.; Zhao, L.; Shan, B. lncRNA SNHG5 Modulates Endometrial Cancer Progression via the miR-25-3p/BTG2 Axis. J. Oncol. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Yang, C.-H.; Zhang, X.-Y.; Zhou, L.-N.; Wan, Y.; Song, L.-L.; Gu, W.-L.; Liu, R.; Ma, Y.-N.; Meng, H.-R.; Tian, Y.-L.; et al. LncRNA SNHG8 Participates in the Development of Endometrial Carcinoma through Regulating c-MET Expression by miR-152. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1629–1637. [Google Scholar]
- Park, S.-A.; Kim, L.K.; Kim, Y.T.; Heo, T.-H.; Kim, H.J. Long Non-coding RNA Steroid Receptor Activator Promotes the Progression of Endometrial Cancer via Wnt/β-Catenin Signaling Pathway. Int. J. Biol. Sci. 2020, 16, 99–115. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.-L.; Sun, K.-X.; Liu, Y.; Guan, X.; Zong, Z.-H.; Zhao, Y. LncRNA TDRG1 Enhances Tumorigenicity in Endometrial Carcinoma by Binding and Targeting VEGF-A protein. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Sun, X.; Liu, H.; Li, P. Knockdown of lncRNA TDRG1 Inhibits Tumorigenesis in Endometrial Carcinoma Through the PI3K/AKT/mTOR Pathway. OncoTargets Ther. 2019, ume 12, 10863–10872. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Zhang, Y.; Hu, Y.; Shen, X.; Zhu, W. Long Non-Coding RNA TUG1 Promotes Endometrial Cancer Development via Inhibiting miR-299 and miR-34a-5p. Oncotarget 2017, 8, 31386–31394. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Lang, B.; Ao, C.N.; Meng, L. Long Non-Coding RNA Tumor Suppressor Candidate 7 Advances Chemotherapy Sensitivity of Endometrial Carcinoma through Targeted Silencing of miR-23b. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, D.; Zhang, F.; Li, M.; Wan, Q. Long Noncoding RNA TUSC7 Inhibits Cell Proliferation, Migration and Invasion by Regulating SOCS4 (SOCS5) Expression through Targeting miR-616 in Endometrial Carcinoma. Life Sci. 2019, 231, 116549. [Google Scholar] [CrossRef]
- Peng, J.-T.; Li, M.-C. A Functional Cis-EQTL Locus in LncRNA ZNRD1-AS1 Contributes to the Susceptibility of Endometrial Cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7802–7807. [Google Scholar] [CrossRef]
- Konno, Y.; Dong, P.; Xiong, Y.; Suzuki, F.; Lu, J.; Cai, M.; Watari, H.; Mitamura, T.; Hosaka, M.; Hanley, S.J.B.; et al. MicroRNA-101 Targets EZH2, MCL-1 and FOS to Suppress Proliferation, Invasion and Stem Cell-like Phenotype of Aggressive Endometrial Cancer Cells. Oncotarget 2014, 5, 6049–6062. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Li, Q.; Zhu, P. MiR-101 Reduces Cell Proliferation and Invasion and Enhances Apoptosis in Endometrial Cancer via Regulating PI3K/Akt/mTOR. Cancer Biomark. 2021, 21, 179–186. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhao, C.; Jia, H. MicroRNA-101 Inhibits Angiogenesis via COX-2 in Endometrial Carcinoma. Mol. Cell. Biochem. 2018, 448, 61–69. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B. miR-101-3p Induces Autophagy in Endometrial Carcinoma Cells by Targeting EZH2. Arch. Gynecol. Obstet. 2018, 297, 1539–1548. [Google Scholar] [CrossRef]
- Du, J.; Zhang, F.; Zhang, L.; Jia, Y.; Chen, H. MicroRNA-103 Regulates the Progression in Endometrial Carcinoma through ZO-1. Int. J. Immunopathol. Pharmacol. 2019, 33. [Google Scholar] [CrossRef]
- Zhao, Z.-N.; Bai, J.-X.; Zhou, Q.; Yan, B.; Qin, W.-W.; Jia, L.-T.; Meng, Y.-L.; Jin, B.-Q.; Yao, L.-B.; Wang, T.; et al. TSA Suppresses miR-106b-93-25 Cluster Expression through Downregulation of MYC and Inhibits Proliferation and Induces Apoptosis in Human EMC. PLoS ONE 2012, 7, e45133. [Google Scholar] [CrossRef]
- Tang, W.; Li, J.; Liu, H.; Zhou, F.; Liu, M. MiR-106a Promotes Tumor Growth, Migration, and Invasion by Targeting BCL2L11 in Human Endometrial Adenocarcinoma. Am. J. Transl. Res. 2017, 9, 4984–4993. [Google Scholar]
- Zavesky, L.; Jandakova, E.; Turyna, R.; Langmeierova, L.; Weinberger, V.; Minar, L. Supernatant versus Exosomal Urinary MicroRNAs. Two Fractions with Different Outcomes in Gynaecological Cancers. Neoplasma 2016, 63, 121–132. [Google Scholar] [CrossRef]
- Huang, C.; Hu, G. Shikonin Suppresses Proliferation and Induces Apoptosis in Endometrioid Endometrial Cancer Cells via Modulating miR-106b/PTEN/AKT/mTOR Signaling Pathway. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. miR-107-5p Promotes Tumor Proliferation and Invasion by Targeting Estrogen Receptor α in Endometrial Carcinoma. Oncol. Rep. 2018, 41, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, Y.; Xu, W.; Chen, J.; Xu, C.; Wei, X.; Fang, D.; Feng, Y. miR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biother. Radiopharm. 2016, 31, 225–231. [Google Scholar] [CrossRef]
- Li, L.; Qu, Y.W.; Li, Y.P. Over-Expression of miR-1271 Inhibits Endometrial Cancer Cells Proliferation and Induces Cell B by Targeting CDK1. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2816–2822. [Google Scholar] [PubMed]
- Tian, Y.; Chen, Y.-Y.; Han, A.-L. MiR-1271 Inhibits Cell Proliferation and Metastasis by Targeting LDHA in Endometrial Cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5648–5656. [Google Scholar] [PubMed]
- Dong, P.; Karaayvaz, M.; Jia, N.; Kaneuchi, M.; Hamada, J.; Watari, H.; Sudo, S.; Ju, J.; Sakuragi, N. Mutant p53 Gain-of-Function Induces Epithelial–Mesenchymal Transition through Modulation of the miR-130b–ZEB1 Axis. Oncogene 2012, 32, 3286–3295. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, T.; Huang, Y. MicroRNA-134 Suppresses Endometrial Cancer Stem Cells by Targeting POGLUT1 and Notch Pathway Proteins. FEBS Lett. 2014, 589, 207–214. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.-H.; Shan, T.; Aguilera-Barrantes, I.; Wang, L.-S.; Huang, T.H.-M.; Rader, J.S.; Sheng, X.; Huang, Y.-W. miR-137 is a Tumor Suppressor in Endometrial Cancer and Is Repressed by DNA Hypermethylation. Lab. Investig. 2018, 98, 1397–1407. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Jiang, Y.; Wan, Y.; Zhou, S.; Cheng, W. Tumor-Suppressor Role of miR-139-5p in Endometrial Cancer. Cancer Cell Int. 2018, 18, 51. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Ma, Z.; Gong, W.; Yu, L. miR-142 Suppresses Endometrial Cancer Proliferation In Vitro and In Vivo by Targeting Cyclin D1. DNA Cell Biol. 2019, 38, 144–150. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Ti, H.; Zhao, J.; Wang, Y.; Li, T.; Zhang, B. Down-Regulation of miR-145 and miR-143 Might Be Associated with DNA Methyltransferase 3B Overexpression and Worse Prognosis in Endometrioid Carcinomas. Hum. Pathol. 2013, 44, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, D.; Shi, H.; Bian, Y.; Guo, R. MiR-143 Inhibits Endometrial Cancer Cell Proliferation and Metastasis by Targeting MAPK1. Oncotarget 2017, 8, 84384–84395. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Xin, H.; Jiang, J.; Younglai, E.; Sun, S.; Wang, H. Up-Regulation of microRNA-145 Promotes Differentiation by Repressing OCT4 in Human Endometrial Adenocarcinoma Cells. Cancer 2011, 117, 3989–3998. [Google Scholar] [CrossRef]
- Men, Y.; Zhang, L.; Ai, H. MicroRNA-145-5p Over-Expression Suppresses Proliferation, Migration and Invasion and Promotes B of Human Endometrial Cancer Cells by Tar-Geting Dual Specific Phosphatase 6. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 61–66. (In Chinese) [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Li, L.; Su, Y. miR-148b Functions as a Tumor Suppressor by Targeting Endoplasmic Reticulum Metallo Protease 1 in Human Endometrial Cancer Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 27, 81–88. [Google Scholar] [CrossRef]
- Li, B.; Lu, W.; Qu, J.; Ye, L.; Du, G.; Wan, X. Loss of Exosomal miR-148B from Cancer-Associated Fibroblasts Promotes Endometrial Cancer Cell Invasion and Cancer Metastasis. J. Cell. Physiol. 2019, 234, 2943–2953. [Google Scholar] [CrossRef]
- Tsuruta, T.; Kozaki, K.-I.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 Is a Tumor Suppressor microRNA That Is Silenced by DNA Hypermethylation in Endometrial Cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef]
- Widodo; Djati, M.S.; Rifa’I, M. Role of MicroRNAs in Carcinogenesis That Potential for Biomarker of Endometrial Cancer. Ann. Med. Surg. 2016, 7, 9–13. [Google Scholar] [CrossRef]
- Xie, D.; Liang, Y.; Su, Y.; An, Y.; Qu, P. miR-152 Inhibits Proliferation of Human Endometrial Cancer Cells via Inducing G2/M Phase Arrest by Suppressing CDC25B Expression. BioMed. Pharmacother. 2018, 99, 299–305. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, Y.-A.; Choi, J.-J.; Song, T.; Song, S.Y.; Lee, Y.-Y.; Lee, J.-W.; Kim, T.-J.; Kim, B.-G.; Bae, D.-S. Angiotensin II Type I Receptor and miR-155 in Endometrial Cancers: Synergistic Antiproliferative Effects of Anti-miR-155 and Losartan on Endometrial Cancer Cells. Gynecol. Oncol. 2012, 126, 124–131. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wan, X.-H.; Sang, G.-Y.; Zhao, J.-D.; Zhu, Q.-Y.; Wang, D.-M. miR-15a-5p Suppresses Endometrial Cancer Cell Growth via Wnt/β-Catenin Signaling Pathway by Inhibiting WNT3A. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4810–4818. [Google Scholar] [PubMed]
- Devor, E.J.; Miecznikowski, J.; Schickling, B.M.; Gonzalez-Bosquet, J.; Lankes, H.A.; Thaker, P.; Argenta, P.A.; Pearl, M.L.; Zweizig, S.L.; Mannel, R.S.; et al. Dysregulation of miR-181c Expression Influences Recurrence of Endometrial Endometrioid Adenocarcinoma by Modulating NOTCH2 Expression: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 147, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Qu, H.; Cong, J.; Dai, H.; Liu, X. MiR-181c Affects Estrogen-Dependent Endometrial Carcinoma Cell Growth by Targeting PTEN. Endocr. J. 2019, 66, 523–533. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, Y.; Jia, C.; Ren, J.; Wang, J.; Li, T. MicroRNA-182 Promotes Tumor Cell Growth by Targeting Transcription Elongation Factor A-like 7 in Endometrial Carcinoma. Cell. Physiol. Biochem. 2013, 32, 581–590. [Google Scholar] [CrossRef]
- Ruan, H.; Liang, X.; Zhao, W.; Ma, L.; Zhao, Y. The Effects of microRNA-183 Promots Cell Proliferation and Invasion by Targeting MMP-9 in Endometrial Cancer. BioMed. Pharmacother. 2017, 89, 812–818. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, R.; Liu, S.; Lin, Q.; Chen, H.; Jiang, Q. MicroRNA-183 Induces Epithelial-Mesenchymal Transition and Promotes Endometrial Cancer Cell Migration and Invasion in by Targeting CPEB1. J. Cell. Biochem. 2018, 119, 8123–8137. [Google Scholar] [CrossRef]
- Yan, H.; Sun, B.; Zhang, Y.; Li, Y.; Huang, C.; Feng, F.; Li, C. Upregulation of miR-183-5p Is Responsible for the Promotion of Apoptosis and Inhibition of the Epithelial-Mesenchymal Transition, Proliferation, Invasion and Migration of Human Endometrial Cancer Cells by Downregulating Ezrin. Int. J. Mol. Med. 2018, 42, 2469–2480. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Fan, X.; Liu, Y.; Feng, Q. Decreased Expression of miR-184 Restrains the Growth and Invasion of Endometrial Carcinoma Cells through CDC25A-Dependent Notch Signaling Pathway. Am. J. Transl. Res. 2019, 11, 755–764. [Google Scholar]
- Yang, C.; Ota-Kurogi, N.; Ikeda, K.; Okumura, T.; Horie-Inoue, K.; Takeda, S.; Inoue, S. MicroRNA-191 Regulates Endometrial Cancer Cell Growth via TET1-Mediated Epigenetic Modulation of APC. J. Biochem. 2020, 168, 7–14. [Google Scholar] [CrossRef]
- Deng, J.; Wang, W.; Yu, G.; Ma, X. MicroRNA-195 Inhibits Epithelial Mesenchymal Transition by Targeting G Protein Coupled Estrogen Receptor 1 in Endometrial Carcinoma. Mol. Med. Rep. 2019, 20, 4023–4032. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, L.; Yue, Q.; Wang, H.; Wang, X.U.; Li, Y.; Chen, R. MiR-195 Inhibits Migration, Invasion and Epithelial-Mesenchymal Transition (EMT) of Endometrial Carcinoma Cells by Targeting SOX4. J. Biosci. 2019, 44, 146. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, X.; Ruan, L.; Fu, J.; Liu, F.; Qu, J. MiR-200a Promotes Epithelial-Mesenchymal Transition of Endometrial Cancer Cells by Negatively Regulating FOXA2 Expression. Die Pharm. 2017, 72, 694–699. [Google Scholar]
- Dai, Y.; Xia, W.; Song, T.; Su, X.; Li, J.; Li, S.; Chen, Y.; Wang, W.; Ding, H.; Liu, X.; et al. MicroRNA-200b Is Overexpressed in Endometrial Adenocarcinomas and EnhancesMMP2Activity by DownregulatingTIMP2in Human Endometrial Cancer Cell Line HEC-1A Cells. Nucleic Acid Ther. 2013, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-A.; Lee, J.-W.; Choi, J.-J.; Jeon, H.-K.; Cho, Y.; Choi, C.; Kim, T.-J.; Lee, N.W.; Kim, B.-G.; Bae, D.-S. The Interactions between MicroRNA-200c and BRD7 in Endometrial Carcinoma. Gynecol. Oncol. 2012, 124, 125–133. [Google Scholar] [CrossRef]
- Li, F.; Liang, A.; Lv, Y.; Liu, G.; Jiang, A.; Liu, P. MicroRNA-200c Inhibits Epithelial-Mesenchymal Transition by Targeting the BMI-1 Gene Through the Phospho-AKT Pathway in Endometrial Carcinoma Cells In Vitro. Med. Sci. Monit. 2017, 23, 5139–5149. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, M.; Liu, W.; Chen, H.; Cai, T.; Xiong, H.; Sheng, X.; Liu, S.; Peng, J.; Wang, F.; et al. Estrogen Affects the Negative Feedback Loop of PTENP1-miR200c to inhibit PTEN Expression in the Development of Endometrioid Endometrial Carcinoma. Cell Death Dis. 2018, 10, 4. [Google Scholar] [CrossRef]
- Chen, P.; Xing, T.; Wang, Q.; Liu, A.; Liu, H.; Hu, Y.; Ji, Y.; Song, Y.; Wang, D. MicroRNA-202 Inhibits Cell Migration and Invasion through Targeting FGF2 and Inactivating Wnt/β-Catenin Signaling in Endometrial Carcinoma. Biosci. Rep. 2019, 39, 20190680. [Google Scholar] [CrossRef]
- Chung, T.; Lau, T.; Cheung, T.; Yim, S.; Lo, K.; Siu, N.; Chan, L.; Yu, M.; Kwong, J.; Doran, G.; et al. Dysregulation of microRNA-204 Mediates Migration and Invasion of Endometrial Cancer by Regulating FOXC1. Int. J. Cancer 2011, 130, 1036–1045. [Google Scholar] [CrossRef]
- Bao, W.; Wang, H.-H.; Tian, F.-J.; He, X.-Y.; Qiu, M.-T.; Wang, J.-Y.; Zhang, H.-J.; Wang, L.-H.; Wan, X.-P. A TrkB–STAT3–miR-204-5p Regulatory Circuitry Controls Proliferation and Invasion of Endometrial Carcinoma Cells. Mol. Cancer 2013, 12, 155. [Google Scholar] [CrossRef]
- Guo, S.; Yang, J.; Wu, M.; Xiao, G. Clinical Value Screening, Prognostic Significance and Key Pathway Identification of miR-204-5p in Endometrial Carcinoma: A study based on the Cancer Genome Atlas (TCGA), and bioinformatics analysis. Pathol. Res. Pr. 2019, 215, 1003–1011. [Google Scholar] [CrossRef]
- Su, N.; Qiu, H.; Chen, Y.; Yang, T.; Yan, Q.; Wan, X. miR-205 Promotes Tumor Proliferation and Invasion through Targeting ESRRG in Endometrial Carcinoma. Oncol. Rep. 2013, 29, 2297–2302. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, X.; Li, Y.; Zhao, M. MiR-205 Inhibits Cell Apoptosis by Targeting Phosphatase and Tensin Homolog Deleted on Chromosome Ten in Endometrial Cancer Ishikawa Cells. BMC Cancer 2014, 14, 440. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Ha, C.-F.; Bai, Z.-M.; Li, H.-N.; Xiong, Y.; Jiang, J. Overexpression of microRNA-205 Predicts Lymph Node Metastasis and Indicates an Unfavorable Prognosis in Endometrial Cancer. Oncol. Lett. 2016, 12, 4403–4410. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, H. miR-205 Inhibits Cell Growth by Targeting AKT-mTOR Signaling in Progesterone-Resistant Endometrial Cancer Ishikawa Cells. Oncotarget 2017, 8, 28042–28051. [Google Scholar] [CrossRef]
- Jin, C.; Liang, R. miR-205 Promotes Epithelial-Mesenchymal Transition by Targeting AKT Signaling in Endometrial Cancer Cells. J. Obstet. Gynaecol. Res. 2015, 41, 1653–1660. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Wang, C.; Zhang, S.; Wang, Z.; Li, M.; Wang, Y.; Wang, X.; Yang, X. HDAC6, modulated by miR-206, Promotes Endometrial Cancer Progression through the PTEN/AKT/mTOR Pathway. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; He, Y. MicroRNA-21-5p Promotes Epithelial to Mesenchymal Transition by Targeting SRY-box 17 in Endometrial Cancer. Oncol. Rep. 2020, 43, 1897–1905. [Google Scholar] [CrossRef]
- Gao, X.; Cai, Y.; An, R. miR-215 Promotes Epithelial to Mesenchymal Transition and Proliferation by Regulating LEFTY2 in Endometrial Cancer. Int. J. Mol. Med. 2018, 42, 1229–1236. [Google Scholar] [CrossRef]
- Li, X.-C.; Hai, J.-J.; Tan, Y.-J.; Yue, Q.-F.; Liu, L.-J. MiR-218 Suppresses Metastasis and Invasion of Endometrial Cancer via Negatively Regulating ADD2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1408–1417. [Google Scholar]
- Liu, B.; Che, Q.; Qiu, H.; Bao, W.; Chen, X.; Lu, W.; Li, B.; Wan, X. Elevated MiR-222-3p Promotes Proliferation and Invasion of Endometrial Carcinoma via Targeting ERα. PLoS ONE 2014, 9, e87563. [Google Scholar] [CrossRef]
- Huang, K.; Dong, X.; Sui, C.; Hu, D.; Xiong, T.; Liao, S.; Zhang, H. MiR-223 Suppresses Endometrial Carcinoma Cells Prolif-eration by Targeting IGF-1R. Am. J. Transl. Res. 2014, 6, 841–849. [Google Scholar] [PubMed]
- Jiang, F.-Z.; He, Y.-Y.; Wang, H.-H.; Zhang, H.-L.; Zhang, J.; Yan, X.-F.; Wang, X.-J.; Che, Q.; Ke, J.-Q.; Chen, Z.; et al. Mutant p53 Induces EZH2 Expression and Promotes Epithelial-Mesenchymal Transition by Disrupting p68-Drosha Complex Assembly and Attenuating MiR-26a Processing. Oncotarget 2015, 6, 44660–44674. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS Serum-Derived Exosomal miR-27a-5p Stimulates Endometrial Cancer Cells Migration and Invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, J.; Yu, T.; You, S.; Zhang, Y.; Hu, L. miR-27b-3p/MARCH7 Regulates Invasion and Metastasis of Endometrial Cancer Cells through Snail-Mediated Pathway. Acta Biochim. Biophys. Sin. 2019, 51, 492–500. [Google Scholar] [CrossRef]
- Jiang, T.; Sui, D.; You, D.; Yao, S.; Zhang, L.; Wang, Y.; Zhao, J.; Zhang, Y. MiR-29a-5p Inhibits Proliferation and Invasion and Induces Apoptosis in Endometrial Carcinoma via Targeting TPX2. Cell Cycle 2018, 17, 1268–1278. [Google Scholar] [CrossRef]
- Kong, J.; He, X.; Wang, Y.; Li, J. Effect of microRNA-29b on Proliferation, Migration, and Invasion of Endometrial Cancer Cells. J. Int. Med. Res. 2019, 47, 3803–3817. [Google Scholar] [CrossRef]
- Chen, H.-X.; Xu, X.-X.; Tan, B.-Z.; Zhang, Z.; Zhou, X.-D. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell. Physiol. Biochem. 2017, 41, 933–946. [Google Scholar] [CrossRef]
- Li, L.; Shou, H.; Wang, Q.; Liu, S. Investigation of the Potential Theranostic Role of KDM5B/miR-29c Signaling Axis in Paclitaxel Resistant Endometrial Carcinoma. Gene 2019, 694, 76–82. [Google Scholar] [CrossRef]
- Ma, J.; Li, D.; Kong, F.-F.; Yang, D.; Yang, H.; Ma, X.-X. miR-302a-5p/367-3p-HMGA2 Axis Regulates Malignant Processes during Endometrial Cancer Development. J. Exp. Clin. Cancer Res. 2018, 37, 19. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, H.; Xu, X.; Xun, Q.; Yu, D.; Ling, J.; Guo, F.; Yan, Y.; Shi, J. microRNA-30c Negatively Regulates Endometrial Cancer Cells by Targeting Metastasis-Associated Gene-1. Oncol. Rep. 2011, 27, 807–812. [Google Scholar] [CrossRef]
- Kong, X.; Xu, X.; Yan, Y.; Guo, F.; Li, J.; Hu, Y.; Zhou, H.; Xun, Q. Estrogen Regulates the Tumour Suppressor MiRNA-30c and Its Target Gene, MTA-1, in Endometrial Cancer. PLoS ONE 2014, 9, e90810. [Google Scholar] [CrossRef]
- Xu, X.; Kong, X.; Liu, T.; Zhou, L.; Wu, J.; Fu, J.; Wang, Y.; Zhu, M.; Yao, S.; Ding, Y.; et al. Metastasis-Associated Protein 1, Modulated by miR-30c, Promotes Endometrial Cancer Progression through AKT/mTOR/4E-BP1 Pathway. Gynecol. Oncol. 2019, 154, 207–217. [Google Scholar] [CrossRef]
- Shu, S.; Liu, X.; Xu, M.; Gao, X.; Chen, S.; Zhang, L.; Li, R. MicroRNA-320a Acts as a Tumor Suppressor in Endometrial Carcinoma by Targeting IGF-1R. Int. J. Mol. Med. 2019, 43, 1505–1512. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Li, Y.; Yu, X.; Sun, Q.; Li, A.; Kong, Y. eIF4E-Related miR-320a and miR-340-5p Inhibit Endometrial Carcinoma Cell Metastatic Capability by Preventing TGF-β1-Induced Epithelial Mesenchymal Transition. Oncol. Rep. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Xu, N.; Wang, M.-J.; Liu, Q. miR-326 Regulates EMT and Metastasis of Endometrial Cancer through Targeting TWIST1. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3787–3793. [Google Scholar]
- Gao, Y.; Qian, H.; Tang, X.; Du, X.; Wang, G.; Zhang, H.; Ye, F.; Liu, T. Superparamagnetic Iron Oxide Nanoparticle-Mediated Expression of miR-326 Inhibits Human Endometrial Carcinoma Stem Cell Growth. Int. J. NanoMed. 2019, ume 14, 2719–2731. [Google Scholar] [CrossRef]
- Dou, X.; Chen, X.; Zhou, Q.; Wen, M.; Zhang, S.; Zhang, S. miR-335 Modulates Numb Alternative Splicing via Targeting RBM10 in Endometrial Cancer. Kaohsiung J. Med. Sci. 2020, 36, 171–177. [Google Scholar] [CrossRef]
- Xie, W.; Qin, W.; Kang, Y.; Zhou, Z.; Qin, A. MicroRNA-340 Inhibits Tumor Cell Proliferation and Induces Apoptosis in Endometrial Carcinoma Cell Line RL 95-2. Med. Sci. Monit. 2016, 22, 1540–1546. [Google Scholar] [CrossRef]
- Schirmer, U.; Doberstein, K.; Rupp, A.-K.; Bretz, N.P.; Wuttig, D.; Kiefel, H.; Breunig, C.; Fiegl, H.; Müller-Holzner, E.; Zeillinger, R.; et al. Role of miR-34a as a Suppressor of L1CAM in Endometrial Carcinoma. Oncotarget 2014, 5, 462–472. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, K.E. The Significance of miR-34a Expression in Endometrial Carcinogenesis: Correlation With Expression of p16 and Ki-67 Proteins in Endometrial Cancers. J. Cancer Prev. 2015, 20, 268–274. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Wang, W.; Huang, K.; Wang, Y.; Li, J.; Yang, X. MicroRNA-34a Inhibits Cells Proliferation and Invasion by Downregulating Notch1 in Endometrial Cancer. Oncotarget 2017, 8, 111258–111270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Meng, W.; Zeng, J.; Hu, H.; Lu, L. MiR-34c Oligonucleotide Enhances Chemosensitivity of Ishikawa Cell to Cisplatin by Inducing Apoptosis. Cell Biol. Int. 2013, 37, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, H.; Huang, Y.; Zhang, Q.; Xue, J.; Liu, Z.; Zheng, F. miR-34c Plays a Role of Tumor Suppressor in HEC-1-B Cells by Targeting E2F3 Protein. Oncol. Rep. 2015, 33, 3069–3074. [Google Scholar] [CrossRef]
- Liu, B.-L.; Sun, K.-X.; Zong, Z.-H.; Chen, S.; Zhao, Y. MicroRNA-372 Inhibits Endometrial Carcinoma Development by Targeting the Expression of the Ras Homolog Gene Family Member C (RhoC). Oncotarget 2016, 7, 6649–6664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, D.; Gao, J.; Shi, Z.; Chi, P.; Meng, Y.; Zou, C.; Wang, Y. MicroRNA-373 Promotes the Development of Endometrial Cancer by Targeting LATS2 and activating the Wnt/β-Catenin Pathway. J. Cell. Biochem. 2019, 120, 8611–8618. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wang, F.; Wan, J. MicroRNA-381 Inhibits Cell Proliferation and Invasion in Endometrial Carcinoma by Targeting the IGF-1R. Mol. Med. Rep. 2017, 17, 4090–4098. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Wu, L. MicroRNA-409 May Function as a Tumor Suppressor in Endometrial Carcinoma Cells by Targeting Smad2. Mol. Med. Rep. 2018, 19, 622–628. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, X.-M.; Li, Q.-H.; Wang, X.-Y.; Li, L.; Xu, M.; Dong, M.; Xiao, Y.-B. MicroRNA-424 May Function as a Tumor Suppressor in Endometrial Carcinoma Cells by Targeting E2F7. Oncol. Rep. 2015, 33, 2354–2360. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, Z.; Wang, W. MicroRNA-424 Suppresses Estradiol-Induced Cell Proliferation via Targeting GPER in Endometrial Cancer Cells. Cell. Mol. Biol. 2015, 61, 96–101. [Google Scholar]
- Shu, S.; Liu, X.; Xu, M.; Gao, X.; Fan, J.; Liu, H.; Li, R. MicroRNA-424 Regulates Epithelial-Mesenchymal Transition of Endometrial Carcinoma by Directly Targeting Insulin-like Growth Factor 1 Receptor. J. Cell. Biochem. 2019, 120, 2171–2179. [Google Scholar] [CrossRef]
- Lu, K.; Lin, J.; Jiang, J. Osthole Inhibited Cell Proliferation and Induced Cell Apoptosis through Decreasing CPEB2 Expression via Up-regulating miR-424 in Endometrial Carcinoma. J. Recept. Signal Transduct. 2020, 40, 89–96. [Google Scholar] [CrossRef]
- Ye, W.; Xue, J.; Zhang, Q.; Li, F.; Zhang, W.; Chen, H.; Huang, Y.; Zheng, F. MiR-449a Functions as a Tumor Suppressor in Endometrial Cancer by Targeting CDC25A. Oncol. Rep. 2014, 32, 1193–1199. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, A.-Y.; Xu, C.; Song, K.-Q.; Wang, W.-J.; Yin, X.; Di, W.; Hong, Z.-B.; Qiu, L.-H. MicroRNA-449a Inhibits Tumor Metastasis through AKT/ERK1/2 Inactivation by Targeting Steroid Receptor Coactivator (SRC) in Endometrial Cancer. J. Cancer 2019, 10, 547–555. [Google Scholar] [CrossRef]
- Wu, A.-Y.; Hu, Y.; Cang, W.; Li, D.; Wang, W.-J.; Tian, Q.; Gu, L.-Y.; Zhang, N.; Ji, F.; Qiu, L.-H. Suppressive effect of microRNA-449a on the NDRG1/PTEN/AKT Axis Regulates Endometrial Cancer Growth and Metastasis. Exp. Cell Res. 2019, 382, 111468. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Wang, T.; Zhu, W.; Zhou, X. miR-494-3p Promotes the Progression of Endometrial Cancer by Regulating the PTEN/PI3K/AKT pathway. Mol. Med. Rep. 2018, 19, 581–588. [Google Scholar] [CrossRef]
- Tan, A.; Luo, R.; Ruan, P. miR-495 Promotes Apoptosis and Inhibits Proliferation in Endometrial Cells via Targeting PIK3R1. Pathol. Res. Pr. 2019, 215, 594–599. [Google Scholar] [CrossRef]
- Chen, S.; Sun, K.-X.; Liu, B.-L.; Zong, Z.-H.; Zhao, Y. MicroRNA-505 Functions as a Tumor Suppressor in Endometrial Cancer by Targeting TGF-α. Mol. Cancer 2016, 15, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Y.; Zhang, X.; Xiao, N.; Jiang, T.; Zhu, S.; Wang, E.; Chen, C. miR-522 Facilitates the Prosperities of Endometrial Carcinoma Cells by Directly Binding to Monoamine Oxidase B. Kaohsiung J. Med. Sci. 2019, 35, 598–606. [Google Scholar] [CrossRef]
- Bing, L.; Hong, C.; Li-Xin, S.; Wei, G. MicroRNA-543 Suppresses Endometrial Cancer Oncogenicity via Targeting FAK and TWIST1 Expression. Arch. Gynecol. Obstet. 2014, 290, 533–541. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Liu, Y. Targeting Thyroid Receptor Interacting Protein 6 by MicroRNA-589-5p Inhibits Cell Proliferation, Migration, and Invasion in Endometrial Carcinoma. Cancer Biother. Radiopharm 2019, 34, 529–536. [Google Scholar] [CrossRef]
- Sun, X.; Dongol, S.; Qiu, C.; Xu, Y.; Sun, C.; Zhang, Z.; Yang, X.; Zhang, Q.; Kong, B. miR-652 Promotes Tumor Proliferation and Metastasis by Targeting RORA in Endometrial Cancer. Mol. Cancer Res. 2018, 16, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Sun, K.-X.; Xiu, Y.-L.; Liu, B.-L.; Feng, M.-X.; Sang, X.-B.; Zhao, Y. MicroRNA-93 Promotes Epithelial–Mesenchymal Transition of Endometrial Carcinoma Cells. PLoS ONE 2016, 11, e0165776. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Deng, K.; Liu, X.; Dai, M.; Chen, X.; Chen, J.; Chen, J.; Huang, Y.; Dai, S.; Chen, J. Molecular Mechanism and Role of microRNA-93 in Human Cancers: A Study Based on Bioinformatics Analysis, Meta-Analysis, and Quantitative Polymerase Chain Reaction Validation. J. Cell. Biochem. 2019, 120, 6370–6383. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-B. MicroRNA-93-5p/IFNAR1 Axis Accelerates Metastasis of Endometrial Carcinoma by Activating the STAT3 Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5657–5666. [Google Scholar] [PubMed]
- Zhou, Z.; Xu, Y.-P.; Wang, L.-J.; Kong, Y. miR-940 Potentially Promotes Proliferation and Metastasis of Endometrial Carcinoma through Regulation of MRVI1. Biosci. Rep. 2019, 39, 20190077. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, H.; Meng, Y.; Kuang, Y. miR-944 Acts as a Prognostic Marker and Promotes the Tumor Progression in Endometrial Cancer. BioMed. Pharmacother. 2017, 88, 902–910. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhang, X.; Lin, Y.; Luo, T.; Xiao, Z.; Zhou, Q. A dual PI3K/AKT/mTOR Signaling Inhibitor miR-99a Suppresses Endometrial Carcinoma. Am. J. Transl. Res. 2016, 8, 719–731. [Google Scholar]
- Ala, U. Competing Endogenous RNAs, Non-Coding RNAs and Diseases: An Intertwined Story. Cells 2020, 9, 1574. [Google Scholar] [CrossRef]
- Lou, W.; Ding, B.; Fu, P. Pseudogene-Derived lncRNAs and Their miRNA Sponging Mechanism in Human Cancer. Front. Cell Dev. Biol. 2020, 8, 85. [Google Scholar] [CrossRef]
- Kumar, S.; Cheng, X.; Klimasauskas, S.; Sha, M.; Posfai, J.; Roberts, R.J.; Wilson, G.G. The DNA (cytosine-5) Methyltransferases. Nucleic Acids Res. 1994, 22, 1–10. [Google Scholar] [CrossRef]
- Deaton, A.M.; Bird, A. CpG Islands and the Regulation of Transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Wu, C.; Cui, W.; Wang, L. DNA Methyltransferases in Cancer: Biology, Paradox, Aberrations, and Targeted Therapy. Cancers 2020, 12, 2123. [Google Scholar] [CrossRef]
- Ghabreau, L.; Roux, J.P.; Niveleau, A.; Mokni, M.; Frappart, L. Correlation between the DNA Global Methylation Status and Progesterone Receptor Expression in Normal Endometrium, Endometrioid Adenocarcinoma and Precursors. Virchows Arch. 2004, 445, 129–134. [Google Scholar] [CrossRef]
- Berger, S.L. The Complex Language of Chromatin Regulation during Transcription. Nat. Cell Biol. 2007, 447, 407–412. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Flaus, A.; Downs, J.A.; Owen-Hughes, T. Histone Isoforms and the Oncohistone Code. Curr. Opin. Genet. Dev. 2021, 67, 61–66. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Z.; Zhao, H.; Bao, S.; Sun, J. A Novel lncRNA-Focus Expression Signature for Survival Prediction in Endometrial Carcinoma. BMC Cancer 2018, 18, 39. [Google Scholar] [CrossRef]
- Ouyang, D.; Li, R.; Li, Y.; Zhu, X. A 7-lncRNA Signature Predict Prognosis of Uterine Corpus Endometrial Carcinoma. J. Cell. Biochem. 2019, 120, 18465–18477. [Google Scholar] [CrossRef]
- Tang, H.; Wu, Z.; Zhang, Y.; Xia, T.; Liu, D.; Cai, J.; Ye, Q. Identification and Function Analysis of a Five-Long Noncoding RNA Prognostic Signature for Endometrial Cancer Patients. DNA Cell Biol. 2019, 38, 1480–1498. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, Z.; Shen, X.; Tian, X.; Ye, Q. Identification of a Multi-RNA-Type-Based Signature for Recurrence-Free Survival Prediction in Patients with Uterine Corpus Endometrial Carcinoma. DNA Cell Biol. 2020, 39, 615–630. [Google Scholar] [CrossRef]
- Zeng, Z.; Cheng, J.; Ye, Q.; Zhang, Y.; Shen, X.; Cai, J.; Li, M. A 14-Methylation-Driven Differentially Expressed RNA as a Signature for Overall Survival Prediction in Patients with Uterine Corpus Endometrial Carcinoma. DNA Cell Biol. 2020, 39, 975–991. [Google Scholar] [CrossRef]
- Ahsen, M.E.; Boren, T.P.; Singh, N.K.; Misganaw, B.; Mutch, D.G.; Moore, K.N.; Backes, F.J.; McCourt, C.K.; Lea, J.S.; Miller, D.S.; et al. Sparse Feature Selection for Classification and Prediction of Metastasis in Endometrial Cancer. BMC Genom. 2017, 18, 233. [Google Scholar] [CrossRef]
- Rai, R.; Fatima, I.; Essel, K.; Chandra, V. Molecular Diagnosis of Uterine Cancer. In Molecular Diagnostics in Cancer Patients; Shukla, K.K., Sharma, P., Misra, S., Eds.; Springer: Singapore, 2019; pp. 305–321. ISBN 9789811358760. [Google Scholar]
- Gulìa, C.; Signore, F.; Gaffi, M.; Gigli, S.; Votino, R.; Nucciotti, R.; Bertacca, L.; Zaami, S.; Baffa, A.; Santini, E.; et al. Y RNA: An Overview of Their Role as Potential Biomarkers and Molecular Targets in Human Cancers. Cancers 2020, 12, 1238. [Google Scholar] [CrossRef]
| Long non Coding RNA Name | Expression Level | Described Functions | miR Interactions | Other Functional Interactions in EC | References |
|---|---|---|---|---|---|
| ABHD11-AS1 | up | a, b | cyclin D1, CDK1, CDK2, CDK4, Bcl-xl, VEGFA,p16 | [33] | |
| AL161431.1 | up | a | miR-1252-5p | MAPK | [34] |
| ASlnc04080 | up | a, b | at least 19 genes | [35] | |
| BANCR | up | b | MMP1/2, ERK, MAPK | [36] | |
| C2orf48 | up | n/a | miR-183 | CCNB1 | [37] |
| CARLo-5 | up | a, c | CDK, MMP2/9, | [38] | |
| CCAT1 | up | n/a | miR-181a-5p | [39,40] | |
| CCAT2 | up | a, c | miR-216b | Bcl-2 | [41] |
| CDKN2B-AS | up | d | miR-125a-5p | Bcl-2, MRP4 | [42] |
| CHL1-AS1 | up | n/a | miR-6076 | CHL1 | [43] |
| circ_0002577 | up | a, b, e | miR-197 | CTNND1 | [44,45] |
| circ_0061140 | up/down | a | miR-149-5p | STAT3 | [46] |
| DANCR | up | b | miR-214 | [47] | |
| DCST1-AS1 | down | c | miR-92a-3p | Notch1 | [48] |
| DLEU1 | up | b, e | miR-490 | Bcl-2, BAX, E-cadherin, N-cadherin, Snail, vimentin, CASP-3, SP1, PI3K, AKT1, p70S6K, rpS6, GSK3B, STAT3, Bcl-xl, mTOR | [49,50] |
| FER1L4 | down | c | PTEN, AKT | [51,52] | |
| FRMD6-AS2 | down | a, c | FRMD6 | [53] | |
| GAS5 | down | b | miR-103, miR-222-3p | p27, PTEN | [54,55] |
| H19 | up | a, b, c, e | miR-20b-5p, miR-124-3p, miR-612 | HIF-1α, AXL, PCNA, Snail, HOXA10, E-cadherin, ITGB3 | [56,57,58,59,60,61] |
| HAND2-AS1 | down | c | NMU | [62] | |
| HOTAIR | up/down | b, f | miR-646 | PRB, NPM1, Beclin-1, MDR, P-gp, PTEN, PI3K | [63,64,65,66,67,68] |
| HOTAIRM1 | up | a, c, e | HOXA1 | [69] | |
| HOXB-AS1 | up | a, c | miR-149-3p | Wnt10b, β-catenin, cyclin D1, c-Myc | [70] |
| LA16c-313D11.11 | down | a, c | miR-205-5p | PTEN, PI3K | [71] |
| LINC00261 | down | a, c | miR-27a, miR-96, miR-153, miR-182, miR-183 | FOXO1 | [72] |
| LINC00483 | up | b | miR-183, miR-192 | CCNB1, GRHL1 | [37] |
| LINC00672 | down | d | p53, LASP1 | [73] | |
| LINC00958 | up | c | miR-761 | DOLPP1 | [74] |
| LINC01016 | up | n/a | miR-302a-3p, miR-3130-3p | NFYA, SATB1 | [75] |
| LINC01170 | up | b | AKT | [76] | |
| LINC01220 | up | a, b | MAPK11 | [77] | |
| LINC01410 | up | a, b | miR-23c | CHD7 | [78] |
| LINC-ROR | up | n/a | miR-145 | [79] | |
| LINP1 | up | a, c | PI3K, AKT | [80] | |
| lnc-NA | down | a, b | NR4A1 | [81] | |
| lnc-OC1 | up | b | miR-34a | PD-L1 | [82] |
| lncRNA-ATB | up | a, b, e | miR-126 | CASP-3, Sox2, TGF-b, PIK3R2 | [83] |
| lncRNA-HEIH | up | a, d | MAPK | [84] | |
| lnc-XLEC1 | down | n/a | MBP-1 | [85] | |
| LOC134466 | down | b | miR-196a-5p | TAC1 | [86] |
| LOXL1-AS1 | up | a, b | miR-28-5p | RAP1B | [87] |
| MALAT1 | down | c, e | miR-200c | TGF-B | [88] |
| MEG3 | down | a, f | PI3K, MEG3, Notch1, Hes1 | [89,90] | |
| miR143HG | down | b | miR-125a | p53 | [91] |
| MIR22HG | down | a, b | miR-141-3p | DAPK1 | [92] |
| NEAT1 | up | a | miR-361, miR-144-3p, miR-146b-5p | MEF2D, ROCK1, WNT7A, VEGFA, PDE4B, EZH2, STAT3, KPNA4, LEF1, MMP9, c-Myc | [93,94,95] |
| NIFK-AS1 | down | a | miR-146a | [96] | |
| NR2F1-AS1 | up | a, b, c | miR-363 | SOX4, PI3K, AKT | [97] |
| PCAT1 | up | a, b, c, e | E-cadherin, EZH2, Bcl-2, vimentin, N-cadherin, Bad | [98,99] | |
| PCGEM1 | up | a, c, b | miR-129-5p | STAT3, Bcl-2, survivin, VEGFA, MMP2 | [100] |
| PVT1 | up/down | a, b | miR-195-5p | UPF1, FGFR1, FGF2 | [101,102] |
| RNA-14327.1 | up | a, e | Kca3.1 | [103] | |
| RP11-357H14.17 | up | n/a | miR-24-1-5p, miR-27b, miR-143, miR-204, miR-503, miR-4770 | up to 183 targets | [104] |
| RP11-395G23.3 | down | a, c | miR-205-5p | PTEN, AKT | [105] |
| RP11-89K21.1 | up | n/a | miR-27b, miR-4770, miR-143, miR-204, miR-125a-5p, miR-125b-5p, miR-139-5p, miR-670-3p | up to 183 targets | [104] |
| SNHG16 | up | a | miR-490-3p | HK2 | [106] |
| SNHG5 | down | a, c | miR-25-3p | BTG2 | [107] |
| SNHG8 | up | a | miR-152 | c-MET | [108] |
| SRA | up | a, b, e | EIF4E-BP1, Wnt, β-catenin | [109] | |
| TDRG1 | up | a, b, c | VEGFA, AKT, PI3K, mTOR | [110,111] | |
| TUG1 | up | n/a | miR-34a-5p, miR-299 | [112] | |
| TUSC7 | down | a, e | miR-23b, miR-616 | SOCS4 | [113,114] |
| ZNRD1-AS1 | up | n/a | ZNRD1 | [115] |
| miR Name | Expression Level | Described Functions | Primary Targets | Secondary Targets | References |
|---|---|---|---|---|---|
| miR-101 | down | a, b, c, f | EZH2, MCL-1, FOS, mTOR, COX-2 | VEGF-A, TSP-1, COX-2, PGE2, P450arom | [116,117,118] |
| miR-101-3p | down | f | EZH2 | [119] | |
| miR-103 | up | a | ZO-1 | [120] | |
| miR-106a | up | a, b, c | MYC, BCL2L11 | p21, BIM | [121,122] |
| miR-106b | up/down | a, b | PTEN | AKT, mTOR | [123,124] |
| miR-107-5p | up | a, c | ERα | [125] | |
| miR-10b | up | a, b, c | HOXB3 | [126] | |
| miR-1271 | down | a, c, b | CDK1, LDHA | [127,128] | |
| miR-130b | down | e | ZEB1 | [129] | |
| miR-134 | down | a, c | POGLUT1, Notch | [130] | |
| miR-137 | down | a | EZH2, LSD1 | [131] | |
| miR-139-5p | down | a, c | HOXA10 | [132] | |
| miR-142 | down | a | CCND1 | Ki67 | [133] |
| miR-143 | down | a, c, f | DNMT3B, MAPK1 | [134,135] | |
| miR-145 | down | f | DNMT3B, OCT4 | [134,136] | |
| miR-145-5p | down | a, c, b | DUSP6 | [137] | |
| miR-148b | down | a, c, e, f | ERMP1, DNMT1 | HIF-1, Nrf2 | [138,139] |
| miR-152 | down | a, b, f | DNMT1, E2F3, MET, Rictor, SOS2, NRAS, APC, PIK3R3, SOS1, PTEN, CDC25B | [140,141,142] | |
| miR-155 | up | a | AGTR1 | [143] | |
| miR-15a-5p | down | a | WNT3A | [144] | |
| miR-181c | down | b | PTEN, NOTCH2 | Bax, Bcl-2, AKT, p53, Cyclin D. | [145,146] |
| miR-181d | up | a, b, f | PIK3R3, SOS1, PTEN | [141] | |
| miR-182 | up | a | TCEAL7 | c-Myc, cyclin D1, NFκB | [147] |
| miR-183 | up | a, b, c, e | MMP9, CPEB1 | E-cadherin, vimentin | [148,149] |
| miR-183-5p | down | a, b, c, e | Ezrin | [150] | |
| miR-184 | down | c | CDC25A | NOTCH1/2/3/4, HES1 | [151] |
| miR-191 | up | a | TET1 | [152] | |
| miR-195 | down | c, e | SOX4, GPER | TIMP-2, MMP2/9, PI3K, AKT | [153,154] |
| miR-200a | up | e | FOXA2 | E-cadherin, vimentin | [155] |
| miR-200b | up | c | TIMP2 | MMP2 | [156] |
| miR-200c | up | a, c, e | BRD7, BMI-1, PTEN, PTENP1 | β-catenin, cyclinD1, c-myc, AKT, Slug, N-cadherin, PI3K, E-cadherin | [157,158,159] |
| miR-202 | down | c, e | FGF2 | β-catenin, N-cadherin, vimentin, E-cadherin | [160] |
| miR-204 | down | a, c | FOXC1 | [161] | |
| miR204-5p | down | a, c | TrkB, SF3B1, FBXW7, BRD4 | [162,163] | |
| miR-205 | up | a, b, c, e | ESRRG, PTEN, AKT | E-cadherin, Snail | [164,165,166,167,168] |
| miR-206 | down | a, c | HDAC6 | PTEN, AKT | [169] |
| miR-21-5p | up | e | SOX17 | [170] | |
| miR-215 | up | a, c, d, e | LEFTY2 | [171] | |
| miR-218 | down | c | ADD2 | [172] | |
| miR-222-3p | up | a, c, d | ERα | [173] | |
| miR-223 | down | a | IGF-1R | [174] | |
| miR-25 | up | a, b | p21, BIM | [121] | |
| miR-26a | down | e | EZH2 | N-cadherin, Vimentin, Snail, E-cadherin | [175] |
| miR-27a-5p | up | c | SMAD4 | [176] | |
| miR-27b-3p | down | c | MARCH7 | Snail, Vimentin, E-cadherin | [177] |
| miR-29a-5p | down | a, c, b | TPX2 | [178] | |
| miR-29b | down | a, c, d | PTEN | BAX, Bcl-2, AKT | [179] |
| miR-29b | down | f | VEGFA | MAPK, PI3K, mTOR, Bcl-2 | [180] |
| miR-29c-3p | down | d | KDM5B | [181] | |
| miR-301b | down | e | ZEB1 | [129] | |
| miR-302a-5p | down | c | HMGA2 | [182] | |
| miR-30c | down | a, c | MTA1 | mTOR, 4E-BP1, AKT | [183,184,185] |
| miR-320a | down | a, c, e | eIF4E, IGF-1R | MMP3, MMP9, TGF-β1 | [186,187] |
| miR-326 | down | a, c, e, f | GPR91, TWIST1 | STAT3, VEGF | [188,189] |
| miR-335 | up | a | RBM10 | Numb-L | [190] |
| miR-340 | down | a, b | p27, KIP1, Bax, Casp3 | [191] | |
| miR-340-5p | down | c, e | eIF4E | MMP3, MMP9, TGF-β1 | [186] |
| miR-34a | down | a, c, e | L1CAM, p16, Ki-67, Notch1 | [192,193,194] | |
| miR-34c | down | a, b, c, d | E2F3 | [195,196] | |
| miR-367-3p | down | c | HMGA2 | [182] | |
| miR-372 | down | a, c | RhoC, Cyclin A1, CDK2 | MMP2, MMP9, PARP, Bax | [197] |
| miR-373 | up | a, c, e | LATS2 | Wnt | [198] |
| miR-381 | down | a, c | IGF-1R | AKT, ERK | [199] |
| miR-409 | down | a | Smad2 | [200] | |
| miR-424 | down | a, b, e | E2F7, GPER, IGF-1R, CPEB2 | PI3K, AKT, E-cadherin, vimentin | [201,202,203,204] |
| miR-449a | down | a, b, c | CDC25A, NDRG1, SRC | PTEN, AKT, ERK1/2 | [205,206,207] |
| miR-494-3p | up | a, c | PTEN | PI3K, AKT | [208] |
| miR-495 | down | a, b, f | GSK3B, NRAS, TCF4, PIK3CB, PIK3R3, CCND1, AXIN2, PIK3R1, SOS1, PIK3CA, FOXO3, PTEN | Bcl-2, VEGF, Bax, CASP-3 | [141,209] |
| miR-505 | down | a | TGF-α | MMP2, MMP9, CDK2, Bax, PARP | [210] |
| miR-522 | up | a, c | MAOB | [211] | |
| miR-543 | down | a, c | FAK, TWIST1 | [212] | |
| miR-589-5p | down | a, c | TRIP6 | E-cadherin, N-cadherin, vimentin | [213] |
| miR-652 | up | a, c | RORA | β-catenin | [214] |
| miR-93 | up | a, b, e, f | p21, BIM, FOXA1 | E-cadherin, N-cadherin, MAPK1, RBBP7, Smad7 | [121,215,216] |
| miR-93-5p | up | a, c | IFNR1 | STAT3, MMP9 | [217] |
| miR-940 | up | a, c | MRVI1 | [218] | |
| miR-944 | up | a | CADM2 | [219] | |
| miR-99a | down | a, b, c | AKT1, mTOR | [220] |
| Gene (TGCA/PORTEC4a Classifications) | Interacting lncRNA | Interacting sncRNA |
|---|---|---|
| CTNNB1 (β catenin) | HOXB-AS1, SRA | miR-200c, miR-202, miR-652 |
| FBXW7 | n/a | miR-204-5p |
| L1CAM | n/a | miR-34a |
| PIK3CA | n/a | miR-495 |
| PIK3R1 | n/a | miR-495 |
| PTEN | FER1L4, GAS5, HOTAIR, LA16c 313D11.11, RP11-395G23.3 | mir-106b, miR-152, miR-181c, miR-181d, miR-200c, miR-205, miR-29b, miR-494-3p, miR-495 |
| TP53 | LINC00672, miR143HG | n/a |
| ARID1A, KRAS, MLH1, MLH2, MLH6, PMS2, POLE, PPP2R1A | unknown | unknown |
| Target | Purpose | Expected Analysis Output | Candidate Genes | References |
|---|---|---|---|---|
| Coding gene | Finding gene mutations | Sequence mutation(s) | PTEN, VEGF, TP53, FGF, PIK3CA, Ki-67, β-Catenin, EGFR, RAS-RAF-MEK-ERK pathway, p21, p16, ERBB2, E-Cadherin, ER, PR, Cox-2 | [237] |
| lncRNA (a) | EC marker and identification of potential target genes | Up-/down-regulation | See Table 1 (62 lncRNA) | See Table 1 |
| sncRNA (a) | EC marker and identification of potential target genes | Up-/down-regulation | See Table 1 and Table 2 (127 miR) | See Table 2 |
| lncRNA (b) | EC marker | Up-/down-regulation | ENSG00000260684, ENSG00000229589, ENSG00000224037, ENSG00000235499, ENSG00000224905, ENSG00000260992, ENSG00000248008, ENSG00000234945, ENSG00000182648, ENSG00000253636, ENSG00000233760 | [231] |
| sncRNA (b) | EC marker | Up-/down-regulation | Several tens | [12,26,27,31,236,237] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. https://doi.org/10.3390/ijms22063151
Piergentili R, Zaami S, Cavaliere AF, Signore F, Scambia G, Mattei A, Marinelli E, Gulia C, Perelli F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. International Journal of Molecular Sciences. 2021; 22(6):3151. https://doi.org/10.3390/ijms22063151
Chicago/Turabian StylePiergentili, Roberto, Simona Zaami, Anna Franca Cavaliere, Fabrizio Signore, Giovanni Scambia, Alberto Mattei, Enrico Marinelli, Caterina Gulia, and Federica Perelli. 2021. "Non-Coding RNAs as Prognostic Markers for Endometrial Cancer" International Journal of Molecular Sciences 22, no. 6: 3151. https://doi.org/10.3390/ijms22063151
APA StylePiergentili, R., Zaami, S., Cavaliere, A. F., Signore, F., Scambia, G., Mattei, A., Marinelli, E., Gulia, C., & Perelli, F. (2021). Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. International Journal of Molecular Sciences, 22(6), 3151. https://doi.org/10.3390/ijms22063151











