Genetic Mapping and Identification of the Candidate Gene for White Seed Coat in Cucurbita maxima
Abstract
1. Introduction
2. Results
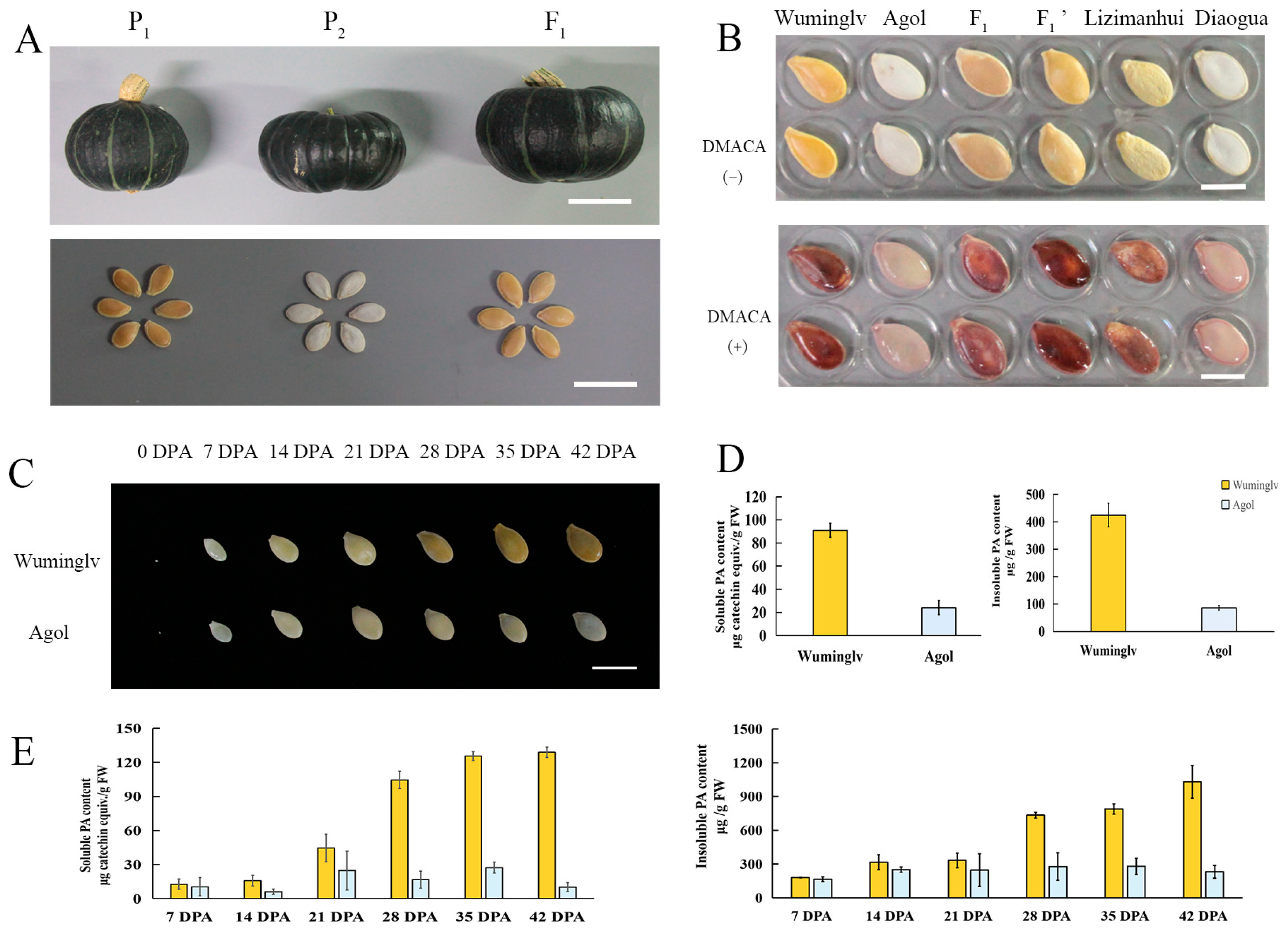
2.1. The Pigment Deposition of Seed Coat in Cucurbita maxima
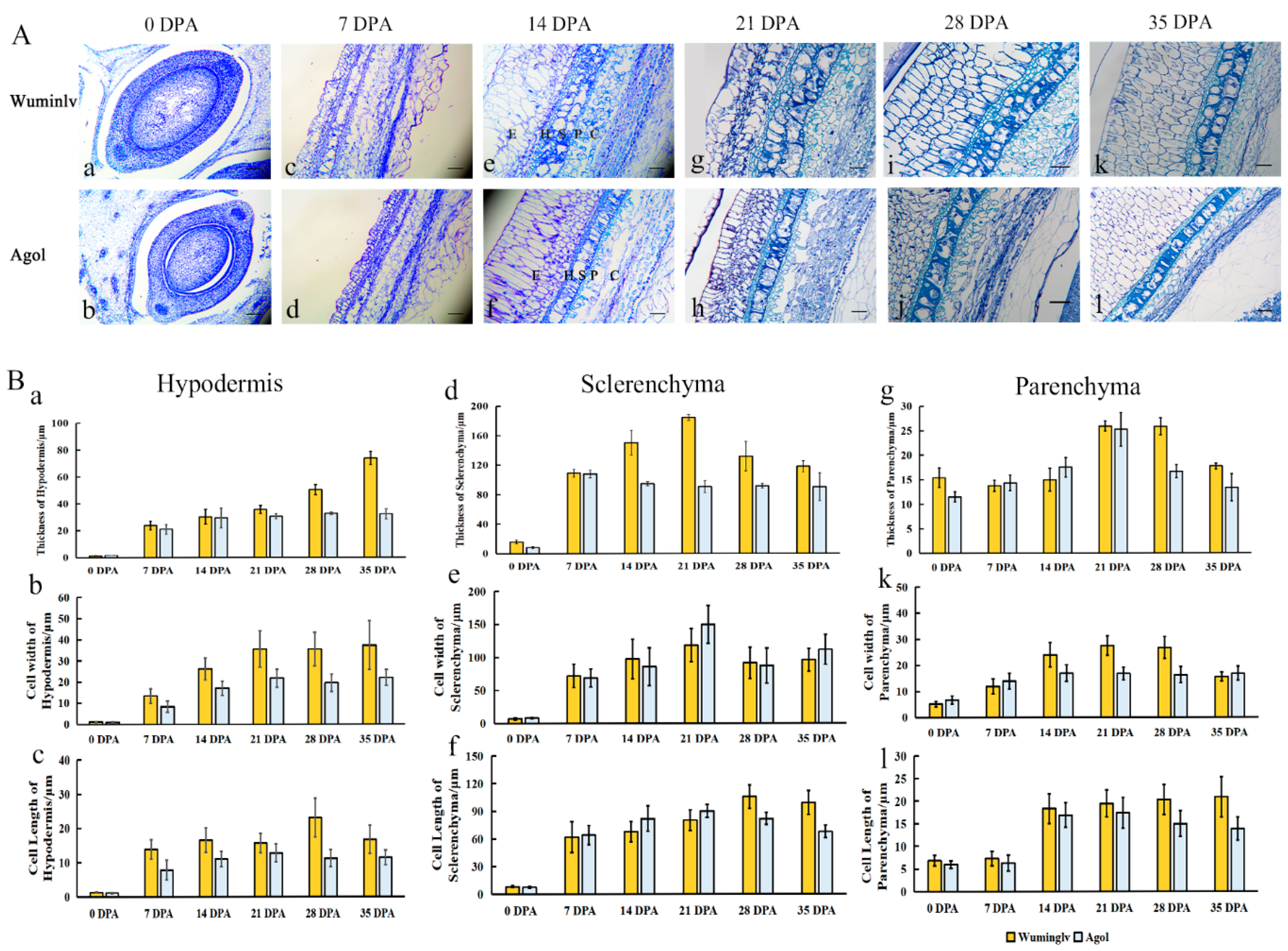
2.2. The Histochemical Analysis of Seed Coat
2.3. Genetic Analysis of Seed Coat Color
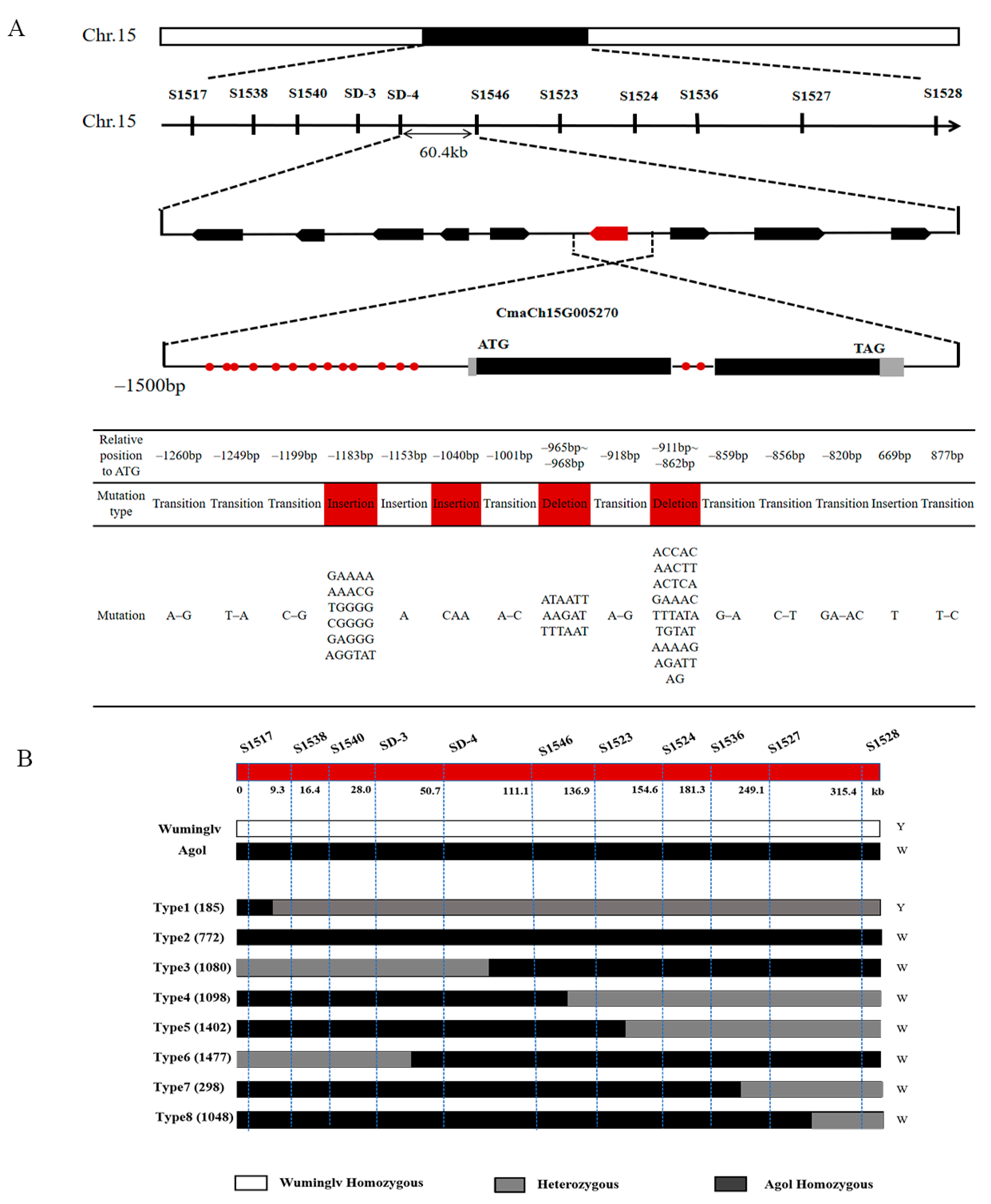
2.4. Map-Based Cloning of White Seed Coat
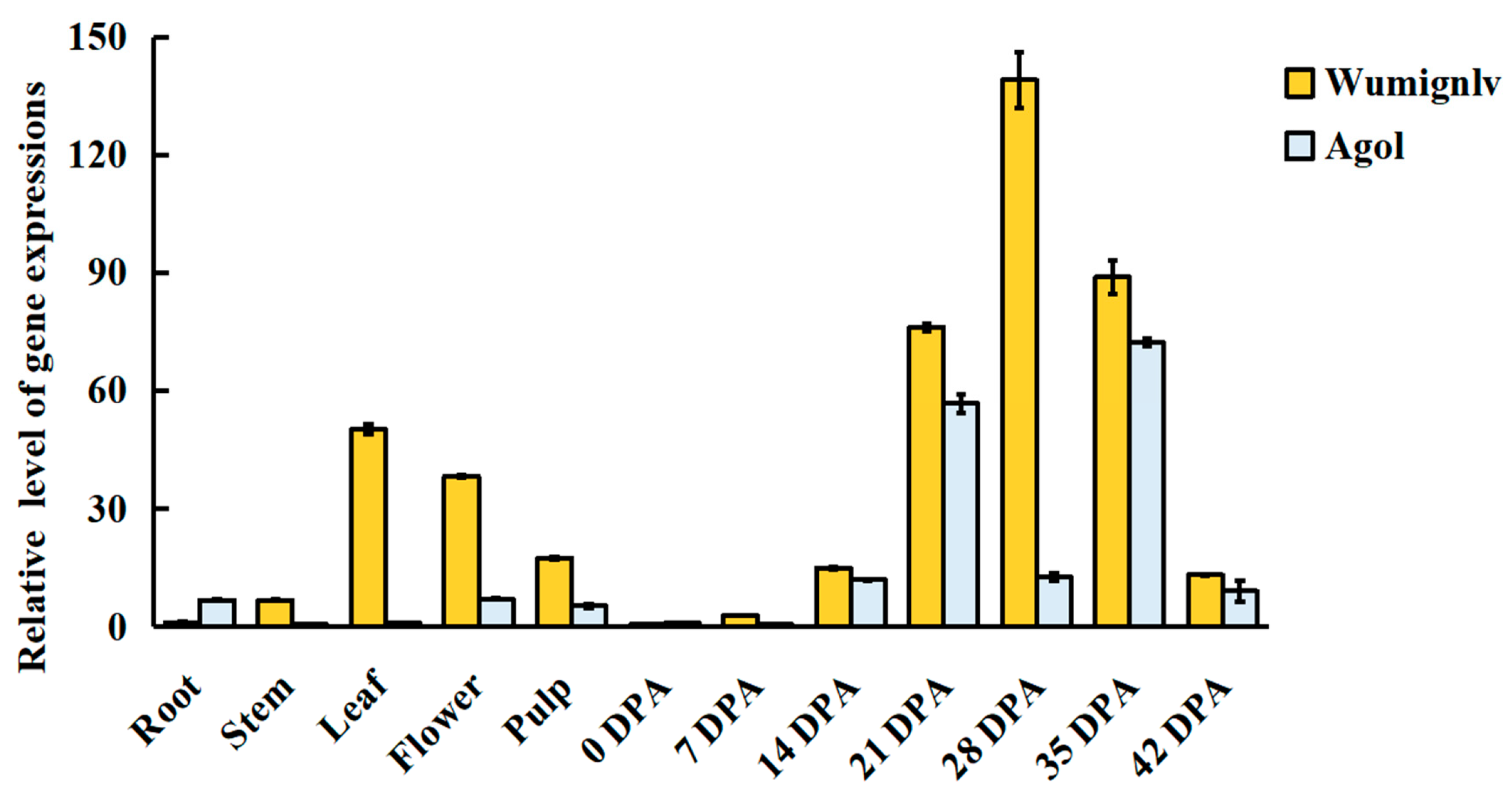
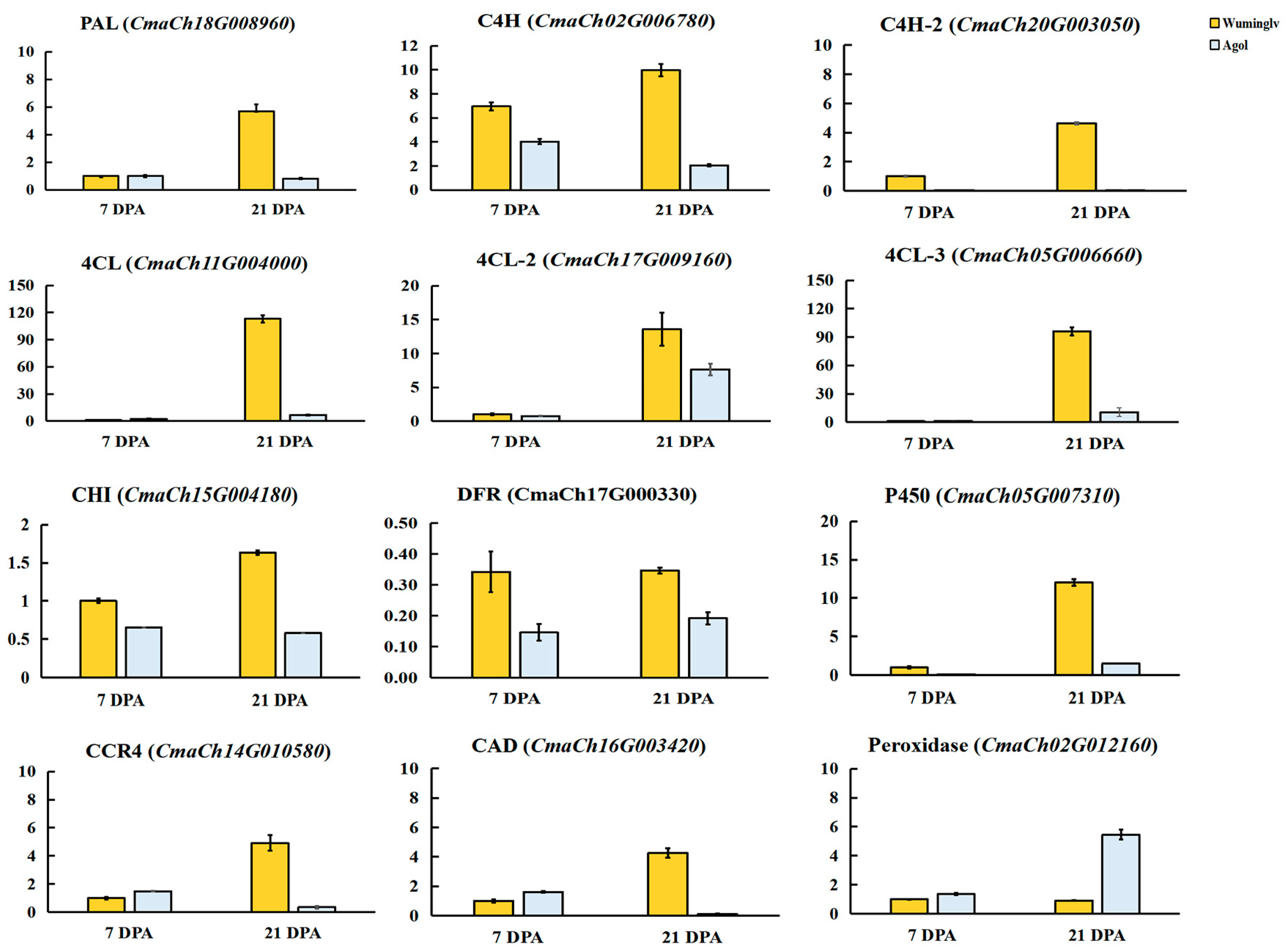
2.5. Candidate Gene and Expression Analysis
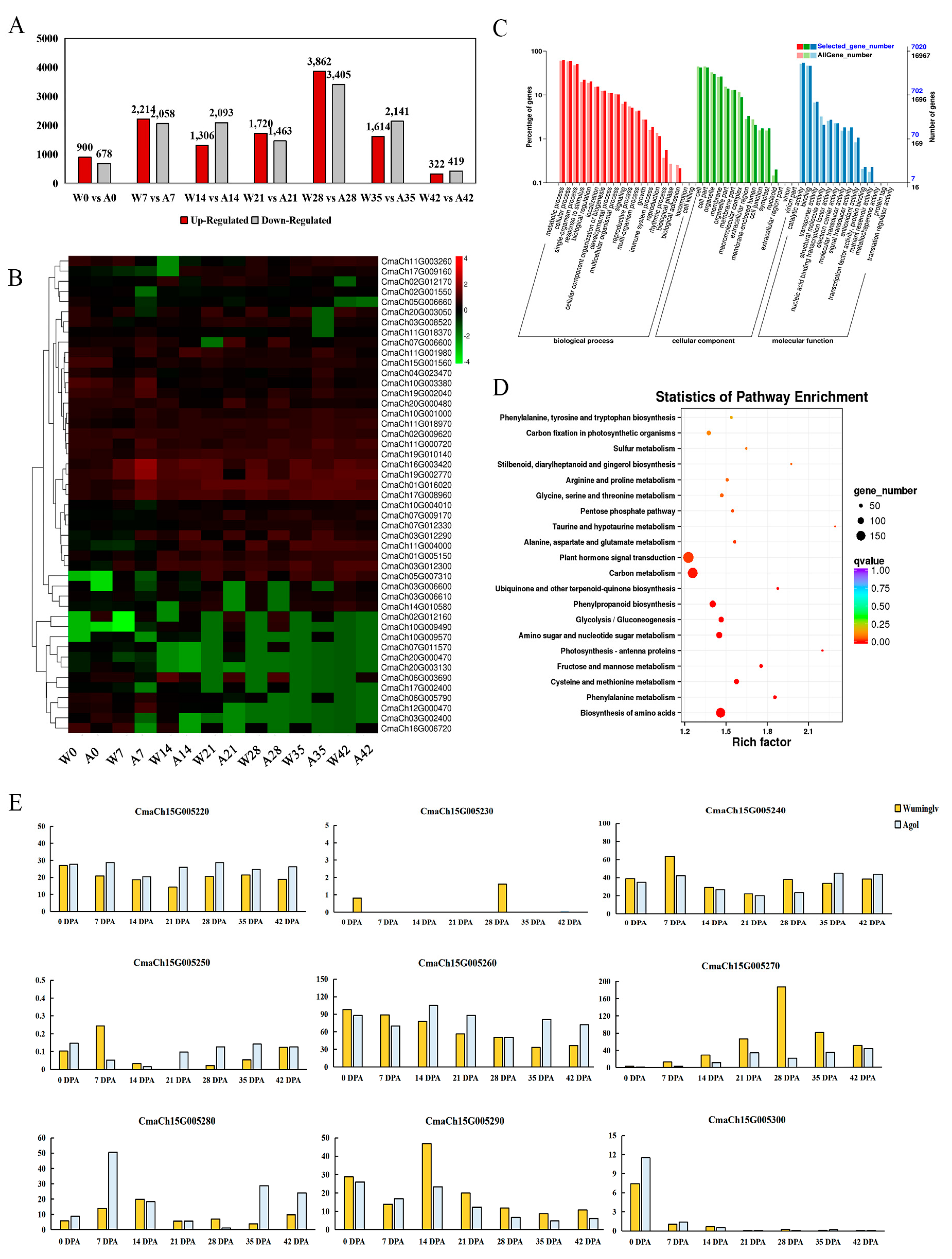
2.6. RNA-Seq Analysis
2.7. Validation of Molecular Marker
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Characterization
4.3. Histochemical Analysis
4.4. DNA and RNA Extraction
4.5. Genetic Analysis
4.6. Map-Based Cloning of wsc Gene
4.7. Candidate Gene Prediction and Validation of Molecular Marker
4.8. RNA-Seq and Spatiotemporal Expression Analysis
4.9. DMACA Staining and PA Determination
4.10. Quantitative Determination for Lignin
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2019, 226, 1240–1255. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.; Satter, M.A. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 2019, 5, e02994. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, A.T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crops Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Gebremeskel, H.; Zhao, S.; He, N.; Yuan, P.; Gong, C.; Mohammed, U.; Liu, W. Genetic mapping and discovery of the candidate gene for black seed coat color in Watermelon (Citrullus lanatus). Front. Plant Sci. 2020, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jun, J.H.; Dixon, R.A. MYB5 and MYB14 play pivotal roles in seed coat polymer biosynthesis in Medicago truncatula. Plant Physiol. 2014, 165, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yu, K.; Cai, S.; Hu, L.; Amoo, O.; Xu, L.; Yang, Y.; Ma, B.; Jiao, Y.; Zhang, C.; et al. Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol. J. 2020, 18, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, M.; Liang, X.; Xu, M.; Liu, X.; Zhang, Y.; Liu, X.; Liu, J.; Gao, Y.; Qu, S.; et al. Quantitative trait loci for seed size variation in Cucurbits—A Review. Front. Plant Sci. 2020, 11, 304. [Google Scholar] [CrossRef]
- Heneidak, S.; Khalik, K. Seed coat diversity in some tribes of Cucurbitaceae: Implications for taxonomy and species iden-tification. Acta Bot. Bras. 2015, 29, 129–142. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.S.; Kim, S.T.; Yoon, W.B.; Han, W.Y.; Kang, I.K.; Choung, M.G. Seed coat color and seed weight contribute differential responses of targeted metabolites in soybean seeds. Food Chem. 2017, 214, 248–258. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Demonsais, L.; Utz-Pugin, A.; Loubéry, S.; Lopez-Molina, L. Identification of tannic cell walls at the outer surface of the endosperm upon Arabidopsis seed coat rupture. Plant J. 2020, 104, 567–580. [Google Scholar] [CrossRef]
- Bezold, T.N.; Loy, J.; Minocha, S.C. Changes in the cellular content of polyamines in different tissues of seed and fruit of a normal and a hull-less seed variety of pumpkin during development. Plant Sci. 2003, 164, 743–752. [Google Scholar] [CrossRef]
- Li, X.; Ge, Y.; Gui, M.; Cui, C.S.; Qu, S.P. Histological analysis of hulled and hull-less squash seed. Coat. Int. J. Agric. Biol. 2013, 15, 1047–1050. [Google Scholar]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef]
- Malenčić, D.; Popović, M.; Miladinović, J. Phenolic content and antioxidant properties of Soybean (Glycine max (L.) Merr.) Seeds. Molecules 2007, 12, 576–581. [Google Scholar] [CrossRef]
- Qu, C.; Fu, F.; Lu, K.; Zhang, K.; Wang, R.; Xu, X.; Wang, M.; Lu, J.; Wan, H.; Zhanglin, T.; et al. Differential accumulation of phenolic compounds and expression of related genes in black- and yellow-seeded Brassica napus. J. Exp. Bot. 2013, 64, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Mansfield, S.D.; Hall, H.C.; Douglas, C.J.; Ellis, B.E. MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant Physiol. 2010, 154, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Peel, G.J.; Sharma, S.B.; Tang, Y.; Dixon, R.A. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc. Natl. Acad. Sci. USA 2008, 105, 14210–14215. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wenger, J.P.; Saathoff, K.; Peel, G.J.; Wen, J.; Huhman, D.; Allen, S.N.; Tang, Y.; Cheng, X.; Tadege, M.; et al. A WD40 repeat protein from Medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant Physiol. 2009, 151, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- DeBolt, S.; Scheible, W.R.; Schrick, K.; Auer, M.; Beisson, F.; Bischoff, V.; Bouvier-Navé, P.; Carroll, A.; Hematy, K.; Li, Y.; et al. Mutations in UDP-Glucose: Sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol. 2009, 151, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Shirley, B.W.; Kubasek, W.L.; Storz, G.; Bruggemann, E.; Koornneef, M.; Ausubel, F.M.; Goodman, H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995, 8, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Zheng, T.; Tan, W.; Yang, H.; Zhang, L.; Li, T.; Liu, B.; Zhang, D.; Lin, H. Regulation of anthocyanin accumulation via MYB75/HAT1/TPL-mediated transcriptional repression. PLoS Genet. 2019, 15, e1007993. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- McKay, J.W. Factor Interaction in Citrullus. Jpn. J. Crop Sci. 1936, 27, 110–112. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Dou, J.; Aslam, A.; Gao, L.; Zhao, S.; He, N.; Liu, W. Construction of a high-density genetic map and mapping of fruit traits in Watermelon (Citrullus Lanatus L.) based on Whole-genome resequencing. Int. J. Mol. Sci. 2018, 19, 3268. [Google Scholar] [CrossRef]
- Paudel, L.; Clevenger, J.; McGregor, C. Chromosomal locations and interactions of four loci associated with seed coat color in Watermelon. Front. Plant Sci. 2019, 10, 788. [Google Scholar] [CrossRef]
- Nath, P.; Khandelwal, R.C. Genetics of seed-coat colour in Citrullus lanatus (Thunb.) Mansf. Theor. Appl. Genet. 1978, 53, 29–31. [Google Scholar] [CrossRef]
- Brown, R.N.; Myers, J.R. A genetic map of Squash (Cucurbita sp.) with randomly amplified polymorphic DNA markers and morphological markers. J. Am. Soc. Hortic. Sci. 2002, 127, 568–575. [Google Scholar] [CrossRef]
- Zraidi, A.; Lelley, T.; Lebeda, A.; Paris, H.S. Genetic map for pumpkin Cucurbita pepo using random amplified polymorphic DNA markers. In Proceedings of the Meeting on Progress in Cucurbit Genetics and Breeding Research Cucurbitaceae, Olomouc, Czech Republic, 12–17 July 2004. [Google Scholar]
- Gong, L.; Stift, G.; Kofler, R.; Pachner, M.; Lelley, T. Microsatellites for the genus Cucurbita and an SSR-based genetic linkage map of Cucurbita pepo L. Theor. Appl. Genet. 2008, 117, 37–48. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, Y.; Sun, H.; Guo, S.; Zhang, F.; Zhang, J.; Zhang, H.; Jia, Z.; Fei, Z.; Xu, Y.; et al. A high-density genetic map for anchoring genome sequences and identifying QTLs associated with dwarf vine in pumpkin (Cucurbita maxima Duch.). BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Montero-Pau, J.; Blanca, J.; Esteras, C.; Martínez-Pérez, E.M.; Gómez, P.; Monforte, A.J.; Cañizares, J.; Picó, B. An SNP-based saturated genetic map and QTL analysis of fruit-related traits in Zucchini using Genotyping-by-sequencing. BMC Genom. 2017, 18, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype stability and unbiased fractionation in the Paleo-Allotetraploid Cucurbita genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Zhou, Y.Y.; Li, J.X.; Yu, T.; Wu, T.Q.; Luo, J.N.; Luo, S.B.; Huang, H.X. A high-density linkage map and QTL mapping of fruit-related traits in pumpkin (Cucurbita moschata Duch.). Sci. Rep. 2017, 7, 12785. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Martí-Gómez, C.; Ferriol, M.; Gómez, P.; Jamilena, M.; Muelleret, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Kim, M.; Jung, J.K.; Shim, E.J.; Chung, S.M.; Park, Y.; Lee, G.P.; Sim, S.C. Genome-wide SNP discovery and core marker sets for assessment of genetic variations in cultivated pumpkin (Cucurbita spp.). Hortic. Res. 2020, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Duan, Y.; Li, H.; Ma, W.; Huang, S.; Sui, X.; Zhang, Z.; Wang, C. A high-density EST-SSR-based genetic map and QTL analysis of dwarf trait in Cucurbita pepo L. Int. J. Mol. Sci. 2018, 19, 3140. [Google Scholar] [CrossRef] [PubMed]
- Kaźmińska, K.; Hallmann, E.; Korzeniewska, A.; Niemirowicz-Szczytt, K.; Bartoszewski, G. Identification of fruit-associated QTLs in Winter Squash (Cucurbita maxima Duchesne) using recombinant inbred lines. Genes 2020, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Fu, Y.; Michael, V.; Meru, G. QTL-seq for identification of loci associated with resistance to Phytophthora crown rot in squash. Sci. Rep. 2020, 10, 5326. [Google Scholar] [CrossRef]
- Sáez, C.; Martínez, C.; Montero-Pau, J.; Esteras, C.; Sifres, A.; Blanca, J.; Ferriol, M.; López, C.; Picó, B. A major QTL located in chromosome 8 of Cucurbita moschata is responsible for resistance to Tomato leaf curl New Delhi virus. Front. Plant Sci. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; LaPlant, K.E.; Mazourek, M.; Gore, M.A.; Smart, C.D. A combined BSA-Seq and linkage mapping approach identifies genomic regions associated with Phytophthora root and crown rot resistance in squash. Theor. Appl. Genet. 2021, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Han, H.; Luo, Y.; Wang, Z.; Yan, C.; Xu, W.; Qu, S. Construction of a high-density genetic map and analysis of seed-related traits using specific length amplified fragment sequencing for Cucurbita maxima. Front. Plant Sci. 2020, 10, 1782. [Google Scholar] [CrossRef]
- Abeynayake, S.W.; Panter, S.; Mouradov, A.; Spangenberg, G. A high-resolution method for the localization of proanthocyanidins in plant tissues. Plant Methods 2011, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, Q.; Mao, H.; Xu, J.; Wang, Y.; Hu, H.; He, S.; Tu, J.; Cheng, C.; Tian, G.; et al. AtDIV2, an R-R-type MYB transcription factor of Arabidopsis, negatively regulates salt stress by modulating ABA signaling. Plant Cell Rep. 2018, 37, 1499–1511. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, S.; Kwon, C.; Yun, H.S. Regulation of cellular VAMP721/722 abundance in Arabidopsis. Plant Signal. Behav. 2019, 14, e1632690. [Google Scholar] [CrossRef]
- Galego, L. Role of DIVARICATA in the control of dorsoventral asymmetry in Antirrhinum flowers. Genes Dev. 2002, 16, 880–891. [Google Scholar] [CrossRef]
- Machemer, K.; Shaiman, O.; Salts, Y.; Shabtai, S.; Sobolev, I.; Belausov, E.; Grotewold, E.; Barg, R. Interplay of MYB factors in differential cell expansion, and consequences for tomato fruit development. Plant J. 2011, 68, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Jeong, S.H.; Kang, Y.M.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, Y.S.; Chang, W.S.; Moon, J.K.; Choung, M.G. Seed maturity differentially mediates metabolic responses in black soybean. Food Chem. 2013, 141, 2052–2059. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Wang, F.; He, J.; Shi, J.; Zheng, T.; Xu, F.; Wu, G.; Liu, R.; Liu, S. Embryonal control of yellow seed coat locus ECY1 is related to Alanine and Phenylalanine metabolism in the seed embryo of Brassica napus. G3 Genes Genomes Genet. 2016, 6, 1073–1081. [Google Scholar] [CrossRef]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Haughn, G.; Chaudhury, A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005, 10, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Loubéry, S.; De Giorgi, J.; Utz-Pugin, A.; Demonsais, L.; Lopez-Molina, L. A maternally deposited endosperm cuticle contributes to the physiological defects of transparent testa seeds. Plant Physiol. 2018, 177, 1218–1233. [Google Scholar] [CrossRef]
- Erfatpour, M.; Navabi, A.; Pauls, K.P. Mapping the non-darkening trait from ‘Wit-rood boontje’ in bean (Phaseolus vulgaris). Theor. Appl. Genet. 2018, 131, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Junk-Knievel, D.C.; Vandenberg, A.; Bett, K.E. Slow darkening in Pinto Bean (Phaseolus vulgaris L.) seed coats is controlled by a single major gene. Crops Sci. 2008, 48, 189–193. [Google Scholar] [CrossRef]
- Perez-Rodriguez, M.; Jaffe, F.W.; Butelli, E.; Glover, B.J.; Martin, C. Development of three different cell types is associated with the activity of a specific MYB transcription factor in the ventral petal of Antirrhinum majus flowers. Development 2005, 132, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Zhou, R.; Li, D.; Liu, A.; Qin, L.; Mmadi, M.A.; Su, R.; Zhang, Y.; Wang, J.; Gao, Y.; et al. A novel motif in the 5′-UTR of an orphan gene ‘Big Root Biomass’ modulates root biomass in sesame. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodríguez-Franco, A.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; Blanco-Portales, R. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria × ananassa fruits. J. Exp. Bot. 2014, 65, 401–417. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Chagné, D.; Carlisle, C.M.; Blond, C.; Volz, R.K.; Whitworth, C.J.; Oraguzie, N.C.; Crowhurst, R.N.; Allan, A.C.; Espley, R.V.; Hellens, R.P.; et al. Mapping a candidate gene (MdMYB10) for red flesh and foliage colour in apple. BMC Genom. 2007, 8, 212. [Google Scholar] [CrossRef]
- Espley, R.V.; Brendolise, C.; Chagné, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P.; et al. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, Y.; Yang, S.; Xu, Y.; Chen, X. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, H.; Lin-Wang, K.; Vimolmangkang, S.; Espley, R.V.; Wang, L.; Allan, A.C.; Han, Y. Transcriptome analysis and transient transformation suggest an ancient duplicated MYB transcription factor as a candidate gene for leaf red coloration in peach. BMC Plant Biol. 2014, 14, 388. [Google Scholar] [CrossRef]
- Tuan, P.A.; Bai, S.; Yaegaki, H.; Tamura, T.; Hihara, S.; Moriguchi, T.; Oda, K. The crucial role of PpMYB10.1 in anthocyanin accumulation in peach and relationships between its allelic type and skin color phenotype. BMC Plant Biol. 2015, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, C.; Waurich, V.; Wagner, H.; Ramos, R.; Oiza, N.; Muñoz, P.; Triviño, J.C.; Caruana, J.; Liu, Z.; Cobo, N.; et al. Allelic variation of MYB10 is the major force controlling natural variation of skin and flesh color in Strawberry (Fragaria spp.) fruit. Plant Cell 2020, 32, 3723–3749. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Fu, Y.; Zhou, Y.; Cheng, M.; Deng, M.; Li, M.; Zhu, T.; Yang, J.; Guo, X.; Gui, L.; et al. Myb10-D confers PHS-3D resistance to pre-harvest sprouting by regulating NCED in ABA biosynthesis pathway of wheat. New Phytol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Liu, C.; Xiao, X.; Dixon, R.A. The transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyandin and anthocyanin pigmentation in Medicago truncatula. Plant Cell 2015, 27, 2860–2879. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Erfatpour, M.; Pauls, K.P. A R2R3-MYB gene-based marker for the non-darkening seed coat trait in pinto and cranberry beans (Phaseolus vulgaris L.) derived from ‘Wit-rood boontje’. Theor. Appl. Genet. 2020, 133, 1977–1994. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in Blood Oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.E.; Garcia-Lor, A.; Licciardello, C.; Licciardello, G.; Hill, L.; Recupero, G.R.; Keremane, M.L.; Ramadugu, C.; Krueger, R.; Xu, Q.; et al. Changes in anthocyanin production during domestication of Citrus. Plant Physiol. 2017, 173, 2225–2242. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Ramadugu, C.; Durand-Hulak, M.; Celant, A.; Reforgiato, R.G.; Froelicher, Y.; Martin, C. Noemi controls production of flavonoid pigments and fruit acidity and illustrates the domestication routes of modern citrus varieties. Curr. Biol. 2019, 29, 158–164.e2. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotté, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; Costa de Oliveira, A.; Caporali, E.; Koroney, A.S. The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- Mizzotti, C.; Ezquer, I.; Paolo, D.; Rueda-Romero, P.; Guerra, R.F.; Battaglia, R.; Rogachev, I.; Aharoni, A.; Kater, M.M.; Caporali, E. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014, 10, e1004856. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R13–R36. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Dyckmans, J.; Flessa, H.; Brinkmann, K.; Mai, C.; Polle, A. Carbon and nitrogen dynamics in acid detergent fibre lignins of beech (Fagus sylvatica L.) during the growth phase. Plant Cell Environ. 2002, 25, 469–478. [Google Scholar] [CrossRef]
| L* | a* | b* | E | |
|---|---|---|---|---|
| P1 (yellow) | 68.89 ± 3.40 | 12.45 ± 6.02 | 25.14 ± 6.68 | 75.32 ± 3.95 |
| P2 (white) | 86.71 ± 6.08 | 0.98 ± 0.96 | 4.44 ± 3.05 | 86.89 ± 5.90 |
| F1 (P1 × P2) (yellow) | 66.13 ± 4.69 | 11.46 ± 2.12 | 19.74 ± 5.41 | 70.82 ± 5.03 |
| F1′ (P1 × P2) (yellow) | 67.03 ± 4.08 | 11.13 ± 2.58 | 19.54 ± 5.04 | 70.83 ± 5.41 |
| F2 (separation) | 59.63~94.71 | 0.04~17.28 | 1.67~30.46 | 64.53~94.80 |
| BC1 (yellow) | 68.71 ± 6.62 | 11.94 ± 4.40 | 21.43 ± 5.24 | 72.41 ± 6.23 |
| BC2 (separation) | 60.89~96.27 | 0.01~15.05 | 0.87~21.83 | 64.17~96.27 |
| P3 (yellow) | 65.35 ± 4.96 | 7.59 ± 3.66 | 19.39 ± 3.46 | 68.89 ± 5.28 |
| P4 (white) | 82.67 ± 3.07 | 3.57 ± 1.58 | 15.13 ± 4.53 | 83.79 ± 2.62 |
| F1 (P3 × P4) (yellow) | 64.66 ± 4.29 | 13.61 ± 2.45 | 21.95 ± 2.59 | 69.58 ± 4.02 |
| F1’ (P3 × P4) (yellow) | 64.28 ± 5.11 | 13.09 ± 2.44 | 23.82 ± 2.42 | 69.48 ± 4.81 |
| Population | Number of Plants | Expected Ratio | Chi Square Test | ||
|---|---|---|---|---|---|
| Yellow Seed Coat | White Seed Coat | Total | |||
| P1 | 5 | 0 | 5 | ||
| P2 | 0 | 6 | 6 | ||
| F1 | 13 | 0 | 13 | ||
| F1’ | 13 | 0 | 13 | ||
| F2 | 282 | 95 | 377 | 3:1 | χc2 = 0.002 < 0.052 = 3.84 |
| BC1 | 109 | 0 | 109 | ||
| BC2 | 65 | 56 | 121 | 1:1 | χc2 = 0.17 < 0.052 = 3.84 |
| Gene ID | Location in Chr.15 | Gene Function Annotation | Homologs in At | Gene Description in TAIR10 |
|---|---|---|---|---|
| CmaCh15G005220 | 2407955~2413296 | Vesicle-associated membrane protein 727 | AT3G54300 | Synaptobrevin-like protein family |
| CmaCh15G005230 | 2413306~2414285 | Unknown protein | – | – |
| CmaCh15G005240 | 2415047~2420995 | AMMECR1 family | AT2G38710 | AMMECR1 family |
| CmaCh15G005250 | 2421828~2424505 | Multiple myeloma tumor-associated protein, putative | AT3G52220 | Leukocyte immunoglobulin-like receptor family protein |
| CmaCh15G005260 | 2424963~2427834 | F1F0-ATPase inhibitor protein | AT5G04750 | F1F0-ATPase inhibitor protein |
| CmaCh15G005270 | 2428976~2431637 | MYB transcription factor | AT5G04760 | R-R-type MYB protein which plays negative roles in salt stress and is required for ABA signaling in Arabidopsis |
| CmaCh15G005280 | 2441111~2445124 | Cationic amino acid transporter 7 | AT5G04770 | Cationic amino acid transporter (CAT) subfamily of amino acid polyamine choline transporters |
| CmaCh15G005290 | 2449424~2462027 | Pentatricopeptide repeat-containing protein | AT5G04780 | Pentatricopeptide repeat (PPR) superfamily protein |
| CmaCh15G005300 | 2463445~2466291 | Double Clp-N motif-containing P-loop nucleoside triphosphate hydrolases superfamily protein, putative | AT3G52490 | SMAX1 and SMAX1-like protein that has weak similarity to AtHSP101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, M.; Shu, Q.; Ma, W.; Sun, T.; Xiang, C.; Wang, C.; Duan, Y. Genetic Mapping and Identification of the Candidate Gene for White Seed Coat in Cucurbita maxima. Int. J. Mol. Sci. 2021, 22, 2972. https://doi.org/10.3390/ijms22062972
Shi Y, Zhang M, Shu Q, Ma W, Sun T, Xiang C, Wang C, Duan Y. Genetic Mapping and Identification of the Candidate Gene for White Seed Coat in Cucurbita maxima. International Journal of Molecular Sciences. 2021; 22(6):2972. https://doi.org/10.3390/ijms22062972
Chicago/Turabian StyleShi, Yuzi, Meng Zhang, Qin Shu, Wei Ma, Tingzhen Sun, Chenggang Xiang, Changlin Wang, and Ying Duan. 2021. "Genetic Mapping and Identification of the Candidate Gene for White Seed Coat in Cucurbita maxima" International Journal of Molecular Sciences 22, no. 6: 2972. https://doi.org/10.3390/ijms22062972
APA StyleShi, Y., Zhang, M., Shu, Q., Ma, W., Sun, T., Xiang, C., Wang, C., & Duan, Y. (2021). Genetic Mapping and Identification of the Candidate Gene for White Seed Coat in Cucurbita maxima. International Journal of Molecular Sciences, 22(6), 2972. https://doi.org/10.3390/ijms22062972







