Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex
Abstract
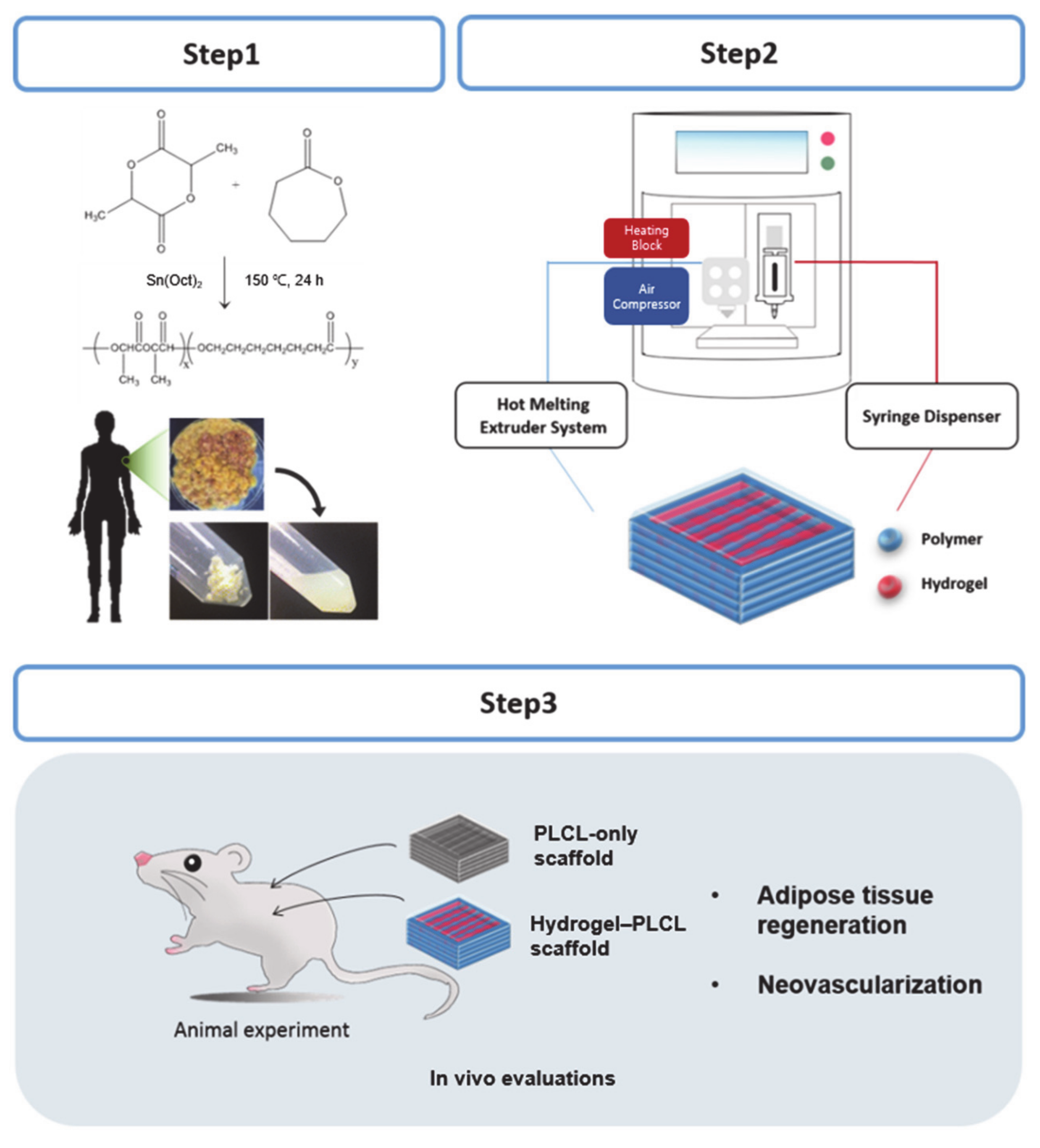
1. Introduction
2. Results and Discussion
2.1. PLCL Characterization
2.2. Characterization of dECM Hydrogel for 3D Priting Ink
2.3. Characterization of the PLCL Scaffolds and the Hydrogel–PLCL Constructs
2.4. In Vivo Studies
2.5. Histological Analysis with Hematoxylin and Eosin (H&E) and Masson’s Trichrome (MT) Staining
2.6. Macrophage Infiltration Analysis
2.7. Analysis of Angiogenesis and Vascularization in the Scaffolds
2.8. Quantitative Analysis by Real-Time Polymerase Chain Reaction
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of PLCL
3.3. Preparation of the Decellularized Extracellular Matrix (dECM)-Based Ink
3.4. Fabrication of the PLCL Scaffolds and the Hydrogel–PLCL Constructs
3.5. Scanning Electron Microscopy (SEM) Analysis
3.6. Mechanical Tests
3.7. In Vivo Experiments
3.8. Evaluation of Adipose Tissue Regeneration with Histological Analysis
3.9. Macrophage and Angiogenesis Assessment of the Constructs
3.10. Adipose Tissue mRNA Expression Analysis by RT-PCR
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benavides, O.M. Amniotic Fluid-Derived Stem Cells as a Source of In Situ Vascularization within Fibrin/Poly (Ethylene Glycol) Hydrogels. Ph.D. Thesis, Rice University, Houston, TX, USA, 2014. [Google Scholar]
- Lequeux, C.; Rodriguez, J.; Boucher, F.; Rouyer, O.; Damour, O.; Mojallal, A.; Auxenfans, C. In vitro and in vivo biocompatibility, bioavailability and tolerance of an injectable vehicle for adipose-derived stem/stromal cells for plastic surgery indications. J. Plast. Reconstr. Aesth. Surg. 2015, 68, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Auclair, E.; Blondeel, P.; Del Vecchio, D.A. Composite breast augmentation: Soft-tissue planning using implants and fat. Plast. Reconstr. Surg. 2013, 132, 558–568. [Google Scholar] [CrossRef]
- Sterodimas, A.; de Faria, J.; Nicaretta, B.; Pitanguy, I. Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J. Plast. Reconstr. Aesth. Surg. 2010, 63, 1886–1892. [Google Scholar] [CrossRef]
- Choi, J.S.; Yang, H.-J.; Kim, B.S.; Kim, J.D.; Lee, S.H.; Lee, E.K.; Park, K.; Cho, Y.W.; Lee, H.Y. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng. Part C Methods 2010, 16, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef]
- Han, T.T.Y.; Toutounji, S.; Amsden, B.G.; Flynn, L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 2015, 72, 125–137. [Google Scholar] [CrossRef]
- Chang, K.-H.; Liao, H.-T.; Chen, J.-P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.R.; Bradley, J.P.; Dickinson, B.; Heller, J.B.; Wasson, K.; O’Hara, C.; Huang, C.; Gabbay, J.; Ghadjar, K.; Miller, T.A. Autologous fat transfer national consensus survey: Trends in techniques for harvest, preparation, and application, and perception of short-and long-term results. Plast. Reconstr. Surg. 2007, 119, 323–331. [Google Scholar] [CrossRef]
- Patrick, C.W., Jr. Adipose tissue engineering: The future of breast and soft tissue reconstruction following tumor resection. Semin. Surg. Oncol. 2000, 19, 302–311. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Klein, A.W.; Rish, D.C. Substances for soft tissue augmentation: Collagen and silicone. J. Dermatol. Surg. Oncol. 1985, 11, 337–339. [Google Scholar] [CrossRef]
- Siggelkow, W.; Klosterhalfen, B.; Klinge, U.; Rath, W.; Faridi, A. Analysis of local complications following explantation of silicone breast implants. Breast 2004, 13, 122–128. [Google Scholar] [CrossRef]
- Patrick, C.W.; Mikos, A.G.; McIntire, L.V. Frontiers in Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Khor, H.L.; Ng, K.W.; Schantz, J.-T.; Phan, T.-T.; Lim, T.C.; Teoh, S.-H.; Hutmacher, D. Poly (ε-caprolactone) films as a potential substrate for tissue engineering an epidermal equivalent. Mater. Sci. Eng. C 2002, 20, 71–75. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nomura, N.; Hanada, S.; Kamakura, S.; Anada, T.; Fuji, T.; Honda, Y.; Masuda, T.; Sasaki, K.; Kokubun, S.; et al. Osteoconductivity of porous titanium having young’s modulus similar to bone and surface modification by OCP. Key Eng. Mater. 2007, 330–332, 951–954. [Google Scholar] [CrossRef]
- Cho, S.-W.; Kim, S.-S.; Rhie, J.W.; Cho, H.M.; Choi, C.Y.; Kim, B.-S. Engineering of volume-stable adipose tissues. Biomaterials 2005, 26, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly (lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.H.; You, H.J.; Kim, S.-H.; Ha Kim, Y.; Min, B.G. Application of an elastic biodegradable poly (L-lactide-co-ε-caprolactone) scaffold for cartilage tissue regeneration. J. Biomater. Sci. Polym. Ed. 2008, 19, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Chung, Y.-I.; Kim, S.H.; Tae, G.; Kim, Y.H.; Rhie, J.W.; Kim, S.-H.; Kim, S.H. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-β1 loaded fibrin–poly (lactide-caprolactone) nanoparticulate complex. Biomaterials 2009, 30, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Kayabolen, A.; Keskin, D.; Aykan, A.; Karslıoglu, Y.; Zor, F.; Tezcaner, A. Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization. Biomed. Mater. 2017, 12, 035007. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Phelps, E.A.; García, A.J. Engineering more than a cell: Vascularization strategies in tissue engineering. Curr. Opin. Biotechnol. 2010, 21, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.; Butler, P.; Seifalian, A.M. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 2005, 26, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, L.; Schiegel, A.K.; Schultze-Mosgau, S.; Wiltfang, J. Different substitute biomaterials as potential scaffolds in tissue engineering. Int. J. Oral Maxillofac. Implants 2006, 21, 225–231. [Google Scholar] [PubMed]
- Cheung, H.K.; Han, T.T.Y.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef]
- Wang, L.; Johnson, J.A.; Zhang, Q.; Beahm, E.K. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013, 9, 8921–8931. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L.; Kraine, M.R.; Simmons, C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achille’s tendon repair in a dog model. J. Biomed. Mater. Res. 1995, 29, 977–985. [Google Scholar] [CrossRef]
- Brown, B.; Lindberg, K.; Reing, J.; Stolz, D.B.; Badylak, S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006, 12, 519–526. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Stewart-Akers, A.M.; Simmons-Byrd, A.; Badylak, S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Jt. Surg. 2007, 89, 621–630. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Poon, C.J.; Cotta, M.V.P.E.; Sinha, S.; Palmer, J.A.; Woods, A.A.; Morrison, W.A.; Abberton, K.M. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013, 9, 5609–5620. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Yook, S.-W.; Jung, H.-D.; Park, C.-H.; Shin, K.-H.; Koh, Y.-H.; Estrin, Y.; Kim, H.-E. Reverse freeze casting: A new method for fabricating highly porous titanium scaffolds with aligned large pores. Acta Biomater. 2012, 8, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Causa, F.; Netti, P.A.; Ambrosio, L. A multi-functional scaffold for tissue regeneration: The need to engineer a tissue analogue. Biomaterials 2007, 28, 5093–5099. [Google Scholar] [CrossRef]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of porous substrates: A review of processes using pore forming agents in the biomaterial field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef]
- Salerno, A.; Di Maio, E.; Iannace, S.; Netti, P. Tailoring the pore structure of PCL scaffolds for tissue engineering prepared via gas foaming of multi-phase blends. J. Porous Mater. 2012, 19, 181–188. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Hu, X.; Yu, W.; You, C.; Hu, H.; Han, C. Promotion of angiogenesis by sustained release of rhGM-CSF from heparinized collagen/chitosan scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 100, 788–798. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Götz, H.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly (L-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef]
- Larrañaga, A.; Sarasua, J.-R. Effect of bioactive glass particles on the thermal degradation behaviour of medical polyesters. Polym. Degrad. Stab. 2013, 98, 751–758. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-based composite scaffold matrices for tissue engineering applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, R.; Garcia-Giralt, N.; Rodriguez, M.; Caceres, E.; Garcia, S.; Gómez Ribelles, J.; Monleon, M.; Monllau, J.C.; Suay, J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. Part A 2008, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Ha, D.-H.; Jang, J.; Han, H.H.; Rhie, J.-W.; Cho, D.-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Kim, B.-S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly (l-lactide-co-ε-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W., III; Ferreira, A. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Durney, A.R.; Anseth, K.S. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006, 12, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, J.C.; Drachenberg, C.B.; Munivenkatappa, R.; Ramos, E.; Nogueira, J.; Sailey, C.; Klassen, D.K.; Haririan, A. Glomerular inflammation in renal allografts biopsies after the first year: Cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation 2010, 90, 1478–1485. [Google Scholar] [CrossRef]
- Tinckam, K.J.; Djurdjev, O.; Magil, A.B. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005, 68, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Expanded applications, shifting paradigms and an improved understanding of host–biomaterial interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-inflammatory CD11c+ CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, Z.; Zhang, Y.; Liu, Q.; Fan, J.; Luo, N.; Dong, X.; Yu, X. Characterization of infiltrating macrophages in high glucose-induced peritoneal fibrosis in rats. Mol. Med. Rep. 2012, 6, S83–S99. [Google Scholar]
- Ng, Y.-Y.; Hou, C.-C.; Wang, W.; Huang, X.R.; Lan, H.Y. Blockade of NFκB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int. 2005, 67, 83–91. [Google Scholar] [CrossRef]
- Nie, J.; Hao, W.; Dou, X.; Wang, X.; Luo, N.; Lan, H.Y.; Yu, X. Effects of Smad7 overexpression on peritoneal inflammation in a rat peritoneal dialysis model. Perit. Dial. Int. 2007, 27, 580–588. [Google Scholar] [CrossRef]
- Wang, W.; Huang, X.R.; Li, A.G.; Liu, F.; Li, J.-H.; Truong, L.D.; Wang, X.J.; Lan, H.Y. Signaling mechanism of TGF-β1 in prevention of renal inflammation: Role of Smad7. J. Am. Soc. Nephrol. 2005, 16, 1371–1383. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef]
- Mokarram, N.; Merchant, A.; Mukhatyar, V.; Patel, G.; Bellamkonda, R.V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 2012, 33, 8793–8801. [Google Scholar] [CrossRef]
- Acker, T.; Beck, H.; Plate, K.H. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and-2 suggests an important role of astrocytes in cerebellar vascularization. Mech. Dev. 2001, 108, 45–57. [Google Scholar] [CrossRef]
- Cremona, O.; Savoia, P.; Marchisio, P.C.; Gabbiani, G.; Chaponnier, C. The alpha 6 and beta 4 integrin subunits are expressed by smooth muscle cells of human small vessels: A new localization in mesenchymal cells. J. Histochem. Cytochem. 1994, 42, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Hyvelin, J.-M.; Rochefort, G.Y.; Lermusiaux, P.; Antier, D.; Awede, B.; Bonnet, P.; Domenech, J.; Eder, V. Undifferentiated mesenchymal stem cells seeded on a vascular prosthesis contribute to the restoration of a physiologic vascular wall. J. Vasc. Surg. 2008, 47, 1313–1321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Zhang, R.; Li, Y.; He, G.; Zhang, D.; Zhang, F. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Mol. Biol. Rep. 2012, 39, 285–293. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.H.; Jung, Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017, 9, 348. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, Y.; Kim, S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 2018, 67, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.-H.; Kim, Y.H.; Kim, S.H. The effects of dynamic and three-dimensional environments on chondrogenic differentiation of bone marrow stromal cells. Biomed. Mater. 2009, 4, 055009. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.E.; Kim, S.H.; Jung, Y. Substance P/dexamethasone-encapsulated PLGA scaffold fabricated using supercritical fluid process for calvarial bone regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3469–3480. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jung, Y.; Kim, S.H. Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng. Part A 2018, 24, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.J.; Collins, J.M.; Gloyn, A.L.; McCarthy, M.I.; Karpe, F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity 2011, 19, 888–892. [Google Scholar] [CrossRef]
- Tan, Q.-W.; Zhang, Y.; Luo, J.-C.; Zhang, D.; Xiong, B.-J.; Yang, J.-Q.; Xie, H.-Q.; Lv, Q. Hydrogel derived from decellularized porcine adipose tissue as a promising biomaterial for soft tissue augmentation. J. Biomed. Mater. Res. Part A 2017, 105, 1756–1764. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, H.S.; Chung, J.J.; Kim, S.H.; Park, J.W.; Lee, K.; Jung, Y. Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. Int. J. Mol. Sci. 2021, 22, 2886. https://doi.org/10.3390/ijms22062886
Lee S, Lee HS, Chung JJ, Kim SH, Park JW, Lee K, Jung Y. Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. International Journal of Molecular Sciences. 2021; 22(6):2886. https://doi.org/10.3390/ijms22062886
Chicago/Turabian StyleLee, Soojin, Hyun Su Lee, Justin J. Chung, Soo Hyun Kim, Jong Woong Park, Kangwon Lee, and Youngmee Jung. 2021. "Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex" International Journal of Molecular Sciences 22, no. 6: 2886. https://doi.org/10.3390/ijms22062886
APA StyleLee, S., Lee, H. S., Chung, J. J., Kim, S. H., Park, J. W., Lee, K., & Jung, Y. (2021). Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. International Journal of Molecular Sciences, 22(6), 2886. https://doi.org/10.3390/ijms22062886






