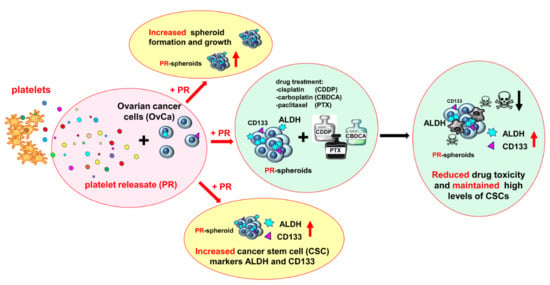
In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel
Abstract
1. Introduction
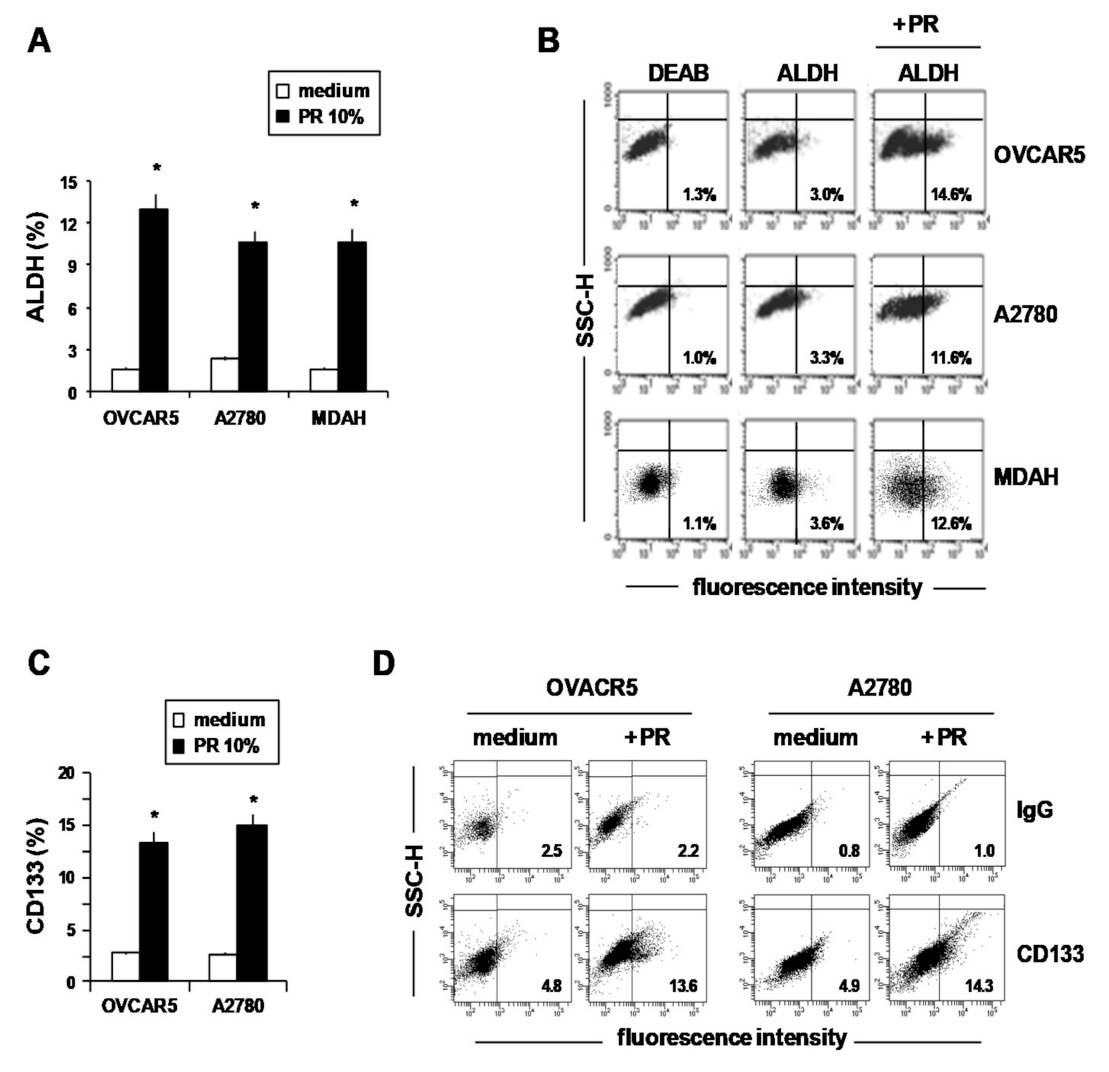
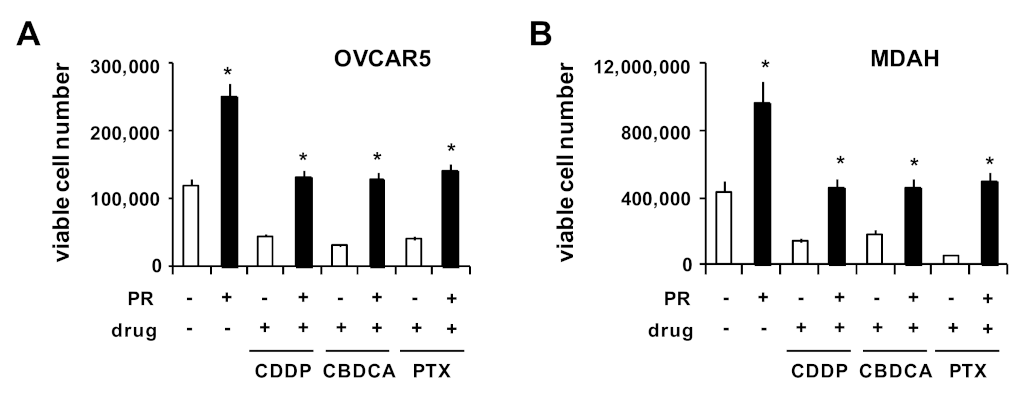
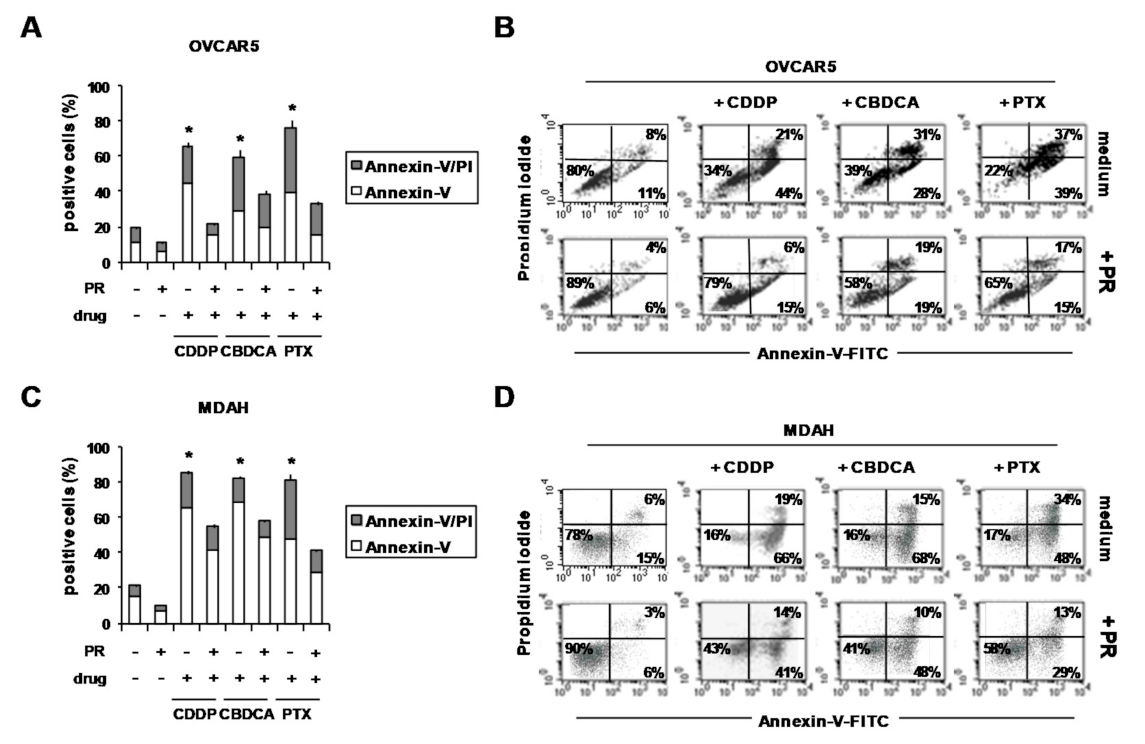
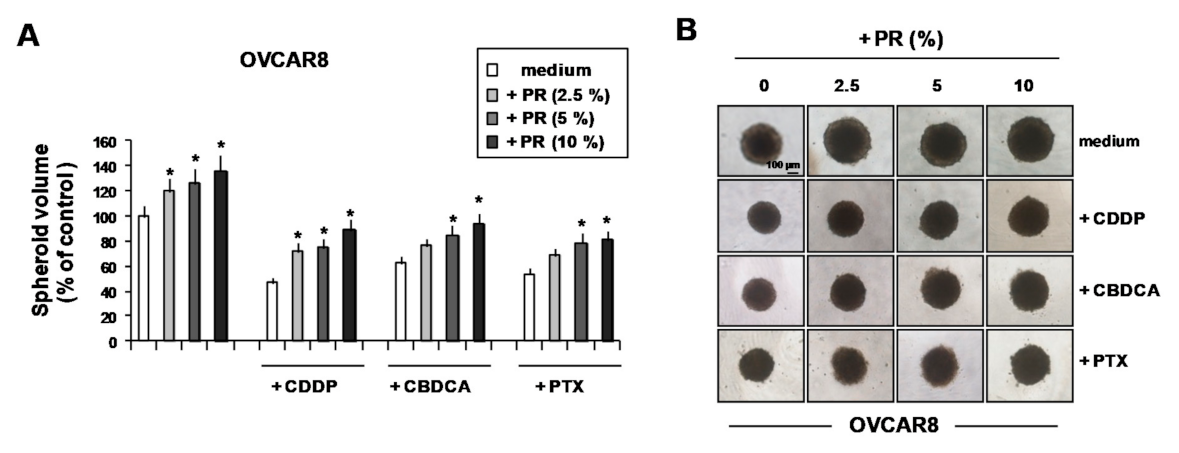
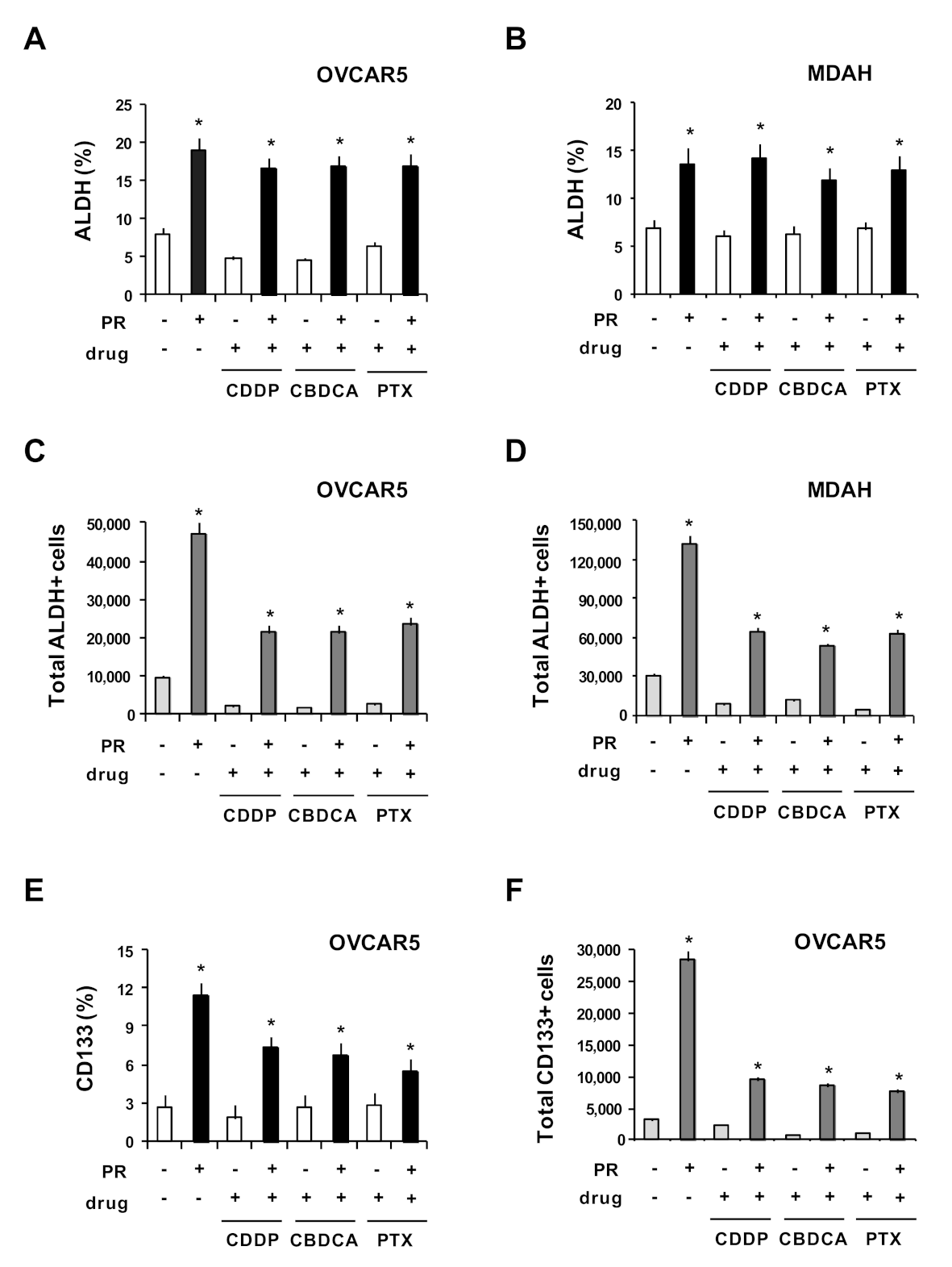
2. Results and Discussion
3. Materials and Methods
3.1. Drugs
3.2. Platelet-Releasate (PR) Production and Cytokine Evaluation
3.3. Cell Lines and Culture Conditions
3.4. Spheroid Formation, Growth and Treatment
3.5. Flow Cytometry
3.6. Statistics
4. Conclusions
Authors Contributions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belluco, C.; Forlin, M.; DelRio, P.; Rega, D.; Degiuli, M.; Sofia, S.; Olivieri, M.; Pucciarelli, S.; Zuin, M.; De Manzoni, G.; et al. Elevated platelet count is a negative predictive and prognostic marker in locally advanced rectal cancer undergoing neoadjuvant chemoradiation: A retrospective multi-institutional study on 965 patients. BMC Cancer 2018, 18, 1094. [Google Scholar] [CrossRef]
- Liu, S.; Fang, J.; Jiao, D.; Liu, Z. Elevated Platelet Count Predicts Poor Prognosis in Breast Cancer Patients with Supraclavicular Lymph Node Metastasis. Cancer Manag. Res. 2020, 12, 6069–6075. [Google Scholar] [CrossRef]
- Catani, M.V.; Savini, I.; Tullio, V.; Gasperi, V. The “Janus Face” of Platelets in Cancer. Int. J. Mol. Sci. 2020, 21, 788. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Bottsford-Miller, J.; Choi, H.-J.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Haemmerle, M.; Nick, A.M.; Pradeep, S.; Zand, B.; Previs, R.A.; et al. Differential Platelet Levels Affect Response to Taxane-Based Therapy in Ovarian Cancer. Clin. Cancer Res. 2015, 21, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. Int. J. Mol. Sci. 2020, 21, 8169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hada, A.; Han, L. Platelet count as a biomarker for monitoring treatment response and disease recurrence in recurrent epithelial ovarian cancer. J. Ovarian Res. 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, S.; Muschel, R.J. Platelets and Metastasis: New Implications of an Old Interplay. Front. Oncol. 2020, 10, 1350. [Google Scholar] [CrossRef]
- Elaskalani, O.; Berndt, M.C.; Falasca, M.; Metharom, P. Targeting Platelets for the Treatment of Cancer. Cancers 2017, 9, 94. [Google Scholar] [CrossRef]
- Huong, P.T.; Nguyen, L.T.; Nguyen, X.-B.; Lee, S.K.; Bach, D.-H. The Role of Platelets in the Tumor-Microenvironment and the Drug Resistance of Cancer Cells. Cancers 2019, 11, 240. [Google Scholar] [CrossRef]
- Franco, A.T.; Ware, J. Pathophysiology 2: The Role of Platelets in Cancer Biology. Cancer Treat. Res. 2019, 179, 37–54. [Google Scholar] [CrossRef]
- Johnson, K.E.; Ceglowski, J.R.; Roweth, H.G.; Forward, J.A.; Tippy, M.D.; El-Husayni, S.; Kulenthirarajan, R.; Malloy, M.W.; Machlus, K.R.; Chen, W.Y.; et al. Aspirin inhibits platelets from reprogramming breast tumor cells and promoting metastasis. Blood Adv. 2019, 3, 198–211. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in Healthy and Disease States: From Biomarkers Discovery to Drug Targets Identification by Proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sun, C.; Li, N.; Shan, W.; Lu, H.; Guo, L.; Guo, E.; Xia, M.; Weng, D.; Meng, L.; et al. Cisplatin-induced CCL5 secretion from CAFs promotes cisplatin-resistance in ovarian cancer via regulation of the STAT3 and PI3K/Akt signaling pathways. Int. J. Oncol. 2016, 48, 2087–2097. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 11. [Google Scholar] [CrossRef]
- Hu, Q.; Hisamatsu, T.; Haemmerle, M.; Cho, M.S.; Pradeep, S.; Rupaimoole, R.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Wong, S.T.; Sood, A.K.; et al. Role of Platelet-Derived Tgfβ1 in the Progression of Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5611–5621. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, X. Platelets are associated with xenograft tumor growth and the clinical malignancy of ovarian cancer through an angiogenesis-dependent mechanism. Mol. Med. Rep. 2015, 11, 2449–2458. [Google Scholar] [CrossRef]
- Orellana, R.; Kato, S.; Erices, R.; Bravo, M.L.; Gonzalez, P.; Oliva, B.; Cubillos, S.; Valdivia, A.; Ibañez, C.; Brañes, J.; et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer 2015, 15, 1–16. [Google Scholar] [CrossRef]
- Cooke, N.M.; Spillane, C.D.; Sheils, O.; O’Leary, J.; Kenny, D. Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion. BMC Cancer 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets increase the proliferation of ovarian cancer cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Casagrande, N.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical Activity of the Liposomal Cisplatin Lipoplatin in Ovarian Cancer. Clin. Cancer Res. 2014, 20, 5496–5506. [Google Scholar] [CrossRef] [PubMed]
- Borghese, C.; Casagrande, N.; Corona, G.; Aldinucci, D. Adipose-Derived Stem Cells Primed with Paclitaxel Inhibit Ovarian Cancer Spheroid Growth and Overcome Paclitaxel Resistance. Pharmaceutics 2020, 12, 401. [Google Scholar] [CrossRef]
- Durante, C.; Agostini, F.; Abbruzzese, L.; Toffola, R.T.; Zanolin, S.; Suine, C.; Mazzucato, M. Growth factor release from platelet concentrates: Analytic quantification and characterization for clinical applications. Vox Sang. 2013, 105, 129–136. [Google Scholar] [CrossRef]
- Borghese, C.; Agostini, F.; Durante, C.; Colombatti, A.; Mazzucato, M.; Aldinucci, N. Clinical-grade quality platelet-rich plasma releasate (PRP-R/SRGF) from CaCl 2 -activated platelet concentrates promoted expansion of mesenchymal stromal cells. Vox Sang. 2016, 111, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Vicinanza, C.; Di Cintio, F.; Battiston, M.; Lombardi, E.; Golinelli, G.; Durante, C.; Toffoli, G.; Dominici, M.; Mazzucato, M. Adipose mesenchymal stromal/stem cells expanded by a GMP compatible protocol displayed improved adhesion on cancer cells in flow conditions. Ann. Transl. Med. 2020, 8, 533. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-Like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yamawaki, K.; Ishiguro, T.; Yoshihara, K.; Ueda, H.; Sato, A.; Ohata, H.; Yoshida, Y.; Minamino, T.; Okamoto, K.; et al. ALDH-Dependent Glycolytic Activation Mediates Stemness and Paclitaxel Resistance in Patient-Derived Spheroid Models of Uterine Endometrial Cancer. Stem Cell Rep. 2019, 13, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, Z.; Amaya-Padilla, M.A.; Singomat, T.; Binju, M.; Madjid, B.D.; Yu, Y.; Kaur, P. Ovarian cancer stem cells and their role in drug resistance. Int. J. Biochem. Cell Biol. 2019, 106, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, N.; Jacob, F.; Dubrovska, A.; Grünberg, J. Novel Therapeutic Strategies for Ovarian Cancer Stem Cells. Front. Oncol. 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Neal, A.S.; Moatamed, N.A.; Memarzadeh, S. Exploring the Potential of Drug Response Assays for Precision Medicine in Ovarian Cancer. Int. J. Mol. Sci. 2020, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, I.; Grimley, E.; Yang, K.; Hong, L.; Vinogradova, E.V.; Suciu, R.; Kovalenko, I.; Karnak, D.; Morgan, C.A.; Chtcherbinine, M.; et al. A Pan-ALDH1A Inhibitor Induces Necroptosis in Ovarian Cancer Stem-like Cells. Cell Rep. 2019, 26, 3061–3075.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Chen, Y.J.; Fan, M.H.; Liao, Y.J.; Mao, T.L. Characteristics of CD133-Sustained Chemoresistant Cancer Stem-Like Cells in Human Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 6467. [Google Scholar] [CrossRef]
- Kryczek, I.; Liu, S.; Roh, M.; Vatan, L.; Szeliga, W.; Wei, S.; Banerjee, M.; Mao, Y.; Kotarski, J.; Wicha, M.S.; et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int. J. Cancer 2011, 130, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Bai, S.; McLean, K.; Yang, K.; A Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R.K.; et al. Aldehyde Dehydrogenase in Combination with CD133 Defines Angiogenic Ovarian Cancer Stem Cells That Portend Poor Patient Survival. Cancer Res. 2011, 71, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, V.; Hurley, T.D.; Nephew, K.P. Targeting Aldehyde Dehydrogenases to Eliminate Cancer Stem Cells in Gynecologic Malignancies. Cancers 2020, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef]
- Zeineldin, R.; Muller, C.Y.; Stack, M.S.; Hudson, L.G. Targeting the EGF Receptor for Ovarian Cancer Therapy. J. Oncol. 2009, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Xie, H.; Yuan, J.; Jiang, X.-Y.; Yong, J.-H.; Zeng, D.; Dou, Y.-Y.; Xiao, S.-S. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Kaipio, K.; Chen, P.; Roering, P.; Huhtinen, K.; Mikkonen, P.; Östling, P.; Lehtinen, L.; Mansuri, N.; Korpela, T.; Potdar, S.; et al. ALDH1A1-related stemness in high-grade serous ovarian cancer is a negative prognostic indicator but potentially targetable by EGFR/mTOR-PI3K/aurora kinase inhibitors. J. Pathol. 2020, 250, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Granados, M.L.; Hudson, L.G.; Samudio-Ruiz, S.L. Contributions of the Epidermal Growth Factor Receptor to Acquisition of Platinum Resistance in Ovarian Cancer Cells. PLoS ONE 2015, 10, e0136893. [Google Scholar] [CrossRef]
- Poursheikhani, A.; Yousefi, H.; Tavakoli-Bazzaz, J.; Ghaffari, S.H. EGFR Blockade Reverses Cisplatin Resistance in Human Epithelial Ovarian Cancer Cells. Iran. Biomed. J. 2020, 24, 365–373. [Google Scholar] [CrossRef]
- Liefers-Visser, J.; Meijering, R.; Reyners, A.; Van Der Zee, A.; De Jong, S. IGF system targeted therapy: Therapeutic opportunities for ovarian cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef]
- Camblin, A.J.; Tan, G.; Curley, M.D.; Yannatos, I.; Iadevaia, S.; Rimkunas, V.; Mino-Kenudson, M.; Bloom, T.; Schoeberl, B.; Drummond, D.C.; et al. Dual targeting of IGF-1R and ErbB3 as a potential therapeutic regimen for ovarian cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Snyder, C.S.; Wang, A.; McLean, K.; Zamarin, D.; Buckanovich, R.J.; Mehta, G. Carcinoma-Associated Mesenchymal Stem Cells Promote Chemoresistance in Ovarian Cancer Stem Cells via PDGF Signaling. Cancers 2020, 12, 2063. [Google Scholar] [CrossRef]
- Alsina-Sanchís, E.; Figueras, A.; Lahiguera, A.; Gil-Martín, M.; Pardo, B.; Piulats, J.M.; Martí, L.; Ponce, J.; Matias-Guiu, X.; Vidal, A.; et al. TGFβ Controls Ovarian Cancer Cell Proliferation. Int. J. Mol. Sci. 2017, 18, 1658. [Google Scholar] [CrossRef] [PubMed]
- Roane, B.M.; Arend, R.C.; Birrer, M.J. Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer. Cancers 2019, 11, 668. [Google Scholar] [CrossRef]
- Roane, B.M.; Meza-Perez, S.; Katre, A.A.; Goldsberry, W.N.; Randall, T.D.; Norian, L.A.; Birrer, M.J.; Arend, R.C. Neutralization of TGFβ Improves Tumor Immunity and Reduces Tumor Progression in Ovarian Carcinoma. Mol. Cancer Ther. 2021, 20, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 Signaling Promotes Invasion and Migration of CD133+Ovarian Cancer Stem-Like Cells via NF-κB-Mediated MMP-9 Upregulation. STEM CELLS 2012, 30, 2309–2319. [Google Scholar] [CrossRef]
- Pasquier, J.; Gosset, M.; Geyl, C.; Hoarau-Véchot, J.; Chevrot, A.; Pocard, M.; Mirshahi, M.; Lis, R.; Rafii, A.; Touboul, C. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol. Cancer 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Varas-Godoy, M.; Rice, G.; Illanes, S.E. The Crosstalk between Ovarian Cancer Stem Cell Niche and the Tumor Microenvironment. Stem Cells Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Dolznig, H.; Rupp, C.; Puri, C.; Haslinger, C.; Schweifer, N.; Wieser, E.; Kerjaschki, D.; Garin-Chesa, P. Modeling colon adenocarcinomas in vitro a 3D co-culture system induces cancer-relevant pathways upon tumor cell and stromal fibroblast interaction. Am. J. Pathol. 2011, 179, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Cafferty, F.H.; Macnair, A.; Ring, A.; E Langley, R. The role of aspirin in the prevention of ovarian, endometrial and cervical cancers. Women’s Health 2020, 16, 1745506520961710. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande, N.; Borghese, C.; Agostini, F.; Durante, C.; Mazzucato, M.; Colombatti, A.; Aldinucci, D. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. Int. J. Mol. Sci. 2021, 22, 3019. https://doi.org/10.3390/ijms22063019
Casagrande N, Borghese C, Agostini F, Durante C, Mazzucato M, Colombatti A, Aldinucci D. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. International Journal of Molecular Sciences. 2021; 22(6):3019. https://doi.org/10.3390/ijms22063019
Chicago/Turabian StyleCasagrande, Naike, Cinzia Borghese, Francesco Agostini, Cristina Durante, Mario Mazzucato, Alfonso Colombatti, and Donatella Aldinucci. 2021. "In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel" International Journal of Molecular Sciences 22, no. 6: 3019. https://doi.org/10.3390/ijms22063019
APA StyleCasagrande, N., Borghese, C., Agostini, F., Durante, C., Mazzucato, M., Colombatti, A., & Aldinucci, D. (2021). In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. International Journal of Molecular Sciences, 22(6), 3019. https://doi.org/10.3390/ijms22063019