The Effects of Tgfb1 and Csf3 on Chondrogenic Differentiation of iPS Cells in 2D and 3D Culture Environment
Abstract
1. Introduction
2. Results
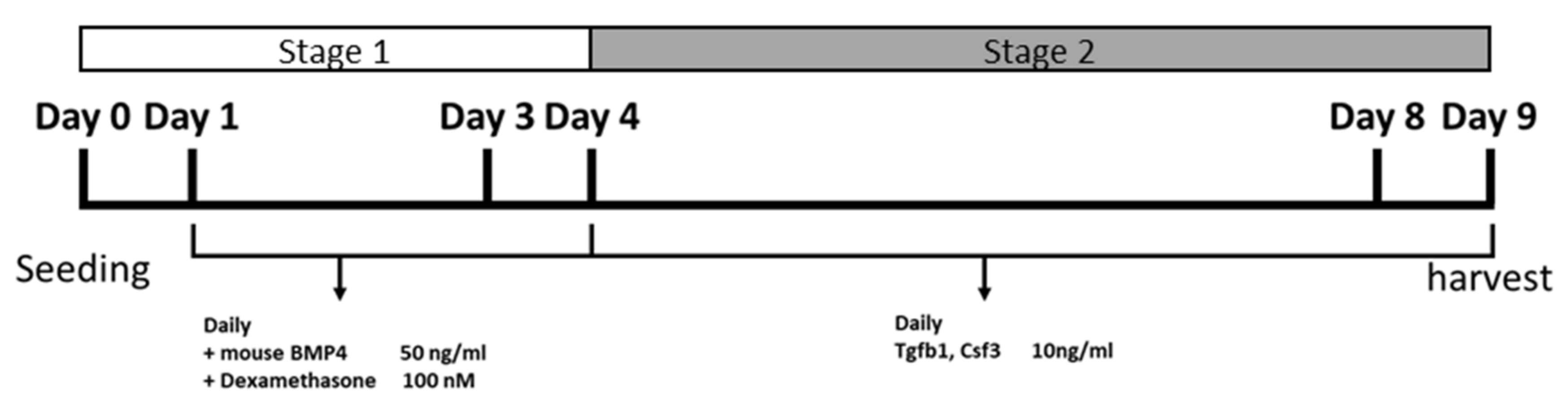
2.1. The Strategy to Induce Chondrogenic Differentiation in iPS Cells
2.2. Morphology of iPS Cultures at Different Stages of Chondrogenic Differentiation
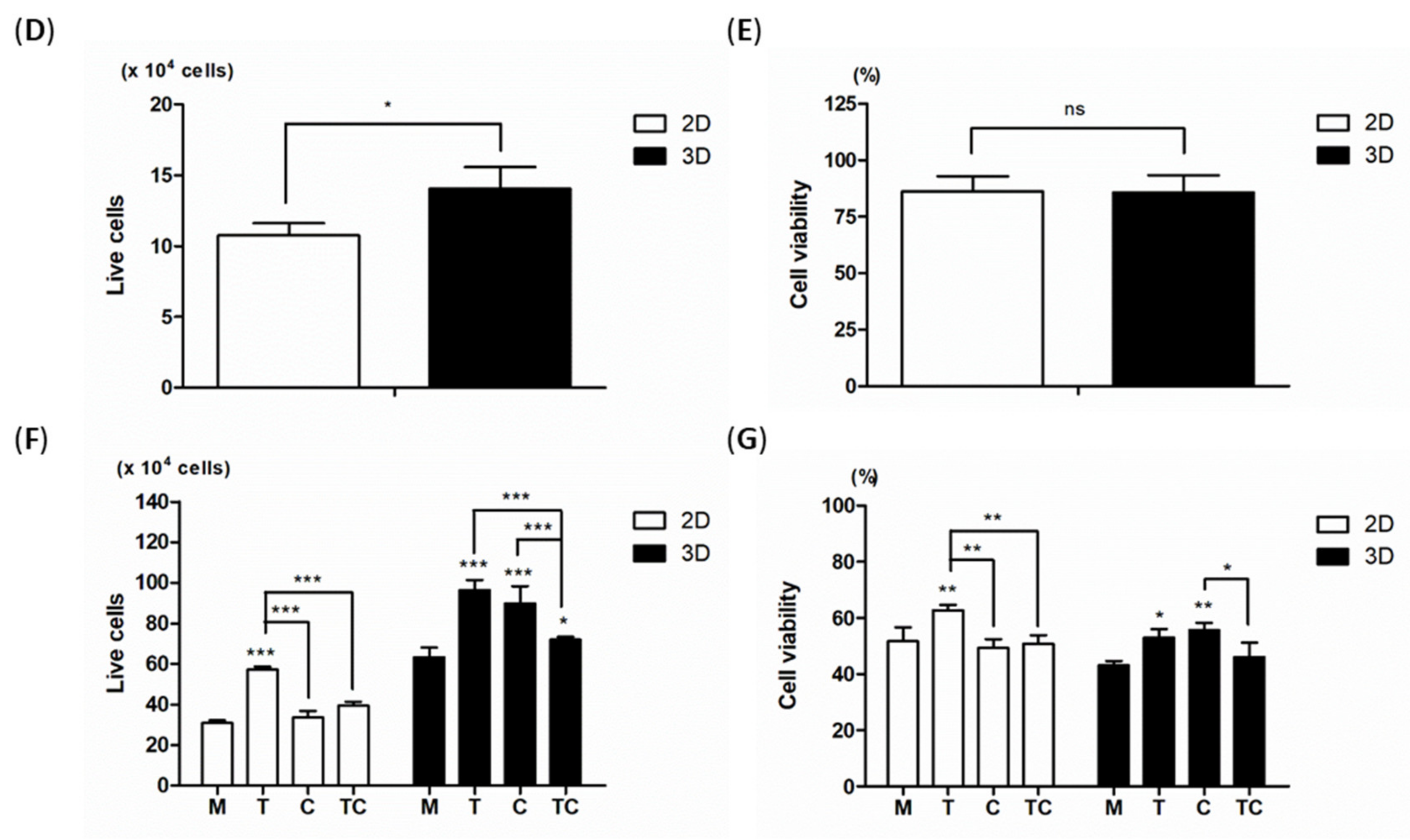
2.3. Proliferation Rates and Viability of Cell in iPS Cultures at Different Stages of the Protocol
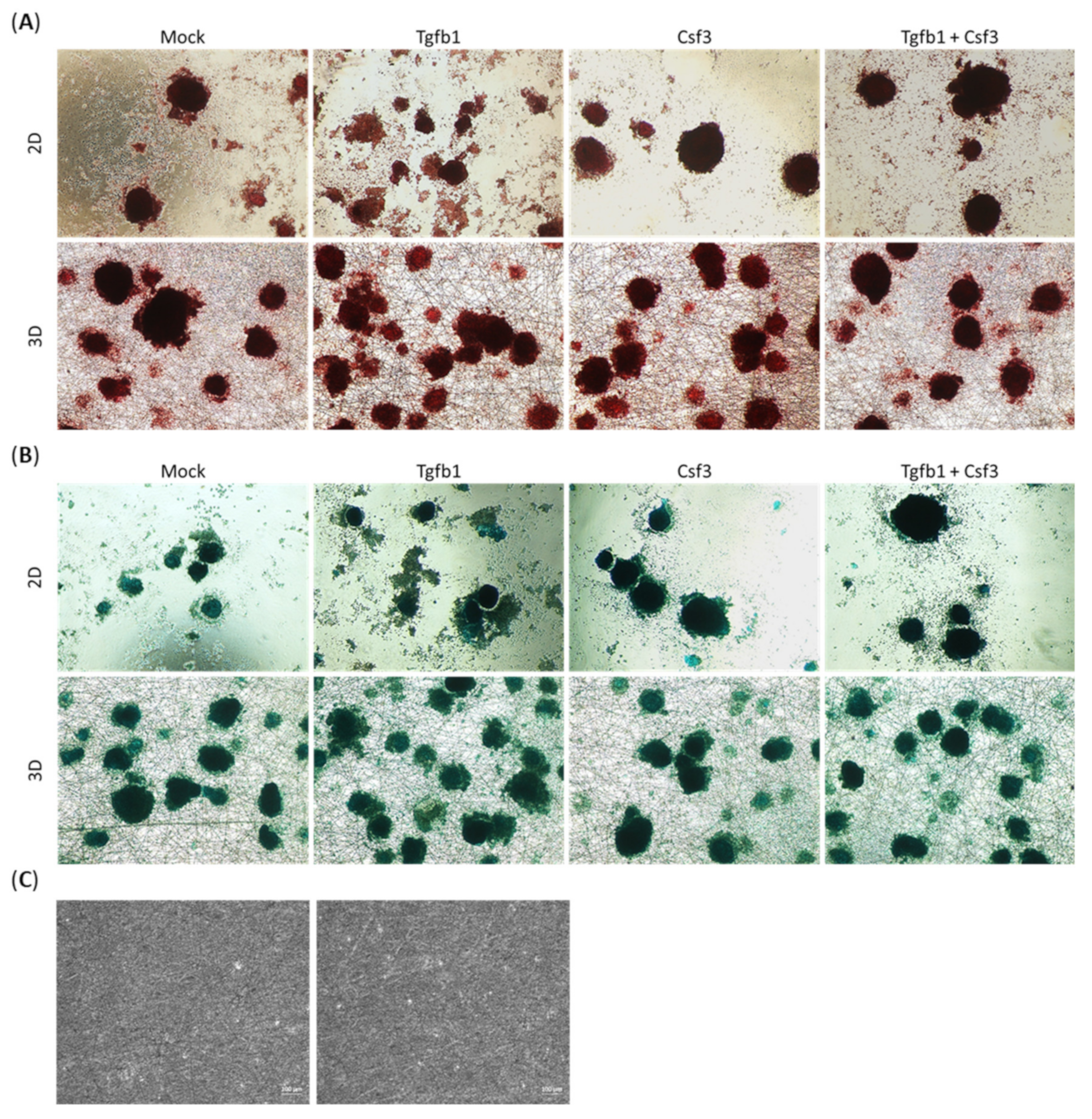
2.4. The Accumulation of Sulfated Glycosaminoglycans
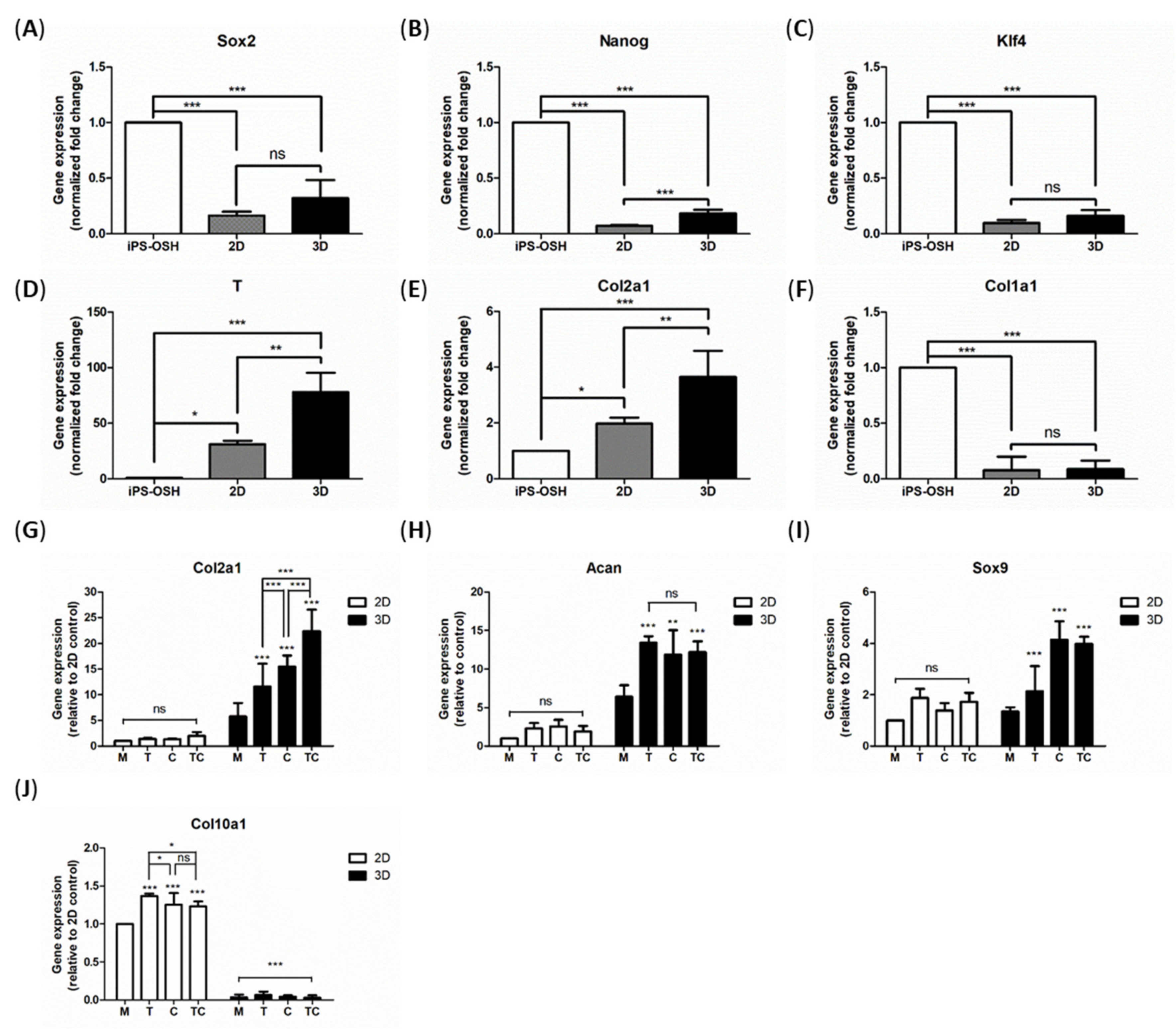
2.5. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Culture of Mouse Embryonic Fibroblast Cells
4.2. Culture of the iPS-OSH Cells
4.3. Differentiation of the iPS-OSH Cells into Chondrocytes
4.4. Cell Proliferation and Cell Viability
4.5. Cell Morphology
4.6. Safranin O and Alcian Blue Staining
4.7. Real-Time PCR
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iPSC | Induced pluripotent stem cell |
| Tgfb1 | transforming growth factor β1 |
| Csf3 | colony stimulating factor 3 (granulocyte) |
| Col1a1 | Collagen Type I Alpha 1 Chain |
| Col2a1 | Collagen Type II Alpha 1 Chain |
| Acan | Aggrecan |
References
- Merceron, C.; Portron, S.; Masson, M.; Lesoeur, J.; Fellah, B.H.; Gauthier, O.; Geffroy, O.; Weiss, P.; Guicheux, J.; Vinatier, C. The effect of two- and three-dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant. 2011, 20, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y. The prevalence and burden of arthritis. Rheumatology 2002, 41 (Suppl. S1), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Roos, E.M. Clinical update: Treating osteoarthritis. Lancet 2007, 370, 2082–2084. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Lamba, D.A.; Gust, J.; Reh, T.A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 2009, 4, 73–79. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Z.J.; Oldenburg, M.; Ayala, M.; Zhang, S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 2008, 26, 55–63. [Google Scholar] [CrossRef]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Markway, B.D.; Weekes, K.J.; McCarthy, H.E.; Johnstone, B. Physioxia Promotes the Articular Chondrocyte-Like Phenotype in Human Chondroprogenitor-Derived Self-Organized Tissue. Tissue Eng. Part A 2018, 24, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Suchorska, W.M.; Augustyniak, E.; Richter, M.; Trzeciak, T. Comparison of Four Protocols to Generate Chondrocyte-Like Cells from Human Induced Pluripotent Stem Cells (hiPSCs). Stem Cell Rev. Rep. 2017, 13, 299–308. [Google Scholar] [CrossRef]
- Yamashita, A.; Nishikawa, S.; Rancourt, D.E. Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. PLoS ONE 2010, 5, e10998. [Google Scholar] [CrossRef]
- Roberts, S.; Menage, J.; Sandell, L.J.; Evans, E.H.; Richardson, J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 2009, 16, 398–404. [Google Scholar] [CrossRef]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Sun, H.; Sanchez-Adams, J.; Leong, K.W.; Guilak, F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef]
- Smeriglio, P.; Lai, J.H.; Yang, F.; Bhutani, N. 3D Hydrogel Scaffolds for Articular Chondrocyte Culture and Cartilage Generation. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Fu, R.H.; Wu, D.C.; Hsu, C.Y.; Chang, C.H.; Lee, W.; Lee, Y.D.; Liu, C.H.; Chien, Y.J.; Lin, S.Z.; et al. Mouse-induced pluripotent stem cells generated under hypoxic conditions in the absence of viral infection and oncogenic factors and used for ischemic stroke therapy. Stem Cells Dev. 2014, 23, 421–433. [Google Scholar] [CrossRef]
- Marmotti, A.; Bonasia, D.E.; Bruzzone, M.; Rossi, R.; Castoldi, F.; Collo, G.; Realmuto, C.; Tarella, C.; Peretti, G.M. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: An in vitro study of the chondrocyte migration and the influence of TGF-beta1 and G-CSF. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1819–1833. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Liu, J.; Nie, H.; Xu, Z.; Niu, X.; Guo, S.; Yin, J.; Guo, F.; Li, G.; Wang, Y.; Zhang, C. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS ONE 2014, 9, e111566. [Google Scholar] [CrossRef]
- Teramura, T.; Onodera, Y.; Mihara, T.; Hosoi, Y.; Hamanishi, C.; Fukuda, K. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell. Reprogram. 2010, 12, 249–261. [Google Scholar] [CrossRef]
- Waese, E.Y.; Stanford, W.L. One-step generation of murine embryonic stem cell-derived mesoderm progenitors and chondrocytes in a serum-free monolayer differentiation system. Stem Cell Res. 2011, 6, 34–49. [Google Scholar] [CrossRef]
- Chua, K.H.; Aminuddin, B.S.; Fuzina, N.H.; Ruszymah, B.H. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur. Cell Mater. 2005, 9, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Sakaguchi, Y.; Shoji, E.; Nishino, T.; Maki, I.; Sakai, H.; Hanaoka, K.; Kakizuka, A.; Sehara-Fujisawa, A. In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells. PLoS ONE 2012, 7, e47078. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, L.L.; Kuo, P.Y.; Chang, J.L.; Wang, Y.J.; Hung, S.C. Apoptosis in chondrogenesis of human mesenchymal stem cells: Effect of serum and medium supplements. Apoptosis 2010, 15, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; van Vlierberghe, S.; van Nieuwenhove, I.; Baudisch, F.; Graulus, G.J.; Benecke, V.; Alberti, K.; Neumann, H.G.; Rychly, J.; Martins, J.C.; et al. Gelatin-Based Hydrogels Promote Chondrogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro. Materials 2014, 7, 1342. [Google Scholar] [CrossRef]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Yang, S.L.; Harnish, E.; Leeuw, T.; Dietz, U.; Batchelder, E.; Wright, P.S.; Peppard, J.; August, P.; Volle-Challier, C.; Bono, F.; et al. Compound screening platform using human induced pluripotent stem cells to identify small molecules that promote chondrogenesis. Protein Cell 2012, 3, 934–942. [Google Scholar] [CrossRef]


| Gene | NCBI Reference Sequence | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Gapdh | NM_008084.3 | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Col1a1 | NM_007742.4 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| Col2a1 | NM_031163.3 | GGGAATGTCCTCTGCGATGAC | GAAGGGGATCTCGGGGTTG |
| Col10a1 | NM_009925.4 | TTCTGCTGCTAATGTTCTTGACC | GGGATGAAGTATTGTGTCTTGGG |
| Acan | NM_007424.2 | CCTGCTACTTCATCGACCCC | AGATGCTGTTGACTCGAACCT |
| T | NM_009309.2 | GCTTCAAGGAGCTAACTAACGAG | CCAGCAAGAAAGAGTACATGGC |
| Sox2 | NM_011443.4 | AGGGCTGGACTGCGAACTG | TTTGCACCCCTCCCAATTC |
| Sox9 | NM_011448.4 | AGTACCCGCATCTGCACAAC | ACGAAGGGTCTCTTCTCGCT |
| Nanog | NM_001289828.1 | GAGCTATAAGCAGGTTAAGACC | TGCTGAGCCCTTCTGAA |
| Klf4 | NM_010637.3 | CCTTTCAGTGCCAGAAGT | ACTACGTGGGATTTAAAAGTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Tsai, C.-H.; Lin, T.-L.; Liu, S.-P. The Effects of Tgfb1 and Csf3 on Chondrogenic Differentiation of iPS Cells in 2D and 3D Culture Environment. Int. J. Mol. Sci. 2021, 22, 2978. https://doi.org/10.3390/ijms22062978
Wang C-H, Tsai C-H, Lin T-L, Liu S-P. The Effects of Tgfb1 and Csf3 on Chondrogenic Differentiation of iPS Cells in 2D and 3D Culture Environment. International Journal of Molecular Sciences. 2021; 22(6):2978. https://doi.org/10.3390/ijms22062978
Chicago/Turabian StyleWang, Chie-Hong, Chun-Hao Tsai, Tsung-Li Lin, and Shih-Ping Liu. 2021. "The Effects of Tgfb1 and Csf3 on Chondrogenic Differentiation of iPS Cells in 2D and 3D Culture Environment" International Journal of Molecular Sciences 22, no. 6: 2978. https://doi.org/10.3390/ijms22062978
APA StyleWang, C.-H., Tsai, C.-H., Lin, T.-L., & Liu, S.-P. (2021). The Effects of Tgfb1 and Csf3 on Chondrogenic Differentiation of iPS Cells in 2D and 3D Culture Environment. International Journal of Molecular Sciences, 22(6), 2978. https://doi.org/10.3390/ijms22062978






