Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities
Abstract
1. Introduction
1.1. Differentiated Thyroid Cancer
1.2. Anaplastic Thyroid Cancer
1.3. Medullary Thyroid Cancer
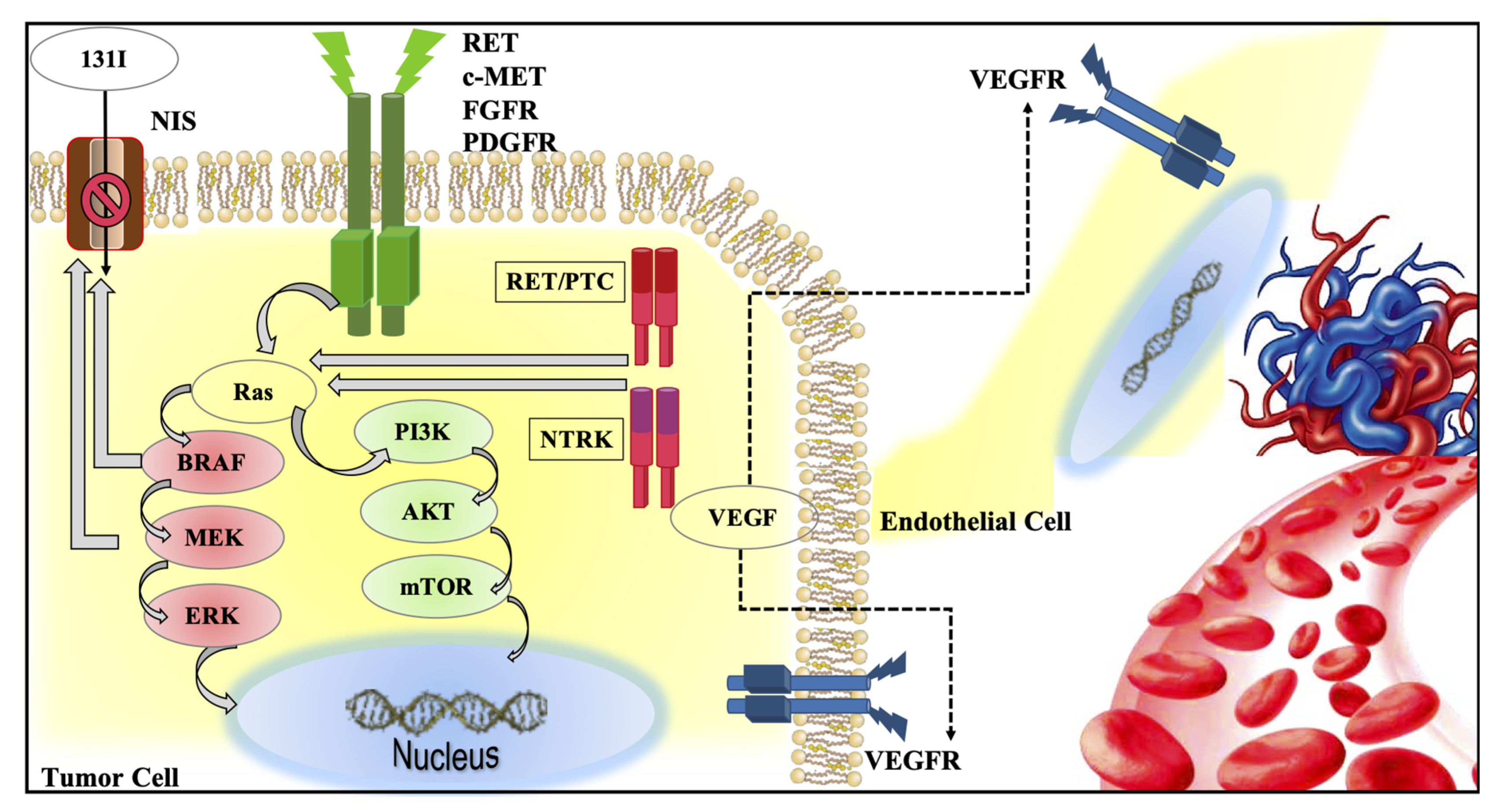
2. Intracellular Signaling Pathways in Thyroid Cancer
2.1. MAPK and PI3K Pathways
2.2. Other Genetic Alteration in Thyroid Cancer
2.3. Immune Microenvironment
3. Current Available Systemic Therapies for the Treatment of Advanced and Multimetastatic Thyroid Cancer
3.1. Systemic Treatment in DTC and PDTC: Multikinase Inhibitor Approved Drugs
3.2. Systemic Treatment in MTC: Multikinase Inhibitor Approved Drugs
3.3. Systemic Treatment in DTC, PDTC, and ATC: Specific Inhibitors of NTRK-Fusions
3.4. Systemic Treatment in DTC, PDTC, ATC, and MTC: Specific Inhibitors of RET Oncogene Alterations
3.5. Systemic Treatment in DTC, PDTC, and ATC: Specific Inhibitors of BRAFV600E Mutation
4. Drugs under Evaluation
4.1. Ongoing Studies on Follicular-Derived TC
4.2. Redifferentiation Treatment in DTC
4.3. Ongoing Studies on MTC
4.4. Immunotherapy: The Present and the Future
5. Open Issues
6. Conclusions
Funding
Conflicts of Interest
References
- Institute, N.C. Surveillance Epidemiology and End Results Program: SEER Stat Facts: Thyroid Cancer. Available online: https://seer.cancer.gov/statfacts/html/thyro.html (accessed on 16 March 2021).
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, Q.; Davies, L. Differentiated Thyroid Cancer Incidence. In Surgery of the Thyroid and Parathyroid Glands; Elsevier: Amsterdam, The Netherlands, 2021; pp. 174–180.e2. [Google Scholar]
- Eustatia-Rutten, C.F.A.; Corssmit, E.P.M.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Survival and death causes in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 313–319. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Bae, M.R.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018, 87, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hiltzik, D.; Carlson, D.L.; Tuttle, R.M.; Chuai, S.; Ishill, N.; Shaha, A.; Shah, J.P.; Singh, B.; Ghossein, R.A. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: A clinicopathologic study of 58 patients. Cancer 2006, 106, 1286–1295. [Google Scholar] [CrossRef]
- Volante, M.; Collini, P.; Nikiforov, Y.E.; Sakamoto, A.; Kakudo, K.; Katoh, R.; Lloyd, R.V.; LiVolsi, V.A.; Papotti, M.; Sobrinho-Simoes, M.; et al. Poorly differentiated thyroid carcinoma: The Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am. J. Surg. Pathol. 2007, 31, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimpasic, T.; Ghossein, R.; Carlson, D.L.; Chernichenko, N.; Nixon, I.; Palmer, F.L.; Lee, N.Y.; Shaha, A.R.; Patel, S.G.; Tuttle, R.M.; et al. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986-2009 memorial Sloan-Kettering cancer center experience. Thyroid 2013, 23, 997–1002. [Google Scholar] [CrossRef]
- Ho, A.S.; Luu, M.; Barrios, L.; Balzer, B.L.; Bose, S.; Fan, X.; Walgama, E.; Mallen-St. Clair, J.; Alam, U.; Shafqat, I.; et al. Prognostic Impact of Histologic Grade for Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 2020, 28, 1731–1739. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef]
- Schlumberger, M.; Challeton, C.; De Vathaire, F.; Travagli, J.P.; Gardet, P.; Lumbroso, J.D.; Francese, C.; Fontaine, F.; Ricard, M.; Parmentier, C. Radioactive Iodine Treatment and External Radiotherapy for Lung and Bone Metastases from Thyroid Carcinoma. J. Nucl. Med. 1996, 37, 598–605. [Google Scholar]
- Bernier, M.O.; Leenhardt, L.; Hoang, C.; Aurengo, A.; Mary, J.Y.; Menegaux, F.; Enkaoua, E.; Turpin, G.; Chiras, J.; Saillant, G.; et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2001, 86, 1568–1573. [Google Scholar] [CrossRef]
- Dupuy, D.E.; Monchik, J.M.; Decrea, C.; Pisharodi, L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001, 130, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jung, S.L.; Kim, B.S.; Ahn, K.J.; Choi, H.S.; Lim, D.J.; Kim, M.H.; Bae, J.S.; Kim, M.S.; Jung, C.K.; et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J. Radiol. 2014, 15, 817–826. [Google Scholar] [CrossRef]
- Lewis, B.D.; Hay, I.D.; Charboneau, J.W.; McIver, B.; Reading, C.C.; Goellner, J.R. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am. J. Roentgenol. 2002, 178, 699–704. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Chang, H.; Kim, B.W.; Lim, C.Y.; Lee, Y.S.; Chang, H.S.; Park, C.S. Long-term outcomes of ethanol injection therapy for locally recurrent papillary thyroid cancer. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3497–3501. [Google Scholar] [CrossRef] [PubMed]
- Eustatia-Rutten, C.F.A.; Romijn, J.A.; Guijt, M.J.; Vielvoye, G.J.; Van Den Berg, R.; Corssmit, E.P.M.; Pereira, A.M.; Smit, J.W.A. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 3184–3189. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, S.; Lorenzoni, A.; Boni, G.; Rubello, D.; Elisei, R.; Mariani, G. Anaplastic thyroid cancer: Prevalence, diagnosis and treatment. Minerva Endocrinol. 2008, 33, 341–357. [Google Scholar] [PubMed]
- Prasongsook, N.; Kumar, A.; Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.; Molina, J.; Garces, Y.; Ma, D.; Neben Wittich, M.A.; Rubin, J.; et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2017, 102, 4506–4514. [Google Scholar] [CrossRef]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef]
- GHARIB, H.; McCONAHEY, W.M.; TIEGS, R.D.; BERGSTRALH, E.J.; GOELLNER, J.R.; GRANT, C.S.; van HEERDEN, J.A.; SIZEMORE, G.W.; HAY, I.D. Medullary Thyroid Carcinoma: Clinicopathologic Features and Long-Term Follow-Up of 65 Patients Treated During 1946 Through 1970. Mayo Clin. Proc. 1992, 67, 934–940. [Google Scholar] [CrossRef]
- Schlumberger, M.; Bastholt, L.; Dralle, H.; Jarzab, B.; Pacini, F.; Smit, J.W.A. 2012 European Thyroid Association Guidelines for Metastatic Medullary Thyroid Cancer. Eur. Thyroid J. 2012, 1, 5–14. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; MacHens, A.; Moley, J.F.; Pacini, F.; et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Ringe, K.I.; Panzica, M.; Von Falck, C. Thermoablation of Bone Tumors. In RoFo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG: Stuttgart, Germany, 2016. [Google Scholar]
- Eisele, R.M. Advances in local ablation of malignant liver lesions. World J. Gastroenterol. 2016, 22, 3885–3891. [Google Scholar] [CrossRef]
- Roche, A.; Girish, B.V.; de Baère, T.; Baudin, E.; Boige, V.; Elias, D.; Lasser, P.; Schlumberger, M.; Ducreux, M. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur. Radiol. 2003, 13, 136–140. [Google Scholar] [CrossRef]
- Lupoli, G.A.; Fonderico, F.; Fittipaldi, M.R.; Colarusso, S.; Panico, A.; Cavallo, A.; Di Micco, L.; Lupoli, G. The role of somatostatin analogs in the management of medullary thyroid carcinoma. J. Endocrinol. Investig. 2003, 26, 72–74. [Google Scholar]
- Salavati, A.; Puranik, A.; Kulkarni, H.R.; Budiawan, H.; Baum, R.P. Peptide Receptor Radionuclide Therapy (PRRT) of Medullary and Nonmedullary Thyroid Cancer Using Radiolabeled Somatostatin Analogues. Semin. Nucl. Med. 2016, 46, 215–224. [Google Scholar] [CrossRef]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh, M.; Zadeh-Vakili, A.; Azizi, F.; Foroughi, F.; Akhavan, M.M.; Hedayati, M. Altered Epigenetic Mechanisms in Thyroid Cancer Subtypes. Mol. Diagn. Ther. 2018, 22, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007, 246, 466–470. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Pieruzzi, L.; Tacito, A.; Molinaro, E.; Agate, L.; Bottici, V.; Casella, F.; Ugolini, C.; Materazzi, G.; et al. Classical point mutations of RET, BRAF and RAS oncogenes are not shared in papillary and medullary thyroid cancer occurring simultaneously in the same gland. J. Endocrinol. Investig. 2017, 40, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef]
- Censi, S.; Cavedon, E.; Bertazza, L.; Galuppini, F.; Watutantrige-Fernando, S.; De Lazzari, P.; Nacamulli, D.; Pennelli, G.; Fassina, A.; Iacobone, M.; et al. Frequency and significance of Ras, Tert promoter, and Braf mutations in cytologically indeterminate thyroid nodules: A monocentric case series at a tertiary-level Endocrinology unit. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Mian, C.; Fugazzola, L.; Cosci, B.; Romei, C.; Barollo, S.; Cirello, V.; Bottici, V.; Marconcini, G.; Rosa, P.M.; et al. Evidence of a low prevalence of ras mutations in a large medullary thyroid cancer series. Thyroid 2013, 23, 50–57. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; Di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular alterations in thyroid cancer: From bench to clinical practice. Genes 2019, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, P.; Ji, M.; Guan, H.; Studeman, K.; Jensen, K.; Vasko, V.; El-Naggar, A.K.; Xing, M.M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J. Clin. Endocrinol. Metab. 2008, 93, 3106–3116. [Google Scholar] [CrossRef]
- Ricarte-Filho, J.C.; Ryder, M.; Chitale, D.A.; Rivera, M.; Heguy, A.; Ladanyi, M.; Janakiraman, M.; Solit, D.; Knauf, J.A.; Tuttle, R.M.; et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009, 69, 4885–4893. [Google Scholar] [CrossRef]
- Paes, J.E.; Ringel, M.D. Dysregulation of the Phosphatidylinositol 3-Kinase Pathway in Thyroid Neoplasia. Endocrinol. Metab. Clin. N. Am. 2008, 37, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. Ret gene fusions in malignancies of the thyroid and other tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saenko, V.; Yamashita, S.; Mitsutake, N. Radiation-induced thyroid cancers: Overview of molecular signatures. Cancers 2019, 11, 1290. [Google Scholar] [CrossRef]
- Taccaliti, A.; Silvetti, F.; Palmonella, G.; Boscaro, M. Genetic Alterations in Medullary Thyroid Cancer: Diagnostic and Prognostic Markers. Curr. Genom. 2011, 12, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Krampitz, G.W.; Norton, J.A. RET gene mutations (genotype and phenotype) of multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma. Cancer 2014, 120, 1920–1931. [Google Scholar] [CrossRef]
- Chernock, R.D.; Hagemann, I.S. Molecular pathology of hereditary and sporadic medullary thyroid carcinomas. Am. J. Clin. Pathol. 2015, 143, 768–777. [Google Scholar] [CrossRef]
- Greco, A.; Miranda, C.; Pierotti, M.A. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol. Cell. Endocrinol. 2010, 321, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016, 1. [Google Scholar] [CrossRef]
- Yakushina, V.D.; Lerner, L.V.; Lavrov, A.V. Gene fusions in thyroid cancer. Thyroid 2018, 28, 158–167. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K.; Xing, M.M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011, 71, 4403–4411. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Tang, S.J.; Sun, G.H.; Sun, K.H. CXCR7 mediates TGFβ1-promoted EMT and tumor-initiating features in lung cancer. Oncogene 2016, 35, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Yang, H.; Yip, L.; Ohori, N.P.; McCoy, K.L.; Stang, M.T.; Hodak, S.P.; Nikiforova, M.N.; Carty, S.E.; Nikiforov, Y.E. PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of papillary carcinoma. Thyroid 2014, 24, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Koenig, R.J. Pax-8-PPAR-γ 3 fusion protein in thyroid carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef]
- Manzella, L.; Stella, S.; Pennisi, M.S.; Tirrò, E.; Massimino, M.; Romano, C.; Puma, A.; Tavarelli, M.; Vigneri, P. New insights in thyroid cancer and p53 family proteins. Int. J. Mol. Sci. 2017, 18, 1325. [Google Scholar] [CrossRef]
- Sastre-Perona, A.; Santisteban, P. Role of the Wnt pathway in thyroid cancer. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Mirzaei, N.; Christian, M.; Sastre, M. Activation of the Wnt/β-catenin pathway represses the transcription of the β-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J. 2015, 29, 623–635. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, A.; Folstad, A.; Fenton, C.; Dinauer, C.A.; Tuttle, R.M.; Conran, R.; Francis, G.L. Infiltration of Differentiated Thyroid Carcinoma by Proliferating Lymphocytes Is Associated with Improved Disease-Free Survival for Children and Young Adults1. J. Clin. Endocrinol. Metab. 2001, 86, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, Y.; Guo, Y.; Wen, S. Immune microenvironment of thyroid cancer. J. Cancer 2020, 11, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Liu, X.; Jin, H.; Zhang, G.; Zhang, Q.; Yu, J. Regulatory T cells and plasmacytoid dendritic cells contribute to the immune escape of papillary thyroid cancer coexisting with multinodular non-toxic goiter. Endocrine 2013, 44, 172–181. [Google Scholar] [CrossRef]
- French, J.D.; Weber, Z.J.; Fretwell, D.L.; Said, S.; Klopper, J.P.; Haugen, B.R. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2325–2333. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, P.Z.; Chen, X.; Ge, M.; Zhang, Y.; Guo, Z.; Wang, J.; Shi, F.; Zhang, J.; Cheng, Y.; et al. Anlotinib treatment in locally advanced or metastatic medullary thyroid carcinoma: A multicenter, randomized, double-blind, placebo-controlled phase IIB trial. J. Clin. Oncol. 2019, 37, 6019. [Google Scholar] [CrossRef]
- Qing, W.; Fang, W.Y.; Ye, L.; Shen, L.Y.; Zhang, X.F.; Fei, X.C.; Chen, X.; Wang, W.Q.; Li, X.Y.; Xiao, J.C.; et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid 2012, 22, 905–910. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef]
- Na, K.J.; Choi, H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr. Relat. Cancer 2018, 25, 523–531. [Google Scholar] [CrossRef] [PubMed]
- French, J.D. Immunotherapy for advanced thyroid cancers — rationale, current advances and future strategies. Nat. Rev. Endocrinol. 2020, 16, 629–641. [Google Scholar] [CrossRef]
- Gupta-Abramson, V.; Troxel, A.B.; Nellore, A.; Puttaswamy, K.; Redlinger, M.; Ransone, K.; Mandel, S.J.; Flaherty, K.T.; Loevner, L.A.; O’Dwyer, P.J.; et al. Phase II trial of sorafenib in advanced thyroid cancer. J. Clin. Oncol. 2008, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Kloos, R.T.; Ringel, M.D.; Knopp, M.V.; Hall, N.C.; King, M.; Stevens, R.; Liang, J.; Wakely, P.E.; Vasko, V.V.; et al. Phase II trial of sorafenib in metastatic thyroid cancer. J. Clin. Oncol. 2009, 27, 1675–1684. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; De La Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic diff erentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Sherman, E.J.; Dunn, L.A.; Ho, A.L.; Baxi, S.S.; Ghossein, R.A.; Fury, M.G.; Haque, S.; Sima, C.S.; Cullen, G.; Fagin, J.A.; et al. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer 2017, 123, 4114–4121. [Google Scholar] [CrossRef] [PubMed]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef]
- Mooney, C.J.; Nagaiah, G.; Fu, P.; Wasman, J.K.; Cooney, M.M.; Savvides, P.S.; Bokar, J.A.; Dowlati, A.; Wang, D.; Agarwala, S.S.; et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid 2009, 19, 233–240. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Schlumberger, M.; Jarzab, B.; Martins, R.G.; Pacini, F.; Robinson, B.; McCaffrey, J.C.; Shah, M.H.; Bodenner, D.L.; Topliss, D.; et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated t. Cancer 2015, 121, 2749–2756. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Berdelou, A.; Borget, I.; Godbert, Y.; Nguyen, T.; Garcia, M.E.; Chougnet, C.N.; Ferru, A.; Buffet, C.; Chabre, O.; Huillard, O.; et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid 2018. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Piovesan, A.; Durante, C.; Bregni, M.; Castagna, M.G.; Zovato, S.; Giusti, M.; Ibrahim, T.; Puxeddu, E.; Fedele, G.; et al. Real-world efficacy and safety of lenvatinib: Data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Giani, C.; Valerio, L.; Bongiovanni, A.; Durante, C.; Grani, G.; Ibrahim, T.; Mariotti, S.; Massa, M.; Pani, F.; Pellegriti, G.; et al. Safety and Quality-of-Life Data from an Italian Expanded Access Program of Lenvatinib for Treatment of Thyroid Cancer. Thyroid 2020. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Kim, J.W.; Lee, I.J.; Jeon, T.J.; Chang, H.; Kim, B.W.; Lee, Y.S.; Chang, H.S.; Park, C.S. Survival With Lenvatinib for the Treatment of Progressive Anaplastic Thyroid Cancer: A Single-Center, Retrospective Analysis. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; INAGAKI, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. Phase II study of lenvatinib in patients with differentiated, medullary, and anaplastic thyroid cancer: Final analysis results. J. Clin. Oncol. 2016, 34, 6088. [Google Scholar] [CrossRef]
- Wells, S.A.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Fox, E.; Widemann, B.C.; Chuk, M.K.; Marcus, L.; Aikin, A.; Whitcomb, P.O.; Merino, M.J.; Lodish, M.; Dombi, E.; Steinberg, S.M.; et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2b associated medullary thyroid carcinoma. Clin. Cancer Res. 2013, 19, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.; Bottici, V.; Matrone, A.; Piaggi, P.; Viola, D.; Cappagli, V.; Agate, L.; Molinaro, E.; Ciampi, R.; Tacito, A.; et al. Medullary thyroid cancer treated with vandetanib: Predictors of a longer and durable response. Endocr. Relat. Cancer 2020, 27, 97–110. [Google Scholar] [CrossRef]
- Ramos, H.E.; Hecht, F.; Berdelou, A.; Borget, I.; Leboulleux, S.; Baudin, E.; Schlumberger, M. Long-term follow-up and safety of vandetanib for advanced medullary thyroid cancer. Endocrine 2020, 71, 434–442. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Tan, D.S.W.; Lassen, U.N.; Albert, C.M.; Kummar, S.; van Tilburg, C.; Dubois, S.G.; Geoerger, B.; Mascarenhas, L.; Federman, N.; Basu-Mallick, A.; et al. Larotrectinib efficacy and safety in TRK fusion cancer: An expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann. Oncol. 2018, 29, ix23. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Drilon, A.; Farago, A.F.; Brose, M.S.; McDermott, R.; Sohal, D.; Oh, D.-Y.; Almubarak, M.; Bauman, J.; Chu, E.; et al. 1916P Larotrectinib treatment of advanced TRK fusion thyroid cancer. Ann. Oncol. 2020, 31, S1086. [Google Scholar] [CrossRef]
- Groussin, L.; Clerc, J.; Huillard, O. Larotrectinib-Enhanced Radioactive Iodine Uptake in Advanced Thyroid Cancer. N. Engl. J. Med. 2020, 383, 1686–1687. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- John, T.; Chiu, C.-H.; Cho, B.C.; Fakih, M.; Farago, A.F.; Demetri, G.D.; Goto, K.; Doebele, R.C.; Siena, S.; Drilon, A.; et al. 364O Intracranial efficacy of entrectinib in patients with NTRK fusion-positive solid tumours and baseline CNS metastases. Ann. Oncol. 2020, 31, S397–S398. [Google Scholar] [CrossRef]
- Carlomagno, F.; Guida, T.; Anaganti, S.; Vecchio, G.; Fusco, A.; Ryan, A.J.; Billaud, M.; Santoro, M. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004, 23, 6056–6063. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET -Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Bradford, D.; Larkins, E.; Mushti, S.L.; Rodriguez, L.; Skinner, A.M.; Helms, W.S.; Price, L.S.L.; Fourie Zirkelbach, J.; Li, Y.; Liu, J.; et al. FDA Approval Summary: Selpercatinib for the Treatment of Lung and Thyroid cancers with RET Gene Mutations or Fusions. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hernando, J.; Tarasova, V.; Hu, M.I.; Sherman, E.J.; Brose, M.S.; Robinson, B.; Tahara, M.; Wirth, L.J.; Sashegyi, A.; Soldatenkova, V.; et al. 1927TiP LIBRETTO-531: Selpercatinib in patients with treatment (Tx)-naïve RET-mutant medullary thyroid cancer (MTC). Ann. Oncol. 2020, 31, S1091. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Subbiah, V.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Brose, M.S.; Curigliano, G.; Leboulleux, S.; Zhu, V.W.; Keam, B.; et al. 1913O Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET mutation-positive medullary thyroid cancer (RET+ MTC). Ann. Oncol. 2020, 31, S1084. [Google Scholar] [CrossRef]
- Brose, M.S.; Cabannilas, M.E.; Sherman, S.I.; Cohen, E.E.W.; Wirth, L.J.; Riehl, T.; Yue, H.; Sherman, E.J. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1272–1282. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Subbiah, V.; Cabanillas, M.E.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Keam, B.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Keam, B.; Kreitman, R.J.; Wainberg, Z.A.; Cabanillas, M.E.; Cho, D.C.; Italiano, A.; Stein, A.; Cho, J.Y.; Schellens, J.H.M.; Wen, P.Y.; et al. Updated efficacy and safety data of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated anaplastic thyroid cancer (ATC). Ann. Oncol. 2018, 29, viii645–viii646. [Google Scholar] [CrossRef]
- Shah, M.H.; Wei, L.; Wirth, L.J.; Daniels, G.A.; De Souza, J.A.; Timmers, C.D.; Sexton, J.L.; Beshara, M.; Nichols, D.; Snyder, N.; et al. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J. Clin. Oncol. 2017, 35, 6022. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Brose, M.S.; Holland, J.; Ferguson, K.C.; Sherman, S.I. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid 2014, 24, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; De Souza, J.A.; Geyer, S.; Wirth, L.J.; Menefee, M.E.; Liu, S.V.; Shah, K.; Wright, J.; Shah, M.H. Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: Results of a multicenter phase II international thyroid oncology group trial. J. Clin. Oncol. 2017, 35, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Qin, S.-K.; Li, Z.-Y.; Yang, H.; Fu, W.; Li, S.-H.; Chen, W.-X.; Gao, Z.-R.; Miao, W.-B.; Xu, H.-Q.; et al. LBA89 A randomized multicentered phase III study to evaluate apatinib in subjects with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer. Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, W.; Chi, Y.; Li, C.; Du, C.-X.; Li, S.; Wang, J.; Li, L.; Wang, F. Safety, pharmacokinetic, and antitumor activity of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Clin. Oncol. 2015, 33, e13586. [Google Scholar] [CrossRef]
- Ruan, X.; Shi, X.; Dong, Q.; Yu, Y.; Hou, X.; Song, X.; Wei, X.; Chen, L.; Gao, M. Antitumor effects of anlotinib in thyroid cancer. Endocr. Relat. Cancer 2019, 26, 153–164. [Google Scholar] [CrossRef]
- Chi, Y.; Gao, M.; Zhang, Y.; Shi, F.; Cheng, Y.; Guo, Z.; Ge, M.; Qin, J.; Zhang, J.; Li, Z.; et al. Anlotinib in locally advanced or metastatic radioiodine-refractory differentiated thyroid carcinoma: A randomized, double-blind, multicenter phase II trial. Ann. Oncol. 2020, 31, S1347. [Google Scholar] [CrossRef]
- Handkiewicz-Junak, D.; Roskosz, J.; Hasse-Lazar, K.; Szpak-Ulczok, S.; Puch, Z.; Kukulska, A.; Olczyk, T.; Piela, A.; Paliczka-Cieslik, E.; Jarzab, B. 13-cis-retinoic acid re-differentiation therapy and recombinant human thyrotropin-aided radioiodine treatment of non-Functional metastatic thyroid cancer: A single-center, 53-patient phase 2 study. Thyroid Res. 2009, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Van Der Pluijm, G.; Karperien, M.; Stokkel, M.P.M.; Pereira, A.M.; Morreau, J.; Kievit, J.; Romijn, J.A.; Smit, J.W.A. Lithium as adjuvant to radioiodine therapy in differentiated thyroid carcinoma: Clinical and in vitro studies. Clin. Endocrinol. 2006, 64, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer. N. England J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Dedecjus, M.; Wirth, L.J.; Tuttle, R.; Tennaval, J.S.K.; Carroll, D.; Hovey, T.; Thakre, B.F.J. ASTRA: A phase III, randomized, placebo-controlled study evaluating complete remission rate (CRR) with short-course selumetinib plus adjuvant radioactive iodine (RAI) in patients (pts) with differentiated thyroid cancer (DTC). In Proceedings of the 88th Annual Meeting of the American Thyroid Association, Washington, DC, USA, 3–7 October 2018. Abstract short call oral 8, 2018. [Google Scholar]
- Dunn, L.A.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Vemurafenib redifferentiation of BRAF mutant, Rai-refractory thyroid cancers. J. Clin. Endocrinol. Metab. 2019, 104, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.M.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 2015, 21, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Jaber, T.; Waguespack, S.G.; Cabanillas, M.E.; Elbanan, M.; Vu, T.; Dadu, R.; Sherman, S.I.; Amit, M.; Santos, E.B.; Zafereo, M.; et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J. Clin. Endocrinol. Metab. 2018, 103, 3698–3705. [Google Scholar] [CrossRef]
- De Falco, V.; Buonocore, P.; Muthu, M.; Torregrossa, L.; Basolo, F.; Billaud, M.; Gozgit, J.M.; Carlomagno, F.; Santoro, M. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98. [Google Scholar] [CrossRef]
- Mologni, L.; Redaelli, S.; Morandi, A.; Plaza-Menacho, I.; Gambacorti-Passerini, C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol. Cell. Endocrinol. 2013, 377, 1–6. [Google Scholar] [CrossRef]
- Drilon, A.E.; Zhai, D.; Rogers, E.; Deng, W.; Zhang, X.; Ung, J.; Lee, D.; Rodon, L.; Graber, A.; Zimmerman, Z.F.; et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J. Clin. Oncol. 2020, 38, 3616. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.C.; Prawira, A.; De Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; Aix, S.P.; Lin, C.C.; Ramlau, R.; Butler, M.O.; Delord, J.P.; Gelderblom, H.; Ascierto, P.A.; et al. PD-1 blockade in anaplastic thyroid carcinoma. J. Clin. Oncol. 2020, 38, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Yin, J.; Foote, R.L.; Kasperbauer, J.L.; Rivera, M.; Asmus, E.; Garces, N.I.; Janus, J.R.; Liu, M.; Ma, D.J.; et al. A Phase 2 Study of Pembrolizumab Combined with Chemoradiotherapy as Initial Treatment for Anaplastic Thyroid Cancer. Thyroid 2019, 29, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Tsai, C.J.; Zhi, W.I.; Fetten, J.V.; Wu, V.; Ho, A.L.; Riaz, N.; Pfister, D.G.; Lee, N.Y. Pilot study combining PD-L1 antibody durvalumab (D) with CTLA-4 antibody tremelimumab (T) and stereotactic body radiotherapy (SBRT) to treat metastatic anaplastic thyroid cancer (ATC). J. Clin. Oncol. 2019, 37, 6088. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Ruf, J.; Duyster, J.; Thomusch, O.; Miething, C.; Zielke, A. 1915P The lenvatinib/pembrolizumab combination induces long lasting and complete responses in patients with metastatic anaplastic or poorly differentiated thyroid carcinoma: Results from a retrospective study and first results from the prospective phase I. Ann. Oncol. 2020, 31, S1085. [Google Scholar] [CrossRef]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L.D. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Marinelli, S.; Negrini, G.; Menetti, S.; Benevento, F.; Bolondi, L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Ther. Adv. Gastroenterol. 2016, 9, 240–249. [Google Scholar] [CrossRef]
- Kucharz, J.; Dumnicka, P.; Kusnierz-Cabala, B.; Demkow, T.; Wiechno, P. The correlation between the incidence of adverse events and progression-free survival in patients treated with cabozantinib for metastatic renal cell carcinoma (mRCC). Med Oncol. 2019, 36. [Google Scholar] [CrossRef] [PubMed]
- Cappagli, V.; Moriconi, D.; Bonadio, A.G.; Giannese, D.; La Manna, G.; Egidi, M.F.; Comai, G.; Vischini, G.; Bottici, V.; Elisei, R.; et al. Proteinuria is a late-onset adverse event in patients treated with cabozantinib. J. Endocrinol. Investig. 2021, 44, 95–103. [Google Scholar] [CrossRef]
- Tahara, M.; Schlumberger, M.; Elisei, R.; Habra, M.A.; Kiyota, N.; Paschke, R.; Dutcus, C.E.; Hihara, T.; McGrath, S.; Matijevic, M.; et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur. J. Cancer 2017, 75, 213–221. [Google Scholar] [CrossRef]
- Tiedje, V.; Ting, S.; Walter, R.F.; Herold, T.; Worm, K.; Badziong, J.; Zwanziger, D.; Schmid, K.W.; Führer, D. Prognostic markers and response to vandetanib therapy in sporadic medullary thyroid cancer patients. Eur. J. Endocrinol. 2016, 175, 173–180. [Google Scholar] [CrossRef]
- Fugazzola, L.; Muzza, M.; Pogliaghi, G.; Vitale, M. Intratumoral genetic heterogeneity in papillary thyroid cancer: Occurrence and clinical significance. Cancers 2020, 12, 383. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef]
- Hass, R.; von der Ohe, J.; Ungefroren, H. Impact of the tumor microenvironment on tumor heterogeneity and consequences for cancer cell plasticity and stemness. Cancers 2020, 12, 3716. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Schütz, E.; Beck, J.; Kanzow, P.; Plowman, P.N.; Weiss, G.J.; Walson, P.D. Using circulating cell-free DNA to monitor personalized cancer therapy. Crit. Rev. Clin. Lab. Sci. 2017, 54, 205–218. [Google Scholar] [CrossRef]

| Drugs/ Clinical Trials | VEGFR-1 | VEGFR-2 | VEGFR-3 | c-KIT | RET | PDGFR | FGFR | EGFR | Others |
|---|---|---|---|---|---|---|---|---|---|
| Lenvatinib (Schlumberger et al.) | + | + | + | + | + | + | + | - | RET-KIF5B rearrangements |
| Sorafenib (Brose et al.) | - | + | + | + | + | + | - | - | Raf, FLT3 |
| Vandetanib (Wells et al.) | - | + | - | + | + | - | - | + | RET-KIF5B rearrangements |
| Cabozantinib (Elisei et al.) | - | + | - | + | + | - | - | - | MET, RET-KIF5B rearrangements |
| Larotrectinib (Drilon et al.) | - | - | - | - | - | - | - | - | TRK1 |
| Entrectinib (Doebele et al.) | - | - | - | - | - | - | - | - | TRK, ALK, ROS1 |
| Selpercatinib (Wirth et al.) | - | - | - | - | + | - | - | - | - |
| Pralsetinib (NCT03037385) | - | - | - | - | + | - | - | - | - |
| Vemurafenib (Brose et al.) (Hytman et al.) | - | - | - | - | - | - | - | - | BRAFV600E |
| Dabrafenib (Falchook et al.) | - | - | - | - | - | - | - | - | BRAFV600E |
| Adverse Events (All Grade) | Lenvatinib (%) | Sorafenib (%) | Vandetanib (%) | Cabozantinib (%) | Selpercatinib (%) | Pralsetinib (%) | Larotrectinib (%) | Entrectinib (%) |
|---|---|---|---|---|---|---|---|---|
| Hypertension | 68 | 41 | 32 | 33 | 43 | 40 | 11 | NR |
| Diarrhea | 59 | 69 | 56 | 63 | 38 | 34 | 22 | 35 |
| Skin rash | 15 | 50 | 45 | 19 | NR | 24 * | NR | 11 |
| Anorexia | 49 | 32 | 21 | 46 | NR | 15 | 13 | 13 |
| Fatigue | 59 | 50 | 24 | 41 | 38 | 38 | 37 | 48 |
| Nausea | 41 | 20 | 33 | 43 | 35 | 17 | 29 | 34 |
| Weight loss | 46 | 47 | 10 | 48 | NR | NR | NR | NR |
| QT prolongation | 8 | NR | 14 | NR | 19 | NR | NR | 3.1 |
| Hand-foot syndrome | 32 | 76 | NR | 50 | NR | NR | NR | NR |
| Weight gain | NR | NR | NR | NR | 25 | NR | 15 | 25 |
| Increased aspartate aminotransferase level | 0.4 ** | 23 | NR | 86 | 57 | 69 | 45 | 44 |
| Increased alanine aminotransferase level | 0.4 ** | 26 | NR | 86 | 51 | 43 | 45 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, L.; Cappagli, V.; Valerio, L.; Giani, C.; Viola, D.; Puleo, L.; Gambale, C.; Minaldi, E.; Campopiano, M.C.; Matrone, A.; et al. Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities. Int. J. Mol. Sci. 2021, 22, 3117. https://doi.org/10.3390/ijms22063117
Lorusso L, Cappagli V, Valerio L, Giani C, Viola D, Puleo L, Gambale C, Minaldi E, Campopiano MC, Matrone A, et al. Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities. International Journal of Molecular Sciences. 2021; 22(6):3117. https://doi.org/10.3390/ijms22063117
Chicago/Turabian StyleLorusso, Loredana, Virginia Cappagli, Laura Valerio, Carlotta Giani, David Viola, Luciana Puleo, Carla Gambale, Elisa Minaldi, Maria Cristina Campopiano, Antonio Matrone, and et al. 2021. "Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities" International Journal of Molecular Sciences 22, no. 6: 3117. https://doi.org/10.3390/ijms22063117
APA StyleLorusso, L., Cappagli, V., Valerio, L., Giani, C., Viola, D., Puleo, L., Gambale, C., Minaldi, E., Campopiano, M. C., Matrone, A., Bottici, V., Agate, L., Molinaro, E., & Elisei, R. (2021). Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities. International Journal of Molecular Sciences, 22(6), 3117. https://doi.org/10.3390/ijms22063117






