Predialysis and Dialysis Therapies Differently Affect Nitric Oxide Synthetic Pathway in Red Blood Cells from Uremic Patients: Focus on Peritoneal Dialysis
Abstract
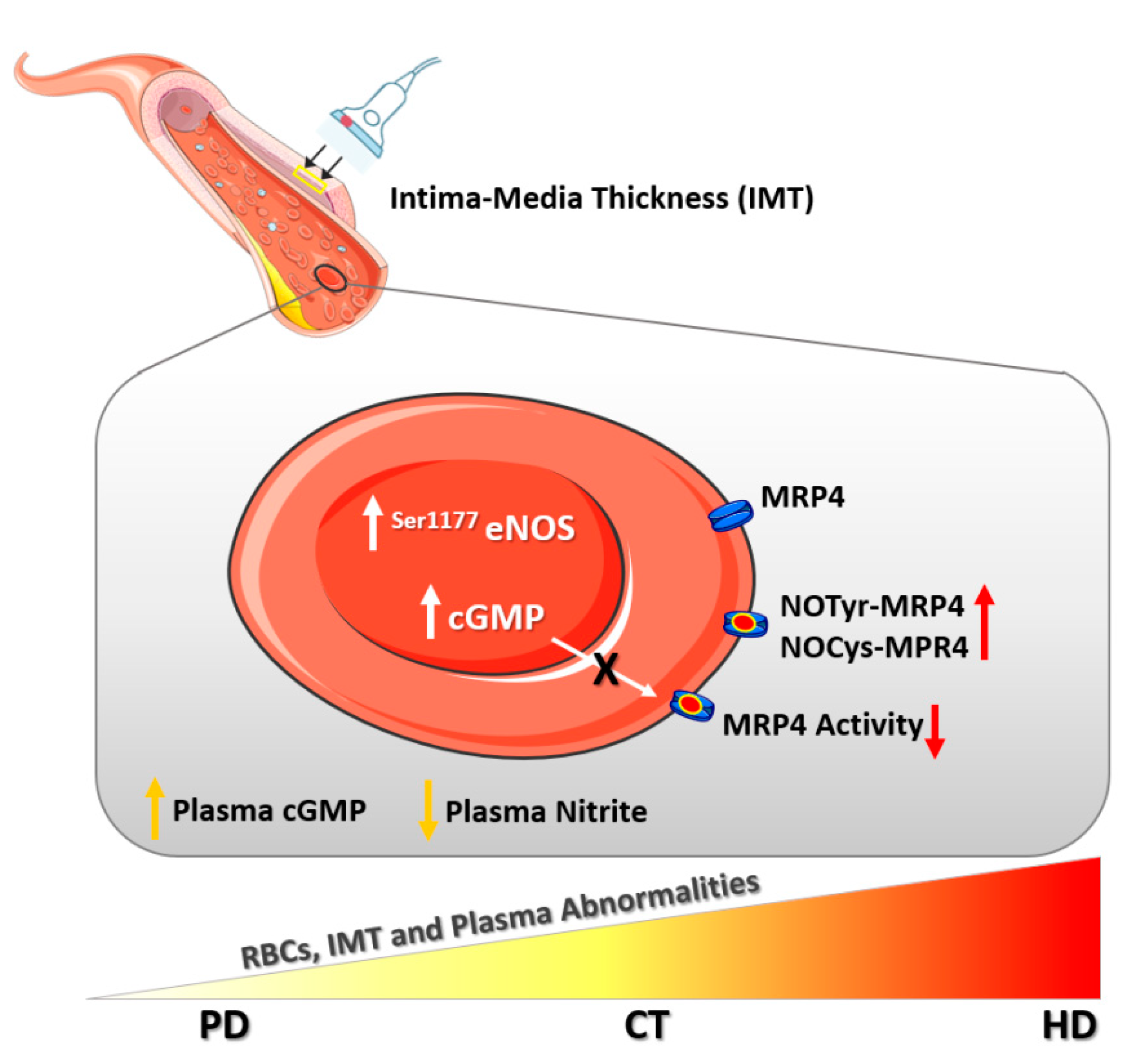
1. Introduction
2. Results
2.1. Main Clinical and Biochemical Characteristics of the Study Population
2.2. RBC-NOS Activation
2.3. cGMP Levels in RBCs
2.4. MRP4 Nitration and Nitrosylation Levels in RBCs
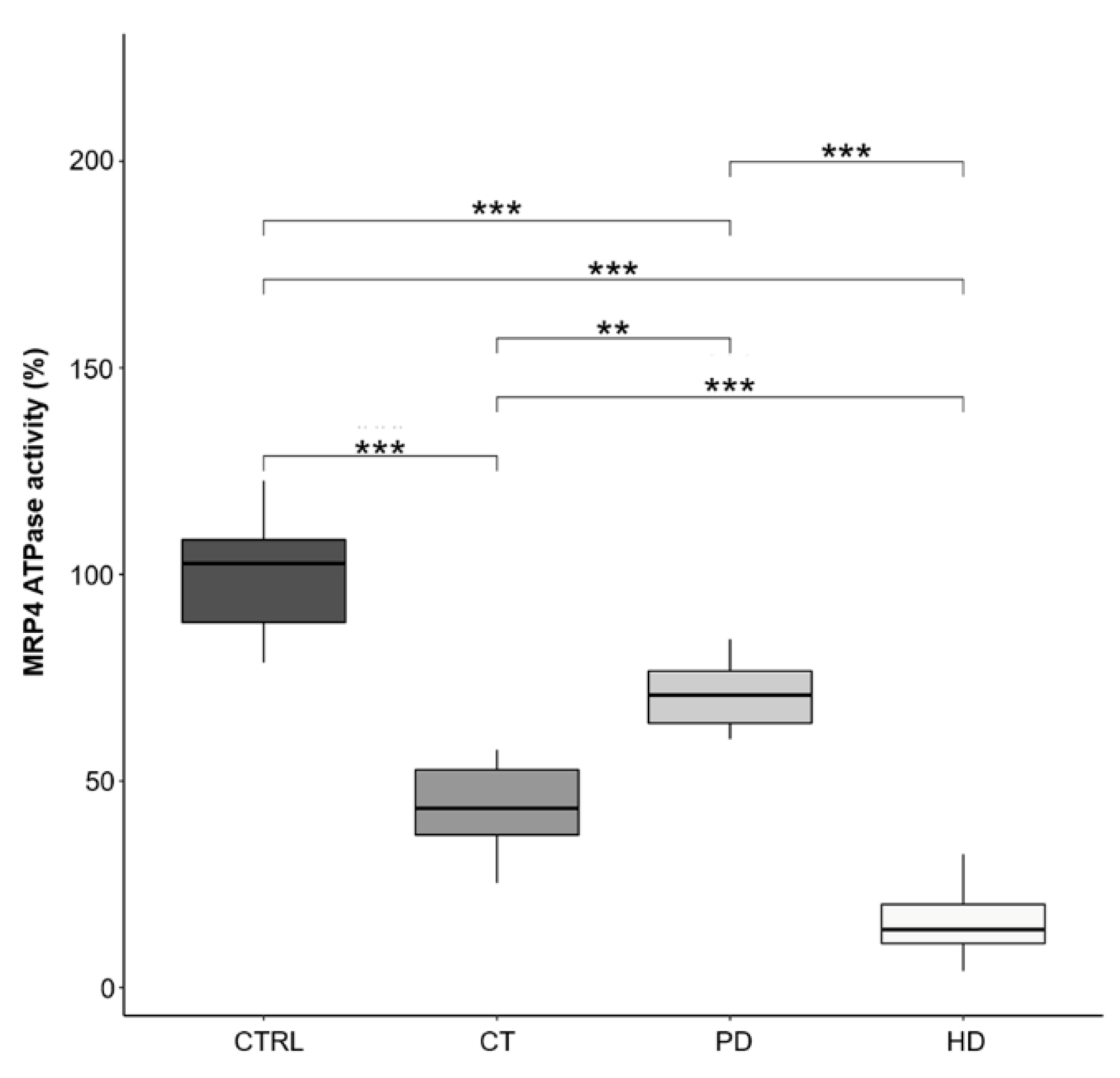
2.5. MRP4-ATPase Activity in RBCs
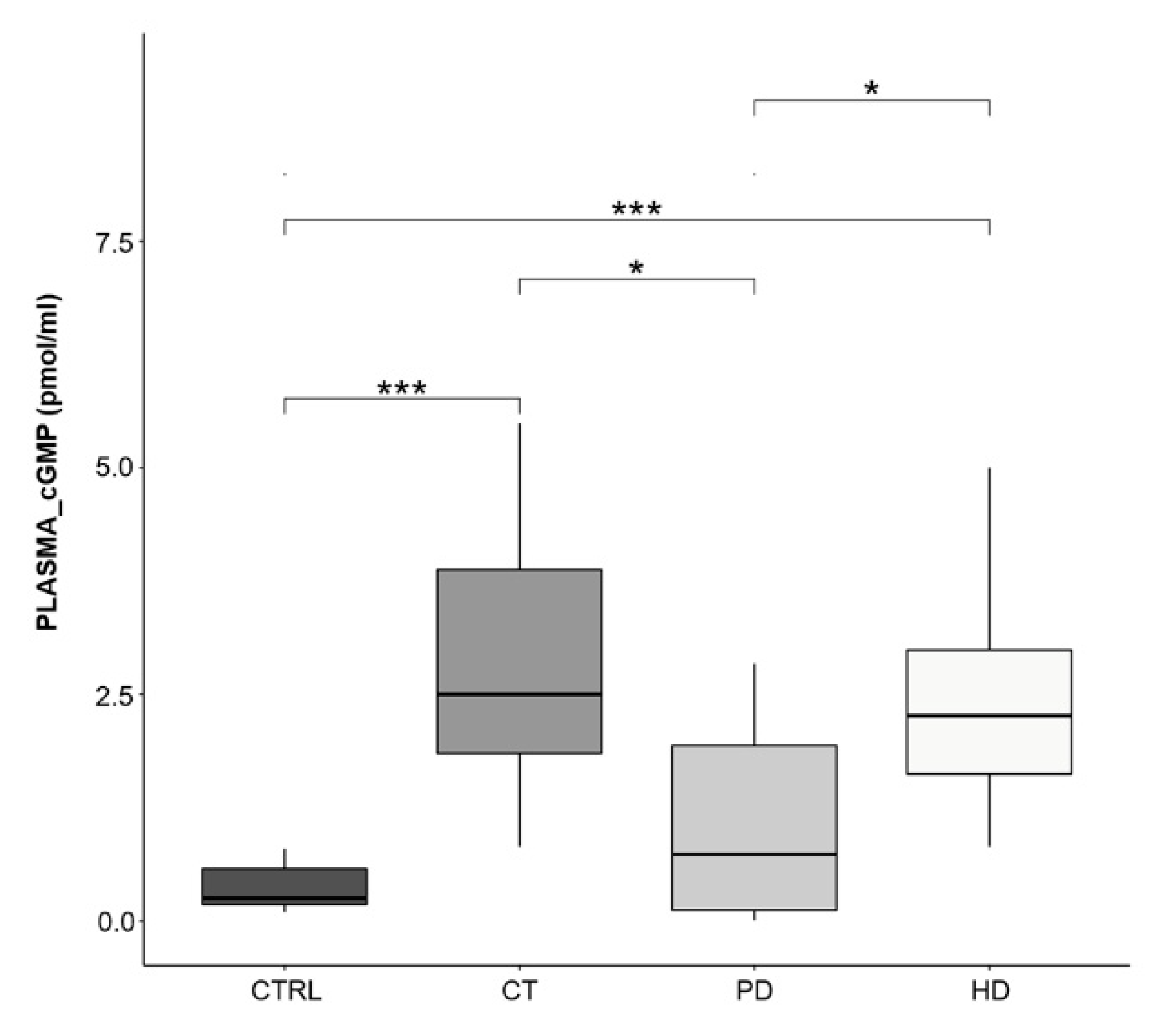
2.6. Plasma cGMP Levels
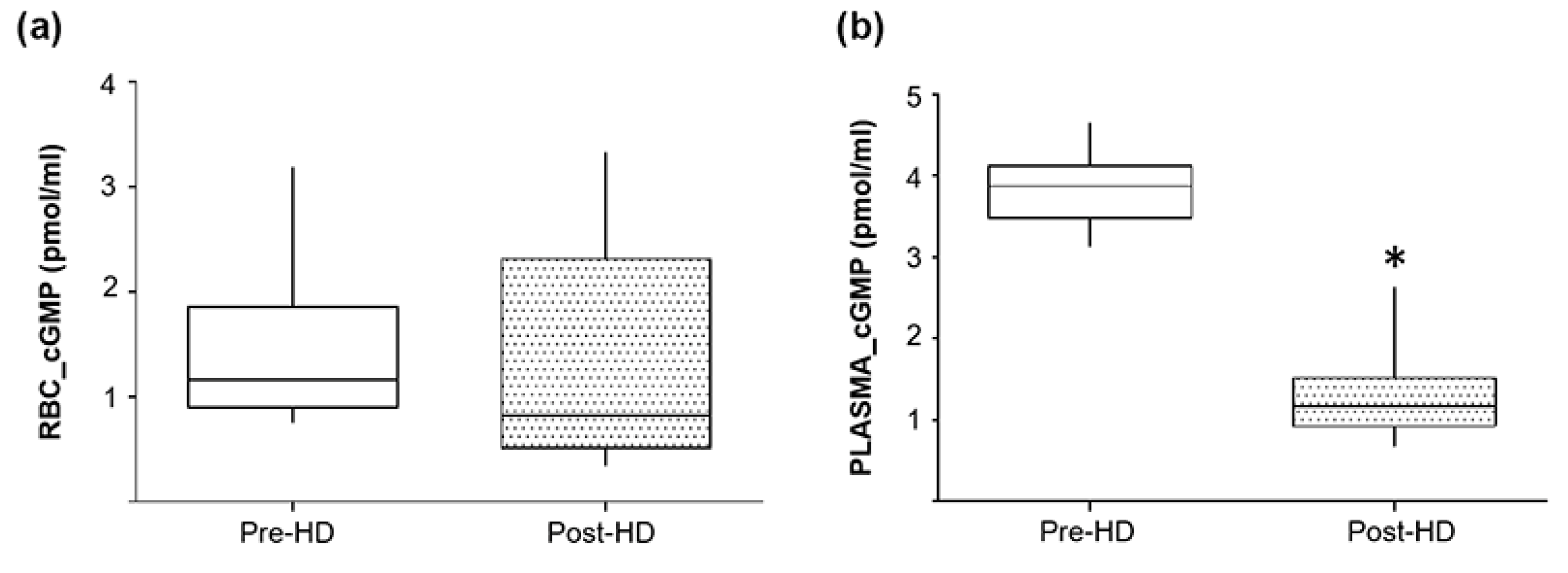
2.7. Pre- and Post-Hemodialysis cGMP Levels in Plasma and RBCs from ESRD Patients
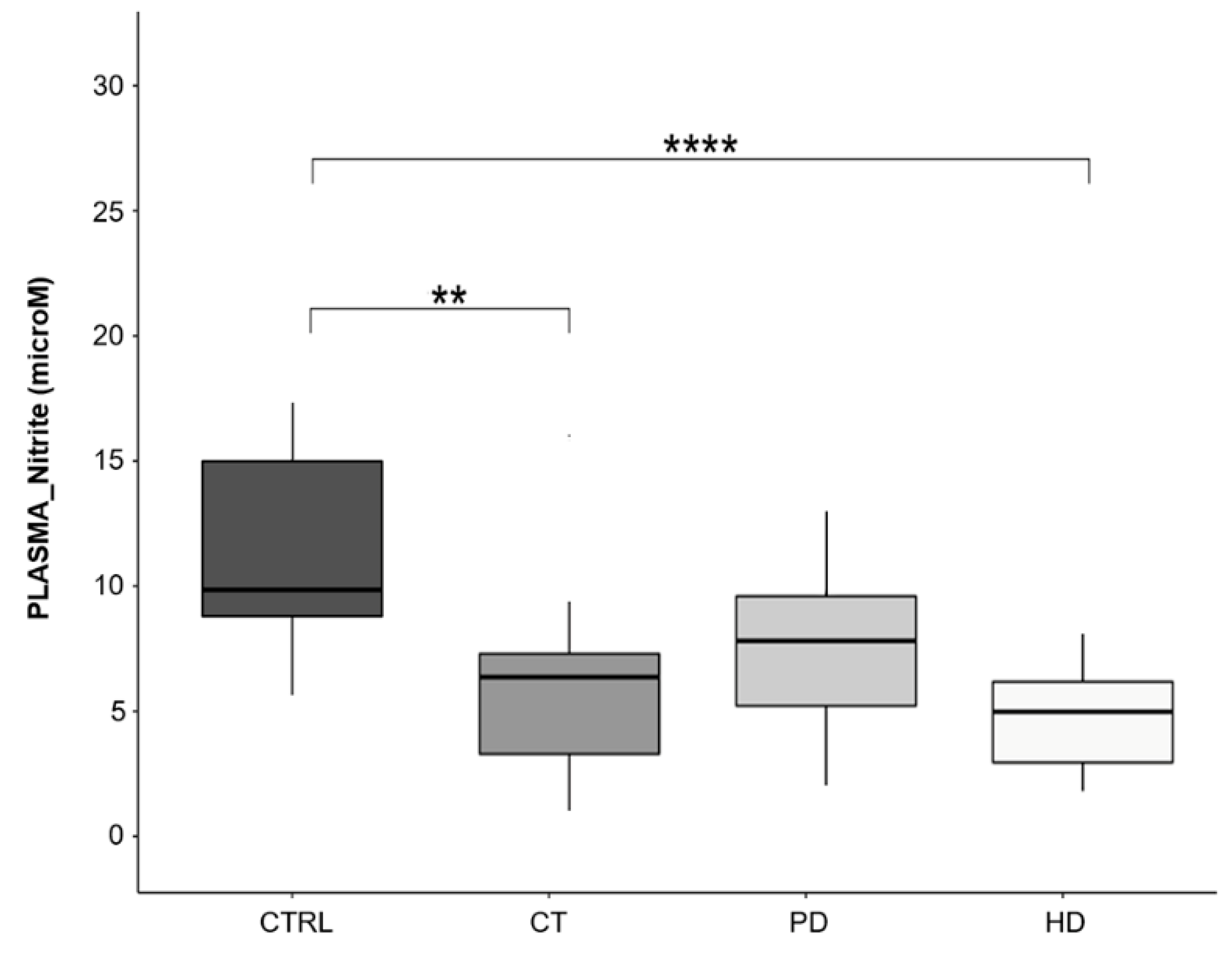
2.8. Plasma Nitrite Levels
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Collection
4.3. Materials and Antibodies
4.4. Flow Cytometry Analysis
4.5. Evaluation of cGMP Levels
4.6. Western Blot Analysis
4.7. MRP4 ATPase Activity
4.8. Assessment of Plasma Nitrite Levels
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mozos, I. Mechanisms Linking Red Blood Cell Disorders and Cardiovascular Diseases. BioMed. Res. Int. 2015, 2015, 682054. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of Anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Özüyaman, B.; Grau, M.; Kelm, M.; Merx, M.W.; Kleinbongard, P. RBC NOS: Regulatory mechanisms and therapeutic aspects. Trends Mol. Med. 2008, 14, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Özüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Sansone, R.; Kuhnle, G.G.C.; Thasian-Sivarajah, S.; Krenz, T.; Horn, P.; Krisp, C.; Wolters, D.; Heiß, C.; et al. Human red blood cells at work: Identification and visualization of erythrocytic eNOS activity in health and disease. Blood 2012, 120, 4229–4237. [Google Scholar] [CrossRef]
- Di Pietro, N.; Giardinelli, A.; Sirolli, V.; Riganti, C.; Di Tomo, P.; Gazzano, E.; Di Silvestre, S.; Panknin, C.; Cortese-Krott, M.M.; Csonka, C.; et al. Nitric oxide synthetic pathway and cGMP levels are altered in red blood cells from end-stage renal disease patients. Mol. Cell. Biochem. 2016, 417, 155–167. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahorán, S.; Kövamees, O.; Nordin, F.; Gonzalez, A.E.U.; Alvarsson, M.; Östenson, C.-G.; Andersson, D.C.; et al. Erythrocytes From Patients With Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, X.; Mahdi, A.; Zhou, Z.; Tratsiakovich, Y.; Jiao, T.; Kiss, A.; Kövamees, O.; Alvarsson, M.; Catrina, S.-B.; et al. Red Blood Cells in Type 2 Diabetes Impair Cardiac Post-Ischemic Recovery through an Arginase-Dependent Modulation of Nitric Oxide Synthase and Reactive Oxygen Species. JACC Basic Transl. Sci. 2018, 3, 450–463. [Google Scholar] [CrossRef]
- Eligini, S.; Porro, B.; Lualdi, A.; Squellerio, I.; Veglia, F.; Chiorino, E.; Crisci, M.; Garlaschè, A.; Giovannardi, M.; Werba, J.-P.; et al. Nitric Oxide Synthetic Pathway in Red Blood Cells Is Impaired in Coronary Artery Disease. PLoS ONE 2013, 8, e66945. [Google Scholar] [CrossRef]
- Tsuda, K. Red blood cell abnormalities and hypertension. Hypertens. Res. 2020, 43, 72–73. [Google Scholar] [CrossRef]
- Bonomini, M.; Pandolfi, A.; Sirolli, V.; Arduini, A.; Di Liberato, L.; Di Pietro, N. Erythrocyte Alterations and Increased Cardiovascular Risk in Chronic Renal Failure. Nephro Urol. Mon. 2017, 9, e45866. [Google Scholar] [CrossRef]
- Siqueira, M.A.D.S.; Brunini, T.M.; Pereira, N.R.; Martins, M.A.; Moss, M.B.; Santos, S.F.; Lugon, J.R.; Mendes-Ribeiro, A.C. Increased nitric oxide production in platelets from severe chronic renal failure patients. Can. J. Physiol. Pharmacol. 2011, 89, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Debari, V.A.; Bennun, A. Cyclic GMP in the human erythrocyte. Intracellular levels and transport in normal subjects and chronic hemodialysis patients. Clin. Biochem. 1982, 15, 219–221. [Google Scholar] [CrossRef]
- Grau, M.; Mozar, A.; Charlot, K.; Lamarre, Y.; Weyel, L.; Suhr, F.; Collins, B.; Jumet, S.; Hardy-Dessources, M.-D.; Romana, M.; et al. High red blood cell nitric oxide synthase activation is not associated with improved vascular function and red blood cell deformability in sickle cell anaemia. Br. J. Haematol. 2014, 168, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Brinkmann, C.; Bloch, W.; Grau, M. Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells. PLoS ONE 2015, 10, e0125206. [Google Scholar] [CrossRef]
- Da Silva, C.D.; Brunini, T.M.; Reis, P.F.; Moss, M.B.; Santos, S.F.; Roberts, N.B.; Ellory, J.C.; Mann, G.E.; Mendes-Ribeiro, A.C. Effects of nutritional status on the L-arginine-nitric oxide pathway in platelets from hemodialysis patients. Kidney Int. 2005, 68, 2173–2179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, H.; Pritchard, K.A.; Jr, K.A.P. Targeted Increases in Endothelial Cell Superoxide Anion Production Stimulate eNOS-Dependent Nitric Oxide Production, Not Uncoupled eNOS Activity. Arter. Thromb. Vasc. Biol. 2008, 28, 1580–1581. [Google Scholar] [CrossRef][Green Version]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef]
- Wu, C.-P.; Woodcock, H.; Hladky, S.B.; Barrand, M.A. cGMP (guanosine 3′,5′-cyclic monophosphate) transport across human erythrocyte membranes. Biochem. Pharmacol. 2005, 69, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Chan, C.T.; Blankestijn, P.J.; Dember, L.M.; Gallieni, M.; Harris, D.C.; Lok, C.E.; Mehrotra, R.; Stevens, P.E.; Wang, A.Y.-M.; Cheung, M.; et al. Dialysis initiation, modality choice, access, and prescription: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 96, 37–47. [Google Scholar] [CrossRef]
- Jaar, B.G.; Gimenez, L.F. Dialysis Modality Survival Comparison: Time to End the Debate, It’s a Tie. Am. J. Kidney Dis. 2018, 71, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Kayalar, A.O.; Basturk, T.; Koc, Y.; Yilmaz, F.; Caglayan, F.B.; Sakaci, T.; Ahbap, E.; Ünsal, A. Comparison of Long-term Complications in Patients on Haemodialysis and Peritoneal Dialysis Longer than 10 Years. J. Clin. Diagn. Res. 2016, 10, OC05-8. [Google Scholar] [CrossRef]
- Zhan, X.; Yang, M.; Chen, Y.; Zhang, L.; Yan, C.; Wang, Y. Comparison of risk of stroke in patients treated with peritoneal dialysis and hemodialysis: A systematic review and meta-analysis. Ren. Fail. 2019, 41, 650–656. [Google Scholar] [CrossRef]
- Zazzeroni, L.; Pasquinelli, G.; Nanni, E.; Cremonini, V.; Rubbi, I. Comparison of Quality of Life in Patients Undergoing Hemodialysis and Peritoneal Dialysis: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2017, 42, 717–727. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Jeon, Y.; Park, Y.; Kim, Y.S.; Kang, S.-W.; Yang, C.W.; Kim, N.-H.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; et al. Better Quality of Life of Peritoneal Dialysis compared to Hemodialysis over a Two-year Period after Dialysis Initiation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Bellasi, A.; Di Lullo, L.; Raggi, P. Is peritoneal dialysis superior to hemodialysis as far as cardiovascular risk? Another unsolved dilemma for maintenance dialysis. Atherosclerosis 2020, 307, 75–77. [Google Scholar] [CrossRef]
- Ng, C.H.; Ong, Z.H.; Sran, H.K.; Wee, T.B. Comparison of cardiovascular mortality in hemodialysis versus peritoneal dialysis. Int. Urol. Nephrol. 2020, 1–9. [Google Scholar] [CrossRef]
- Selby, N.M.; Kazmi, I. Peritoneal dialysis has optimal intradialytic hemodynamics and preserves residual renal function: Why isn’t it better than hemodialysis? Semin. Dial. 2019, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Banshodani, M.; Kawanishi, H.; Moriishi, M.; Shintaku, S.; Tsuchiya, S. Association between Dialysis Modality and Cardiovascular Diseases: A Comparison between Peritoneal Dialysis and Hemodialysis. Blood Purif. 2019, 49, 302–309. [Google Scholar] [CrossRef]
- Kawada, T. Risk of hemodialysis against peritoneal dialysis for chronic heart failure in patients with end-stage renal disease. Int. J. Cardiol. 2016, 223, 360. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, W.; Verbeke, F.; Vanholder, R. Cardiovascular disease in haemodialysis and peritoneal dialysis: Arguments pro peritoneal dialysis. Nephrol. Dial. Transplant. 2006, 22, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conran, N.; Oresco-Santos, C.; Acosta, H.C.; Fattori, A.; Saad, S.T.O.; Costa, F.F. Increased soluble guanylate cyclase activity in the red blood cells of sickle cell patients. Br. J. Haematol. 2004, 124, 547–554. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Mergia, E.; Kramer, C.M.; Lückstädt, W.; Yang, J.; Wolff, G.; Panknin, C.; Bracht, T.; Sitek, B.; Pernow, J.; et al. Identification of a soluble guanylate cyclase in RBCs: Preserved activity in patients with coronary artery disease. Redox Biol. 2018, 14, 328–337. [Google Scholar] [CrossRef]
- Hon, W.-M.; Lee, J.-C.; Lee, K.-H. Effect of Hemodialysis on Plasma Nitric Oxide Levels. Artif. Organs 2000, 24, 387–390. [Google Scholar] [CrossRef]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58, S47–S59. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free. Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, T.C.; Wood, J.; Garthwaite, J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc. Natl. Acad. Sci. USA 2001, 99, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Adderley, S.P.; Thuet, K.M.; Sridharan, M.; Bowles, E.A.; Stephenson, A.H.; Ellsworth, M.L.; Sprague, R.S. Identification of cytosolic phosphodiesterases in the erythrocyte: A possible role for PDE5. Med. Sci. Monit. 2011, 17, CR241–CR247. [Google Scholar] [CrossRef]
- Petrov, V.; Fagard, R.; Lijnen, P. Human erythrocytes contain Ca2+, calmodulin-dependent cyclic nucleotide phosphodiesterase which is involved in the hydrolysis of cGMP. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 387–393. [Google Scholar] [CrossRef]
- Ravarotto, V.; Simioni, F.; Pagnin, E.; Davis, P.A.; Calò, L.A. Oxidative stress—Chronic kidney disease—Cardiovascular disease: A vicious circle. Life Sci. 2018, 210, 125–131. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef] [PubMed]
- Devynck, M.-A.; Simon, A.; Pernollet, M.-G.; Chironi, G.; Gariepy, J.; Rendu, F.; Levenson, J. Plasma cGMP and Large Artery Remodeling in Asymptomatic Men. Hypertension 2004, 44, 919–923. [Google Scholar] [CrossRef]
- Wever, R.; Boer, P.; Hijmering, M.; Stroes, E.; Verhaar, M.; Kastelein, J.; Versluis, K.; Lagerwerf, F.; Rijn, H.; Van Koomans, H.; et al. Nitric Oxide Production Is Reduced in Patients With Chronic Renal Failure. Arter. Thromb. Vasc. Biol. 1999, 19, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Baylis, C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000, 58, 1261–1266. [Google Scholar] [CrossRef]
- Wagner, L.; Riggleman, A.; Erdely, A.; Couser, W.; Baylis, C. Reduced nitric oxide synthase activity in rats with chronic renal disease due to glomerulonephritis. Kidney Int. 2002, 62, 532–536. [Google Scholar] [CrossRef][Green Version]
- Thuraisingham, R.C.; Yaqoob, M.M. Oxidative consumption of nitric oxide: A potential mediator of uremic vascular disease. Kidney Int. 2003, 63, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.M.; Balogun, M.O.; Akintomide, A.O.; Ayoola, O.O.; Mene-Afejuku, T.O.; Ogunlade, O.; Okunola, O.O.; Lawal, A.O.; Akinsola, A. Carotid Intima-Media Thickness: A Surrogate Marker for Cardiovascular Disease in Chronic Kidney Disease Patients. Clin. Med. Insights Cardiol. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Maruhashi, T.; Fujii, Y.; Idei, N.; Fujimura, N.; Mikami, S.; Kajikawa, M.; Matsumoto, T.; Kihara, Y.; Chayama, K.; et al. Intima-Media Thickness of Brachial Artery, Vascular Function, and Cardiovascular Risk Factors. Arter. Thromb. Vasc. Biol. 2012, 32, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Pencina, M.J.; Bs, A.M.; Pencina, K.M.; Brown, L.S.; Wolf, P.A.; Sr, R.B.D. Associations of Carotid Artery Intima-Media Thickness (IMT) With Risk Factors and Prevalent Cardiovascular Disease. J. Ultrasound Med. 2010, 29, 1759–1768. [Google Scholar] [CrossRef] [PubMed]



| CKD | |||||
|---|---|---|---|---|---|
| CTRL (N = 20) | CT (N = 20) | PD (N = 20) | HD (N = 20) | p-Value | |
| Gender (female/male) | 10/10 | 10/10 | 6/14 | 9/11 | |
| Age | 53 ± 11 | 64 ± 16 | 56 ± 22 | 65 ± 15 | ns |
| BMI | 24.8 ± 4.2 | 24.5 ± 4.1 | 26.9 ± 4.7 | 26.1 ± 5.9 | ns |
| Systolic Blood Pressure (mmHg) | 113 ± 11.2 | 138 ± 18.9 * | 140 ± 20.8 * | 127 ± 21.8 * | ˂0.05 |
| Diastolic Blood Pressure (mmHg) | 71 ± 8.4 | 76 ± 11 | 78 ± 10 | 72 ± 14 | ns |
| IMT | 0.64 ± 0.1 | 0.95 ± 0.3 * | 0.86 ± 0.3 | 0.99 ± 0.2 * | ˂0.05 |
| Hematocrit (%) | 43.6 ± 4.3 | 36 ± 4.9 * | 35.7 ± 3 * | 34.8 ± 4.1 * | <0.05 |
| Hemoglobin (g/dL) | 14.4 ± 1.5 | 11.4 ± 1.6 * | 11.3 ± 1 * | 10.7 ± 1.2 * | ˂0.05 |
| Creatinine (mg/dL) | 0.83 ± 0.2 | 4.08 ± 3 * | 9.93 ± 4 * § | 8.37 ± 2 * § | ˂0.005 |
| Albumin (g/dL) | 4.54 ± 0.51 | 3.98 ± 0.58 * # | 3.49 ± 0.40 * | 3.89 ± 0.45 * # | ˂0.05 |
| Total Proteins (g/dL) | 7.57 ± 0.55 | 6.89 ± 0.94 * # | 6.48 ± 0.66 * | 6.59 ± 0.55 * | ˂0.05 |
| Glycemia (mg/dL) | 88.8 ± 13.2 | 82.7 ± 15.4 | 93.2 ± 34.6 | 91.4 ± 35.2 | ns |
| Triglycerides (mg/dL) | 127.1 ± 49 | 132.8 ± 62 * | 144.1 ± 69 * | 154.1 ± 85 * | ˂0.05 |
| Total Cholesterol (mg/dL) | 167.8 ± 42.7 | 168.8 ± 51.9 | 152.2 ± 37.8 | 143.8 ± 31.9 | ns |
| Cholesterol HDL (mg/dL) | 55.5 ± 21.1 | 52.3 ± 16.9 | 43.8 ± 13.4 | 48.7 ± 22.9 | ns |
| Cholesterol LDL (mg/dL) | 88.7 ± 36.9 | 90.2 ± 39.4 | 77.3 ± 27.8 | 64.3 ± 21.6 | ns |
| CRP (mg/dL) | 0.30 ± 0.12 | 0.70 ± 0.75 * | 0.80 ± 1.03 * | 1.12 ± 1.60 * | ˂0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmerini, C.; Piscitani, L.; Bologna, G.; Riganti, C.; Lanuti, P.; Mandatori, D.; Di Liberato, L.; Di Fulvio, G.; Sirolli, V.; Renda, G.; et al. Predialysis and Dialysis Therapies Differently Affect Nitric Oxide Synthetic Pathway in Red Blood Cells from Uremic Patients: Focus on Peritoneal Dialysis. Int. J. Mol. Sci. 2021, 22, 3049. https://doi.org/10.3390/ijms22063049
Palmerini C, Piscitani L, Bologna G, Riganti C, Lanuti P, Mandatori D, Di Liberato L, Di Fulvio G, Sirolli V, Renda G, et al. Predialysis and Dialysis Therapies Differently Affect Nitric Oxide Synthetic Pathway in Red Blood Cells from Uremic Patients: Focus on Peritoneal Dialysis. International Journal of Molecular Sciences. 2021; 22(6):3049. https://doi.org/10.3390/ijms22063049
Chicago/Turabian StylePalmerini, Carola, Luca Piscitani, Giuseppina Bologna, Chiara Riganti, Paola Lanuti, Domitilla Mandatori, Lorenzo Di Liberato, Giorgia Di Fulvio, Vittorio Sirolli, Giulia Renda, and et al. 2021. "Predialysis and Dialysis Therapies Differently Affect Nitric Oxide Synthetic Pathway in Red Blood Cells from Uremic Patients: Focus on Peritoneal Dialysis" International Journal of Molecular Sciences 22, no. 6: 3049. https://doi.org/10.3390/ijms22063049
APA StylePalmerini, C., Piscitani, L., Bologna, G., Riganti, C., Lanuti, P., Mandatori, D., Di Liberato, L., Di Fulvio, G., Sirolli, V., Renda, G., Pipino, C., Marchisio, M., Bonomini, M., Pandolfi, A., & Di Pietro, N. (2021). Predialysis and Dialysis Therapies Differently Affect Nitric Oxide Synthetic Pathway in Red Blood Cells from Uremic Patients: Focus on Peritoneal Dialysis. International Journal of Molecular Sciences, 22(6), 3049. https://doi.org/10.3390/ijms22063049












